ABSTRACT
Which subgroups patients with muscle-invasive bladder cancer (MIBC) could benefit most from adjuvant chemotherapy (ACT) is blurred. Here we tried to stratify MIBC patients with tumor infiltrating mast cells (TIMs), explore the prognostic and predictive value of TIMs, and provide possible cellular explanations. We selected 259 MIBC patients who underwent radical cystectomy from two independent clinical centers between 2002 and 2014. TIMs were evaluated and prognostic and predictive value was assessed. The CIBERSORT method, Gene Set Enrichment Analysis (GSEA) and differential gene expression analyses were performed to explore the possible cellular mechanisms. TIMs infiltration was distinct between stromal and epithelial area of MIBC specimens. Patients with higher stromal TIMs had a significant worse overall survival and recurrence free survival (HR = 2.228, 95%CI: 1.467–3.550; P = 0.001 and HR = 1.984, 95%CI: 1.105–3.374; P = 0.016). More importantly, pT2 patients with low stromal TIMs tended to have a lower risk of death and recurrence after ACT (HR = 0.233, 95%CI: 0.020–0.814; P = 0.033 and HR = 0.180, 95%CI: 0.022–0.722; P = 0.031). A negative correlativity between TIMs and CD8 + T cells was identified on TCGA-BLCA cohort. Immunohistochemistry results validated that high stromal TIMs were negatively correlated with CD8 + T cells (Spearman’s rho = -0.215, P < 0.001). Differential gene expression suggested that low TIMs might represent a state of immune activation in MIBC. To conclude, high stromal TIMs infiltration was an independent unfavorable prognosticator for MIBC patients. Patients with low stromal TIMs might benefit the most from ACT, especially in pT2 stage.
Keywords: adjuvant chemotherapy, bladder cancer, mast cells, overall survival, prognosis, recurrence-free survival
Introduction
Patients suffered from MIBC have worse prognosis and limited treatment options. Latest clinical guideline recommends radical cystectomy as the major treatment for MIBC. After surgery, cisplatin-based combination therapy was considered conventionally as first-line chemotherapy to improve clinical outcomes. However, applying adjuvant chemotherapy (ACT) after radical cystectomy was still under debate.1 Several meta-analyses of ACT trials revealed that a relative low rate of 23–25% reduction in risk of death, and were questioned about study population or lack of individual data.2-4 Thus, an improved stratification of bladder cancer is urgently needed to predict more accurately on patient prognosis and chemotherapy treatment response.
There is a rising awareness that various tumor infiltrating immune cells could interact with cancer cells, reform tumor microenvironment, and even affect chemotherapeutic efficacy.5,6 Current studies revealed that tumor infiltrating mast cells (TIMs) could be found in various cancers and acted either pro-tumor or protective role depending on different tumor types.7-9 We also previously reported that high TIMs could serve as a favorable prognosticator in non-metastatic clear-cell renal cell carcinoma.10 Several reviews owed this contradiction to the complicated functions of TIMs in controlling adaptive immunity, inflammation and angiogenesis.7,9
Bladder cancer was long considered to have a strong relationship with immune system, and many immune cells and inflammatory biomarkers in bladder cancer specimens were analyzed.11.However, the clinical role of mast cell in bladder cancer has not been evaluated. In this present study, we found that patients with low stromal TIMs infiltration benefited the most from ACT, especially in pT2 patients. Further differential expression analyses revealed that this good prognosis in low stromal TIMs group might correlated with highly infiltrated CD8 T cells, and various cytokine genes related to immune response were also enriched in low TIMs group, suggesting an immune activation state in MIBC.
Results
Evaluation of TIMs in MIBC tumor tissue
Representative immunohistochemistry images of tryptase were illustrated in Figure 1A. Mast cells were stained clearly within tissues, and the infiltration differed distinctly in tumor stromal and epithelial area. We then separately counted the stromal and epithelial TIMs densities. The cutoff values of stromal and epithelial TIMs were then determined as 20 cells/mm2 and 5 cells/mm2, respectively.
Figure 1.
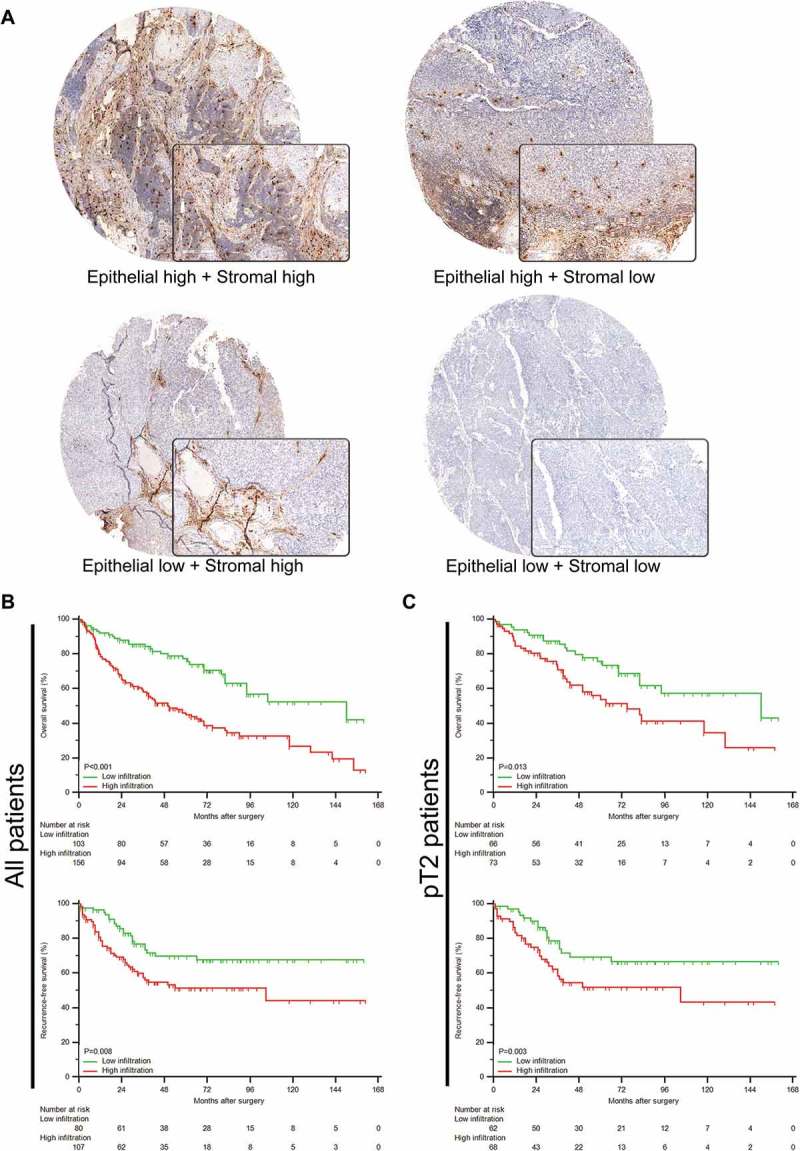
Evaluation of TIMs by immunohistochemistry in MIBC patients. (A) Representative immunohistochemistry images of TIMs infiltration in different tumor tissue compartments. Right bottom pictures showed 200X magnification. (B-C) Kaplan-Meier analyses of OS (top) and RFS (bottom) in all (B) and (C) pT2 patients according to TIMs infiltration in tumor stromal area.
Associations between TIMs and patients’ clinicopathologic features
Patient characteristics and association with both stromal and epithelial TIMs were listed in Table 1. Worse clinical outcomes, such as death and recurrence, had significant positive correlation only with stromal TIMs (P < 0.001 and P = 0.002, respectively), while these factors were not significantly correlated with epithelial TIMs. Stromal TIMs were also positively correlated with pT stage (P = 0.024). Epithelial TIMs were positively correlated with tumor size (P = 0.040), and were negatively correlated with pN stage and ACT applications. Other clinicopathologic factors did not present any statistical significant correlations with stromal or epithelial TIMs.
Table 1.
Patient characteristics and associations with TIM infiltration.
| Patients |
Stromal TIM infiltration |
Epithelial TIM Infiltration |
||||||
|---|---|---|---|---|---|---|---|---|
| Factor | No. | % | Low (n = 103) (<20cells/mm2) |
High (n = 156) (≥20cells/mm2) |
P* | Low (n = 154) (<5cells/mm2) |
High (n = 105) (≥5cells/mm2) |
P* |
| Age at surgery (year) | 0.014† | 0.394† | ||||||
| Median (IQR) | 62 (56–69) | 61 (54–68) | 64 (58–71) | 61 (56–69) | 63 (56–71) | |||
| Gender | 0.861 | 0.784 | ||||||
| Male | 219 | 84.6 | 88 | 131 | 131 | 88 | ||
| Female | 40 | 15.4 | 15 | 25 | 23 | 17 | ||
| Tumor size (cm) | 0.195† | 0.040† | ||||||
| Median (IQR) | 3.5 (2.6–5.0) | 3.5 (2.8–6.0) | 3.5 (2.6–4.0) | 3.5 (2.8–5.3) | 3.5 (2.6–4.0) | |||
| pT stage | 0.024 | 0.104 | ||||||
| pT2 | 139 | 53.7 | 66 | 73 | 88 | 51 | ||
| pT3 | 82 | 31.7 | 25 | 57 | 41 | 41 | ||
| pT4 | 38 | 14.6 | 12 | 26 | 25 | 13 | ||
| pN stage | 0.192 | 0.010 | ||||||
| pN0 | 214 | 82.6 | 89 | 125 | 135 | 79 | ||
| pN+ | 45 | 17.4 | 14 | 31 | 19 | 26 | ||
| Grade | 0.325 | 0.188 | ||||||
| Low grade | 36 | 13.9 | 17 | 19 | 25 | 11 | ||
| High grade | 223 | 86.1 | 86 | 137 | 129 | 94 | ||
| Lymphovascular invasion | 0.162 | 0.702 | ||||||
| Absent | 132 | 51.0 | 58 | 74 | 80 | 52 | ||
| Present | 127 | 49.0 | 45 | 82 | 74 | 53 | ||
| Adjuvant chemotherapy | 0.062 | 0.049 | ||||||
| Applied | 119 | 45.9 | 40 | 79 | 63 | 56 | ||
| Not applied | 140 | 54.1 | 63 | 77 | 91 | 49 | ||
| Events | ||||||||
| Death | 123 | 47.1 | 31 | 91 | <0.001 | 70 | 52 | 0.519 |
| Recurrence | 105 | 40.5 | 30 | 75 | 0.002 | 63 | 42 | 0.884 |
Abbreviations: TIM: tumor-infiltrated mast cells, IQR: interquartile range.
*Fisher’s exact test was used when table data did not meet the requirement of Chi-square test.
† Mann-Whitney U test.
Patients with higher stromal TIMs had worse prognosis
We then analyzed the prognostic value of both stromal and epithelial TIMs in MIBC patients via Kaplan-Meier curves. In Figure 1B, patients with higher stromal TIMs were more likely to experience death (P < 0.001) and recurrence (P = 0.008). Moreover, subgroup analyses showed that in pT2 stage, higher stromal TIMs remained significant in predicting OS and RFS (P = 0.013 and P = 0.003; Figure 1C upper and bottom, respectively). However, epithelial TIMs could not stratify prognosis for all patients (Supplementary Figure 1B) and pT2 patients (Supplementary Figure 1C). Thus, stromal TIMs might present higher prognostic value than epithelial TIMs.
Stromal TIMs infiltration was an independent prognosticator
Cox proportional hazard models were applied to further evaluate the prognostic value of TIMs in MIBC patients. In univariate analyses (Supplementary Table 1), stromal TIMs infiltration was a potential prognostic factor in both OS and RFS (P = 0.001 and P = 0.005, respectively). While epithelial TIMs failed in both endpoints. Notably, ACT application did not reach statistical significance within Cox models (OS: P = 0.602 and RFS: P = 0.302). As listed in Table 2, stromal TIMs were identified as an independent prognosticator for both OS (P = 0.001) and RFS (P = 0.016), with estimated hazard ratios as 2.228 (95%CI, 1.467 to 3.550) and 1.848 (95%CI, 1.105 to 3.374), respectively. Thus, higher stromal TIMs could independently predict the postoperative prognosis of MIBC patients.
Table 2.
Multivariate Cox regression analyses of clinicopathological features and TIMs for overall survival and recurrence-free survival.
| Factor | HR (95% CI) | P |
|---|---|---|
| Overall survival | ||
| Age at surgery (year) | 1.021 (0.998–1.043) | 0.052 |
| pT stage | 0.673 | |
| pT3 vs. pT2 | 1.176 (0.720–1.865) | 0.508 |
| pT4 vs. pT2 | 1.213 (0.730–2.071) | 0.422 |
| pN stage (pN1+ vs. pN0) | 2.457 (1.411–4.200) | 0.002 |
| Grade (high vs. low) | 2.090 (1.183–4.302) | 0.020 |
| LVI (present vs. absent) | 1.145 (0.732–1.696) | 0.607 |
| TIMs infiltration, stromal (high vs. low) | 2.228 (1.467–3.550) | 0.001 |
| Recurrence-free survival | ||
| pT stage (pT3 vs. pT2) | 1.070 (0.612–1.866) | 0.806 |
| Grade (high vs. low) | 3.655 (1.502–13.531) | 0.004 |
| TIMs infiltration, stromal (high vs. low) | 1.984 (1.105–3.374) | 0.016 |
Abbreviation: HR: Hazard Ratio; CI: confidence interval; LVI: lyphmovascular invasion; TIM: tumor-infiltrated mast cells.
All HR and 95%CI were calculated from 1000 bootstrap samples protected from overfitting.
Our previous research also identified that tumor infiltrating neutrophils (TINs) as an independent unfavorable prognosticator in bladder cancer,12 we thus try to compare the prognostic value of TINs and TIMs. As shown in Supplementary Table 2, c-indices of TIMs and TINs were calculated, and the differences were not statistic significant. These results showed that there were no significant prognostic value differences between TIMs and TINs.
Predictive value of TIMs with response to ACT for MIBC patients
We next sought to explore whether the efficacy of ACT was different according to stromal TIMs. As exhibited in Supplementary Figure 2A and 2D, applying ACT could not stratify patients’ OS or RFS (P = 0.600 and P = 0.298, respectively). Further subgroup analyses indicated that ACT application could prolong both OS and RFS in patients with low stromal TIMs (Supplementary Figure 2C and 2F, P = 0.032 and P = 0.011, respectively). However, no statistical significance was reached in patients with high stromal TIMs (Supplementary Figure 2B and 2E, P = 0.135 and P = 0.578, respectively). The same phenomena were also observed when we narrowed the subgroup to pT2 patients for OS and pT2N0 patients for RFS. As represented in Figure 2C and 2F, in pT2 patients with low stromal TIMs, applying ACT could improve both OS and RFS (P = 0.026 and P = 0.043, respectively). However, neither entire pT2 patients (Figure 2A and 2D) nor pT2 patients with high stromal TIMs presented significant prolonged OS and RFS after receiving ACT (Figure 2B and 2E).
Figure 2.
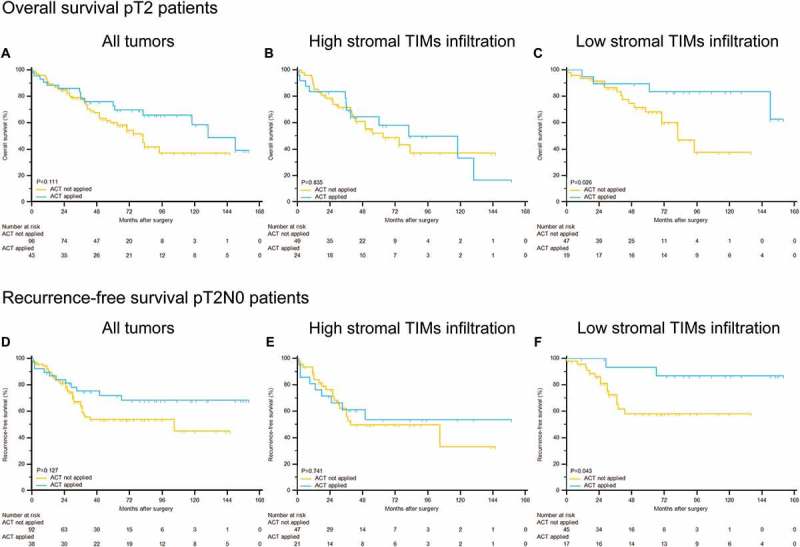
Stromal TIMs density could predict adjuvant chemotherapy effectiveness in different subgroups of pT2 MIBC patients. Kaplan-Meier analyses of OS in (A) all pT2 patients, (B) high stromal TIMs infiltration patients and (C) low stromal TIMs infiltration patients; of RFS in (D) all pT2N0 patients, (E) high stromal TIMs infiltration patients and (F) low stromal TIMs infiltration patients according to adjuvant chemotherapy applications.
Moreover, Cox regression analyses were also applied to calculate the effect of ACT within different subgroups. As displayed in Table 3, ACT application was a strong protective factor for OS in pT2 patients with low stromal TIMs (P = 0.033; HR, 95%CI: 0.233, 0.020 to 0.814), which was consistent with Kaplan-Meier curves (Figure 2C). In all patients with low TIMs, ACT application also reached significance for OS (P = 0.029; HR, 95%CI: 0.414, 0.163 to 0.926). Similarly, applying ACT could decrease recurrence risk in all patients with low stromal TIMs and pT2 patients with low stromal TIMs (P = 0.014; HR, 95%CI: 0.238, 0.052 to 0.662 and P = 0.031; HR, 95%CI: 0.180, 0.022 to 0.722, respectively). Consequently, our findings suggested that pT2 patients with low stromal TIMs could benefit the most from cisplatin-based combination ACT after surgery.
Table 3.
Risk of mortality and recurrence in different subgroup patients receiving adjuvant chemotherapy or not according to stromal TIMs infiltration.
| Factor | Number (%) | HR (95%CI); P | Pinteraction* |
|---|---|---|---|
| Overall survival | |||
| All MIBC patients | |||
| ACT (yes vs. no) | 119/140 (45.9/54.1) | 1.100 (0.773–1.571); 0.615 | 0.036 |
| ACT in high stromal TIMs subgroup (yes vs. no) | 79/77 (50.6/49.4) | 1.374 (0.911–2.119); 0.120 | |
| ACT in low stromal TIMs subgroup (yes vs. no) | 40/63 (38.8/61.2) | 0.414 (0.163–0.926): 0.029 | |
| pT2 patients | |||
| ACT (yes vs. no) | 43/96 (30.9/69.1) | 0.599 (0.286–1.101); 0.116 | 0.023 |
| ACT in high stromal TIMs subgroup (yes vs. no) | 24/49 (32.9/67.1) | 0.887 (0.398–1.781); 0.834 | |
| ACT in low stromal TIMs subgroup (yes vs. no) | 19/47 (28.8/71.2) | 0.233 (0.020–0.814); 0.033 | |
| Recurrence-free survival | |||
| All MIBC patients | |||
| ACT (yes vs. no) | 78/110 (41.5/58.5) | 0.776 (0.460–1.262); 0.296 | 0.025 |
| ACT in high stromal TIMs subgroup (yes vs. no) | 47/60 (43.9/56.1) | 1.198 (0.645–2.104); 0.571 | |
| ACT in low stromal TIMs subgroup (yes vs. no) | 31/50 (38.3/61.7) | 0.238 (0.052–0.662); 0.014 | |
| pT2 patient | |||
| ACT (yes vs. no) | 38/93 (29.0/71.0) | 0.582 (0.257–1.095); 0.127 | 0.002 |
| ACT in high stromal TIMs subgroup (yes vs. no) | 21/47 (30.9/69.1) | 0.874 (0.341–1.923); 0.748 | |
| ACT in low stromal TIMs subgroup (yes vs. no) | 17/46 (27.0/73.0) | 0.180 (0.022–0.722); 0.031 |
Abbreviation: HR: Hazard Ratio; CI: confidence interval; ACT: adjuvant chemotherapy; TIM: tumor-infiltrated mast cell; MIBC: muscle-invasive bladder cancer.
All HR and 95%CI were calculated from 1000 bootstrap samples protected from overfitting.
*P represents the interaction of the ACT and expression of TIMs infiltration
CD8 T cell was correlated with TIMs infiltration
We next sought to explore the cellular explanations for favorable prognostic and predictive value of low TIMs. As depicted in Figure 3A, in low TIMs group, activated DCs were less infiltrated, while CD8 T cells and M1 macrophages were significantly increased. Spearman’s correlation coefficients between TIMs and other infiltrated immune cells were calculated. Figure 3B showed the heat map of calculated Spearman’s rho and the corresponding P values were listed on the right chart. CD8 T cells and activated memory CD4 T cells were negatively correlated with TIMs with a relatively strong Spearman’s rho −0.245 and −0.274, respectively. We then performed GSEA to narrow down potential immune lineages change related to low TIMs. As illustrated in Figure 3C, T cell receptor signaling pathways and antigen processing and presentation pathways were the top two significantly enriched pathways in low TIMs group (Top 20 enriched pathway were listed in Figure 3D). These results suggested that high CD8 T cells infiltration might correlate with low TIMs.
Figure 3.
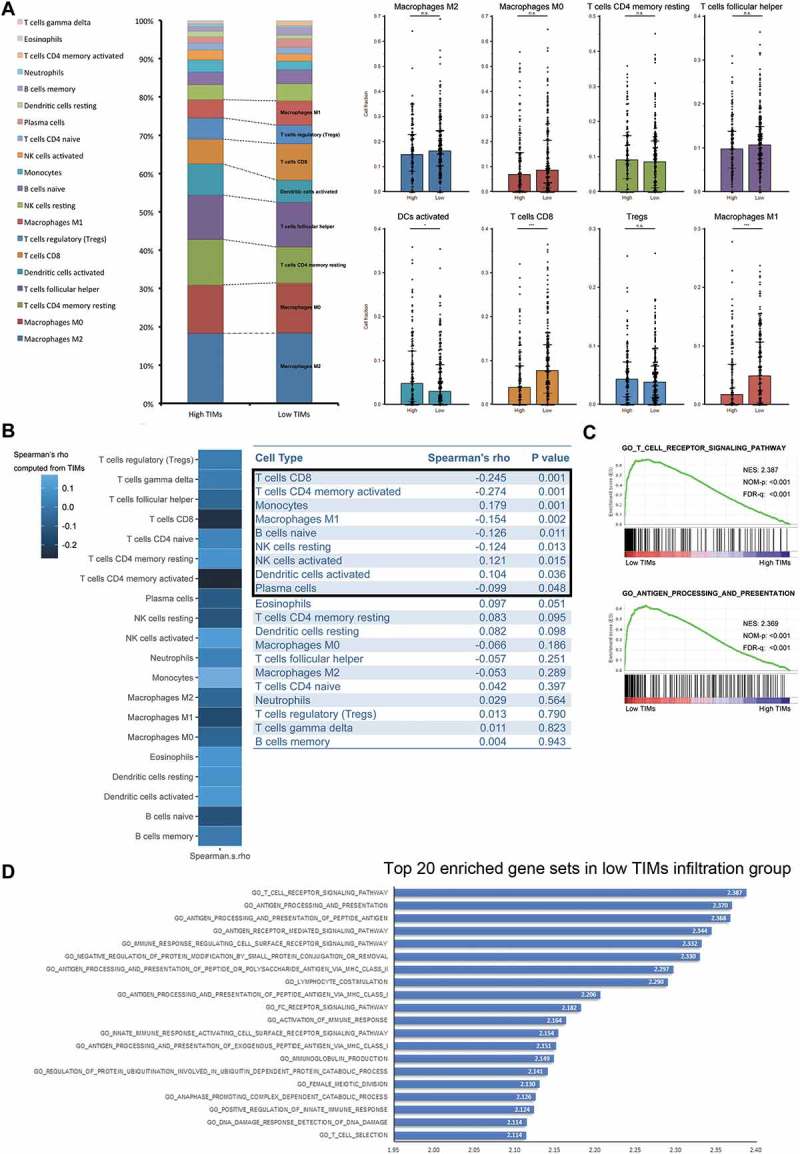
Differences of immune cell infiltration and biological process pathways according to TIMs infiltration in MIBC. (A) Frequency of immune lineages based on TIMs infiltration (left). Barplots (right) showed major abundant immune cells distribution according to TIMs. (*p < 0.05, **p < 0.01, and ***p < 0.001 by Mann-Whitney U test). Barplots show median ± quartile. (B) Heatmap showed Spearman’s correlation coefficients computed from analyses among different cell types and TIMs (left). Charts (right) listed detailed Spearman’s rho and corresponding P values. (C) Top two enriched biology pathways related with immune response in low TIMs infiltration groups. Gene Otology Biological Process gene sets from MSigDB were used. 1000 random sample permutations were performed. NES: Normalized Enrichment Score; NOM-p: Nominal P Value; FDR-q: False discovery rate. (D) Top 20 enriched gene sets in low TIMs group derived from GSEA. Gene Otology Biological Process gene sets from MSigDB were used. 1000 random sample permutations were performed.
Low tumoral TIMs infiltration represented immune activation state
After explorations on TCGA MIBC set, we then tried to validate the negative correlativity between TIMs and CD8 T cells on constructed TMAs. CD8 T cell infiltration was evaluated via immunohistochemistry. As demonstrated in Figure 4A, stromal TIMs might present a negative relationship with CD8 T cells. Barplot in Figure 4B also showed increased CD8 T cell density in low stromal TIMs group compared with high stromal TIMs group (P = 0.003). Correlation analyses validated that only stromal TIMs was negatively correlated with CD8 T cell density with a Spearman’s rho −0.215 (Figure 4B, bottom; P < 0.001).
Figure 4.
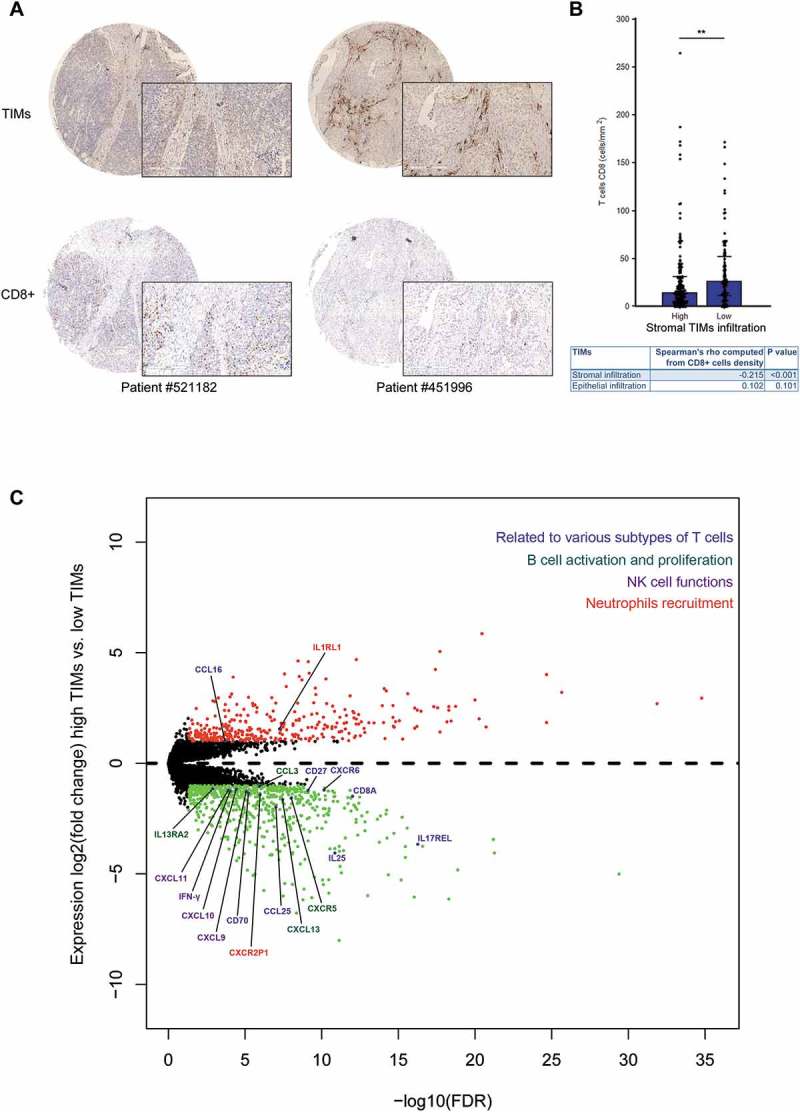
CD8 + T cells were correlated with TIMs infiltration in MIBC. (A) Representative immunohistochemistry images of high (left bottom) and low (right bottom) CD8+ cells infiltration in corresponding low (left upper) and high stromal (right upper) TIMs infiltration patients. Original, 48X; magnification, 200X. (B) Barplots showed frequencies of CD8+ cells density according to stromal TIMs infiltration. (*p < 0.05, **p < 0.01, and ***p < 0.001 by Mann-Whitney U test.). Barplots show median ± quartile. Chart (bottom) listed detailed Spearman’s rho and corresponding P values computed from correlation analyses among CD8+ cell density and stromal or epithelial TIMs density subgroups. (C) Volcano plot presented differential gene expression involved in multiple immune cells reaction between high and low TIMs groups. Gene in red or green were significantly differential expressed (defined as FDR-adjusted p-value ≤ 0.05 and fold change of at least 2x).
To bring insight into TIMs possible roles in MIBC, further differential gene expression analyses were performed. As illustrated in Figure 4C, low tumoral TIMs infiltration group were enriched of genes involved in multiple immune cell activation and recruitments, such as T cell (CD27, CD70, CD8A, CXCR6, CCL25), B cell (CXCL13 and CXCR5), neutrophils (CXCR2P1).13,14 We also observed that IFN-gamma and its stimulated chemokines (CXCL9, CXCL10 and CXCL11), which promoted NK and T cells, were highly enriched in low tumoral TIMs infiltration group as well.15 These results suggested that low tumoral TIMs infiltration might correlate with a state of immune activation in MIBC tumor environment.
Discussion
Applying ACT for MIBC patients after surgery is long under debate since last century. Latest EAU guideline rated this treatment option as C grade of recommendation for various data flaws of current studies, such as small study population, or differences in the chemotherapy regimens.1 Furthermore, concluded by several reviews, the improvement of clinical outcomes after ACT varied in different RCTs.3,4,16-18 Thus, there is an unanswered clinical question: which patients may actually benefit from ACT? Svatek et al. and Galsky et al. confirmed that applying ACT could benefit MIBC patients, especially with advanced pathological stage.19,20 However, as a conventional staging system, pathological stratification did not pay much attention to the immune reaction between tumor and the host.21 Increasing studies focused on tumor infiltrating immune cells, and raised novel opinions that tumor immune environment may affect the prognosis and even may influence the effect of chemotherapy.5,22 Therefore, incorporating immune cells with current pathological-based stratification could promote prognosis and therapy prediction of MIBC patients. In this present study, we evaluated 259 MIBC patients from two independent clinical institutes. Our results suggested that high stromal TIMs might be an independent unfavorable prognosticator for both OS and RFS of MIBC patients. Further analyses recommended that pT2 MIBC patients with low stromal TIMs could benefit most from ACT with a hazard ratio of 0.233 for OS and 0.180 for RFS (Table 3). Thus, our study provided clinical evidence for selecting appropriate patients for ACT, and explored the clinical significance of TIMs in MIBC.
Recent oncology researches have now focused on intratumoral immune environment. Various types of tumor infiltrating immune cells, and their location, density and functions may play distinct roles in tumor progression and even clinical response to therapy.5,6 As one of the tissue-resident sentinel cells, mast cells were best known for promoting angiogenesis and inflammation, and thus were considered to play a important role in remodeling tumor microenvironment. Crivellato reviewed that tumor-infiltrating mast cells could synthesize and release potent angiogenic factors, such as VEGF.23 In urologic malignancies, several studies revealed that tumor-infiltrating mast cells may correlate with tumor microvessel density and promote tumor angiogenesis.24,25 In accordance with its proinflammatory function, mast cells were also reported to have strong capacity to recruit other immune cells into tumor microenvironment, such as neutrophils, macrophages and eosinophils.26 These abilities for mast cells to impact tumor microenvironment attract attentions to explore its prognostic and predictive value in various tumor.
Besides mast cells, our previous study also confirmed that high TINs infiltration could predict worse outcomes,12 however, the prognosis values of TINs and TIMs in bladder cancer were quite similar, and the differences did not reach statistical significance (Supplementary Table 2). Khazaie argued that mast cells in different sites of tumor, such as intraepithelial and invasive border might represent different pro-tumor functions of TIMs.7 Our immunohistochemistry results also showed that mast cell infiltration was quite different in stromal and epithelial area of tumor specimens. Only stromal TIMs were significantly correlated with pT stage, poor clinic outcome, and presented prognostic and predictive value. These results suggested that stromal TIMs in MIBC might be a potential subset of TIMs.
Opinions of the relationship between chemotherapy and immune system have been renovated. Not only were tumor cells attacked by chemotherapeutic drugs, but also host immune system was influenced by chemotherapy. Intriguingly, the efficacy of chemotherapy might depend on its impact on immune system.6,22 As reported before, mast cells might induce an immunosuppressive environment via impairing CD8 + T cells.27 Consistently, our TCGA and GSEA analysis showed a significant correlativity between low TIMs and high CD8 + T cells (Figure 3). Subsequent validation on TMA verified that stromal low TIMs infiltration was correlated with high density of CD8 + T cells (Figure 4A-B). Further differential gene expression presented various interleukins, chemokines and chemokine receptors gene which were involved in multiple immune cells activation and recruitment were significantly enriched in low TIMs infiltration group (Figure 4C). Combined with these results, low TIMs infiltration might be correlated with an immune activation environment of MIBC. In Figure 3A, we surprisingly observed that low TIM infiltration group had less activated dendritic cells, while activated DCs were considered to be associated with T-cell “inflamed” pheonotype.28 Nonetheless, several studies suggested that high dendritic cells infiltration could predict the progression in bladder cancer.29 Ayari et al also observed that high tumor-infiltrating dendritic cells might represent reduced efficacy of BCG immunotherapy.30 Besides, a recent study indicated that urothelial tumor could promote the overexpression of certain immune checkpoints receptors, such as BTLA and TIM-3, by tumor-infiltrating dendritic cells, and then induce their functional inhibition.31 Thus, in bladder cancer, detailed function of tumor-infiltrating dendritic cells seems vague. We here showed that low stromal TIMs might represent more CD8 + T cells infiltration and consequently promote the efficacy of ACT. Recent studies also raised a subset mast cells as MCregs for their regulatory role in both innate and adaptive immunity.32 However, as the complicated functions of TIMs in tumor microenvironment (reviewed in9), further experimental researches were required to validate these presumptions.
Several limitations of this study should be mentioned. First, this study was retrospective, further prospective research is needed. Second, we failed to acquire enough data related to neo-adjuvant chemotherapy. Lastly, given the sophisticated interaction between TIMs and other immune cells, the exact biologic mechanism under our observations is still unclear.
In conclusion, this study demonstrated that high stromal TIMs infiltration was an independent unfavorable prognosticator for OS and RFS of MIBC patients. Patients with low stromal TIMs were observed retrospectively to have the most favorable outcomes for the application of cisplatin-based combination ACT, especially in pT2 patients. Both database and TMA results revealed the correlativity of low TIMs and high CD8 + T cells infiltration. These findings might promote the personalized prognosis prediction and ACT application of MIBC patients after cystectomy.
Patients and methods
Study patients
This study retrospectively scanned 393 patients who underwent radical cystectomy in Zhongshan Hospital, Fudan University (Shanghai, China) and Fudan University Shanghai Cancer Center (Shanghai, China). The inclusion criterions were: 1) pathological diagnosed as MIBC; 2) no other malignancies; 3) available for tumor tissues and follow-up information. Patients in this study were not involved in any clinical trials, and none of them received additional anti-tumor therapies prior to surgery. After filtrations, 259 eligible MIBC patients were included in this study. Detailed flowchart of patient selections was illustrated in Supplementary Figure 1A.
After surgery, 119 patients (45.9%) were offered cisplatin-based combination chemotherapy and lasted at least one therapeutic cycle. During the follow-up period, physical examination, urine cytology, chest imaging and abdominal ultrasound or CT scan were performed every 3–4 month in the first year, semi-annually in the second year and annually thereafter. All follow-up data was updated at July 2016, and the interquartile range of follow-up was 40 (18–72) months. Interested endpoints were overall survival (OS) and recurrence free survival (RFS), and were calculated from the date of surgery to the date of death from all causes and recurrence, respectively, or to the date of the last follow-up. The Clinical Research Ethics Committee of Zhongshan Hospital and the Ethics Committee of Fudan University Shanghai Cancer Center approved this study, and written consent was obtained from each patient. REMARK criteria were followed in this study.33
Immunohistochemistry and evaluation
Tissue microarray (TMA) was constructed and immunohistochemistry was performed as previously described.34 Formalin-fixed, paraffin-embedded tumor specimens of MIBC patients were obtained from two independent clinic centers. All samples were reviewed histologically by hematoxylin and eosin staining, and representative areas were marked on the paraffin blocks. Duplicate 1.0-mm tissue cores from two different areas were used to construct the TMAs. To perform immunohistochemistry, the TMA sections were deparaffinized in xylene and hydrated to distilled water. After the endogenous peroxidase was inhibited by 3% H2O2 for 30 minutes, the sections were heated in a pressure cooker for 5 minutes in unmasking solution (0.01 M sodium citrate buffer, pH = 6) and then were incubated with 10% normal goat serum for 30 minutes. Anti-tryptase monoclonal antibody (Ab2378, diluted 1:10,000; Abcam) and anti-CD8 monoclonal antibody (clone C8/144B, original dilution; DAKO) was applied overnight in a moist chamber at 4°C to identify mast cells and CD8 + T cells in tissue. After the primary antibody was washed off, DAB detection system was used to explore positive staining. Then the sections were counterstained with hematoxylin, dehydrated, mounted and observed.
TMA slides were analyzed on Leica DM6000 B working station (Leica Microsystems). Two urologic pathologists who were blinded to the clinical and follow-up data accomplished mast cells and CD8 + T cells counting using ImageScope (Aperio Technologies). Mast cell and CD8 + T cell density was counted as cells/mm2, and was categorized into high and low groups. Scorer of reliability analyses were conducted after the two pathologist finished mast cell counting. F tests showed P = 0.890 and P = 0.500 respectively for stromal and epithelial mast cell counting, indicating a good reliability of these two counting results. We then adopted the mean value of the two counting results to conduct further analyses. Cutoff value was determined by X-tile 3.6.1 (Yale University).
Differential expression analysis
The bladder cancer (BLCA) data from The Cancer Genome Atlas (TCGA) was extracted to conduct differential expression analysis in MIBC tissues. The mRNA expression data of TCGA-BLCA were downloaded from cBioPortal (http://www.cbioportal.org/),35,36 and were already normalized to RSEM format. Total 405 samples were analyzed in this study. Gene set enrichment analyses (GSEA) analyses were applied to determine the biological pathway divergences between high and low TIMs. The CIBERSORT method,37 an algorithm to evaluate hematopoietic cell distribution from tumor RNA mixtures, was used to analyze the differential immune cells infiltration distributions. While significant differential gene expression between high and low TIMs groups were explored via EdgeR package.38 The cutoff value of TIMs in BLCA data was determined as median.
Statistical analysis
Associations between TIMs and clinicopathological characteristics of patients were analyzed with chi-square or Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Survival curves were constructed and compared with log-rank test. Cox proportional hazard regression models were established to perform univariate and multivariate analyses. All statistic significance in this study was defined as P < 0.05, and all tests were two-sided. Statistical analyses were performed using IBM SPSS Statistics v21.0 (IBM Corp) and R 3.2.3 (R Foundation for Statistical Computing).
Supplementary Material
Biography
Z. Liu, Y. Zhu and L. Xu for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; J. Zhang, H. Xie, H. Fu, Q. Zhou and Y. Chang for technical and material support; B. Dai and J. Xu for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Funding Statement
This study was supported by grants from National Natural Science Foundation of China [81402082, 81402085, 81471621, 81472227, 81671628 and 31770851], Shanghai Municipal Natural Science Foundation [16ZR1406500], Shanghai Municipal Commission of Health and Family Planning Program [20144Y0223], Guide Project of Science and Technology Commission of Shanghai Municipality [17411963100] and a grant from the Shanghai Cancer Research Charity Center. All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data for this article can be accessed here.
References
- 1.Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J, Rouanne M, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71:462–475. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Advanced Bladder Cancer Meta-analysis C Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48:189–199; discussion 99–201. doi: 10.1016/j.eururo.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Leow JJ, Martin-Doyle W, Rajagopal PS, Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL, Choueiri TK, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66:42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, Sylvester R.. Thoughts on a systematic review and meta-analysis of adjuvant chemotherapy in muscle-invasive bladder cancer. Eur Urol. 2014;66:55–56. doi: 10.1016/j.eururo.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Fridman WH, Pages F, Sautes-Fridman C, Galon J.. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 6.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 7.Khazaie K, Blatner NR, Khan MW, Gounari F, Gounaris E, Dennis K, Bonertz A, Tsai F-N, Strouch MJ, Cheon E, et al. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30:45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Zhang Y, Zhao J, Yang Z, Li D, Katirai F, Huang B. Mast cell: insight into remodeling a tumor microenvironment. Cancer Metastasis Rev. 2011;30:177–184. doi: 10.1007/s10555-011-9276-1. [DOI] [PubMed] [Google Scholar]
- 9.Rigoni A, Colombo MP, Pucillo C. The role of mast cells in molding the tumor microenvironment. Cancer Microenviron. 2015;8:167–176. doi: 10.1007/s12307-014-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu H, Zhu Y, Wang Y, Liu Z, Zhang J, Wang Z, Xie H, Dai B, Xu J, Ye D. Tumor Infiltrating Mast Cells (TIMs) confers a marked survival advantage in nonmetastatic clear-cell renal cell carcinoma. Ann Surg Oncol. 2017;24:1435–1442. [DOI] [PubMed] [Google Scholar]
- 11.Masson-Lecomte A, Rava M, Real FX, Hartmann A, Allory Y, Malats N. Inflammatory biomarkers and bladder cancer prognosis: a systematic review. Eur Urol. 2014;66:1078–1091. doi: 10.1016/j.eururo.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Xu L, Chen L, Fu Q, Liu Z, Chang Y, Lin Z, Xu J. Tumor-infiltrating neutrophils predict benefit from adjuvant chemotherapy in patients with muscle invasive bladder cancer. Oncoimmunology. 2017;6:e1293211. doi: 10.1080/2162402X.2017.1293211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 14.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, Hahn NM, Lerner SP, Mason M, Powles T, et al. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62:523–533. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF, Dreicer R, George DJ, Milowsky MI, Theodorescu D, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol. 2013;63:58–66. doi: 10.1016/j.eururo.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Pectasides D, Pectasides M, Nikolaou M. Adjuvant and neoadjuvant chemotherapy in muscle invasive bladder cancer: literature review. Eur Urol. 2005;48:60–67; discussion 7-8. doi: 10.1016/j.eururo.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Svatek RS, Shariat SF, Lasky RE, Skinner EC, Novara G, Lerner SP, Fradet Y, Bastian PJ, Kassouf W, Karakiewicz PI, et al. The effectiveness of off-protocol adjuvant chemotherapy for patients with urothelial carcinoma of the urinary bladder. Clin Cancer Res. 2010;16:4461–4467. doi: 10.1158/1078-0432.CCR-10-0457. [DOI] [PubMed] [Google Scholar]
- 20.Galsky MD, Stensland KD, Moshier E, Sfakianos JP, McBride RB, Tsao CK, Casey M, Boffetta P, Oh WK, Mazumdar M, et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol. 2016;34:825–832. doi: 10.1200/JCO.2015.64.1076. [DOI] [PubMed] [Google Scholar]
- 21.Bindea G, Mlecnik B, Fridman WH, Pages F, Galon J. Natural immunity to cancer in humans. Curr Opin Immunol. 2010;22:215–222. doi: 10.1016/j.coi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Crivellato E, Nico B, Ribatti D. Mast cells and tumour angiogenesis: new insight from experimental carcinogenesis. Cancer Lett. 2008;269:1–6. doi: 10.1016/j.canlet.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Sari A, Calli A, Cakalagaoglu F, Altinboga AA, Bal K. Association of mast cells with microvessel density in urothelial carcinomas of the urinary bladder. Ann Diagn Pathol. 2012;16:1–6. doi: 10.1016/j.anndiagpath.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Tuna B, Yorukoglu K, Unlu M, Mungan MU, Kirkali Z. Association of mast cells with microvessel density in renal cell carcinomas. Eur Urol. 2006;50:530–534. doi: 10.1016/j.eururo.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasiuk A, Dalton DK, Schpero WL, Stan RV, Conejo-Garcia JR, Noelle RJ. Mast cells impair the development of protective anti-tumor immunity. Cancer Immunol Immunother. 2012;61:2273–2282. doi: 10.1007/s00262-012-1276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweis RF, Spranger S, Bao R, Paner GP, Stadler WM, Steinberg G, Gajewski TF. Molecular drivers of the non-T-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol Res. 2016;4:563–568. doi: 10.1158/2326-6066.CIR-15-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayari C, LaRue H, Hovington H, Caron A, Bergeron A, Tetu B, Fradet V, Fradet Y. High level of mature tumor-infiltrating dendritic cells predicts progression to muscle invasion in bladder cancer. Hum Pathol. 2013;44:1630–1637. doi: 10.1016/j.humpath.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Ayari C, LaRue H, Hovington H, Decobert M, Harel F, Bergeron A, Têtu B, Lacombe L, Fradet Y. Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to bacillus Calmette-Guerin immunotherapy. Eur Urol. 2009;55:1386–1395. doi: 10.1016/j.eururo.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Chevalier MF, Bohner P, Pieraerts C, Lhermitte B, Gourmaud J, Nobile A, Rotman S, Cesson V, Martin V, Legris A-S, et al. Immunoregulation of dendritic cell subsets by inhibitory receptors in urothelial cancer. Eur Urol. 2017;71:854–857. doi: 10.1016/j.eururo.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Frossi B, Gri G, Tripodo C, Pucillo C. Exploring a regulatory role for mast cells: ‘MCregs’? Trends Immunol. 2010;31:97–102. doi: 10.1016/j.it.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 33.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Liu Y, Xu L, An H, Chang Y, Yang Y, Zhang W, Xu J. P2X7 receptor predicts postoperative cancer-specific survival of patients with clear-cell renal cell carcinoma. Cancer Sci. 2015;106:1224–1231. doi: 10.1111/cas.2015.106.issue-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


