Abstract
Efforts to conserve bats in the western United States have long been impeded by a lack of information on their winter whereabouts, particularly bats in the genus Myotis. The recent arrival of white-nose syndrome in western North America has increased the urgency to characterize winter roost habitats in this region. We compiled 4,549 winter bat survey records from 2,888 unique structures across 11 western states. Myotis bats were reported from 18.5% of structures with 95% of aggregations composed of ≤10 individuals. Only 11 structures contained ≥100 Myotis individuals and 6 contained ≥500 individuals. Townsend’s big-eared bat (Corynorhinus townsendii) were reported from 38% of structures, with 72% of aggregations composed of ≤10 individuals. Aggregations of ≥100 Townsend’s big-eared bats were observed at 41 different caves or mines across 9 states. We used zero-inflated negative binomial regression to explore biogeographic patterns of winter roost counts. Myotis counts were greater in caves than mines, in more recent years, and in more easterly longitudes, northerly latitudes, higher elevations, and in areas with higher surface temperatures and lower precipitation. Townsend’s big-eared bat counts were greater in caves, during more recent years, and in more westerly longitudes. Karst topography was associated with higher Townsend’s big-eared bat counts but did not appear to influence Myotis counts. We found stable or slightly-increasing trends over time in counts for both Myotis and Townsend’s big-eared bats from 82 hibernacula surveyed ≥5 winters since 1990. Highly-dispersed winter roosting of Myotis in the western USA complicates efforts to monitor population trends and impacts of disease. However, our results reveal opportunities to monitor winter population status of Townsend’s big-eared bats across this region.
Introduction
Effective conservation relies on knowledge of the whereabouts and ecological needs of animals during critical phases of their annual cycle. Most species of bats in temperate areas spend half or more of each year in hibernation, which allows them to avoid seasonal prey shortages, limit mortality due to concealment and inactivity, and reduce somatic degradation [1]. Hence, availability of winter roosts has long been understood to contribute to patterns of bat community diversity [2]. Hibernation has been an evolutionarily successful strategy for temperate-zone bats, allowing them to persist in cold regions with net-positive population growth rates despite low fecundity and other “slow” life history traits relative to other small mammals [1, 3]. The central importance of hibernation to temperate-zone bat biogeography underscores the need to understand bat overwintering ecology [4]. However, our ability to do so is hampered in many regions by a fundamental lack of information about where bat hibernacula occur [4].
A prominent example of this is in the western United States, where the winter roosts of bats are poorly known [5–7]. Bats are ubiquitous in natural areas throughout the western USA, which commonly support multiple species of bats, and their activity patterns and roost habitats during summer have been the topic of extensive research [8, 9]. This discrepancy between the omnipresence of many species of bats during summer and dearth of information on their winter whereabouts throughout the western USA has long puzzled biologists (e.g., [7]). The prominent exception is for Townsend’s big-eared bats (Corynorhinus townsendii; hereafter COTO) which occur in conspicuous aggregations, mostly in caves and mines, during summer and winter [10–13]. Several recent studies have reported on multi-year monitoring efforts of COTO hibernacula in caves in the Pacific Northwest [14–16]. Winter roosts of big brown bats (Eptesicus fuscus) have been documented in rock crevices in Alberta, Canada [17, 18]. However, information on other species of bats during winter in western North America is largely limited to reviews of winter records [19–21] and reports of bat activity [18, 22, 23].
This lack of information about other species, especially from the genus Myotis, has been of increased concern in recent years given the devastating population impacts of white-nose syndrome (hereafter WNS) which affects bats during hibernation. The outbreak of WNS represents a major threat to multiple bat species because it greatly increases mortality rates, particularly among adults, over the generally low rates thought to be typical during hibernation [1]. As of August 2018, WNS has been confirmed in 11 species of bats in North America and, regionally, has devastated populations of several Myotis species [24–26]. The disease was first observed in a cave in New York in 2006 [27] and in subsequent years spread from its presumed point of introduction into adjoining states and Canadian provinces [24, 28, 29]. The disease is caused by the fungus Pseudogymnoascus destructans that thrives in cold, wet conditions typical of caves [30–32]. Accordingly, efforts to document impacts of the disease and detect its causative agent have primarily focused on surveillance of caves and mines [26, 33].
The impacts to bats in eastern North America were relatively easy to discern because locations of hibernacula were fairly well known and hibernating bats aggregated conspicuously within them [4, 34]. Population-level impacts have been quantified by comparing impacted population sizes against reconstructions of pre-disease population sizes [24, 26, 33, 35]. In fact, the outbreak of WNS appears to have precipitated the first regional estimates of population size for some species of bats [13, 24, 26]. Although a comprehensive database of hibernacula counts was lacking, regional estimates in these studies were possible due to similarities in census methods among states and hibernation behavior of bats within their winter roosts. In particular, many of the affected species tended to roost in large aggregations on exposed surfaces in caves and mines, which made them relatively easy to count.
During the decade-long westward encroachment of WNS there were efforts to describe and convey the dispersed wintering habits of western bat species and resulting challenges of conducting disease surveillance under these circumstances [36, 37]. However, as long as WNS stayed in eastern North America, it was difficult to inspire the institutional urgency needed to compile the survey records necessary to generate baseline assessments of population status for hibernating bats across the western USA. This changed in 2016, when a little brown myotis (Myotis lucifugus) with WNS was discovered in western Washington approximately 1300 km west of the nearest previous detection of the fungus or disease [29]. This jump in the distribution of WNS was unprecedented since its introduction into North America as spread of the disease had previously only occurred between adjoining states or provinces. This jump-dispersal event outpaced epidemiological models that forecast expected arrival in the Pacific Northwest (e.g., [38]), triggering land management and conservation organizations across the west to accelerate surveillance and monitoring activities [39–41]. However, given the different set of species that occur in the West, the vast and rugged landscapes to be searched, and presumed differences in overwintering behaviors of bats in western states [21, 23, 42], emergence of WNS in the Pacific Northwest necessitates a reevaluation of disease transmission pathways as well as the methods and strategies used to date to detect the disease, its causative agent, and its population impacts on bats.
As a first step towards addressing these needs we compiled historical and contemporary records of winter roost surveys conducted across the western USA. Our primary question was: What do we know about the winter roost habits of bats in the western USA and how confident are we they have been reliably characterized? Hence the objectives of this paper were to summarize the current state of knowledge of winter hibernation sites in the western USA including: quantity of survey effort, spatial distribution of survey effort, types of structures used by bats, species detected, size of aggregations, temporal trends in winter counts, and distribution of winter counts along elevational and other important biogeographic gradients. We constructed a large dataset for this effort by canvassing state and federal agencies across 11 states for existing data on bat hibernacula and survey effort during winter. We anticipated records of survey effort would vary among states and that most records would report counts or occurrence of bats but not results of surveys in which bats were not detected. We further anticipated that most survey effort would have focused on caves and mines, aggregations of bats would be smaller than those observed in the eastern USA, and the most common species and largest aggregations would be of COTO. We used the resulting dataset to explore biogeographic trends in hibernacula counts along environmental gradients using regression modeling techniques that may be useful to guide future disease surveillance and other bat conservation activities. We also used a subset of the data representing sites where bats had been counted over multiple years to assess evidence of temporal trends in hibernacula counts.
Materials and methods
Data compilation
We requested data on winter survey records for bats from 11 states: Arizona, California, Colorado, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming. Our primary requests were sent to state wildlife management agencies, natural heritage programs, and state bat working group contacts that were part of the larger Western Bat Working Group network (http://wbwg.org). We also targeted data requests to the USDA Forest Service, Bureau of Land Management, National Park Service, and individual biologists following recommendations from primary requestees or when the number of records received following our primary request was lower than expected. We focused on detections of species in the genus Myotis due to their demonstrated susceptibility to WNS and also on detections of COTO, primarily because this species has been the focus of winter surveys across the western USA [43, 44]. We provided a template spreadsheet that prompted respondents to report structure name, structure type (e.g., cave, mine, building), survey date, survey location, bat presence/absence, count of COTO, count of Myotis, count of species other than COTO or Myotis, total count of bats observed during a survey, and any comments about the survey. Counts or presence of other species were sometimes included, often as comments.
We considered any survey conducted during the months of November through March to be a winter survey. We assigned each survey to the year in which the winter period ended. Structure type and geographic coordinates were sometimes not reported, but we retained these observations and compiled remaining information. We designated each observation as either a count or presence-only survey. A survey was designated as presence-only when it was originally reported as such, based on interpretations of the comments accompanying the survey, or, conservatively, when it was not possible to tell whether a value of 1 for a particular species group represented a true count or was simply a designation of presence. Records that reported zero bats observed were considered counts. When an observation reported presence of multiple species of bats but only a total count for the number of bats, we split the observation into 2 records: a count record that reflected total number of bats observed and a presence record indicating which species were present. We pooled reports of any species of Myotis, confident that most observers would be able to correctly identify bats to the genus-level but not consistently to species, many of which are cryptic even in the hand [45–47].
Spatial resolution of the locations provided for individual structures varied among and sometimes within states. Precise coordinates for individual structures were the most common resolution provided. Some jurisdictions were concerned about providing precise coordinates; for example, some national parks only provided locations for an area within the park where structures occurred. In these cases, a single set of coordinates, representing the centroid of the surveyed area, was used for multiple structures in our compiled database. The Federal Cave Resources Protection Act of 1988 and other federal and state regulations prevent public disclosure of exact cave locations (see [48] for a review).
Data subsets
We explored biogeographic (spatial) patterns of hibernacula counts along broad environmental gradients with a subset of data consisting of a single representative survey per structure. We only considered surveys conducted in caves and mines from years 1990–2017, as older data and data from other structure types were sparse, and had inconsistent locational and other metadata. We generally selected the most recent survey where bats were detected to represent the count for each structure in the spatial analysis. However, if the most recent survey in which bats were detected was earlier than 2006, we used the most recent non-detection survey to represent the structure. We allowed the individual survey that represented the structure to differ according to whether the analysis was for COTO or Myotis. For example, if no Myotis were observed during the most recent survey of a structure in which ≥1 COTO was counted, yet ≥1 Myotis were counted during another survey after 2006, the most recent survey was selected to represent the structure for COTO and the earlier survey represented the structure for Myotis. In 98% of cases, results from the same survey represented the structure for both species. We explored temporal trends in hibernacula counts over time with a different subset of data consisting of replicate counts spanning ≥5 winters conducted in any structure type (e.g., buildings in addition to caves and mines) since 1990. When ≥1 count per winter was conducted, we selected the largest count for inclusion in trend analysis.
Statistical analyses
We explored spatial and temporal trends in the data subsets for Myotis and COTO with zero-inflated negative binomial regression [49]. In our dataset, most counts were recorded at or near zero but some large counts were also reported that extended the distributional tail far to the right. The negative binomial distribution (a link function) provided a more suitable latent model for the over-dispersed (right-skewed) distribution of counts than alternatives including the more familiar Poisson [49]. However, the number of zeros in analyzed subsets were much greater than could be explained with the negative binomial distribution (zero inflation), regardless of the distributional dispersion parameter assumed. Hence we used zero-inflated negative binomial regression, which models zeros as a mixture of binomially-distributed probabilities via a logit link function and negative-binomial counts, some of which could also be zero [49, 50]. We assumed excess zeros in the dataset to be both structural, resulting from our incomplete understanding of winter hibernacula habitat use (i.e., some caves and mines were not used by bats for reasons we do not perceive), and from random variation (i.e., zeros recorded when a structure is suitable but not used), as well as due to imperfect detection. There was not enough information in the dataset to enable formal estimation of detectability (e.g., too few structures with repeat visits within winters).
Our biogeographic (spatial trend) models included covariates for survey year, structure elevation (elevation), mean annual temperature (temperature), mean annual precipitation (precipitation), karst topography (karst), and whether the structure was a cave or mine (mine). Karst was constructed as a binary indicator variable and year, elevation, temperature, and precipitation were centered at zero and standardized to improve computation and interpretation [51, 52]. Count coefficients, when exponentiated, are interpreted as the estimated multiplicative (percent) change in mean count. In our models, with survey data beginning in 1990, the average winter survey occurred in 2008 with a standard deviation of 7 years, and model parameters should be interpreted accordingly (e.g., model intercept is the mean count in 2008 at average elevation, precipitation, temperature, and on non-karst). We constructed and fit zero-inflated negative binomial regression models using the pscl package [53] in the R statistical environment [54].
We extended our negative binomial regression models with a random effect (separate intercepts and slopes) for individual structures to account for repeated counts at individual structures over time (and presumably not independent). We only included year as a covariate, focusing on the conservation concern of potential decline in counts over time across the collection of structures within our dataset that had ≥5 replicate counts. We fit these zero-inflated negative binomial mixed-effects models using the R package glmmADMB [55].
Biogeographic model covariates
We used a GIS to extract 30-year mean temperature and precipitation, elevation, and karst topography for each cave and mine location used in spatial trend models. Gridded climate data were the PRISM Climate Group’s 30-year normals for the period 1981–2010 [56]. Elevation data were from the U.S. Geological Survey National Elevation Dataset (now the National Map) at 30-m resolution (1 arc-second; https://nationalmap.gov/elevation.html). Karst and pseudo-karst (including volcanics) topography data were derived from Weary and Doctor [57]. For Colorado records, we instead used a detailed karst map that was used by the Colorado Bat Conservation Plan [58].
Conservation risk assessment
To assess protection status of hibernacula, we overlaid hibernacula locations with the Protected Areas Database of the United States (PAD-US; [59]). The inventory provides 4 ranks according to conservation and access for public recreation as follows: 1) managed for biodiversity–disturbance events proceed or are mimicked; 2) managed for biodiversity–disturbance events suppressed; 3) managed for multiple uses–subject to extractive (e.g., mining or logging) or off-highway-vehicle use; 4) no known mandate for protection. Some locations were not ranked so we assigned these to the 4th rank. We also overlaid hibernacula locations with the gravitycaves+ winter maximum likelihood predictive model of WNS spread produced by Maher et al. [38] to assess the pattern of Myotis hibernacula size in relation to WNS arrival risk.
Results
We compiled results of 4,549 winter surveys for bats from 2,888 unique structures across 11 states that were reported between the winters of 1916 and 2017 (Table 1). Across all states we categorized 78% of surveys as counts and 22% as presence surveys. Among the surveyed structures for which type was reported, 97% occurred at either caves (39%) or mines (58%); however the relative composition varied among states (Table 1). Mines represented >90% of structures surveyed in Colorado, Nevada and Utah whereas caves represented >83% of structures surveyed in Arizona, Idaho, and New Mexico. Bridges (n = 51, 41 of which were in Oregon) were the only other structure type that represented >1% of the total structures surveyed.
Table 1. Winter survey effort for bats from 11 states.
Number of caves and mines represent minimum estimates of the number of unique structures reported in databases. Number of surveys is greater than sum of unique structures surveyed because some sites were surveyed multiple times.
| State | Year of Earliest Record | Year of Latest Record | Unique Number of Years | 90% Survey Effort (yrs) | No. of Surveys | No. of Counts | No. of Presence Surveys | No. of Caves | No. of Mines | Other Structure Types | Unknown Structure Type | No. of Unique Structures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZ | 2006 | 2014 | 6 | 2008–2014 | 101 | 97 | 4 | 83 | 16 | 0 | 0 | 99 |
| CA | 1938 | 2017 | 43 | 1994–2017 | 1,288 | 988 | 300 | 350 | 188 | 10 | 23 | 571 |
| CO | 1958 | 2016 | 28 | 2000–2016 | 627 | 627 | 0 | 24 | 538 | 0 | 0 | 562 |
| ID | 1932 | 2016 | 29 | 1996–2016 | 297 | 268 | 29 | 174 | 20 | 1 | 0 | 195 |
| MT | 1938 | 2015 | 27 | 1970–2015 | 104 | 97 | 7 | 41 | 26 | 1 | 0 | 68 |
| NM | 1967 | 2016 | 34 | 2002–2016 | 352 | 352 | 0 | 189 | 23 | 0 | 0 | 212 |
| NV | 1966 | 2017 | 11 | 2010–2017 | 412 | 181 | 231 | 21 | 292 | 3 | 0 | 316 |
| OR | 1916 | 2016 | 53 | 1986–2016 | 468 | 406 | 62 | 94 | 29 | 47 | 54 | 224 |
| UT | 1992 | 2017 | 22 | 2000–2017 | 390 | 95 | 295 | 7 | 340 | 0 | 0 | 347 |
| WA | 1938 | 2016 | 48 | 1978–2016 | 251 | 193 | 58 | 63 | 52 | 16 | 14 | 145 |
| WY | 1994 | 2016 | 18 | 1995–2016 | 259 | 258 | 1 | 38 | 103 | 8 | 0 | 149 |
| TOTALS | 1916 | 2017 | 73 | 1994–2017 | 4,549 | 3,561 | 988 | 1,084 | 1,627 | 86 | 91 | 2,888 |
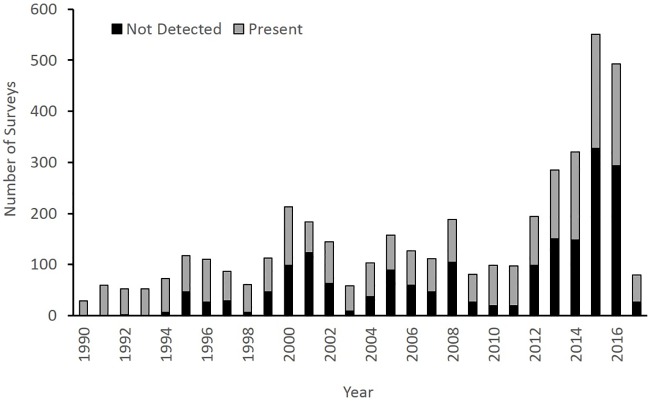
Over 95% of recorded surveys occurred since 1990, reflecting an apparent increase in survey effort and improved record retention. Almost half (43%) of all surveys were compiled after 2011 (Fig 1). Prior to 1990, surveys in which bats were not detected represented only 2 of 217 records. Since 1990 the proportion of records that also recorded non-detection of bats rose steadily with non-detection surveys representing 16% of surveys from 1990–1996, 44% of surveys from 1997–2003 and 2004–2010, and 53% of surveys from 2011–2017.
Fig 1. Number of winter surveys per year and proportion that reported bat presence since 1990 across 11 states.
Note that most states only provided data compilations through 2016 so compiled data 2017 was incomplete.
Overall, Myotis were reported from 19% and COTO was found in 38% of structures searched across 11 states. The proportion of surveyed structures where Myotis were detected varied from 4% in Utah to 44% in Montana. The proportion of surveyed structures where COTO was reported ranged from 22% in Utah to 68% in Washington. In 61% of the structures in which Myotis were found, COTO was also found ranging from 39% of 139 structures in Colorado to 94% of the 62 Myotis hibernacula in Nevada. Across all states, Myotis were found in 19% of 1084 caves and 18% of 1627 mines (Table 2). COTO was detected in 44% of caves and 34% of mines across all states.
Table 2. Proportion of caves and mines occupied by Myotis spp. and Corynorhinus townsendii (COTO) and largest winter count recorded, by structure type, across 11 states.
| Caves | Mines | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| State | Number of Sites | % with Myotis | % with COTO | Largest Myotis Count |
Largest COTO Count | Number | % with Myotis | % with COTO | Largest Myotis Count |
Largest COTO Count |
| AZ | 83 | 9.6 | 31.3 | 500 | 200 | 16 | 18.8 | 25.0 | 1 | 27 |
| CA | 350 | 14.3 | 40.0 | 22 | 699 | 188 | 13.3 | 66.5 | 8 | 80 |
| CO | 24 | 25.0 | 66.7 | 23 | 605 | 538 | 24.7 | 30.7 | 13 | 190 |
| ID | 174 | 17.8 | 40.2 | 804 | 1,932 | 20 | 40.0 | 80.0 | 4 | 83 |
| MT | 41 | 43.9 | 39.0 | 1,738 | 20 | 26 | 46.2 | 73.1 | 14 | 36 |
| NM | 189 | 11.6 | 24.3 | 4,962 | 1,468 | 23 | 13.0 | 13.0 | 5 | 24 |
| NV | 21 | 38.1 | 38.1 | 4 | 24 | 292 | 18.5 | 24.3 | 34 | 455 |
| OR | 94 | 38.3 | 75.5 | 79 | 356 | 29 | 6.9 | 86.2 | 2 | 28 |
| UT | 7 | 42.9 | 85.7 | 3 | 385 | 340 | 2.9 | 20.6 | 6 | 79 |
| WA | 63 | 20.6 | 92.1 | 12 | 300 | 52 | 11.5 | 50.0 | 6 | 50 |
| WY | 38 | 36.8 | 44.7 | 67 | 47 | 103 | 34.0 | 33.0 | 33 | 54 |
| TOTALS | 1,084 | 19.3 | 43.6 | 4,962 | 1,932 | 1,627 | 17.8 | 34.3 | 34 | 455 |
Bats other than COTO and Myotis were reported from 267 surveys (6%), 248 of which were counts. The most frequently reported “other” species was big brown bat. Single individuals represented 61% of the nonzero counts of other species, 88% were counts of ≤3 individuals and 93% reported groups of ≤5 individuals.
Myotis group sizes
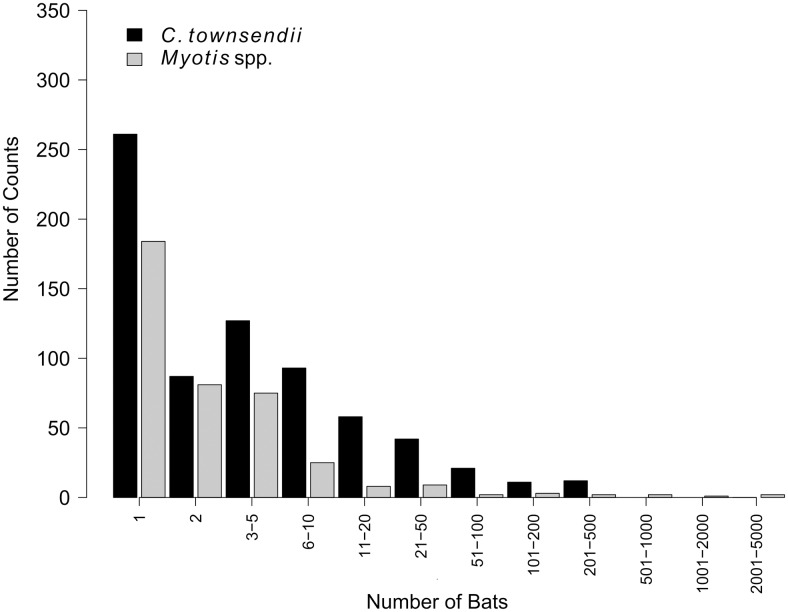
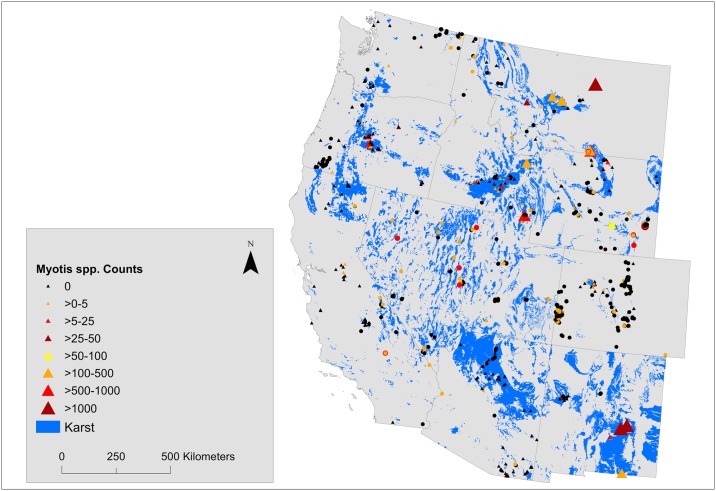
Median nonzero Myotis group size was 2 individuals across all states, ranging from 1 individual in 3 states to 5 individuals in New Mexico (S1 Fig). Single bats accounted for 45% of nonzero counts of Myotis in caves, 86% of observations were of aggregations of ≤5 individuals, and 95% were of ≤10 individuals. Large aggregations of Myotis (>500 individuals) were reported from caves in 4 widely separated states (Arizona, Idaho, Montana, New Mexico; Figs 2 and 3). In all states except Nevada, the largest winter aggregations of Myotis occurred in caves rather than mines. Idaho reported aggregations of 804, 146, 52, 25, and 24 Myotis from 5 separate caves but the remaining 35 structures each contained ≤10 individuals. Montana reported maximum counts of ≥200 wintering Myotis from 5 separate caves. New Mexico reported Myotis hibernacula in 3 caves containing 337–4,962 individuals, while the remaining 22 structures surveyed in New Mexico had maximum Myotis aggregations of ≤18 individuals, 20 of which had ≤10 individuals. In the remaining 7 states, the largest aggregation of Myotis was 79 individuals in Oregon (Table 2).
Fig 2. Frequency of non-zero counts of Corynorhinus townsendii (COTO) and Myotis spp. reported from hibernacula across 11 western states since 1990.
A single, usually the most recent, count was selected to represent each structure.
Fig 3. Hibernacula counts of Myotis spp. in 11 states overlain on maps of karst topography.
Caves are depicted as triangles and mines as circles.
Single bats represented 51% of nonzero counts of Myotis in mines. Of these 94% of counts were of aggregations of ≤5 individuals, and 97% were of ≤10 individuals. The largest aggregations of Myotis in mines were of 34 individuals in Nevada and 33 individuals in Wyoming. Nevada reported 6 of the 11 mines in which ≥10 Myotis were found wintering.
Corynorhinus townsendii group sizes
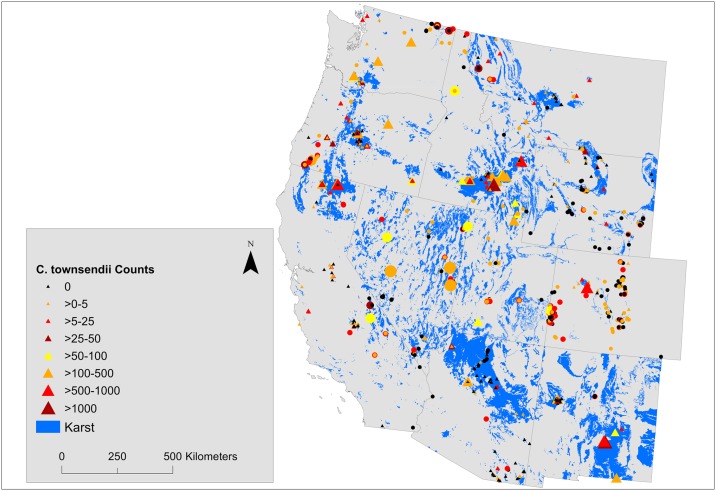
Median nonzero COTO group size was 3 individuals across all states ranging from 1 individual in Colorado to 6 individuals in Idaho (S1 Fig). COTO aggregations in caves were generally greater than in mines. Counts of >500 COTO were reported from 6 caves in 4 different states, the largest of which was 1,932 individuals from a cave in Idaho (Table 2, S1 Fig). Aggregations of ≥100 COTO were observed from 41 different caves across 9 states (Figs 2 and 4). Nevertheless most counts were small. Single bats represented 28% of nonzero counts of COTO in caves, 56% of observations were of aggregations of ≤5 individuals, and 72% were of ≤10 individuals. States with the smallest maximum group sizes of COTO were Montana (36 individuals) and Wyoming (54 individuals), both of which were in mines. COTO were found in aggregations up to 455 individuals during surveys of mines. Aggregations of ≥100 COTO were observed in 6 mines, 5 of which were in Nevada. Single bats accounted for 38% of nonzero COTO counts in mines, 71% of counts were of aggregations of ≤5 individuals, and 83% were of ≤10 individuals.
Fig 4. Observed hibernacula counts of Corynorhinus townsendii in 11 states overlain on maps of karst topography.
Caves are depicted as triangles and mines as circles.
Biogeographic model results
Our regression models estimated that mean counts of Myotis were greater in caves than mines, in more recent years, and in more easterly longitudes, northerly latitudes, higher elevations, and in areas with higher mean annual surface temperatures and lower precipitation (Table 3). Mean counts of COTO were also estimated to be greater in more recent years, in cave locations within karst habitat, and in more westerly longitudes (Table 3).
Table 3. Regression model coefficient estimates for hibernacula counts of Myotis spp. and Corynorhinus townsendii compiled across 11 western states from 1990–2017.
A single, typically the most recent, count per structure was used in the models.
| Myotis spp. | |||
| Parameters | Coefficient | SE | P value |
| Intercept | 1.89 | 0.32 | <0.01 |
| Mines | -2.90 | 0.32 | <0.01 |
| Year | 0.35 | 0.09 | <0.01 |
| Elevation | 0.73 | 0.35 | 0.03 |
| Karst | 0.37 | 0.31 | 0.21 |
| Latitude | 1.03 | 0.34 | <0.01 |
| Longitude | 1.05 | 0.12 | <0.01 |
| Precipitation | -0.33 | 0.12 | 0.01 |
| Temperature | 1.11 | 0.36 | <0.01 |
| C. townsendii | |||
| Parameters | Coefficient | SE | P value |
| Intercept | 2.55 | 0.20 | <0.01 |
| Mines | -1.53 | 0.20 | <0.01 |
| Year | 0.32 | 0.06 | <0.01 |
| Elevation | -0.01 | 0.21 | 0.92 |
| Karst | 0.39 | 0.19 | 0.04 |
| Latitude | 0.01 | 0.21 | 0.96 |
| Longitude | -0.35 | 0.10 | <0.01 |
| Precipitation | 0.00 | 0.06 | 0.98 |
| Temperature | -0.03 | 0.22 | 0.87 |
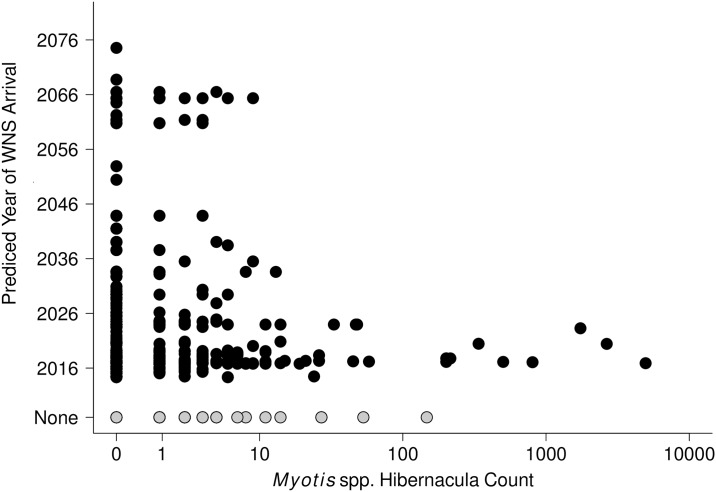
The 25 largest Myotis hibernacula (≥20 bats) all occurred on lands in the lower 2 tiers of protected area status (status 3 and 4; [59]) including the largest, which was in the lowest tier but was on State Trust Lands (S2 Fig). By contrast, the largest COTO hibernacula were distributed across areas with conservation status 2–4 (S2 Fig). Tier 1 areas with the most stringent conservation protections hosted relatively small hibernacula of both COTO (≤20 individuals) and Myotis (≤10 individuals). We found 9 of 10 largest Myotis hibernacula were in areas predicted by Maher et al. [38] to experience earlier arrival of WNS (Fig 5, S3 Fig). Twenty-three of the 31 largest Myotis hibernacula occurred in areas predicted to have WNS exposure by 2025; the remaining 8 were in areas with no predicted WNS establishment.
Fig 5. Size of Myotis spp. hibernacula versus predicted year of WNS infection based on Maher et al. [36].
Temporal trends in counts
We identified 82 individual structures in which ≥5 counts had been recorded since 1990 including 74 caves, 5 mines, 2 buildings and 1 bunker. All states except Arizona and Montana reported at least 1 structure with ≥5 repeat counts (S4 Fig). Repeat counts were conducted at 46 structures in California (44 of them in Lava Beds National Monument) and 16 structures in Oregon. We found evidence for nearly flat (all <1% per year positive rate of change) temporal trends in counts of COTO and Myotis from structures surveyed during ≥5 winters (S5 and S6 Figs). The temporal trend for COTO from the negative-binomial model with random effects was 0.38% per year increase (p <0.001). The trend for Myotis, after removing 23 structures where Myotis had never been detected, was similar at ~0.30% per year (p = 0.0078).
Discussion
Efforts to conserve bats in the western United States have long been impeded by a lack of knowledge of the winter whereabouts of most species. Our results suggest that this has not been for lack of survey effort, at least in caves and mines. We found there has been considerable effort, particularly in the recent past, to survey winter hibernacula of bats in western states. Our database revealed that during 1995–2016 an average of at least 177 surveys per year were conducted across the region and during 2012–2016 the annual average was 369 surveys per year. Only 3 of the 20 hibernacula (15%) that contained ≥30 Myotis, but 30 of the 92 COTO hibernacula (33%) had first records in our database after 2011. This suggests that that recent increased survey effort has generally been effective for locating large COTO hibernacula but less effective for Myotis hibernacula. Survey effort has been relatively well-dispersed geographically and, at least for caves, has focused on areas of karst and volcanic habitat. Encouragingly, most states have been tracking negative surveys, at least in recent years, as opposed to only those surveys where bats had been detected (Fig 1). The inclusion of non-detection records greatly facilitated our biogeographic trend modeling. Structures with more bats tended to be surveyed over a greater number of years. This trend is consistent with patterns observed in northeastern USA and Europe [35] and represents a frugal approach to monitoring for conservation.
We found no evidence of decline in the numbers of bats being counted in routinely surveyed winter roosts, a reassuring finding given that survey effort and tracking of survey results has improved in recent years while the threats facing bats in the western USA, and elsewhere, are increasing [4, 6, 60]. However, it is important to note that most surveys were from either caves or mines. This reflects a bias in that these were where most biologists expected to find hibernating bats during winter. Surveys of caves and mines in the eastern USA have been invaluable for documenting the population status for multiple species of bats including several endangered species [24–26, 33, 61]. However, inference from the data we assembled is that for most Myotis and likely other species (e.g., big brown bats) most individuals do not use caves and mines as winter roosts in the western USA.
Myotis hibernacula
Despite extensive effort to search for bats during winter and our effort to federate these data into a single regional database, the winter whereabouts of Myotis across much of the western United States remains enigmatic. As expected, Myotis were not detected in most structures surveyed and were found in low numbers when they were detected. There were only 11 records of structures that contained ≥100 Myotis and 6 structures that contained ≥500 individuals. Hence our work reinforces results from earlier studies that across the western USA most Myotis individuals do not hibernate in caves and mines [7, 21]. This pattern contrasts strongly with records of Myotis hibernacula in caves and mines of the eastern United States which, prior to the arrival of WNS, often contained thousands of bats [24–26]. We suggest that differences in wintering behavior between eastern and western Myotis, rather than lack of survey effort, explains the paucity of large winter aggregations of Myotis in caves and mines of the western USA. However, our analyses grouped multiple species from the genus Myotis into a single analysis group, which undoubtedly obscured important differences in ecology and behavior and impedes further speculation on differences in hibernation behavior among species.
Although median colony sizes of Myotis were small, our models showed a positive association with year indicating that reported colony sizes have been larger in recent years. However, it is unclear whether colony sizes are truly increasing or whether higher counts in our study resulted from a combination of improved survey effort and better record keeping in recent years. Nevertheless, this result was supported by our analysis of 59 individual structures surveyed during ≥5 winters that also showed a stable-to-slightly increasing trend in colony size of similar magnitude. Hence, best available evidence suggests that Myotis populations that hibernate in caves and mines in western states were stable prior to the invasion of WNS. Although our analyses indicate that few of the largest Myotis hibernacula are currently within protected areas, multiple options are available to protect bats that roost in caves and mines (e.g. gating, seasonal closures).
Our biogeographic models indicated that Myotis counts trended statistically along several of the biogeographic variables examined. An association with eastern longitudes was evident, as by far the largest hibernacula occurred along the eastern extent of our study range. In New Mexico, hibernacula were composed of Myotis velifer that roosted in large numbers in exposed locations within caves. In Montana, the large Myotis roosts were thought to be composed largely of M. lucifugus, which also roosted in exposed locations within caves [62]. The winter roost behavior of M. velifer in New Mexico and M. lucifugus in the northeast portion of our study area contrasts strongly with data from Myotis counts elsewhere in the western USA which were typically small numbers of individuals in crevices within roost structures. This east-west trend in colony size may reflect the increased genetic differentiation that occurs within western populations of M. lucifugus relative to eastern populations [63, 64], or it may be a behavioral response to past population stressors that reduced populations or selected for more dispersed winter roosting behavior [35]. Alternatively it may be due to differences in winter roost habitat availability.
After accounting for associations between higher counts of Myotis with more northerly latitudes and higher elevations, our spatial model also indicated an apparent association with higher surface temperatures and lower precipitation. We used coarse-scale gridded surface temperatures derived from interpolation among weather stations [56], which is decoupled from the actual temperatures experienced by bats within caves and mines during hibernation. As a rule of thumb, cave temperatures are thought to reflect mean annual surface temperatures; however, the microclimate of caves and mines are determined by a complex suite of factors including number, size, and orientation of openings, depth, and airflow patterns [65, 66]. Future efforts that are able to establish actual internal winter temperatures across a large geographically-representative sample of hibernacula structures (e.g., from caves, mines, and building) could provide stronger insights into the relationships between hibernacula size, temperature, and other hibernacula attributes.
Corynorhinus townsendii hibernacula
We found COTO hibernacula to be well distributed throughout the species’ range in the western USA (Fig 4). COTO was found in more than twice as many structures as were Myotis and group sizes were consistently larger than for Myotis. Maximum group sizes we compiled are in accordance with historic estimates for COTO [43]. Smaller maximum group sizes for COTO in Montana and Wyoming are to be expected as this is where the western subspecies reaches the northeastern extent of its distributional range [67]. Counts of COTO were greater in caves than in mines which supports findings from northern Utah that caves represent a more suitable resource for this species [11]. Lava tube caves appear to be particularly important for the species in some regions [12, 13, 16].
Similar to Myotis, we found evidence that colony counts had increased in recent years, possibly as a result of increased effort and improved record keeping, and we found stable-to-slightly increasing counts over time at roosts that had been monitored over multiple years. Hence, indications are that COTO population status across the region has been stable despite the relatively low proportion of hibernacula that occur on formally protected lands. Past concerns about the population status of COTO (e.g., [44]) were likely the primary motivator of hibernacula surveys in western states prior to the current era of WNS. Several studies focused on single structures and smaller geographic extents have also found COTO trends to be stable to increasing at individual sites [14–16]. Our results expand on these narrower inferences with reassuring findings of apparent population stability range-wide. However, it is important to underscore that our inferences are still constrained by a non-random, albeit large, sample with unknown representation of the true distribution and size of the western COTO hibernacula population. Based on our findings, and in contrast to Myotis, we consider it likely that additional medium to large COTO hibernacula remain to be discovered in the western USA.
Characteristics of caves and mines used for bat hibernation
Although we did not find strong and consistent differences between the proportions of caves or mines that provided wintering habitat for at least single individuals, caves hosted larger winter aggregations of both Myotis and COTO than mines across the region. Nevertheless it is important to recognize that the impetus for surveys of caves was likely different than for mines and this may have resulted in dataset bias. Winter surveys of caves were likely motivated by suspected presence of bats, whereas winter mine surveys were often conducted to assess bat use to determine preferred timing and methods for closure; hence counts at caves may be biased high. For example, 96% of structures surveyed in Colorado and 98% in Utah were conducted at mines, many of which were likely selected based on their priority for closure as part of the Abandoned Mine Lands (AML) reclamation program, rather than an expectation that they contained large numbers of bats ([65,68], S1 Text). On the other hand, humans have created an abundance of cave surrogates over the past 150 years in the form of abandoned mines and some of them provide conditions bats seek for overwintering. As an example, the largest winter aggregations of COTO in Montana and Wyoming were in mines and we speculate use of abandoned mines may have allowed the species to extend the northeastern extent of its winter range.
Association of bat abundance and potential disease spread has consistently been related to presence of karst habitat, barren land, or rugged landscapes [35, 38, 69], which is not surprising since this is where most bats have been found during winter. We found an association between higher counts and karst habitat for COTO but not Myotis, but this may be driven by the large number of mines in our dataset, and the fact that only 24% of mines occurred in karst habitat. The karst layer we used for Colorado [58] demonstrated that the regional map of karst habitat we used [57] may be too liberal in its depiction of the type of karst habitat used by bats in other states. Use of higher resolution karst maps may help refine conclusions about karst association, at least for cave hibernacula.
Prospects for winter hibernacula monitoring
For western Myotis, threatened with advancing fronts of WNS from both the Great Plains and western Washington, there is increased urgency to monitor populations and assess expected impacts of disease. Unfortunately few clear biogeographic trends emerged from our modeling efforts that can be used to guide future discoveries. Clearly, the few large Myotis hibernacula identified in our study should continue to be high priorities for monitoring and protection. Additional vigilance at hibernacula in areas where predicted arrival of WNS is earlier [38] is also justifiable. Nevertheless, with few exceptions, our results confirm that Myotis across the western USA do not occur in large aggregations; instead these bats likely hibernate individually or in small groups, in dispersed and inconspicuous locations. Although such roosting behavior may ameliorate the spread of WNS [70] it greatly complicates efforts to monitor population trends and impacts of disease. Alternative strategies could involve the use of radio-telemetry or other ways to locate inconspicuous crevice roosts (e.g. [17, 18, 23, 42, 71, 72]). However, because these methods are likely to reveal only small groups of bats, they may be ineffective for monitoring regional population trends or implementation of regional disease management strategies.
There appear to be more opportunities to continue to use hibernacula surveys of caves, mines, and buildings to monitor COTO trends over time. Future efforts should not only focus on the largest identified hibernacula but also prospect for additional large hibernacula. Furthermore, additional robustness to status and trend estimates could be introduced via statistical sample site selection. For example, a modified dual-frame sampling regime [73, 74] could be employed from which a list of known and accessible hibernacula comprises the list frame and an area frame is used for prospecting and adding new sites into the monitored population. The North American Bat Monitoring Program [75] provides a grid-based areal frame and randomized master sample that could be employed for this purpose.
Data management
Posing similar questions to ours about lack of knowledge on hibernating bats, Twente [7] surveyed over 500 caves and mines and more than 100 buildings in northern Utah, however current databases do not contain these records. Similarly, few of the records from more than 700 structures surveyed in Oregon and Washington [21] or 31 caves surveyed in Idaho [76] during winters of the 1980s appear to have been archived, nor any of the 70 mines surveyed in Nevada during the mid-1990s by Kuenzi et al. [77]. Although, at least some records of mine surveys appear to have been retained, it is clear that many records from 820 structures surveyed in northern Utah during the mid-1990s, including 29 records of COTO wintering in caves [11], are not in current wildlife databases. We compiled few records from the 1970s (n = 45) and 1960s (n = 15) despite recent knowledge of some of these studies [43]. Given limited resources available for bat conservation, it is vital that survey results are archived, both to improve population status assessments over time as well as to prevent inefficiencies via unintended repeat surveys of unoccupied structures. Although efforts to compile results of roost monitoring efforts, including hibernacula, began as early as 1995 [43] an internet-accessible repository of monitoring results has not become a reality up to present. As an interim step toward this goal, we have archived a public version of our dataset (https://irma.nps.gov/DataStore/Reference/Profile/2247583), including covariates, for use by the scientific community. Our data archive removes structure names and provides spatial location precision resolved to the 100 km2 North American Bat Monitoring Program (NABat, [75]) sampling frame grid cell to prevent disclosure of exact locations of hibernacula. Ultimately, we advocate for creation of a database that allows users to upload new, and newly-unearthed historical records via a graphical interface that would allow contributors to archive their data in perpetuity. We acknowledge legal and proprietary concerns surrounding data sharing, particularly locations of sensitive underground sites and vulnerable bat populations, but argue the conservation community can no longer afford to lose occurrence records; a practice that impedes efforts to monitor and conserve bat populations over longer periods (e.g., decades to centuries). A comprehensive catalog of geospatial resources with existing and potential bat hibernacula would also be instrumental in developing an organized approach to monitoring population status of hibernating bats especially in the face of disease or other emerging threats [78].
Supporting information
A single, usually most recent, survey represents each structure. Boxes represent the 1st and 3rd quartiles, horizontal line represents median, whiskers represent 95% of the data and dots represent outliers for each state.
(TIF)
Symbols represent a single count from each structure with triangles representing Myotis spp. and circles representing Corynorhinus townsendii.
(TIF)
Counties are shaded according to predicted year of arrival of White-nose Syndrome (WNS) by Maher et al. (2012). Open red circle indicates King County, Washington where WNS was diagnosed in 2016.
(TIF)
(TIF)
(TIF)
Trends from a single hibernacula in New Mexico are plotted in sub-panel A while the remaining 81 structures are plotted in sub-panel B.
(TIF)
Descriptions of survey efforts by state.
(DOCX)
Acknowledgments
We greatly appreciate the efforts to collect, collate, and archive data included in this study. The following individuals contributed or facilitated data transfer directly to this effort: Raymond Brown, USDA Forest Service Region 6; Joshua Chapman, USDA Forest Service Region 6; Sylvia Copeland, Angie Schmidt, and Nikki Wade, Idaho Department of Fish and Game; Paul Heady, Central Coast Bat Research Group; Paul Johnson, Pinnacles National Park; Nadja Schmidt, Deschutes National Forest; Katrina Smith, Lava Beds National Park; Kelli Van Norman, Bureau of Land Management and USDA Forest Service Region 6; and Masako Wright, Uinta-Wasatch-Cache National Forest. It is also essential that we recognize the countless field biologists, across the many states and decades, who conducted the surveys reported in this paper and provided them to state databases. Their collective knowledge and skill in safely surveying subterranean sites resulted in the discovery and subsequent monitoring of numerous important hibernacula, and contributed greatly to our understanding of winter roosting habitat in the western USA. Some states recognized important contributors individually (S1 Text). Special thanks to Karen Blejwas, Alaska Dept. of Fish & Game, for her assistance and encouragement in the early stages of this project.
Data Availability
Data are publicly available at the URL below. We have removed names of individual structures searched and removed the precise coordinates of the structures to conform with norms of protecting sensitive location information on bat roosts. https://irma.nps.gov/DataStore/Reference/Profile/2247583.
Funding Statement
The authors received no specific funding for the collection of data or preparation of this paper. Authors participated in the preparation of this work in a volunteer capacity or as part of their normal work duties.
References
- 1.Barclay RMR, Harder LD. Life histories of bats: life in the slow lane In: Kunz TH, Fenton MB, editors. Bat Ecology. Chicago: University of Chicago Press; 2003. pp. 209–253. [Google Scholar]
- 2.Humphrey SR. Nursery roosts and community diversity of nearctic bats. J Mammal. 1975; 56: 321–346. [Google Scholar]
- 3.Promislow DEL, Harvey PH. Living fast and dying young: a comparative analysis of life-history variation among mammals. J Zool. 1990; 220: 417–437. [Google Scholar]
- 4.Weller TJ, Cryan PM, O’Shea TJ. Broadening the focus of bat conservation and research in the USA for the 21st century. Endanger Species Res. 2009; 8: 129–145. [Google Scholar]
- 5.Barbour RW, Davis WH. Bats of America. Lexington: University Press of Kentucky; 1969. [Google Scholar]
- 6.Pierson ED. Tall trees, deep holes, and scarred landscapes: conservation biology of North American bats In: Kunz TH, Racey PA, editors. Bat Biology and Conservation. Washington DC: Smithsonian Institute Press; 1998. pp. 309–325. [Google Scholar]
- 7.Twente JW. Environmental problems involving the hiberation [sic] of bats in Utah. Proc Utah Acad Sci Arts and Letters. 1960; pp. 67–71.
- 8.Kalcounis-Rüppell MC, Psyllakis JM, Brigham RM. Tree roost selection by bats: an empirical synthesis using meta-analysis. Wildl Soc Bull. 2005; 33: 1123–1132. [Google Scholar]
- 9.Lacki MJ, Hayes JP, Kurta A, editors. Bats in Forests: Conservation and Management. Baltimore: Johns Hopkins University Press; 2007. [Google Scholar]
- 10.Ingersoll TE, Navo KW, De Valpine P. Microclimate preferences during swarming and hibernation in the Townsend’s big-eared bat, Corynorhinus townsendii. J Mammal. 2010; 91: 1242–1250. [Google Scholar]
- 11.Sherwin RE, Stricklan D, Rogers DS. Roosting affinities of Townsend’s big-eared bat (Corynorhinus townsendii) in northern Utah. J Mammal. 2000; 81: 939–947. [Google Scholar]
- 12.Call RS, Whiting JC, Doering B, Lowe J, Englestead D, Frye J, et al. Maternity roosts of Townsend’s big-eared bats in lava tube caves of southern Idaho. Northwest Sci. 2018; 92:158–165. [Google Scholar]
- 13.Whiting JC, Doering B, Wright G, Englestead DK, Frye JA, Stefanic T. Bat hibernacula in caves of southern Idaho: implications for monitoring and management. West N Am Nat. 2018; 78: 165–173. [Google Scholar]
- 14.Ellison LE. A retrospective survival analysis of Townsend’s big-eared bat (Corynorhinus townsendii) from Washington State. Northwest Nat. 2010; 91: 172–182. [Google Scholar]
- 15.Wainwright JM, Reynolds ND. Cave hibernaculum surveys of a Townsend’s big-eared bat (Corynorhinus townsendii) colony at Mount St. Helens, Washington. Northwest Nat. 2013; 94: 240–244. [Google Scholar]
- 16.Weller TJ, Thomas SC, Baldwin JA. Use of Long-Term Opportunistic Surveys to Estimate Trends in Abundance of Hibernating Townsend’s Big-Eared Bats. J Fish Wildl Manag. 2014; 5: 59–69. [Google Scholar]
- 17.Klüg-Baerwald BJ, Lausen CL, Willis CKR, Brigham RM. Home is where you hang your bat: winter roost selection by prairie-living big brown bats. J Mammal. 2017; 98: 752–760. [Google Scholar]
- 18.Lausen CL, Barclay RMR. Winter bat activity in the Canadian prairies. Can J Zool. 2006; 84: 1079–1086. [Google Scholar]
- 19.Boyles JG, Dunbar MB, Whitaker JO. Activity following arousal in winter by North American vespertilionid bats. Mamm Rev. 2006; 36: 267–280. [Google Scholar]
- 20.Hendricks P. Winter records of bats in Montana. Northwest Nat. 2012; 93: 154–162. [Google Scholar]
- 21.Perkins JM, Barss JM, Peterson J. Winter records of bats in Oregon and Washington. Northwest Nat. 1990; 71: 59–62. [Google Scholar]
- 22.Falxa G. Winter foraging of silver-haired and California Myotis bats in western Washington. Northwest Nat. 2007; 88: 98–100. [Google Scholar]
- 23.Johnson JS, Treanor JJ, Lacki MJ, Baker MD, Falxa GA, Dodd LE, et al. Migratory and winter activity of bats in Yellowstone National Park. JMammal. 2017; 98: 211–221. [Google Scholar]
- 24.Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GG, et al. An emerging disease causes regional population collapse of common North American bat species. Science. 2010; 329: 679–682. 10.1126/science.1188594 [DOI] [PubMed] [Google Scholar]
- 25.Langwig KE, Hoyt JR, Parise KL, Kath J, Kirk D, Frick WF, et al. Invasion dynamics of white-nose syndrome fungus, midwestern United States, 2012–2014. Emerg Infect Dis. 2015; 21: 1023–1026. 10.3201/eid2106.150123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thogmartin WE, King RA, McKann PC, Szymanski JA, Pruitt L. Population-level impact of white-nose syndrome on the endangered Indiana bat. J Mammal. 2012; 93: 1086–1098. [Google Scholar]
- 27.Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, Buckles EL, et al. Bat white-nose syndrome: an emerging fungal pathogen? Science. 2009; 323: 227 10.1126/science.1163874 [DOI] [PubMed] [Google Scholar]
- 28.Hayman DTS, Pulliam JRC, Marshall JC, Cryan PM, Webb CT. Environment, host, and fungal traits predict continental-scale white-nose syndrome in bats. Sci Adv. 2016; 2: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorch JM, Palmer JM, Lindner DL, Ballmann AE, George KG, Griffin K, et al. First detection of bat white-nose syndrome in western North America. mSphere. 2016; 1:1–5. 10.1128/mSphere.00148-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorch JM, Meteyer CU, Behr MJ, Boyles JG, Cryan PM, Hicks AC, et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature. 2011; 480: 376 10.1038/nature10590 [DOI] [PubMed] [Google Scholar]
- 31.Minnis AM, Lindner DL. Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biol. 2013; 117: 638–649. 10.1016/j.funbio.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 32.Verant ML, Boyles JG, Waldrep G Jr., Blehert DS. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS One. 2012; 7: e46280 10.1371/journal.pone.0046280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingersoll T, Sewall B, Amelon S. Improved analysis of long-term monitoring data demonstrates marked regional declines of bat populations in the eastern United States. PLoS One. 2013; 8: e65907 10.1371/journal.pone.0065907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes JP, Ober HK, Sherwin RE. Survey and monitoring of bats In: Kunz TH, Parsons S, editors. Ecological and Behavioral Methods for the Study of Bats. Baltimore: Johns Hopkins University Press; 2009. pp. 112–129. [Google Scholar]
- 35.Frick WF, Puechmaille SJ, Hoyt JR, Nickel BA, Langwig KE, Foster JT, et al. Disease alters macroecological patterns of North American bats. Glob Ecol Biogeogr. 2015; 24: 741–749. [Google Scholar]
- 36.McIntire A, Blejwas K, Gillies K. What do western bats do in winter? Implications for WNS risk assessment and response planning in the West. In: Abstracts of Presented Papers and Posters for the 5th Annual White-nose Syndrome Symposium; 2012 Jun 4–7; Madison, Wisconsin. p. 19. https://www.whitenosesyndrome.org/sites/default/files/resource/wns_symposium_abstracts_2012_final.pdf
- 37.Ormsbee PC, Rodhouse TJ, Hobson J. A tool to assess WNS Risk: prioritize surveillance, population monitoring, and management options across western Landscapes. In: Abstracts of Presented Papers and Posters for 2011 White-nose Syndrome Symposium; 2011 May 17–19; Little Rock, Arkansas. p. 20. https://www.whitenosesyndrome.org/sites/default/files/resource/2011wnssymposiumabstracts.pdf
- 38.Maher SP, Kramer AM, Pulliam JT, Zokan MA, Bowden SE, Barton HD, et al. Spread of white-nose syndrome on a network regulated by geography and climate. Nat Commun. 2012; 3: 13106. [DOI] [PubMed] [Google Scholar]
- 39.Michaels D. A spreading mystery: deadly bat fungus takes a giant leap as white-nose syndrome moves from the East Coast and reaches the West. Outdoor California. 2016; 77: 8–15. [Google Scholar]
- 40.Neubaum DJ, Navo KW, Siemers JL. Guidelines for defining biologically important bat roosts: a case study from Colorado. J Fish Wildl Manag. 2017; 8: 272–282. [Google Scholar]
- 41.Rodhouse TJ, Philippi T, Monahan WB, Castle K. A macroecological perspective on bat conservation in a 2nd-century National Park Service. Ecosphere. 2016; 7: e01576. [Google Scholar]
- 42.Neubaum DJ, O’Shea TJ, Wilson KR. Autumn migration and selection of rock crevices as hibernacula by big brown bats in Colorado. J Mammal. 2006; 87: 470–479. [Google Scholar]
- 43.Ellison LE, O’Shea TJ, Bogan MA, Everette AL, Schneider DM. Existing data on colonies of bats in the United States: summary and analysis of the U.S. Geological Survey’s bat population database. In: O’Shea TJ, Bogan MA, editors. Monitoring Trends in Bat Populations of the United States and Territories: Problems and Prospects. Springfield (VA): U.S. Geological Society, Biological Resources Discipline; 2003. pp. 127–237.
- 44.Pierson ED, Wackenhut MC, Altenbach JS, Bradley P, Call P, Genter DL, et al. Species conservation assessment and conservation strategy for the Townsend’s big-eared bat (Corynorhinus townsendii townsendii and Corynorhinus townsendii pallescens). Boise: Idaho Conservation Effort, Idaho Department of Fish and Game; 1999.
- 45.Rodhouse TJ, Scott SA, Ormsbee PC, Zinck JM. Field identification of Myotis yumanensis and Myotis lucifugus: a morphological evaluation. West N Am Nat. 2008; 68: 437–443. [Google Scholar]
- 46.Rodriguez RM, Ammerman LK. Mitochondrial DNA divergence does not reflect morphological difference between Myotis californicus and Myotis ciliolabrum. J Mammal. 2004; 85: 842–851. [Google Scholar]
- 47.Weller TJ, Scott SA, Rodhouse TJ, Ormsbee PC, Zinck, JM. Field identification of the cryptic vespertilionid bats, Myotis lucifugus and M. yumanensis. Acta Chiropt. 2007; 9: 133–147. [Google Scholar]
- 48.Huppert GN. Legal protection for caves in the United States. Environ Geol. 1995; 26: 121–123. [Google Scholar]
- 49.Hilbe JM. Negative binomial regression. 2nd ed Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 50.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. Berlin: Springer; 2009. [Google Scholar]
- 51.Gelman A, and Hill J. Data analysis using regression and multilevel/hierarchical models. New York: Cambridge University Press; 2007. [Google Scholar]
- 52.Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol. 2010; 1:103–113. [Google Scholar]
- 53.Zeileis A, Kleiber C, Jackman S. Regression Models for Count Data in R. J Stat Softw. 2008; 27(8): 1–25. [Google Scholar]
- 54.R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing. 2017. Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- 55.Skaug H, Fournier D, Bolker B, Magnusson A, Nielsen A. Generalized linear mixed models using ‘AD Model Builder’. R package version 0.8.3.3. 2016. [cited 2018May11]. https://rdrr.io/rforge/glmmADMB/.
- 56.Daly C, Halbleib M, Smith JI, Gibson WP, Doggett WK, Taylor GH, et al. Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int J Climatol. 2008; 28: 2031–2064. http://prism.oregonstate.edu/normals/. [Google Scholar]
- 57.Weary DJ, Doctor DH. Karst in the United States: a digital map compilation and database. 2014. U.S. Geological Survey Open-File Report 2014–1156. 10.3133/ofr20141156. [DOI]
- 58.Navo KW, Neubaum DN, Neubaum MA, editors. Colorado bat conservation plan. 2nd ed. c2018 [cited 2018 August 3]. http://cnhp.colostate.edu/cbwg/consPlan.asp.
- 59.Gergely KJ, McKerrow A. PAD-US—National inventory of protected areas (ver. 1.1, August 2016). U.S. Geological Survey Fact Sheet 2013–3086. http://pubs.usgs.gov/fs/2013/3086/.
- 60.O’Shea TJ, Cryan PM, Hayman DTS, Plowright RK, Streicker DG. Multiple mortality events in bats: a global review. Mamm Rev. 2016; 46: 175–190. 10.1111/mam.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graening GO, Harvey MJ, Puckette WL, Stark RC, Sasse DB, Hensley SL, et al. Conservation status of the endangered Ozark big-eared bat (Corynorhinus townsendii ingens)—a 34-year assessment. Oklahoma Biological Survey. 2011; 11: 1–16. [Google Scholar]
- 62.Hendricks P, Genter DL, Martinez S. Bats of the Azure Cave and the Little Rocky Mountains, Montana. Can Field Nat. 2000; 114: 89–97. [Google Scholar]
- 63.Vonhof MJ, Russell AL, Miller-Butterworth CM. Range-wide genetic analysis of little brown bat (Myotis lucifugus) populations: estimating the risk of spread of white-nose syndrome. PLoS One. 2015; 10: e0128713 10.1371/journal.pone.0128713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilder AP, Kunz TH, Sorenson MD. Population genetic structure of a common host predicts the spread of white-nose syndrome, an emerging infectious disease in bats. Mol Ecol. 2015; 24: 5495–5506. 10.1111/mec.13396 [DOI] [PubMed] [Google Scholar]
- 65.Hayes MA, Schorr RA, Navo KW. Hibernacula selection by Townsend’s Big-Eared Bat in Southwestern Colorado. J Wildl Manage. 2011; 75: 137–143. [Google Scholar]
- 66.Ransome R. The Natural History of Hibernating Bats. Bromley (UK) Christopher Helm Publishers; 1990. [Google Scholar]
- 67.Brown JH. On the relationship between abundance and distribution of species. Am Nat. 1984; 124: 255–279. [Google Scholar]
- 68.Sherwin RE, Gannon WL, Altenbach JS. Managing complex systems simply: understanding inherent variation in the use of roosts by Townsend’s big-eared bat. Wildl Soc Bull. 2003; 31: 62–72. [Google Scholar]
- 69.Flory AR, Kumar S, Stohlgren TJ, Cryan PM. Environmental conditions associated with bat white-nose syndrome mortality in the north-eastern United States. J Appl Ecol. 2012; 49: 680–689. [Google Scholar]
- 70.Langwig KE, Frick WF, Bried JT, Hicks AC, Kunz TH, Kilpatrick AM. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Ecol Lett. 2012; 15: 1050–1057. 10.1111/j.1461-0248.2012.01829.x [DOI] [PubMed] [Google Scholar]
- 71.Moosman PR Jr., Warner DP, Hendren RH, Hosler MJ. Potential for monitoring eastern small-footed bats on talus slopes. Northeast Nat. 2015; 22: NENHC-1–NENHC-13. [Google Scholar]
- 72.Lemen CA, Freeman PW, White JA. Acoustic evidence of bats using rock crevices in winter: A call for more research on winter roosts in North America. Trans Nebr Acad Sci Affil Soc. 2016; 36: 9–13. [Google Scholar]
- 73.Haines DE, Pollock KH. Estimating the number of active and successful bald eagle nests: an application of the dual frame method. Environ Ecol Stat. 1998; 5: 245–256. [Google Scholar]
- 74.Thompson SK. Sampling. New York: John Wiley and Sons; 1992. [Google Scholar]
- 75.Loeb SC, Rodhouse TJ, Ellison LE, Lausen CL, Reichard JD, Irvine KM, et al. A plan for the North American bat monitoring program (NABat). Asheville (NC) U.S. Department of Agriculture Forest Service, Southern Research Station; 2015. Gen. Tech. Rep. SRS-208.
- 76.Genter DL. Wintering bats of the upper Snake River plain: occurrence in lava-tubes caves. Great Basin Nat. 1986; 46: 241–244. [Google Scholar]
- 77.Kuenzi AJ, Downard GT, Morrison ML. Bat distribution and hibernacula use in west central Nevada. Great Basin Nat. 1999; 59: 213–220. [Google Scholar]
- 78.Knudsen GR, Dixon RD, Amelon SK. Potential spread of White-nose syndrome of bats to the Northwest: epidemiological considerations. Northwest Sci. 2013; 87: 292–306. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A single, usually most recent, survey represents each structure. Boxes represent the 1st and 3rd quartiles, horizontal line represents median, whiskers represent 95% of the data and dots represent outliers for each state.
(TIF)
Symbols represent a single count from each structure with triangles representing Myotis spp. and circles representing Corynorhinus townsendii.
(TIF)
Counties are shaded according to predicted year of arrival of White-nose Syndrome (WNS) by Maher et al. (2012). Open red circle indicates King County, Washington where WNS was diagnosed in 2016.
(TIF)
(TIF)
(TIF)
Trends from a single hibernacula in New Mexico are plotted in sub-panel A while the remaining 81 structures are plotted in sub-panel B.
(TIF)
Descriptions of survey efforts by state.
(DOCX)
Data Availability Statement
Data are publicly available at the URL below. We have removed names of individual structures searched and removed the precise coordinates of the structures to conform with norms of protecting sensitive location information on bat roosts. https://irma.nps.gov/DataStore/Reference/Profile/2247583.