Figure 19.
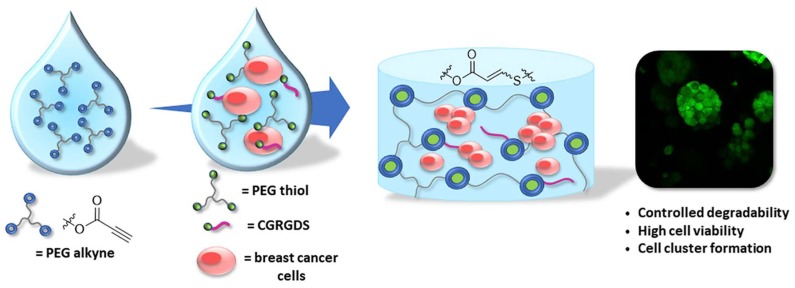
The non-radical nucleophilic addition reaction between multi-armed PEG-thiol and PEG-alkyne produced vinylene sulfide-linked hydrogel for encapsulation of MCF-7 breast cancer cells. An extracellular matrix mimicking peptide CGRGDS is incorporated to the gel through the same type of addition between the thiol group in the cysteine and PEG-alkyne during the network formation. The hydrogel is degradable through hydrolysis of the esters in PEG-alkyne to allow cell growth and formation of cell clusters. Images reproduced with permission from [107]. Copyright 2018 Elsevier.