Abstract
Background:
Following systemic delivery, AAV9-mediated gene expression is significantly increased in ischemic versus non-ischemic muscle, suggesting AAV9 as an attractive vector for treating peripheral arterial disease (PAD). Potential mechanisms underlying ischemia-augmented expression include: 1) increased vascular permeability, and 2) “unmasking” of endogenous AAV9 receptors. Here, we endeavored to reconstitute the ischemic induction of AAV9 in vivo, using local injection of histamine (to increase vascular permeability) and neuraminidase (to desialylate cell surface glycans).
Methods:
Bioassays were performed to optimize the effects of histamine and neuraminidase after intramuscular (IM) injection. Histamine and/or neuraminidase were then injected IM shortly before IV injection of an AAV9 vector expressing luciferase. Luciferase expression was serially assessed with bioluminescence imaging. At study’s end, tissues were harvested for assays of luciferase activity and AAV9 genome copy number to assess AAV-mediated gene expression and transduction, respectively.
Results:
IM injection of either neuraminidase or neuraminidase plus histamine significantly increased both transduction and gene expression, whereas histamine alone had little effect. Pre-injection with neuraminidase increased AAV9-mediated gene delivery by 4–9 fold and luciferase activity by 60–100 fold. Luciferase activity in neuraminidase-injected muscle was >100-fold higher than in any off-target tissue (including heart, liver and brain).
Conclusion:
The ischemic induction of AAV9-mediated gene expression in muscle can largely be reconstituted by pre-injecting neuraminidase IM. This strategy may prove useful in future human gene therapy protocols as a quick and efficient means to selectively target systemically injected AAV9 to localized regions of muscle, thus decreasing the potential for adverse effects in off-target tissues.
Keywords: Adeno-associated virus, gene therapy, receptor, neuraminidase, muscle-specific promoter
Introduction
Adeno-associated viral (AAV) vectors have emerged as a leading delivery vehicle for human gene therapy due to their low pathogenicity and toxicity, safety record and efficacy of gene delivery.[1] Various AAV serotypes have been examined in a wide variety of animal disease models including heart disease,[2] central nervous system disorders,[3] retinopathy,[4] muscular dystrophy,[5] and hemophilia.[6] Further, AAV has shown promise in a number of clinical studies treating hemophilia,[7] mucopolysaccharidosis type IIIA,[8] and inherited retinopathy.[9]
To date, hundreds of AAV serotypes have been identified infecting human and primate species.[10] Among them, AAV8 and AAV9 have emerged as leading serotypes for targeting skeletal and cardiac muscle. AAV9 also shows potential for crossing the blood brain barrier, which could afford therapeutic potential for neurologic disorders or could potentiate untoward side effects if the brain is not the desired target.[11]
In humans, PAD is caused by atherosclerotic blockages that reduce blood flow to the leg(s) and in pre-clinical models PAD is typically approximated by femoral artery ligation and/or excision. Most pre-clinical AAV studies to date on PAD have used local intramuscular (IM) injection. In previous studies, AAV vectors expressing EcSOD from the cytomegalovirus (CMV) promoter were cross-packaged into AAV9 capsids and injected IM into hind-limb muscle just prior to femoral excision/ligation.[12] We found that AAV9-mediated overexpression of EcSOD in ischemic skeletal muscle significantly improved recovery of perfusion ratio and capillary density, while apoptotic nuclei and limb necrosis were decreased.[12] In another study, a combination gene therapy using an AAV vector expressing both FGF2 and Cyr61 from the CMV promoter was tested after IM injection into ischemic hindlimb muscles, and a therapeutic benefit was observed superior to that obtained after IM injection of the corresponding bicistronic plasmid (pC-FGFiCyr).[13] In other studies, hindlimb ischemia was induced in BALB/c mice IM injected with a conditionally silenced AAV vector expressing human vascular endothelial growth factor (hVEGF). They found that AAV mediated gene therapy reversibly activated angiogenic and vasculogenic genes with no apparent adverse side effects.[14] Thus while the IM injection of AAV has shown promise in mice, IM injection is predictably far less effective in humans and results in a far less homogeneous distribution of gene expression due to the >1000-fold increase in muscle mass.[15]
In contrast, the intravenous (IV) administration of AAV vectors has the benefit of offering a perfectly homogeneous distribution of gene expression. One potential concern in the setting of PAD is that systemic delivery to ischemic muscle might be limited in proportion to the reduction in blood flow. Somewhat surprisingly, in a previous study, AAV9-mediated gene expression after IV administration was 30- to 150-fold higher in ischemic vs. non-ischemic muscle.[16] In the current study, we sought to elucidate the mechanism(s) underlying the dramatic improvement in AAV9-mediated gene expression seen in ischemic muscle following systemic delivery.
Many AAV serotypes target glycan residues for their initial interaction with the cell surface. In particular, recent studies have demonstrated that the initial attachment factors/receptors for the AAV9 capsid are cell surface N-linked glycans with terminal β-galactosyl residues.[17, 18] Desialylation of these galactosylated glycans significantly increases cell surface binding and transduction of AAV9, whereas other AAV serotypes such as AAV1 and AAV6 bind more avidly to sialylated N-linked glycans.[17, 19, 20] Previous in vitro studies conducted in sialidase-treated or sialic acid-deficient mutant CHO cells revealed a significant increase in the relative binding potential of AAV9 particles upon desialylation, whereas resialylation of galactosylated glycans on the sialic acid-deficient CHO Lec2 cell line with different sialyltransferases partially blocked AAV9 transduction. In addition, pretreatment of well differentiated human airway epithelial cultures and intranasal instillation of recombinant sialidase in murine airway remarkably enhanced the transduction efficiency of AAV9.[18]
Our previous work demonstrated that terminal N-linked galactosyl residues are predominantly sialylated on normal myofibers, whereas they not only become desialylated, but are also transduced at much higher rates in the setting of hindlimb ischemia.[16] This is consistent with reports that ischemia induces desialylation of cell-surface glycans,[21] thus unmasking the primary receptor for AAV9 and increasing transduction efficiency.
Ischemia is also known to increase vascular permeability,[22] and our previous work had identified increased vascular permeability secondary to ischemia as another potential mechanism that might contribute to the remarkable increase in AAV9-mediated gene expression observed in ischemic vs. non-ischemic hindlimbs.[16] Histamine is well known to increase vascular permeability, and has previously been used to enhance AAV-mediated gene transfer in vivo.[23]
In the current study, we sought to determine whether increased vascular permeability and/or desialylation might function alone or synergistically to increase AAV9-mediated transduction in a mouse hindlimb ischemia model of PAD.
Materials and Methods
Plasmids and AAV vector production
The AAV9.tMCK.PI.eGFP.T2A.ffLuciferase.SV40 vector used in this study was obtained from the Penn Vector Core in the School of Medicine Gene Therapy Program at the University of Pennsylvania. It contains a truncated muscle creatine kinase promoter (tMCK [24]) driving the expression of eGFP and firefly luciferase, linked by the self-cleaving (T2A) peptide sequence. In brief, the Penn Vector Core cross-packaged the AAV2-based vector genomes into AAV9 capsids via the triple transfection of HEK 293 cells, then purified by ammonium sulfate fractionation and iodixanol gradient centrifugation.[25] The titer of the AAV vector (viral genomes/ml) was confirmed by quantitative real-time PCR.[26] The following primers were used for amplifying luciferase: 5’-AGAACTGCCTGCGTGAGATT-3’ (forward) and 5’-AAAACCGTGATGGAATGGAA-3’ (reverse). Known copy numbers (105–108) of a reference plasmid carrying the luciferase cDNA were used to construct standard curves for quantification.
Animal procedures and vector administration
Animal protocols used in this study were approved by the Institutional Animal Care and Use Committee and conformed to the “Guide for the Care and Use of Laboratory Animals” (NIH Publication 85–23, revised 1985). All mice (BALB/c) (15–21 weeks old) were purchased from The Jackson Laboratories (Bar Harbor, ME) and maintained on a 12/12 hr light/dark cycle at 24°C and 60% humidity. The experiments involving histamine and neuraminidase were of 2×2 factorial design and contained four groups: Normal Saline (NS), Histamine (His), Neuraminidase (Neu) and Histamine+Neuraminidase (His+Neu). All mice were anesthetized with an intraperitoneal (IP) injection of sodium pentobarbital (100 mg/kg). The left hindlimb of each mouse then received 3 intramuscular (IM) injections of vehicle (saline), His and/or Neu (20ul each injection, total volume = 60ul). Each left gastrocnemius muscle (GA) was injected proximally and distally (total of 40ul), while each left tibialis anterior muscle (TA) received a single 20ul injection. Five mins or 2h post injection, mice were injected with 150μl of viral stock solution containing 1×1011 viral genome particles via tail vein.
Evans blue dye assay
The Evans blue assay was performed as previously described.[16] Briefly, animals were anesthetized and maintained on 1–1.2% isoflurane in oxygen. Evans blue dye, 100 μl of a 20 mg/ml solution in saline, was injected intra-jugularly 5 min before the mice were injected with NS, histamine and/or neuraminidase, and tissues were harvested 2h later. Injected and contralateral muscles were harvested, weighed, sectioned, and freeze-dried overnight. Eight volumes of formamide (ml/g tissue) were added, and samples were incubated at 60 °C for 2 h to extract Evans blue dye. After an additional 10h at room temperature, the Evans blue dye was collected and absorption at 595 nm was measured on a microplate reader (Model 3550, Bio-Rad, Hercules, CA). Sample concentrations were determined using a calibration curve generated in parallel from dilutions of Evans blue dye in formamide.
Lectin (Ecl and Mal-1) staining
Animals were euthanized 2h after neuraminidase injection. Injected and contralateral muscles were harvested and placed in OCT for snap freezing in liquid nitrogen. Five micron thick cryostat sections were then prepared to compare the distribution of sialylated versus desialylated glycans in treated and non-treated muscles. Staining was performed using biotinlyated Erythrina cristagalli lectin (Ecl) and FITC-labeled Maackia amurensis lectin (Mal-1) (Vector Laboratories, Burlingame, CA). Biotinylated Ecl lectin was visualized using Streptavidin-Alexa Fluor-555 (Invitrogen Carlsbad, CA) as secondary antibody. Quantification of the fluorescence signal intensity of the lectin staining was performed using ImageJ software.
Bioluminescence imaging
Luciferase expression was serially assessed in live mice at 7 and 14 days post AAV9/CK6/Luc injection using an in vivo bioluminescence imaging system (IVIS Spectrum, Caliper Life Sciences, Hopkinton, MA) as described previously.[27]
Quantitative luciferase activity assay
Luciferase activity was measured using the luciferase assay system from Promega Corp (Madison, WI). After bioluminescence imaging and euthanasia at 14 days post vector injection, injected hindlimb muscles were collected from experimental mice. According to the protocol, protein extracts were prepared and luciferase activities (relative light units, RLU) were determined using a luminometer (Berthold Technologies, Bad Wildbad, Germany) and reported in average radiance units after background subtraction.
Determination of AAV vector genome copy number per μg genomic DNA
The AAV2 genomic backbone AAV/CK6/Luc was cross-packaged into AAV9 capsids for injection as described above. Two weeks after vector administration, total genomic DNA from a panel of tissues was prepared using a QIAamp DNA Mini Kit (Qiagen, Inc). Real-time PCR using the Bio-Rad iTaq Universal SYBR Green Supermix was performed on a Bio-Rad CFX Connect system (Hercules, CA, USA). The following primers were used for amplifying the firefly luciferase gene: 5′-AAGATTCAAAGTGCGCTGCTGGTG-3′ (forward) and 5′-TTGCCTGATACCTGGCAGATGGAA-3′ (reverse). The results were expressed as mean AAV vector genome copy numbers per host genome.
Statistical analysis
Data were expressed as mean ± SEM. Statistical analyses of data were performed by Student’s T-test or one-way analysis of variance followed by the Student-Newman-Keuls test, as appropriate. A value of p < 0.05 was considered to be statistically significant.
Results
Effect of histamine dose on vascular permeability
To determine the optimal concentration of histamine for increasing vascular permeability in mouse skeletal muscle, three different doses of histamine (His) were injected into left hindlimb muscle and Evans blue assays were performed. Example images of isolated GA and TA muscles are shown in Supplementary Figure 1.
As shown in Figure 1A, Evans blue dye concentration in treated muscles showed a dose-dependent increase with higher doses of histamine (2uM: 4.58±0.87ug/ml, 100uM: 6.64±0.71ug/ml and 10mM: 8.70±3.24ug/ml), whereas Evans blue concentrations in untreated right hindlimb muscles remained essentially unchanged. However, only the left hindlimb muscles injected with His at 100uM reached statistical significance as compared to Evans blue concentrations in the untreated muscles of the right hindlimb (P<0.05). When the ratios of Evans blue concentrations were compared in His-treated versus untreated muscles, that ratio was higher in the group of muscles treated with 100uM His (3.87±0.60) as compared to the 2uM (2.16±0.52) and 10mM groups (2.35±0.26, Figure 1B). Based on these results, the His concentration of 100uM was selected as the dose to determine the optimal time for tissue harvest following His injection. Towards this end, Evans blue and 100uM His were again injected, and hindlimb muscles were harvested at 1h, 2h, 4h and overnight after the injections. Ex vivo images of TA and GA from the selected time points (Figure 1C) show that the injected left muscles were markedly more blue as compared to the non-injected right muscles. Quantitative analysis of muscle extracts (Figure 1D) showed that Evans blue content in the group of left hindlimb muscles harvested at the 2h time point (7.42±0.44) was significantly higher than in the 1h (5.84±0.43), 4h (4.86±0.68) or overnight groups (5.11±0.40). The ratio of Evans blue dye content in left to right hindlimbs at the 2h time point (3.83±0.61) showed a slight increase as compared to other three groups. Based on these results, the 2h time point was selected as the optimal time for tissue harvest for the remaining experiments.
Figure 1:

Increased permeability from histamine. Evans blue content is higher in left hindlimb following histamine injection. A and B: Evans blue dye was injected IV into normal BALB/c mice, followed 5 min later with three doses of IM histamine injection. GA+TA were harvested for Evans blue assay (*P<0.05 vs. untreated right hindlimb). C, D and E: 100uM histamine was injected and tissues harvested at 1h, 2h, 4h and overnight (*P<0.05 vs. corresponding right hindlimb, N=3/group).
Dose effect of neuraminidase on lectin staining
To optimize the dose of neuraminidase needed to induce desialylation, neuraminidase (Type II) from Vibrio cholera (Sigma Chemical Co, St. Louis, MI) was evaluated at three concentrations (40U/L, 100U/L and 200U/L) in a total volume of 60uL/animal by IM injection into left hindlimbs in the same manner as histamine. Two hours later, the injected GA and TA were harvested for Ecl and Mal-1 staining. Representative fluorescent photomicrographs (Figure 2A) of the neuraminidase (Neu) group showed much less signal in Mal-1 and much more signal in Ecl-stained muscle tissue compared to that found in the negative control group injected with normal saline (NS). When treated with Neu, the ratio of Mal-1/Ecl decreased to a similar extent in the 100U/L (0.26±0.01) and 200U/L (0.26±0.01) groups, but to a lesser extent in the 40U/L group (0.36±0.04, Figure 2B). The Neu concentration of 100U/L was therefore deemed adequate to achieve maximum desialylation and was selected for use in subsequent experiments.
Figure 2:
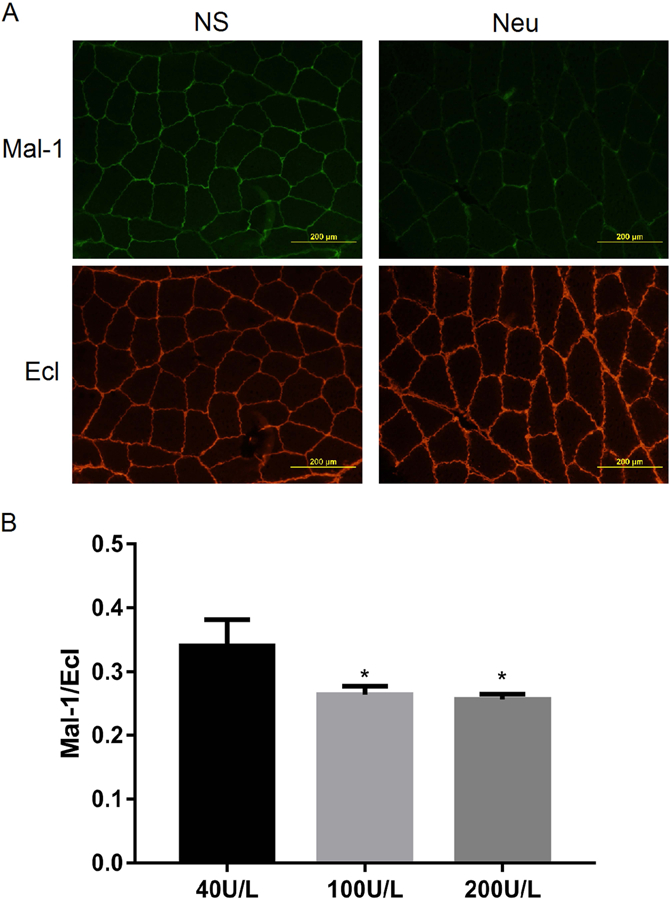
Lectin staining for neuraminidase activity. Mice were injected with three doses of neuraminidase in the left leg, and 2h later tissues were harvested for lectin staining. Representative images of the Ecl and Mal-1 staining in Panel A and quantitative analysis in Panel B (*P<0.05 vs. 40U/L, N=3/group).
Evaluation of potential off-target effects from histamine and neuraminidase
To exclude the possibility that histamine might have off-target effects on desialylation, and conversely that neuraminidase might have an effect on vascular permeability, the optimized doses of histamine and neuraminidase were injected IM in studies of 2×2 factorial design. Briefly, NS, 100uM His, 100U/L Neu and combined His+Neu were injected IM to the skeletal muscle 5 min after the IV injection of Evans blue. Two hours later, mice were humanely euthanized and TA and GA muscles were harvested for Evans blue assay and Mal-1/Ecl staining.
In these experiments, the left hindlimb muscles injected with normal saline (NS) yielded Evans blue contents that were virtually identical to uninjected right hindlimb muscle (Figure 3A). Consistent with our previous results showing that His increased vascular permeability in injected hindlimb muscles, Evans blue content in left hindlimbs (7.09±0.63 ug/ml) in the His group was significantly higher than in uninjected hindlimbs (1.96±0.76 ug/ml, Figure 3B). The combined His+Neu group also showed significantly higher Evans blue content in left hindlimbs (10.48±1.42 ug/ml) than right hindlimbs (3.19±0.59 ug/ml, Figure 3D). When Neu was injected alone, the difference in Evans blue content between left and right hindlimbs did not reach statistical significance (Figure 3C).
Figure 3:
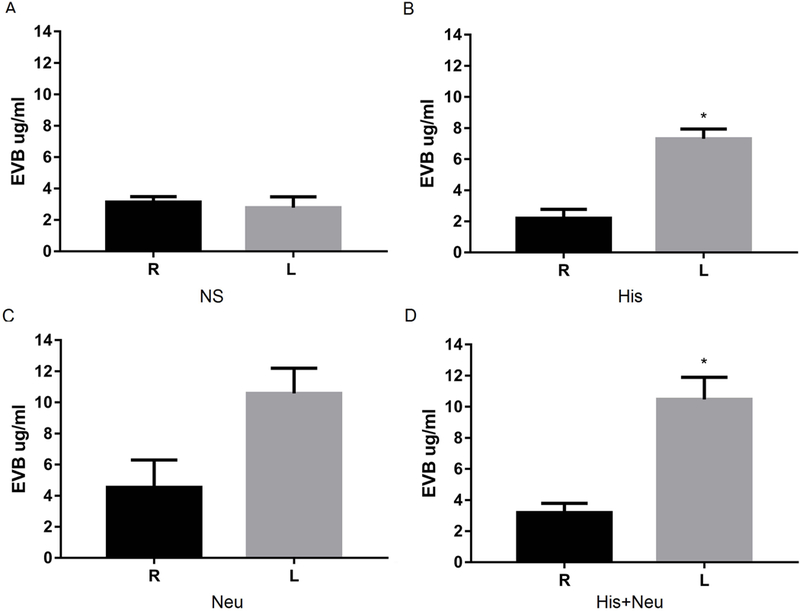
Histamine increases permeability in the injected leg with or without neuraminidase. Normal BALB/c mice were injected IV with Evans blue dye, followed 5 min later with NS (A), His (B), Neu (C) and His+Neu (D) injection (all IM). Mice GA+TA muscles were harvested for Evans blue assay (*P<0.05 L vs. R, N=3/group).
Representative fluorescent photomicrographs of Mal-1 and Ecl staining are shown in Figure 4A. The signal intensities from Mal-1 and Ecl in right (uninjected) and left (injected) hindlimbs were all nearly equivalent in the NS and His groups. However, the Neu and His+Neu groups showed weaker signals after Mal-1 staining and stronger signals after Ecl staining in left (treated) hindlimbs. Quantitative image analysis (Figure 4B) revealed that the ratios of Mal-1 to Ecl in left hindlimbs from the Neu (0.30±0.01) and His+Neu (0.31±0.02) groups were significantly decreased compared to that in the His (0.61±0.04) and NS (0.73±0.08) groups. As anticipated, no significant differences were found between the Mal-1/Ecl ratios in right hindlimbs from any of the four groups. Thus neuraminidase induced desialylation to a similar extent either in the presence or absence of histamine.
Figure 4:
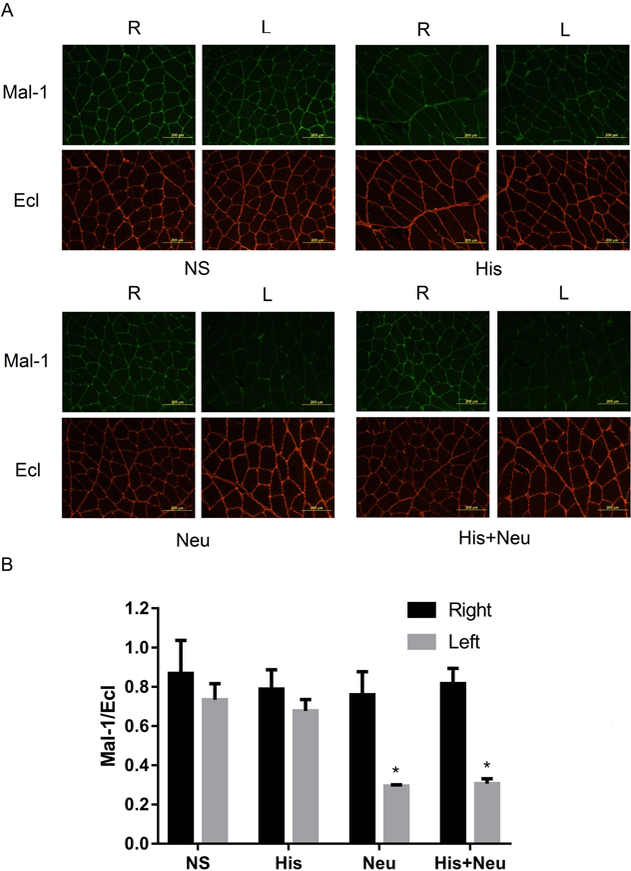
Neuraminidase significantly decreased the ratio of Mal-1 to Ecl with or without histamine in injected hindlimbs. Mice were injected IM with NS, His, Neu and His+Neu. GA+TA were harvested for Ecl and Mal-1 staining. Representative images of Ecl and Mal-1 staining (Panel A) and quantitative analysis of image intensity in each group (Panel B, *P<0.05 vs. NS-L, His-L, or any R-limb, N=3/group).
Desialylation vs. vascular permeability on AAV9-mediated gene delivery in skeletal muscle
In order to compare the effects of “unmasking” the endogenous AAV9 receptor by neuraminidase vs. increased vascular permeability by histamine on AAV9-mediated gene expression, the optimized doses of histamine and neuraminidase were injected IM 2h before the IV injection of an AAV9 vector expressing firefly luciferase in studies of 2×2 factorial design.
At 7 and 14 days post AAV9 injection, luciferase expression was monitored by non-invasive in vivo bioluminescence imaging. In Figure 5A and 5B, as indicated by the color bar, strong bioluminescence signals were observed in the left hindlimbs of mice in the Neu and His+Neu groups, whereas little signal was apparent in the non-injected right hindlimbs, either at 7 and 14 days post AAV9 injection. Moreover, essentially no signal was detected in either the left or right hindlimbs of mice in the His or NS (vehicle control) groups, either at 7 or 14 days post AAV9 injection.
Figure 5:

AAV9-mediated luciferase expression is significantly increased when Neu or His+Neu is injected IM 2h prior to IV injection of AAV9. Mice were IV injected via tail vein with AAV9 2h after IM injection of NS, His, Neu and His+Neu. Seven (Panel A) and 14 days later (Panel B), bioluminescence imaging of luciferase activity was performed. Quantitative analyses of average radiance in select regions of interest are plotted on a log scale in Panel C (*P<0.05 L vs. contralateral R hindlimb, N=4–6/group). Dashed line (#) indicates mean background radiance of control mice injected only with luciferin.
Region of interest (ROI) analysis was then used to determine the average radiance of photons emitted by each hindlimb, as shown in Figure 5C. Consistent with the raw images in Figure 5A, 7 days post AAV9 injection the Neu group demonstrated a 15-fold higher average radiance (p/s/cm2/sr) in left hindlimbs (21,333,333±4,202,354) than right hindlimbs (1,380,000±370,390). Similarly, the His+Neu group showed 30-fold more average radiance in left hindlimbs (19,200,000±7,110,204) than in right hindlimbs (639,000±171,286). The His group showed no significant difference in average radiance between left (936,650±155,768) and right hindlimbs (829,417±316,831), similar to the results obtained from left (1,741,074±785,919) and right hindlimbs (629,300±277,610) in the NS group. Average radiance in positive hindlimbs increased modestly from 7 to 14 days post AAV9 injection, with average radiance in the left hindlimbs in the Neu group (47,173,333±9,796,272) and His+Neu (66,622,000±24,428,537) groups reaching values that were elevated by 20–29 fold compared to the right hindlimbs, respectively (2,317,167±743,799 for Neu right, 2,297,000±432,532 for His+Neu right). In contrast, little or no difference was found between left and right hindlimbs in the His (2,556,567±1,370,456 vs. 1,044,983±512,759) or NS (2,311,500±593,645 vs. 874,750±438,568) groups, respectively. Thus in the presence or absence of histamine, treatment with neuraminidase increased luciferase signal intensity between 15–30 fold in left hindlimbs compared to right hindlimbs both at 7 and 14 days post AAV9 injection. Interestingly, a trend towards doubling was observed in average radiance values from the Neu-treated groups between Days 7 and 14 day post-injection, indicating that AAV9-mediated gene expression may still be increasing in this model during this time period.
Bioluminescence imaging (BLI) provides for the serial, non-invasive assessment of luciferase activity over time. However, BLI results can be influenced by tissue depth and the differential absorption of photons by various tissues. To exclude these variables, the serial BLI results were augmented by traditional luciferase assays of tissue lysates obtained at the terminal time point. These tissue lysates were also assayed for AAV9 genome copy number by qPCR to assess the efficiency of transduction.
As shown in Figure 6A, LGA muscles in both the Neu group (205,309,457±40,058,390) and the His+Neu group (242,726,083±87,672,902) demonstrated 60–100 fold higher luciferase activities as compared to the contralateral RGA muscles (2,030,928±618,339 for Neu right, 4,269,576±1,271,464 for His+Neu right). The LTA muscles in the Neu and His+Neu groups also showed approximately 80 fold higher luciferase activity than in the contralateral RTA muscles. The remaining two groups showed activity levels in the LGA and LTA muscles that varied little from those found in the untreated contralateral right hindlimbs.
Figure 6:
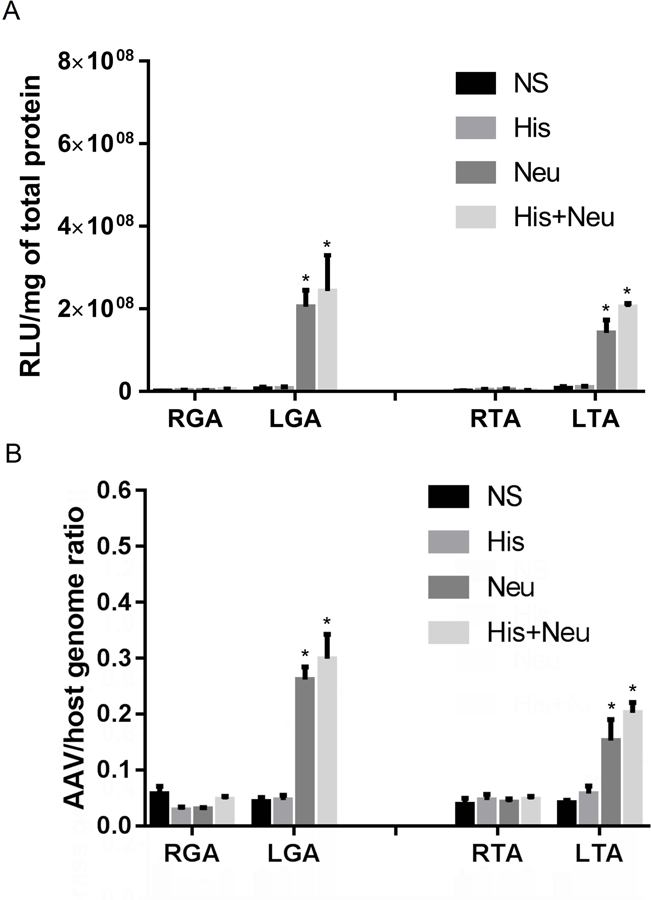
Intramuscular Neu and His+Neu injection 2h before systemic administration of AAV9 increase both reporter gene expression and AAV/host genome ratio. Luciferase enzymatic activity (Panel A) and AAV/host genome ratios (Panel B) in right (R) and left (L) GA and TA muscles 14 days after IV injection of AAV9 are shown (*P<0.05 L vs. contralateral R hindlimb, N=4–6/group).
AAV9 genome copy numbers (Figure 6B) as measured by qPCR showed trends for each group that largely paralleled results from the assays for luciferase activity. For example, neuraminidase increased genome copy numbers by 8.7-fold in LGA vs. RGA muscles, and by 3.6-fold in LTA vs. RTA muscles, both in the presence or absence of histamine.
Figure 7 shows quantitative measurements of luciferase activity and AAV/host genome ratios in LGA muscles from the four treatment groups as compared to a panel of seven other organs, all harvested 14 days post AAV9 injection. Note that LGA extracts from muscles treated with either Neu or His+Neu yielded >100 fold more luciferase activity than the off-target tissues with the next highest luciferase activities (heart and liver), which in turn were approximately 5–10 fold higher than the remaining off-target tissues (including brain). From Figure 7B, it can be seen that the ratio of AAV/host genomes was nearly 10-fold higher in the liver than in the intended target muscle tissue (LGA) treated with either Neu or His+Neu. Thus while the Neu and His+Neu treatment regimens increased the AAV/host genome ratios by approximately 6-fold as compared to NS control-treated muscle, the overall transduction rate (as measured by genome ratio) in treated LGA muscle was still only ~one-tenth of that seen in the liver. As expected, the effects of injecting Neu and His+Neu into muscle tissue were localized to those muscles that were injected, with little or no effect in off-target tissues. This held true for both the levels of transgene expression (as reflected by luciferase activity) and for the levels of AAV transduction (as reflected by AAV/host genome ratio).
Figure 7:
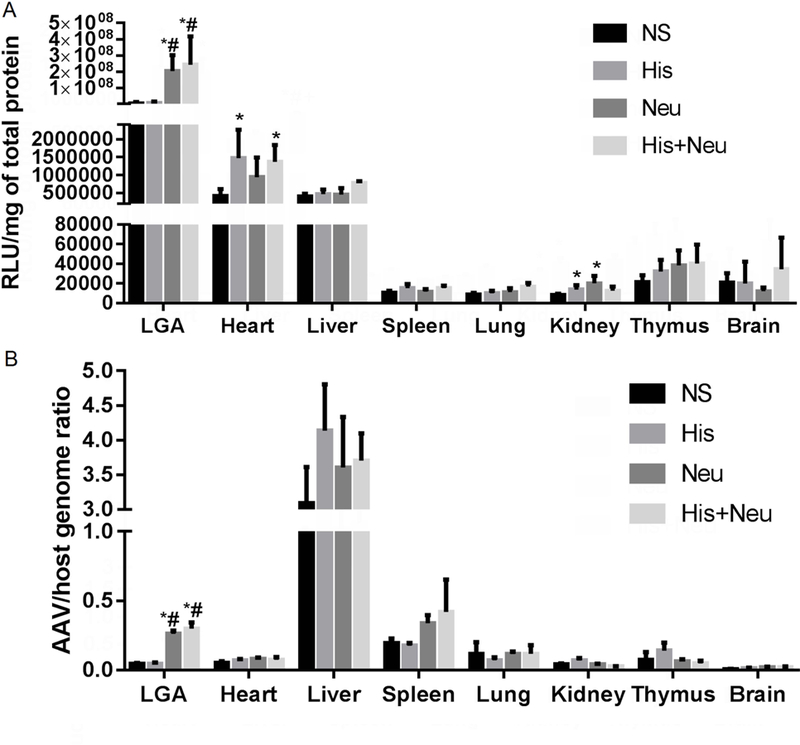
Biodistribution of AAV9-mediated gene expression versus transduction in various tissues. In Panel A, intramuscular injection of Neu or His+Neu 2h before the systemic administration of AAV9 significantly increased luciferase activity in targeted LGA muscles over those injected with NS (vehicle) or His alone. Off-target gene expression was detected at much lower levels (100–400 fold lower) in the heart and liver, whereas luciferase activity was negligible in the spleen, lung, kidney, thymus and brain. In Panel B, AAV/host genome ratios in LGA muscles from the four treatment groups are compared to a panel of seven other organs 14 days after IV injection of AAV9 carrying a muscle specific promoter. Intramuscular injection of Neu or His+Neu 2h before the systemic administration of AAV9 significantly increased AAV/host genome ratios in targeted LGA muscles over those injected with NS (vehicle) or His alone. Off-target AAV9 genomes were detected at similar levels in the spleen, and at much higher levels (10–30 fold higher) in the liver, whereas genome ratios remained consistently low in all other tissues examined (heart, lung, kidney, thymus and brain (*P<0.05 vs NS, #P<0.05 vs His, N=4–6/group).
In order to further characterize the dynamics of the effects of histamine and neuraminidase upon AAV9-mediated transduction and gene expression, we shortened the time between histamine/neuraminidase treatment and AAV injection from 2h to 5 min. In this set of experiments, we also explored the possibility that a needle stick alone (without the injection of normal saline) might impact the efficiency of AAV9-mediated transduction. Quantitative analysis of average radiance values (Figure 8C) showed that average radiance in the left hindlimbs in the Neu (91,080,000±11,370,337) and His+Neu (73,724,000±21,665,988) groups at 14 days post AAV9 injection were increased by 50–60 fold compared to the respective right hindlimbs (5,595,600±3,194,891 for Neu right, 4,424,600±1,390,606 for His+Neu right). In agreement with the previous experiment shown in Figure 5, little difference was noted between left and right hindlimbs in mice treated with either a needle-stick or with His.
Figure 8:
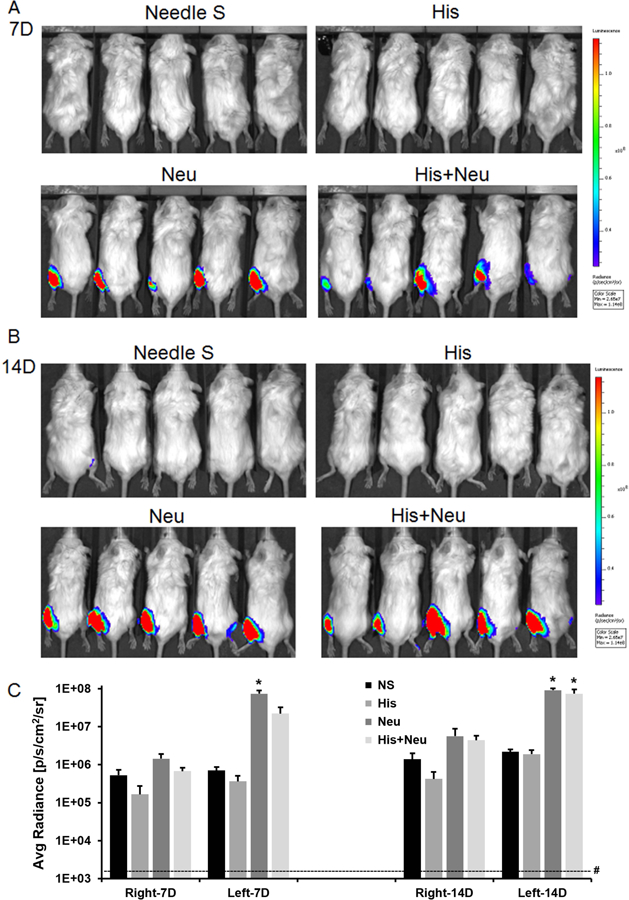
AAV9-mediated luciferase expression is significantly increased when Neu or His+Neu is injected IM 5 min prior to IV injection of AAV9. Mice were IV injected via tail vein with AAV9 5min after IM injection of NS, His, Neu and His+Neu. Seven (Panel A) and 14 days later (Panel B), bioluminescence imaging of luciferase was performed. Quantitative analyses of average radiance in select regions of interest are plotted on a log scale in Panel C (*P<0.05 L vs. contralateral R hindlimb, N=5/group). Dashed line (#) indicates mean background radiance of control mice injected only with luciferin.
Finally, the quantitative analysis of luciferase activity in Figure 9A showed that the Neu and His+Neu groups had much higher luciferase expression in LGA and LTA than RGA and RTA, or from hindlimbs in His and NeedleS groups. The AAV9 genome copy numbers in this experiment were also entirely consistent with the values obtained when the time between histamine/neuraminidase treatment and AAV injection was 2h (Figure 6B) rather than 5 min (Figure 9B).
Figure 9:
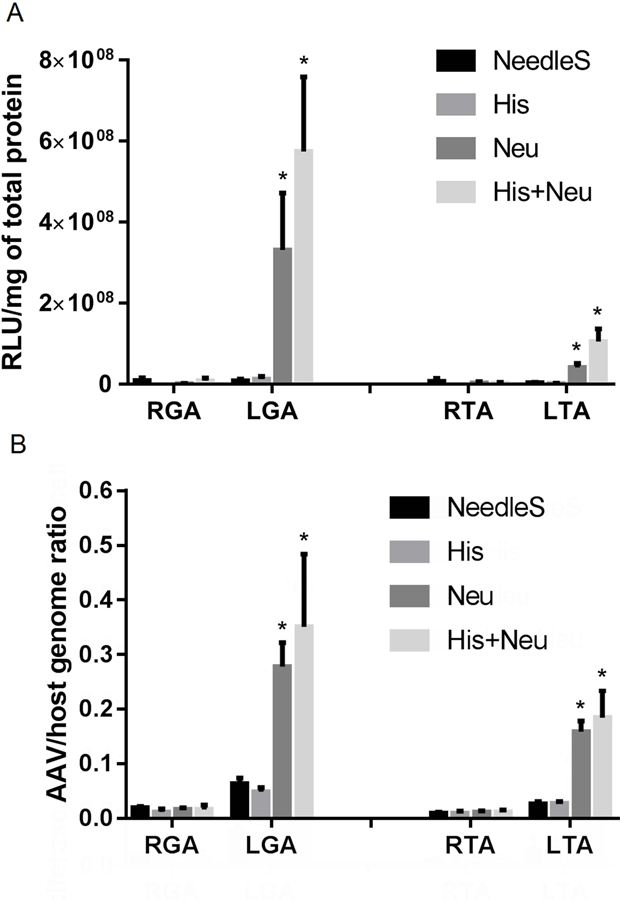
Intramuscular Neu and His+Neu injection 5 min before systemic administration of AAV9 increase both reporter gene expression and AAV/host genome ratio. Luciferase enzymatic activity (Panel A) and AAV/host genome ratios (Panel B) in right (R) and left (L) GA+TA 14 days after IV injection of AAV9 are shown (*P<0.05 L vs. contralateral R hindlimb, N=5/group).
Discussion
For the treatment of PAD, systemic delivery of AAV9 offers an attractive option for selectively targeting therapeutic gene expression to ischemic muscle tissue. In previous work, our group showed that AAV9 vectors harboring a muscle-specific promoter selectively target luciferase activity to ischemic muscle in the mouse hindlimb, with gene expression in ischemic left gastrocnemius (LGA) muscles enhanced by 34-fold over the fully perfused contralateral RGA.[16] This same study also included experiments with Evans blue dye and fluorescently labeled lectins to identify vascular permeability and desialylation of the cell-surface glycans as potential mechanisms underlying the selective targeting of AAV9 to ischemic muscle.
In the current study, we explored the possibility of reconstituting the ischemic induction of AAV9-mediated transduction using natural biologic interventions known to increase vascular permeability (histamine) and to desialylate cell-surface glycans (neuraminidase). In studies of 2×2 factorial design, we investigated the effects of the intramuscular (IM) injection of neuraminidase and histamine (alone and in combination) on the efficacy of systemically administered AAV9 vectors carrying a muscle-specific reporter gene. Our initial experiments were focused on optimizing the doses of histamine (Figure 1) and neuraminidase administration (Figure 2). This was deemed necessary to develop an experimental time line that was compatible with the co-administration of both histamine and neuraminidase. Additional experiments were performed with surrogate assays relevant to AAV9 transduction to confirm that histamine still increased vascular permeability, even when co-injected with neuraminidase (Figure 3); and that neuraminidase still mediated desialylation, even when co-injected with histamine (Figure 4). Neuraminidase has previously been co-instilled locally with AAV9 to improve in vivo gene transfer to the lung in mice[17], and has been injected systemically to alter the distribution of systemically administered AAV[28], but the potential of intramuscularly-injected neuraminidase to bolster the efficacy of systemically-injected AAV9 had yet to be explored.
After suitable conditions were identified, we undertook experiments to quantitatively assess the impact of intramuscularly injected histamine and neuraminidase (alone and in combination) on the transduction of hindlimb muscle by systemically injected AAV9. Initial results were obtained using in vivo bioluminescence imaging (BLI), which may be less accurate and precise than tissue-based assays but has the advantage of being non-invasive, thus enabling longitudinal studies (Figures 5&8). We found that comparisons of left to right hindlimbs by average radiance values at 7 days post-AAV injection were highly predictive of the 14 day results, even though luciferase activity in most groups roughly doubled over this same time period. This finding is consistent with our previous study in ischemic mouse hindlimbs, where average radiance levels at 14 days post-injection trended higher than the 7 day results.[16]
Tissue-based assays to determine luciferase activity and genome copy numbers were conducted after animals were humanely euthanized following the last BLI session on Day 14 post-injection. The results of the in vitro luciferase assays (Figures 6A&9A) were largely in accord with those obtained from BLI in vivo on Day 14 (Figures 5C&8C). However, the ratios of left to right hindlimb luciferase activity were consistently higher by in vitro luciferase assay (60–100 fold differences) than by in vivo BLI (15–30 fold differences). This is not surprising given that the dynamic range of in vitro luciferase assays can be extended by sample dilution, whereas no analogous optimization step is possible in vivo using BLI. Tissue attenuation of light penetration can also be expected to limit the dynamic range of BLI as compared to a relatively transparent in vitro sample assayed in a luminometer.[29] The qPCR results showed that AAV/host genome ratios in left (treated) vs. right (untreated) hindlimbs largely paralleled results from the luciferase assays. That is, there was little difference between the negative control and His groups, and every group treated with Neu or His+Neu showed a significantly elevated ratio of AAV to host genomes in treated vs. untreated (contralateral) hindlimbs. A consistent finding, however, was that the increase in AAV/host genome ratios in the Neu and His+Neu groups (3.6–8.7 fold in Figure 7B) was inadequate to fully account for the dramatic increase in luciferase activity in the Neu and His+Neu groups (60–100 fold in Figure 6A). Assuming a linear response between AAV9 gene dose and reporter gene expression[30], this result suggests that Neu may enhance AAV9-mediated gene expression by other mechanisms beyond the increase in transduction rate due to desialylation and exposure/activation of the primary AAV9 receptor/attachment factor. Given the results of the current experiments that included histamine, this additional mechanism would not appear to involve vascular permeability. Another noteworthy finding from these experiments was that the ratio of reporter gene expression to AAV genomes was approximately 5000-fold higher in the Neu-treated LGA muscles than in livers from those same animals, highlighting the high degree of tissue specificity conferred by the modified muscle creatine kinase promoter.
The mechanisms underlying AAV cellular binding, uptake, and subcellular trafficking are extremely complex and have yet to be fully elucidated, but the recent discovery of the AAV receptor (AAVR) in an unbiased genetic screen has yielded new insight.[31] The AAVR was shown to play an essential role in infection by multiple AAV serotypes (including AAV1, AAV2, AAV3b, AAV5, AAV6, AAV8 and AAV9) by mediating rapid endocytosis from the plasma membrane and trafficking to the trans-Golgi network. Subsequent work showed that AAVR is an N-linked glycosylated membrane protein that uses the host cell’s endosomal pathway to cycle between the plasma membrane and the trans-Golgi network.[32] The finding of N-linked glycosylation on the AAVR opens the possibility that it may function in both the initial attachment and the secondary internalization of AAV9 capsids. One might speculate that this potential duality of function in the AAVR may help explain why ischemia and desialylation have such dramatic impacts on the efficiency of AAV9-mediated gene expression.
While the results of our current study may yield insight into new avenues for localizing and enhancing the potential of AAV9-mediated gene expression in skeletal muscle, this work is not without limitation. In particular, while these studies were undertaken to elucidate potential mechanisms responsible for the ischemic induction of AAV9 mediated gene expression,[16] it must be acknowledged that the Neu injection model described here is only an animal model of desialylation and in no way mimics the full spectrum of hypoxic, free-radical and pro-inflammatory mechanisms operative in human PAD. Thus while every attempt was made to apply Neu at a dose that induced a change in Mal-1/Ecl ratio similar to that observed in the mouse hindlimb ischemia model of PAD,[16] it remains to be seen whether the chronic, partial ischemia characteristic of human PAD elicits similar changes in Mal-1/Ecl ratio that might predict enhanced susceptibility to AAV9-mediated gene expression. Similarly, although this study failed to demonstrate any advantage of using histamine to enhance vascular permeability, previous studies have demonstrated enhanced transgene expression after infusing recombinant AAV2 into the femoral artery in conjunction with histamine-induced endothelial permeabilization.[23] Experimental variables such AAV serotype, vector dose, mode and timing of administration, etc. may explain why histamine largely failed to enhance AAV9-mediated gene expression in the current study, even while Evans blue assays confirmed a marked increase in vascular permeability.
In conclusion, the current results provide a number of interesting findings: First, the enzymatic activity of neuraminidase significantly enhances AAV9-mediated gene expression in skeletal muscle following systemic delivery in vivo by “un-masking” the endogenous AAV9 receptor. Second, in vivo reconstitution experiments indicate that the mechanism of receptor un-masking dominates over any potential effect of histamine on vascular permeability. Third, simple IM injection of neuraminidase 5 min or 2 h prior to IV injection of AAV9 recapitulates the effect of ischemic induction and potentiates transgene expression by 60–100 fold (based on quantitative luciferase assays of tissue lysates). Fourth, consistent with its mechanism of action in un-masking the endogenous AAV9 receptor, IM injection of neuraminidase also increased the ratio of AAV to host genomes in target muscle tissue. Fifth, the combination of the AAV9 capsid and the muscle-specific tMCK promoter is highly selective in targeting Neu-treated muscle tissue, with levels of gene expression that are 60–100 fold higher than in un-treated muscle or in the next most vulnerable off-target tissues (heart and liver). While several hurdles remain, these findings could potentially be used in the future to make muscle-directed human gene therapy more robust and practical by lowering the required dose of AAV9 by some 60–100 fold, while at the same time reducing the potential for undesirable transgene expression in off-target tissues.
Supplementary Material
Acknowledgements
This work is supported by 1R01 HL116455 to BHA and BAF from the NIH/NHLBI, and by the Chinese Scholarship Council (201506270091 to HZ).
Footnotes
Conflicts of Interest Statement
The authors have no conflicts of interest to disclose.
References
- 1.Samulski RJ, Muzyczka N AAV-mediated gene therapy for research and therapeutic purposes. Annual Review of Virology 2014; 1: 427–451 [DOI] [PubMed] [Google Scholar]
- 2.Hammoudi N, Ishikawa K, Hajjar RJ Adeno-associated virus-mediated gene therapy in cardiovascular disease. Current Opinion in Cardiology 2015; 30: 228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murlidharan G, Samulski RJ, Asokan A Biology of adeno-associated viral vectors in the central nervous system. Frontiers in Molecular Neuroscience 2014; 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buch PK, Bainbridge JW, Ali RR AAV-mediated gene therapy for retinal disorders: from mouse to man. Gene therapy 2008; 15: 849. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain JR, Chamberlain JS Progress toward gene therapy for Duchenne muscular dystrophy. Mol Ther 2017; 25: 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.High KA, Anguela XM Adeno-associated viral vectors for the treatment of hemophilia. Human Molecular Genetics 2016; 25: R36–R41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. The New England journal of medicine 2014; 371: 1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tardieu M, Zerah M, Husson B, et al. Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying human SGSH and SUMF1 cDNAs in children with mucopolysaccharidosis type IIIA disease: results of a phase I/II trial. Hum Gene Ther 2014; 25: 506–516 [DOI] [PubMed] [Google Scholar]
- 9.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. The Lancet 2017; 390: 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm D, Zolotukhin S E pluribus unum: 50 years of research, millions of viruses, and one goal--tailored acceleration of AAV evolution. Mol Ther 2015; 23: 1819–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duque S, Joussemet B, Riviere C, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther 2009; 17: 1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saqib A, Prasad K- MR, Katwal AB, et al. Adeno-associated virus serotype 9-mediated overexpression of extracellular superoxide dismutase improves recovery from surgical hind-limb ischemia in BALB/c mice. J Vasc Surg 2011; 54: 810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebas B, Galley J, Renaud-Gabardos E, et al. Therapeutic benefits and adverse effects of combined proangiogenic gene therapy in mouse critical leg ischemia. Ann Vasc Surg 2017; 40: 252–261 [DOI] [PubMed] [Google Scholar]
- 14.Boden J, Lassance-Soares RM, Wang H, et al. Vascular regeneration in ischemic hindlimb by adeno-associated virus expressing conditionally silenced vascular endothelial growth factor. Journal of the American Heart Association 2016; 5: e001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annex BH Therapeutic angiogenesis for critical limb ischaemia. Nature reviews Cardiology 2013; 10: 387–396 [DOI] [PubMed] [Google Scholar]
- 16.Katwal AB, Konkalmatt PR, Piras BA, et al. Adeno-associated virus serotype 9 efficiently targets ischemic skeletal muscle following systemic delivery. Gene therapy 2013; 20: 930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell CL, Vandenberghe LH, Bell P, et al. The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice. The Journal of clinical investigation 2011; 121: 2427–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen S, Bryant KD, Brown SM, et al. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. The Journal of biological chemistry 2011; 286: 13532–13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Miller E, Agbandje-McKenna M, et al. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. Journal of virology 2006; 80: 9093–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao W, Chirmule N, Berta SC, et al. Gene therapy vectors based on adeno-associated virus type 1. Journal of virology 1999; 73: 3994–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanetti L, Vignini A, Raffaelli F, et al. Sialic acid and sialidase activity in acute stroke. Disease Markers 2008; 25: 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korthuis RJ, Granger DN, Townsley MI, et al. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circulation Research 1985; 57: 599. [DOI] [PubMed] [Google Scholar]
- 23.Greelish JP, Su LT, Lankford EB, et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nature medicine 1999; 5: 439–443 [DOI] [PubMed] [Google Scholar]
- 24.Sahenk Z, Galloway G, Clark KR, et al. AAV1.NT-3 gene therapy for charcot-marie-tooth neuropathy. Mol Ther 2013; 22: 511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ried MU, Girod A, Leike K, et al. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. Journal of virology 2002; 76: 4559–4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad KM, Xu Y, Yang Z, et al. Topoisomerase inhibition accelerates gene expression after adeno-associated virus-mediated gene transfer to the mammalian heart. Mol Ther 2007; 15: 764–771 [DOI] [PubMed] [Google Scholar]
- 27.Wu JC, Inubushi M, Sundaresan G, et al. Optical imaging of cardiac reporter gene expression in living rats. Circulation 2002; 105: 1631–1634 [DOI] [PubMed] [Google Scholar]
- 28.Shen S, Bryant KD, Sun J, et al. Glycan binding avidity determines the systemic fate of adeno-associated virus type 9. Journal of virology 2012; 86: 10408–10417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badr CE Bioluminescence imaging: basics and practical limitations. Methods in molecular biology 2014; 1098: 1–18 [DOI] [PubMed] [Google Scholar]
- 30.Prasad KM, Xu Y, Yang Z, et al. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene therapy 2011; 18: 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillay S, Meyer NL, Puschnik AS, et al. An essential receptor for adeno-associated virus infection. Nature 2016; 530: 108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillay S, Zou W, Cheng F, et al. AAV serotypes have distinctive interactions with domains of the cellular receptor AAVR. Journal of virology 2017; 91: 10.1128/jvi.00391-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


