Abstract
Glypicans are a group of cell surface glycoproteins in which heparan sulfate glycosaminoglycan chains are covalently linked to a protein core. The glypican gene family is broadly conserved across animal species and plays important roles in biological processes. Glypicans can function as co-receptors for multiple signaling molecules known for regulating cell growth, motility and differentiation. Some members of the glypican family, including glypican 2 (GPC2) and glypican 3 (GPC3), are expressed in childhood cancers and liver cancers, respectively. Antibody-based therapies targeting glypicans are being investigated in preclinical and clinical studies, with the goal of treating solid tumors that do not respond to standard therapies. These studies may establish glypicans as a new class of therapeutic targets for treating cancer.
Keywords: glypican, Wnt signaling, liver cancer, childhood cancer, antibody-based therapy
Glypicans as Potential Cancer Targets
Glypicans are proteoglycans with heparan sulfate (HS) chains that are linked to the cell surface via a glycosylphosphatidylinositol (GPI) anchor. Glypicans have the ability to regulate cellular morphology and cellular behaviors including survival and differentiation; thus, it is not surprising that changes in glypicans expression have been reported in various human cancers. The abnormal expression of glypicans regulates tumor proliferation and progression by modulating Wnt [1, 2], hedgehog (Hh) [3] and other signaling pathways.
Selecting appropriate targets in solid tumors is challenging. Recent studies show that glypican 2 (GPC2) [4, 5] and glypican 3 (GPC3) [6] are highly specific for tumor tissues. More importantly, GPC2 and GPC3-targeted antibody-based therapies have eliminated established tumors in preclinical studies, and unleashed the potential of targeting glypicans for cancer therapy.
Expression of Glypicans in Cancer
Glypicans are predominantly expressed during embryonic development. Since they regulate a variety of growth and survival factors during morphogenesis, recent reports indicate that changes in glypican expression may be associated with tumorigenesis and progression of cancers.
GPC1
Expression of GPC1 has been linked with proliferation, angiogenesis and metastasis of pancreatic cancer cells [7, 8]. GPC1 is overexpressed in breast cancer and modulates the effects of heparin-binding growth factors in breast cancer cells [9]. The expression of GPC1 is also upregulated in esophageal squamous cell carcinoma (ESCC) and correlated with poor survival [10]. Additionally, GPC1 overexpression is found in gliomas and might play a role in angiogenesis [11, 12].
GPC2
GPC2 is one of several mRNA transcripts highly expressed in multiple childhood cancers, including neuroblastoma [13]. An ideal therapeutic target may have limited expression on normal human tissues. Recent studies demonstrate that the expression of GPC2 protein is significantly increased in neuroblastoma and undetectable in normal tissues, including the brain, heart, lung and kidney, indicating that GPC2 is a suitable tumor antigen in neuroblastoma [4, 5].
GPC3
GPC3 is expressed in over 70% of hepatocellular carcinoma (HCC) [14], the most common type of liver cancer accounting for approximately 90% of all cases [15]. Notably, its expression is not detected in non-malignant tissues [16]. Moreover, GPC3 expression is correlated with poor prognosis in HCC patients [17]. Thus, GPC3 is a suitable biomarker and prognostic factor of HCC, and an attractive target for HCC therapy. The overexpression of GPC3 has also been reported in hepatoblastoma, lung squamous cell carcinoma (LSCC), testicular and ovarian yolk sac tumors, melanoma, ovarian clear cell carcinoma and other cancers [18–23].
The most common isoform of GPC3 is isoform 2, which encodes a 70-kDa core protein with 580 amino acids; additionally, three other variants have been detected that encode alternative spliced forms [24]. Given these findings, expression analysis of GPC3 (or other glypicans) isoforms in normal tissues and cancers may be important for the development of therapeutic intervention or diagnostics in the era of precision medicine.
In addition, several studies revealed significant intratumoral heterogeneity of GPC3 expression levels in HCC tissues [25–27]. These observations raise a critical issue that the immunoreactivity found in a small needle biopsy specimen may not represent the overall level of GPC3 expression within tumor. Thus, acquisition of multiple samples from different regions of tumor sites are important to understand the heterogeneous expression of glypicans in cancer. The impact of such diversity on clinical outcomes such as response to glypican-targeted therapies remains largely unknown and is an area of research worthy of intensive efforts.
Expression of GPC3 may also inhibit cell proliferation. . A loss-of-function mutation in GPC3 causes Simpson-Golabi-Behmel syndrome (SGBS), an X-linked disorder characterized by pre- and post-natal overgrowth. GPC3-deficient mice (GPC3−/−) also display similar phenotypic features of SGBS [28]. In GPC3 transgenic mice, overexpression of GPC3 in hepatocytes suppresses hepatocyte proliferation and liver regeneration [29].
GPC4/5/6
Similar to GPC1, an increased expression of GPC4 has been reported in pancreatic cancer [30]. GPC5 is overexpressed in rhabdomyosarcoma and stimulates rhabdomyosarcoma cell proliferation [31–33]. However, GPC5 expression is downregulated in breast and prostate cancer [34, 35], indicating GPC5 may have distinct roles in different cancer types. Although one report suggests that GPC5 is upregulated in non-small cell lung cancer (NSCLC) [36], more studies demonstrate that GPC5 is a tumor suppressor in NSCLC and low expression of GPC5 is associated with poor survival in lung adenocarcinoma [37, 38]. Additionally, GPC6 expression is significantly higher in gastric cancer tissues compared to that in normal tissues [39]. Overexpression of GPC6 has also been detected in ovarian cancer [40].
To summarize, glypicans are expressed in abnormally high levels in various types of cancers, suggesting that cell surface glypicans may emerge as a new group of therapeutic targets in cancer.
Glypicans in Cancer
Glypicans are essential components of the extracellular matrix (ECM). They are involved in many important biological and pathological processes by mediating cell-ECM and cell-cell interactions. The most significant characteristic of glypicans is that they contain various numbers of HS chains close to the cell membrane; this unique structure affords glypicans the ability of to rescue and sequester different growth factors, morphogens, chemokines and cytokines. Glypicans concentrate these factors and form a gradient around the ECM and cell membrane, which facilitates the recognition of cell membrane receptors with reduced threshold. In addition, glypicans specifically bind to several factors through the identified domain within their HS chains as well as the core protein. In this sense, glypicans act as essential co-receptors and trigger the activation of intracellular signaling including Wnt, Hh, FGF, and many other signaling pathways.
Wnt Signaling
Wnt signaling plays pivotal roles in many biological and pathological processes, including embryonic development, differentiation, cell polarity and tumorigenesis. It has been demonstrated that glypicans act as Wnt modulators in cancer. For example, GPC2 can positively regulate canonical Wnt signaling in neuroblastoma, as silencing GPC2 inactivates Wnt/β-catenin signaling and reduces the expression of target genes [5]. Although GPC3 is a tumor-promoting gene in HCC by activating Wnt/β-catenin signaling [24, 41, 42], GPC3-induced regulation on Wnt signaling is based on the specific cellular environment. Studies showed that depletion of GPC3 inhibited non-canonical Wnt/JNK signaling, while concomitantly activated canonical Wnt/β-catenin signaling [28]. In murine mammary adenocarcinoma cells, GPC3 inhibits canonical Wnt signals that are involved in cell proliferation and survival, whereas activates non-canonical Wnt signals that determine cell morphology and migration [43]. Moreover, a recent study has shown that GPC4 is co-localized with different Wnts to lipid raft and non-lipid raft microdomains and thereby regulates distinct Wnt signaling pathways [44]. In lung adenocarcinoma and prostate cancer, GPC5 inhibits Wnt/β-catenin signaling to suppress tumor cell proliferation, migration, invasion and epithelial-mesenchymal transition (EMT) [38, 45, 46]. GPC6 promotes tumor cell growth and invasiveness by inhibiting canonical Wnt signaling and activating Wnt5a/JNK/p38 MAPK signaling [47].
GPC3 interacts with both Wnt and frizzled (FZD) to form a tripartite complex and triggers downstream signaling (Figure 1). Both the core protein and the HS chains may be involved in GPC3-modulated Wnt signaling. Previous reports indicate that GPC3 HS chains may not be required for Wnt binding [41], but are essential for interaction with FZD [2]. Our studies identified a Wnt-recognizing domain on the HS chains of GPC3 using a HS-specific antibody that blocks GPC3 and Wnt3a interaction [1, 48]. This unique Wnt-binding region is at least four disaccharides in length with 6-O sulfation or shorter when 3-O sulfation is present. Sulfatase 2 (SULF2), an enzyme with 6-O-desulfatase activity is upregulated in HCC. Desulfation by SULF2 releases HS-stored Wnts and may activate canonical Wnt signaling. In addition, we and others have shown that the core protein of GPC3 without HS also bound to Wnt [41, 49], although further structural and functional studies will be necessary to reveal the precise Wnt binding domain on the protein core of GPC3 or other glypicans.
Figure 1. GPC3 Promotes Wnt Signaling through the Core Protein and HS Chains.
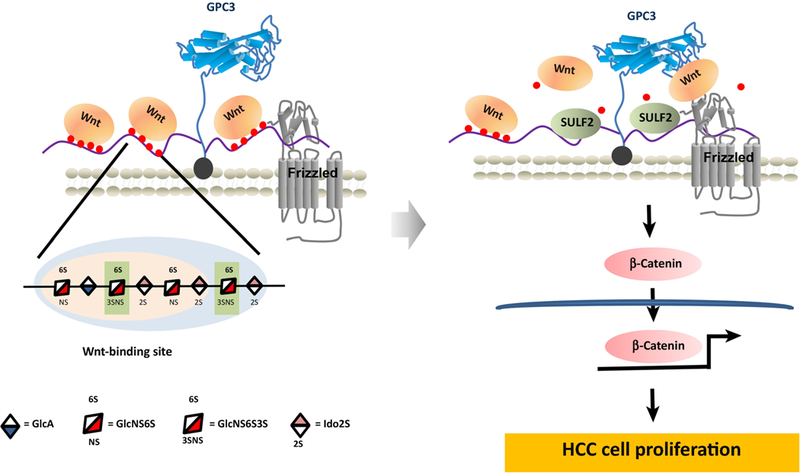
GPC3 interacts with frizzled through HS chains. Meanwhile, the HS chains also contain Wnt specific binding motif which needs 6-O-sulfation with 4 disaccharides in length (the light blue region). When an additional 3-O-sulfation presents, the region that recognized by Wnt could as short as 3 disaccharides in length (the light apricot region). GPC3 rescues circulating Wnt and acts as a Wnt storage site on cell surface through the HS chains. When SULF2 reduces the modification of the 6-O-sulfation on HS chains, the stored Wnt is released and forms a tripartite complex with glypican-3 core protein and frizzled, therefore activates canonical Wnt signaling, and promotes HCC cell proliferation.
R-spondin (RSPO) proteins emerged as important growth factors that drive the renewal of epithelial stem cells in many adult vertebrate tissues [50]. They amplify target cell sensitivity to Wnt ligands by increasing Wnt receptor levels [51, 52]. Translocations and mutations in regulators of RSPO have been reported in multiple cancers (e.g. colon cancer) [53, 54]. More importantly, a recent study has shown that GPC4 and GPC6 are required for RSPO-enhanced Wnt signaling in response to physiologically relevant low levels of Wnt [55]. RSPOs can potentiate Wnt signals through interactions between HS chains and the thrombospondin type 1 (TSP)/basic region (BR) domains of RSPOs [56].
Hedgehog (Hh) Signaling
Glypicans exhibit opposing roles in Hh signaling, which is important for embryonic morphogenesis [3, 57]. Among these roles, GPC3 is a potent inhibitor of Hh signaling (Figure 2). Loss of function mutations of GPC3 causes SGBS, partially due to hyper-activation of the Hh pathway [58, 59]. GPC3 binds to sonic hedgehog (Shh) and indian hedgehog (Ihh) with high affinity in a HS-independent manner, resulting in a strong competition with Patched (Ptc) for Hh binding [60]. The HS chains of GPC3 are required for low-density-lipoprotein receptor related protein-1 (LRP1) mediated-glypican/Hh complex endocytosis [61]. GPC5 and GPC6 show stimulating effects on Hh activation. In contrast to GPC3, GPC5 binds to both Hh and Ptc through its glycosaminoglycan chains and promotes Hh signaling in rhabdomyosarcoma cells, possibly by facilitating or stabilizing the interaction between Hh and Ptc1 [31]. Recently, GPC6 has been reported to stimulate Hh signaling by interacting with Hh and Ptc1 through its core protein and glycosaminoglycan chains, respectively [62].
Figure 2. GPC3 Inhibits Hedgehog Signaling by Competing with Patched for Hh Binding.

GPC3 binds to Shh and Ihh with high affinity. LRP1 promotes glypican-3/Hh complex internalization in a HS dependent way. The internalized Hh is transported to lysosome for degradation. Therefore, patched without Hh binding inhibits Smoothened activation and blocks Hedgehog signaling.
Fibroblast Growth Factor (FGF) Signaling
FGF is one of the heparin-binding growth factors. Many studies reveal that glypicans modulate FGF signaling during tumor cell proliferation. In breast and pancreatic cancer, GPC1 is upregulated and promotes tumor mitogenic signaling by modulating many heparin-binding growth factors, including FGF2 [7, 9]. There is evidence demonstrating GPC1 cooperates with type V collagen to concentrate FGF2 at the cell-ECM interface, thereby affects ECM stability and tumor cell proliferation [63]. GPC1 also contributes to the enhanced signaling of FGF-2/FGFR1c in glioma cells, likely due to highly elevated 2-O-sulfation and 6-O-sulfation-containing disaccharides [12]. Additionally, GPC1 is upregulated in glioma vessels, causing enhanced FGF signaling and glioma angiogenesis [11]. In GPC1 knockout KRAS-driven genetic mouse model of pancreatic ductal adenocarcinoma, GPC1−/− tumor cells show less invasive properties in response to FGF2 treatment compared to tumor cells derived from wild type mice [8]. Silencing of GPC4 results in attenuated DNA synthesis in response to FGF2 treatment in HEK293T cells [64]. Lastly, GPC5 increases rhabdomyosarcoma cell proliferation by enhancing the intracellular FGF2 signaling and altering the cellular distribution of FGF2 [33].
Cancer Therapies Targeting Glypicans
Therapies Targeting GPC1
As previously mentioned, GPC1 is overexpressed in 98% of ESCC and its overexpression is associated with poor prognosis and chemoresistance [10]. The anti-GPC1 antibody, named 1–12, was isolated from chicken and recognizes both human and mouse GPC1 [65]. The chicken/mouse chimeric monoclonal antibody (mAb) with mouse IgG2a Fc domains induces 70% tumor growth inhibition in both ESCC xenograft models and patient-derived tumor (PDX) xenograft models in immunodeficient mice.
Antibody-drug-conjugates (ADC) are agents that consist of a highly cytotoxic small molecule linked covalently to a monoclonal antibody that recognizes a cell-surface antigen. Previous studies demonstrated regression of uterine cervical squamous cell carcinoma and uterine cervical adenocarcinoma after treatment with an ADC composed of an anti-GPC1 antibody (01a033) conjugated to monomethyl auristatin F (MMAF), an antitubulin agent that inhibits cell division [66].
Therapies Targeting GPC2
Recent studies show that GPC2 is overexpressed in neuroblastoma [4, 5]. Human single domain antibodies (sdAbs), e.g., LH7 against GPC2 were isolated from a phage-displayed human single domain antibody library. SdAbs could potentially target cryptic epitopes normally hidden for conventional antibodies. Other advantages of sdAbs include a small size, high solubility, thermal stability, refolding capacity, and good tissue penetration [67].
We developed two types of antibody therapeutics, immunotoxin and chimeric antigen receptor (CAR), to target GPC2 [5]. Immunotoxin is a chimeric protein composed of an antibody fragment fused to a toxin, such as the 38 kDa truncated fragment of Pseudomonas exotoxin (PE38). The immunotoxin LH7-PE38 inhibits neuroblastoma growth in mice [5]. CARs are synthetic receptors that target T cells to cell-surface antigens and augment T-cell function and persistence. CD19-targeted CAR T cells proved that engineered immune cells can serve as a powerful new class of cancer therapeutics [68, 69]; however, selecting appropriate targets in solid tumors is challenging. The tumor-specific expression of GPC2 makes it an attractive target for CAR T-cell therapy. In a recent study, we have showed that CAR T cells targeting GPC2 eliminated tumors in a disseminated neuroblastoma mouse model [5].
In addition, a GPC2-targeted ADC (D3-GPC2-PBD) was developed by conjugating an anti-GPC2 antibody (D3) with pyrrolobenzodiazepine (PBD) dimers [4]. D3-GPC2-PBD is efficacious in a neuroblastoma PDX model. Overall, GPC2 is a promising immunotherapeutic target for the treatment of neuroblastoma [4, 5].
Therapies Targeting GPC3
GPC3 is the most studied target, especially in HCC. Various therapeutic strategies were developed to target GPC3. Peptide vaccines of GPC3 have been extensively studied in recent years. In a Phase II clinical trial, major histocompatibility complex (MHC) class I restricted GPC3 peptides were given to HCC patients after surgery [70]. The GPC3 peptide vaccine improved the 1-year recurrence-free rate in patients with GPC3-expressing tumors. Interestingly, these MHC class I restricted peptides induce CD4+ T cell responses in nearly two thirds of patients resulting in prolonged 3-year and 5-year survival. Given the importance of CD4+ T cell induction, another study developed MHC class II restricted GPC3-derived long peptides (GPC3-LPs) to bind antigen-presenting cells (APC) and induced peptide-specific CD4+ T cell responses from most healthy donors [71]. Additionally, liposome-coupled GPC3-derived peptide (pGPC3-lipsome) was investigated for its antitumor potential [72]. Peptide-specific CD8+ T cells (often called cytotoxic T lymphocytes or CTLs) were induced by pGPC3-liposome vaccine. The pGPC3-liposome vaccine inhibits GPC3-expressing tumor growth. Furthermore, infusion of GPC3-coupled lymphocytes induces robust GPC3-specific antibody and T cell responses, and effectively inhibits proliferation of established HCC tumors by 70% in mice [73].
Several therapeutic anti-GPC3 antibodies have been developed as well. GC33 (mouse mAb) [74] and hYP7 (humanized mouse mAb) [75, 76] recognize the C-lobe of GPC3, and inhibit HCC tumor growth in animals by nearly 50% [74, 77]. Human single domain antibody HN3 targeting the N-lobe of GPC3 [6], and human antibody HS20 targeting the HS chains were also isolated [1, 6]. HN3 and HS20 inhibit HCC tumor growth in mice to approximately 80% and 67%, respectively. The GC33 antibody was well tolerated in a Phase I clinical trial in HCC patients [78], but did not show significant clinical benefit in a randomized Phase II clinical trial [79], suggesting that anti-GPC3 antibodies alone may have limited therapeutic effects in patients.
GPC3-targeted immunotoxins have also been evaluated in preclinical studies. HN3-PE38 immunotoxin regress HCC xenograft tumors in mice at 0.6 mg/kg dosage [49]. To reduce side effects and immunogenicity, great efforts have then been made to generate various forms of immunotoxins [80–82]. HN3-mPE24 is well tolerated in mice even at a dosage of 5 mg/kg and it induces significant tumor regression and increases survival in mice bearing HCC xenografts [80].
Photoimmunotherapy (PIT) employs a mAb-phototoxic phthalocyanine dye, IR700 conjugate, that is activated by near infrared (NIR) light and causes necrotic cell death. A recent study showed that PIT with IR700-YP7 and IR700-HN3 suppressed HCC tumor growth in mice by 40% [77, 83]. Although PIT is less effective than immunotoxins in mouse models, it uses a different mechanism of action and enables direct visualization of treatment.
Moreover, approaches that redirect T cells, including bispecific antibodies and CAR-T cell therapies, have been extensively explored. Bispecific antibodies can redirect T cells to tumor cells by engaging CD3 in the T cell and antigen in the tumor cell. Anti-GPC3/CD3 bispecific T cell-redirecting antibody (ERY974) regress GPC3-expressing solid tumors in mice [84], and is currently in Phase I clinical trial (NCT02748837i). GPC3-targeted CAR T cells have been shown to eliminate GPC3-expressing LSCC [85] and HCC cells [86]. Given the reported promising findings, several clinical trials using GPC3-targeted CAR T cells are underway (NCT02723942ii, NCT02395250iii, NCT03198546iv, NCT02876978v).
In addition, GPC3-targeted CAR natural killer (NK) cells were generated by expressing a GPC3-specific CAR in NK cells [70]. Unlike CAR T cells, mature CAR NK cells have short lifespans and are expected to exhaust shortly after tumor lysis, with a turnover time of ~1–2 weeks [87]. Mature CAR NK cell grafting also has little risk of graft-versus-host disease (GvHD) as compared to CAR T cells [87, 88]. NK-92 is a highly cytotoxic NK cell line and can be continuously and homogeneously expanded; therefore, it has the potential to be developed into off-the-shelf products. In addition, NK-92 infusion was safe in clinical testing [89]. GPC3-targeted CAR NK cells inhibit 90% of GPC3-expressing HCC xenograft growth [90]. Primary NK cells overexpressing anti-GPC3 CAR are also cytotoxic to GPC3-expressing cells.
Lastly, a group of researchers generated a T cell receptor (TCR) targeting GPC3 [91]. They identified a dominant TCR (P1-1) that specifically bound a GPC3 peptide on HLA-A2. P1-1 expressing T cells killed HCC in vitro; however, they only delayed the growth of HCC xenograft tumors in mice [91].
Overall, the emerging new options of glypican-targeted therapies are summarized in Figure 3, and the ongoing preclinical and clinical developments are listed in Table 1 and 2, demonstrating the breath of scientific interest surrounding glypicans as therapeutic targets in cancer therapy.
Figure 3. Antibody-based Therapies Targeting Glypicans in Cancer.
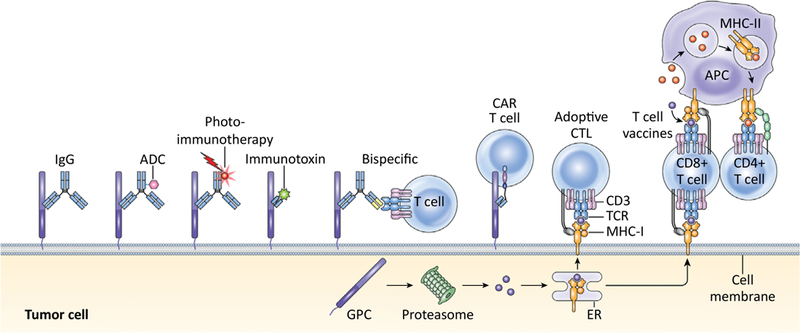
ADC: antibody- drug-conjugate; CAR: chimeric antigen receptor; CTL: cytotoxic T lymphocyte; TCR: T- cell receptor; MHC: major histocompatibility complex; APC: antigen-presenting cell; GPC: glypican; ER: endoplasmic reticulum (adapted with permission from [96]).
Table 1.
Cancer Therapies Targeting Glypicans in Preclinical Development
| Target | Tumor type | Antibody | Format | Efficacy in animals |
Reference |
|---|---|---|---|---|---|
| GPC1 | ESCC | 1–12 | IgG | Tumor growth inhibition |
[65] |
| Cervical cancer | c01a033 | ADC | Tumor regression |
[66] | |
| GPC2 | Neuroblastoma | LH7 | Immunotoxin | Tumor growth inhibition |
[5] |
| CAR T | Tumor regression |
||||
| D3 | ADC | Tumor regression |
[4] | ||
| GPC3 | HCC | NA | LC/GPC3+ | Tumor growth inhibition |
[73] |
| NA | Vaccine | Tumor growth inhibition |
[72] | ||
| HN3 | IgG1 | Tumor growth inhibition |
[6] | ||
| HS20 | IgG1 | Tumor growth inhibition |
[1, 95] | ||
| hYP7 | IgG1 | Tumor growth inhibition |
[76] | ||
| HN3 | Immunotoxin | Tumor regression |
[49, 80] | ||
| YP7 | Immunotoxin | Tumor growth inhibition |
[49] | ||
| GC33 | CAR T | Tumor regression |
[86] | ||
| ERY974 | Bispecific antibody |
Tumor regression |
[84] | ||
| hu9F2 | CAR NK | Tumor growth inhibition |
[90] | ||
| NA | TCR | Tumor growth inhibition |
[91] | ||
| GPC3-positive tumor cells |
YP7 | PIT | Tumor growth inhibition |
[77, 83] | |
| HN3 | PIT | Tumor growth inhibition |
[83] | ||
| LSCC | GC33 | CAR T | Tumor regression |
[85] |
Table 2.
Clinical Development of GPC3-targeted Therapies
| Tumor type |
Antibody | Format | Phase | Status as of June 2018 |
Results | Reference |
|---|---|---|---|---|---|---|
| HCC | NA | Vaccine | II | Completed | Lowered the 1-year recurrence-free rate |
[70] |
| HCC | GC33 | IgG1 | II | Completed | No clinical benefit | [78, 79] |
| Solid tumors |
ERY974 | Bispecific antibody |
I | Recruiting | N/A | NCT02748837i |
| HCC | GC33 | CAR T | I | Ongoing but not recruiting |
N/A | NCT02723942ii |
| LSCC | GC33 | CAR T | I | Recruiting | N/A | NCT02876978v |
Concluding Remarks
We have limited knowledge about the safety and efficacy of targeting tumor antigens to treat cancer, specifically in solid tumors. The potential damage in healthy tissues expressing targetable tumor antigens is of great concern. Thus, more work to determine the safety and efficacy profiles of such therapies is critical. Structural and functional analysis of glypicans and their role in pathobiological processes, particularly cancer development, provide an opportunity to identify a group of new tumor antigens in liver, pancreatic and childhood cancers. Building on recent advances in the development of GPC2 and GPC3-specific antibodies, immunotherapy could make a significant contribution in the field of glypican-targeted anticancer therapeutics. Continued efforts are underway to overcome critical barriers in developing successful T-cell immunotherapies for solid tumor (see Outstanding Questions). Additionally, the potential of newly developed single domain antibodies offer improved tissue penetration, buried functional epitope, and expression capacity affording advances in the field. Despite challenges, ongoing preclinical and clinical studies will establish and validate glypicans as new therapeutic targets for treating cancer.
Figure I, Text Box 1. Glypicans Are Highly Conserved in Animals.
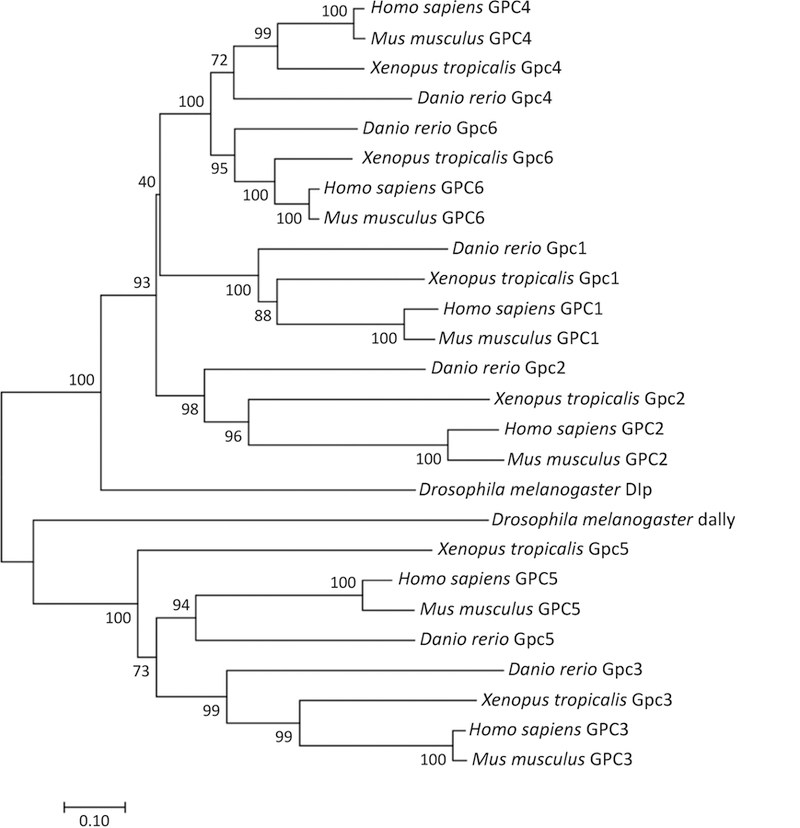
Phylogenetic comparison was carried out for glypicans protein sequences from Homo sapiens, Mus musculus, Xenopus tropicalis, Danio rerio and Drosophila melanogaster. The human genome has six glypican family members that can be grouped into two subfamilies: GPC1/2/4/6 and GPC3/5. Phylogenetic comparison reveals that all mouse, frog and fish proteins cluster with human orthologs. There are two glypicans–Dally and Dally-like protein (Dlp) in Drosophila melanogaster. Dally is an ortholog of the human GPC3/5 subfamily, and Dlp is an ortholog of the GPC1/2/4/6 subfamily. The evolutionary tree was constructed using Neighbor-Joining method in MEGA7 software using the Poisson model and partially deleted dataset. 1000 bootstrap replications were used as a test of phylogeny and the values are indicated next to the branch. Branch length corresponds to evolutionary distances that denote the number of amino acid substitutions per site. Scale bar: 0.1.
Figure II, Text Box 2. Structure of Dlp in Drosophila melanogaster and GPC1 in Humans.
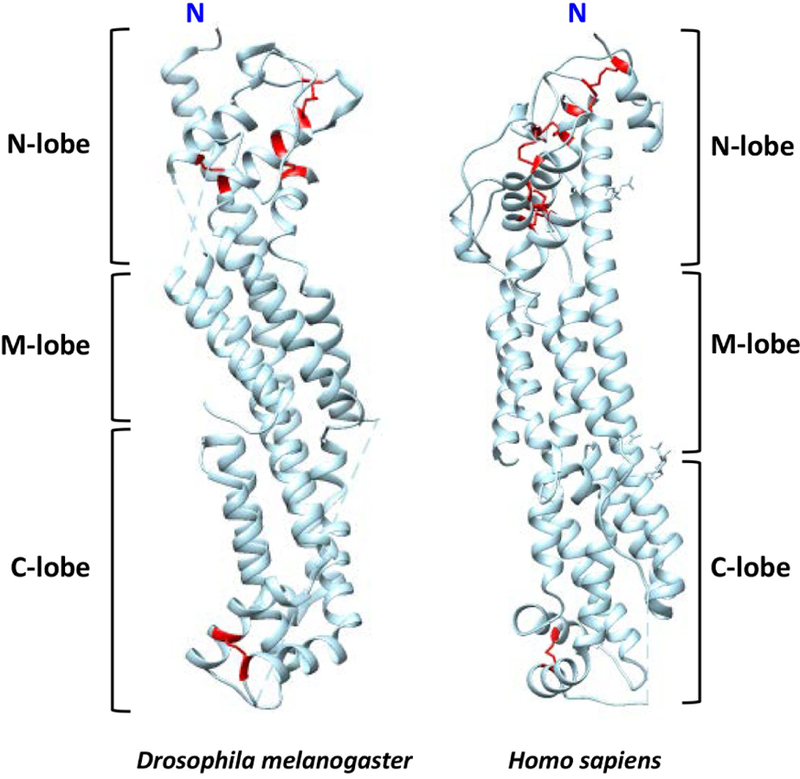
The termini are labeled, and the N, M, and C lobes are indicated. The N-, M-, and C-lobes are named according to their relative spatial position in the protein. The disulfide bonds are indicated (red). Both proteins show cylindrical-like structures that are conserved between Drosophila melanogaster and humans.
Highlights.
Selection of specific tumor antigens for cancer therapy remains a major challenge. Glypicans are expressed in cancers and embryonic tissues during development; however, their expressions are strictly suppressed in most adult normal tissues, which make glypicans attractive tumor-specific antigens.
The most significant structural characteristic of glypicans is their various numbers of heparan sulfate chains close to the cell membrane. This unique structure endows glypicans the capability to rescue and sequester different growth factors, morphogens, chemokines and cytokines in physiological conditions. In this sense, glypicans act as essential co-receptors and trigger the activation of Wnt, Hh, FGF and many other signaling pathways.
Unlike conventional full-size antibodies, single domain antibodies can bind to cavities on antigens, e.g., the buried active sites of enzymes. Together with other advantages including their small size, thermal stability, and good tissue penetration, single domain antibodies may become a new direction for next-generation antibody-based therapies.
Glypican-targeted antibody-based therapies including: vaccine, immunotoxin, bispecific antibody and engineered T-cell therapy, are being intensively investigated in many preclinical and clinical studies. Efforts are now aimed at overcoming immunosuppressive microenvironment, improving T cell infiltration and persistence by developing new strategies and combination of existing therapies.
Outstanding Questions.
How can high-throughput sequencing, such as single-cell sequencing, be utilized to provide a better understanding of heterogeneity of glypicans expression and identify new mutations/isoforms of glypicans in patients for precision medicine?
Can we develop biomarkers that can be used to identify patients who are the most likely to benefit from glypican-targeted therapy?
Which combination treatments are most likely to act synergistically with therapies targeting tumor-specific glypicans?
How can we better mobilize the immune system against glypicans to penetrate the highly immunosuppressive tumor microenvironment and eradicate solid tumors?
Text Box 1-. Evolution of Glypicans.
Glypicans are highly conserved during animal evolution. Here, we summarize the glypicans in various major species (Figure I, Text Box 1). The human genome has six glypican family members (GPC1–6) that can be grouped into two subfamilies: GPC1/2/4/6 and GPC3/5 that share approximately 25% amino acid identity [57]. Within the first subfamily, GPC4 and GPC6 are closely related, while GPC1 and GPC2 form a more divergent clade (Figure I, Text Box 1). Phylogenetic comparison reveals that all mouse, frog and fish proteins cluster with human orthologs
Of additional interest, invertebrate genomes typically contain two glypicans, where many vertebrates have six (or more) glypican genes, likely due to single gene and whole genome duplications. For example, there are two glypicans–Dally and Dally-like protein (Dlp) in Drosophila melanogaster. Dally is an ortholog of the human GPC3/5 subfamily, and Dlp is an ortholog of the GPC1/2/4/6 subfamily.
Text Box 2-. Structure of Glypicans.
All glypicans share an N-terminal secretory signal peptide, 14 evolutionarily conserved cysteine residues, an HS attachment domain near the C terminus, and a hydrophobic domain near the C terminus for the addition of the GPI anchor. Most glypicans are subjected to proteolytic cleavage in vitro and in vivo. The 30–40 kDa cleavage product generated from the N-terminus of the protein core remains attached to its C-terminal half by one or more disulfide bonds [92].
The core protein structures of Dlp and GPC1 have been previously reported [93, 94]. Despite only 25% of sequence identity, both proteins show cylindrical-like structures that are conserved between Drosophila melanogaster and humans (Figure II, Text Box 2). The N-, M-, and C-lobes are named according to their relative spatial position in the protein (Figure II, Text Box 2). Out of the 14 evolutionarily conserved cysteine residues, 12 are located in the N-lobe. The N-lobe is followed by a middle segment named M-lobe, which is stabilized by two conserved hydrophobic centers that connect the α-helices and also contains the C terminus of the protein. The C-lobe is the last part of the structure that contains a long loop that is processed by furin proteases in many of the glypican core proteins.
Acknowledgments
We thank NCI Editorial Board, NIH Library Editing Service, Dr. Bryan Fleming (NCI), Madeline B. Torres, MD. (NCI), and Aarti Kolluri (NCI) for editorial assistance. This research was supported by the Intramural Research Program of NIH, NCI (Z01 BC010891 and ZIA BC010891) (to M.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The National Cancer Institute (NCI) holds patent rights to anti-GPC2 and anti-GPC3 antibodies in many jurisdictions, including the United States [e.g., U.S. Patent 9,409,994, U.S. Patent 9,206,257, U.S Patent 9,304,364, U.S. Patent 9,932,406, U.S. Patent Application 62/716,169, and U.S. Patent Application 62/369,861], China, Japan, South Korea, Singapore and Europe. Claims cover the antibodies themselves, as well as conjugates that utilize the antibodies, such as recombinant immunotoxins (RITs), antibody drug conjugates (ADCs), bispecific antibodies and modified T cell receptors (TCRs)/chimeric antigen receptors (CARs), and vectors expressing these constructs. Anyone interested in licensing these antibodies can contact Dr. Mitchell Ho for additional information.
Resources
References
- 1.Gao W et al. (2014) Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology 60 (2), 576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capurro M et al. (2014) Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. J Cell Sci 127 (Pt 7), 1565–75. [DOI] [PubMed] [Google Scholar]
- 3.Filmus J and Capurro M (2014) The role of glypicans in Hedgehog signaling. Matrix Biol 35, 248–52. [DOI] [PubMed] [Google Scholar]
- 4.Bosse KR et al. (2017) Identification of GPC2 as an Oncoprotein and Candidate Immunotherapeutic Target in High-Risk Neuroblastoma. Cancer Cell 32 (3), 295–309 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li N et al. (2017) Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proc Natl Acad Sci U S A 114 (32), E6623–E6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng M et al. (2013) Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci U S A 110 (12), E1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aikawa T et al. (2008) Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells. J Clin Invest 118 (1), 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whipple CA et al. (2012) A KrasG12D-driven genetic mouse model of pancreatic cancer requires glypican-1 for efficient proliferation and angiogenesis. Oncogene 31 (20), 2535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuda K et al. (2001) Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res 61 (14), 5562–9. [PubMed] [Google Scholar]
- 10.Hara H et al. (2016) Overexpression of glypican-1 implicates poor prognosis and their chemoresistance in oesophageal squamous cell carcinoma. Br J Cancer 115 (1), 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao D et al. (2003) Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J Biol Chem 278 (18), 16045–53. [DOI] [PubMed] [Google Scholar]
- 12.Su G et al. (2006) Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am J Pathol 168 (6), 2014–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orentas RJ et al. (2012) Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Front Oncol 2, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumhoer D et al. (2008) Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol 129 (6), 899–906. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM et al. (2016) Hepatocellular carcinoma. Nat Rev Dis Primers 2, 16018. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi N et al. (2005) The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol 18 (12), 1591–8. [DOI] [PubMed] [Google Scholar]
- 17.Shirakawa H et al. (2009) Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 100 (8), 1403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aviel-Ronen S et al. (2008) Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod Pathol 21 (7), 817–825. [DOI] [PubMed] [Google Scholar]
- 19.He H et al. (2009) Frequent expression of glypican-3 in Merkel cell carcinoma: an immunohistochemical study of 55 cases. Appl Immunohistochem Mol Morphol 17 (1), 40–6. [DOI] [PubMed] [Google Scholar]
- 20.Maeda D et al. (2009) Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod Pathol 22 (6), 824–32. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsura T et al. (2004) Identification of glypican-3 as a novel tumor marker for melanoma. Clin Cancer Res 10 (19), 6612–21. [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka K et al. (2007) Immunohistochemical study of glypican 3 in thyroid cancer. Oncology 73 (5–6), 389–94. [DOI] [PubMed] [Google Scholar]
- 23.Zynger DL et al. (2010) Glypican 3 has a higher sensitivity than alpha-fetoprotein for testicular and ovarian yolk sac tumour: immunohistochemical investigation with analysis of histological growth patterns. Histopathology 56 (6), 750–7. [DOI] [PubMed] [Google Scholar]
- 24.Ho M and Kim H (2011) Glypican-3: a new target for cancer immunotherapy. Eur J Cancer 47 (3), 333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anatelli F et al. (2008) Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy. Am J Clin Pathol 130 (2), 219–23. [DOI] [PubMed] [Google Scholar]
- 26.Wang HL et al. (2008) Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch Pathol Lab Med 132 (11), 1723–8. [DOI] [PubMed] [Google Scholar]
- 27.Yorita K et al. (2011) Prognostic significance of circumferential cell surface immunoreactivity of glypican-3 in hepatocellular carcinoma. Liver Int 31 (1), 120–31. [DOI] [PubMed] [Google Scholar]
- 28.Song HH et al. (2005) The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem 280 (3), 2116–25. [DOI] [PubMed] [Google Scholar]
- 29.Liu B et al. (2010) Suppression of liver regeneration and hepatocyte proliferation in hepatocyte-targeted glypican 3 transgenic mice. Hepatology 52 (3), 1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao J et al. (2018) Targeting glypican-4 overcomes 5-FU resistance and attenuates stem cell-like properties via suppression of Wnt/beta-catenin pathway in pancreatic cancer cells. J Cell Biochem [DOI] [PubMed]
- 31.Li F et al. (2011) Glypican-5 stimulates rhabdomyosarcoma cell proliferation by activating Hedgehog signaling. J Cell Biol 192 (4), 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura R et al. (2013) Characterization of genetic lesions in rhabdomyosarcoma using a high-density single nucleotide polymorphism array. Cancer Sci 104 (7), 856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson D et al. (2007) Role for amplification and expression of glypican-5 in rhabdomyosarcoma. Cancer Res 67 (1), 57–65. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C et al. (2016) Prognostic significance of GPC5 expression in patients with prostate cancer. Tumour Biol 37 (5), 6413–8. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C et al. (2011) A lung cancer gene GPC5 could also be crucial in breast cancer. Mol Genet Metab 103 (1), 104–5. [DOI] [PubMed] [Google Scholar]
- 36.Li Y et al. (2013) The overexpression of glypican-5 promotes cancer cell migration and is associated with shorter overall survival in non-small cell lung cancer. Oncol Lett 6 (6), 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X et al. (2013) Glypican-5 is a novel metastasis suppressor gene in non-small cell lung cancer. Cancer Lett 341 (2), 265–73. [DOI] [PubMed] [Google Scholar]
- 38.Yuan S et al. (2016) GPC5, a novel epigenetically silenced tumor suppressor, inhibits tumor growth by suppressing Wnt/beta-catenin signaling in lung adenocarcinoma. Oncogene 35 (47), 6120–6131. [DOI] [PubMed] [Google Scholar]
- 39.Dinccelik-Aslan M et al. (2015) Diagnostic and prognostic significance of glypican 5 and glypican 6 gene expression levels in gastric adenocarcinoma. Mol Clin Oncol 3 (3), 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karapetsas A et al. (2015) Overexpression of GPC6 and TMEM132D in Early Stage Ovarian Cancer Correlates with CD8+ T-Lymphocyte Infiltration and Increased Patient Survival. Biomed Res Int 2015, 712438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capurro MI et al. (2005) Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 65 (14), 6245–54. [DOI] [PubMed] [Google Scholar]
- 42.Gao W and Ho M (2011) The role of glypican-3 in regulating Wnt in hepatocellular carcinomas. Cancer Rep 1 (1), 14–19. [PMC free article] [PubMed] [Google Scholar]
- 43.Stigliano I et al. (2009) Glypican-3 regulates migration, adhesion and actin cytoskeleton organization in mammary tumor cells through Wnt signaling modulation. Breast Cancer Res Treat 114 (2), 251–62. [DOI] [PubMed] [Google Scholar]
- 44.Sakane H et al. (2012) Localization of glypican-4 in different membrane microdomains is involved in the regulation of Wnt signaling. J Cell Sci 125 (Pt 2), 449–60. [DOI] [PubMed] [Google Scholar]
- 45.Wang S et al. (2016) Glypican-5 suppresses Epithelial-Mesenchymal Transition of the lung adenocarcinoma by competitively binding to Wnt3a. Oncotarget 7 (48), 79736–79746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y et al. (2017) Overexpression of Glypican 5 (GPC5) Inhibits Prostate Cancer Cell Proliferation and Invasion via Suppressing Sp1-Mediated EMT and Activation of Wnt/beta-Catenin Signaling. Oncol Res [DOI] [PMC free article] [PubMed]
- 47.Yiu GK et al. (2011) NFAT promotes carcinoma invasive migration through glypican-6. Biochem J 440 (1), 157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao W et al. (2016) Epitope mapping by a Wnt-blocking antibody: evidence of the Wnt binding domain in heparan sulfate. Sci Rep 6, 26245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao W et al. (2015) Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis. Nat Commun 6, 6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Lau W et al. (2014) The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev 28 (4), 305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao HX et al. (2012) ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485 (7397), 195–200. [DOI] [PubMed] [Google Scholar]
- 52.Koo BK et al. (2012) Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488 (7413), 665–9. [DOI] [PubMed] [Google Scholar]
- 53.Giannakis M et al. (2014) RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet 46 (12), 1264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Storm EE et al. (2016) Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature 529 (7584), 97–100. [DOI] [PubMed] [Google Scholar]
- 55.Lebensohn AM et al. (2016) Comparative genetic screens in human cells reveal new regulatory mechanisms in WNT signaling. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lebensohn AM and Rohatgi R (2018) R-spondins can potentiate WNT signaling without LGRs. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filmus J et al. (2008) Glypicans. Genome Biol 9 (5), 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Capurro MI et al. (2009) Overgrowth of a mouse model of Simpson-Golabi-Behmel syndrome is partly mediated by Indian hedgehog. EMBO Rep 10 (8), 901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filmus J and Capurro M (2008) The role of glypican-3 in the regulation of body size and cancer. Cell Cycle 7 (18), 2787–90. [DOI] [PubMed] [Google Scholar]
- 60.Capurro MI et al. (2008) Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell 14 (5), 700–11. [DOI] [PubMed] [Google Scholar]
- 61.Capurro MI et al. (2012) LRP1 mediates Hedgehog-induced endocytosis of the GPC3-Hedgehog complex. J Cell Sci 125 (Pt 14), 3380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Capurro M et al. (2017) Glypican-6 promotes the growth of developing long bones by stimulating Hedgehog signaling. J Cell Biol 216 (9), 2911–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang G et al. (2017) alpha3 Chains of type V collagen regulate breast tumour growth via glypican-1. Nat Commun 8, 14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng C et al. (2012) MicroRNA-125a inhibits cell growth by targeting glypican-4. Glycoconj J 29 (7), 503–11. [DOI] [PubMed] [Google Scholar]
- 65.Harada E et al. (2017) Glypican-1 targeted antibody-based therapy induces preclinical antitumor activity against esophageal squamous cell carcinoma. Oncotarget 8 (15), 24741–24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsuzaki S et al. (2018) Anti-glypican-1 antibody-drug conjugate exhibits potent preclinical antitumor activity against glypican-1 positive uterine cervical cancer. Int J Cancer 142 (5), 1056–1066. [DOI] [PubMed] [Google Scholar]
- 67.Wesolowski J et al. (2009) Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol 198 (3), 157–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grupp SA et al. (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368 (16), 1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kochenderfer JN et al. (2010) Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116 (19), 3875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawada Y et al. (2016) Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 5 (5), e1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sayem MA et al. (2016) Identification of glypican-3-derived long peptides activating both CD8(+) and CD4(+) T cells; prolonged overall survival in cancer patients with Th cell response. Oncoimmunology 5 (1), e1062209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwama T et al. (2016) Vaccination with liposome-coupled glypican-3-derived epitope peptide stimulates cytotoxic T lymphocytes and inhibits GPC3-expressing tumor growth in mice. Biochem Biophys Res Commun 469 (1), 138–143. [DOI] [PubMed] [Google Scholar]
- 73.Wu Q et al. (2017) A Novel Vaccine Targeting Glypican-3 as a Treatment for Hepatocellular Carcinoma. Mol Ther 25 (10), 2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakano K et al. (2009) Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun 378 (2), 279–84. [DOI] [PubMed] [Google Scholar]
- 75.Phung Y et al. (2012) High-affinity monoclonal antibodies to cell surface tumor antigen glypican-3 generated through a combination of peptide immunization and flow cytometry screening. MAbs 4 (5), 592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang YF and Ho M (2016) Humanization of high-affinity antibodies targeting glypican-3 in hepatocellular carcinoma. Sci Rep 6, 33878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanaoka H et al. (2015) Photoimmunotherapy of hepatocellular carcinoma-targeting Glypican-3 combined with nanosized albumin-bound paclitaxel. Nanomedicine (Lond) 10 (7), 1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu AX et al. (2013) First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res 19 (4), 920–8. [DOI] [PubMed] [Google Scholar]
- 79.Yen C-J et al. (2014) Randomized phase II trial of intravenous RO5137382/GC33 at 1600 mg every other week and placebo in previously treated patients with unresectable advanced hepatocellular carcinoma (HCC; NCT01507168). Journal of Clinical Oncology 32 (15_suppl), 4102–4102.25403208 [Google Scholar]
- 80.Wang C et al. (2017) Construction of an immunotoxin, HN3-mPE24, targeting glypican-3 for liver cancer therapy. Oncotarget 8 (20), 32450–32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weldon JE et al. (2013) A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol Cancer Ther 12 (1), 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazor R et al. (2017) Elimination of murine and human T-cell epitopes in recombinant immunotoxin eliminates neutralizing and anti-drug antibodies in vivo. Cell Mol Immunol 14 (5), 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanaoka H et al. (2015) Glypican-3 targeted human heavy chain antibody as a drug carrier for hepatocellular carcinoma therapy. Mol Pharm 12 (6), 2151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ishiguro T et al. (2017) An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med 9 (410). [DOI] [PubMed] [Google Scholar]
- 85.Li KS et al. (2016) Adoptive immunotherapy using T lymphocytes redirected to glypican-3 for the treatment of lung squamous cell carcinoma. Oncotarget 7 (3), 2496–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao H et al. (2014) Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 20 (24), 6418–28. [DOI] [PubMed] [Google Scholar]
- 87.Glienke W et al. (2015) Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol 6, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suck G et al. (2016) NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother 65 (4), 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arai S et al. (2008) Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 10 (6), 625–32. [DOI] [PubMed] [Google Scholar]
- 90.Yu M et al. (2018) Development of GPC3-Specific Chimeric Antigen Receptor-Engineered Natural Killer Cells for the Treatment of Hepatocellular Carcinoma. Mol Ther 26 (2), 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dargel C et al. (2015) T Cells Engineered to Express a T-Cell Receptor Specific for Glypican-3 to Recognize and Kill Hepatoma Cells In Vitro and in Mice. Gastroenterology 149 (4), 1042–52. [DOI] [PubMed] [Google Scholar]
- 92.De Cat B et al. (2003) Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol 163 (3), 625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim MS et al. (2011) Structure of the protein core of the glypican Dally-like and localization of a region important for hedgehog signaling. Proc Natl Acad Sci U S A 108 (32), 13112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Svensson G et al. (2012) Crystal structure of N-glycosylated human glypican-1 core protein: structure of two loops evolutionarily conserved in vertebrate glypican-1. J Biol Chem 287 (17), 14040–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao W et al. (2015) Human Monoclonal Antibody Targeting the Heparan Sulfate Chains of Glypican-3 Inhibits HGF-Mediated Migration and Motility of Hepatocellular Carcinoma Cells. PLoS One 10 (9), e0137664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang YF et al. (2017) Glypican-3 as a Target for Immune Based Therapy in Hepatocellular Carcinoma. Immunotherapy of Hepatocellular Carcinoma, Springer Nature, chapter 7, 103–119. (Book Reference) [Google Scholar]


