ABSTRACT
Heat is a major stressor during exercise, though its value in driving adaptation is not well understood. Muscle heating can upregulate pathways facilitating protein synthesis and could thereby enhance effects of exercise training, however, few studies have investigated this possibility. We examined whether heating active muscle during resistance training differentially affected physical and functional adaptations. Within a randomised contralateral-limb control study, ten healthy, resistance-untrained individuals (21 ± 3 y; 5 female) completed 30 sessions of progressive resistance training (12 weeks), performing 4 × 8 unilateral knee extensions at 70% of 1RM. One randomly-allocated thigh was heated during, and for 20 min after, each session using an electric pad eliciting muscle temperatures of >38 °C (HOT); the contralateral limb remained unheated (CON). Training intensity was progressed using 4-weekly strength assessments. Quadricep lean mass (measured using DXA) increased by 15 ± 7% in HOT (p = 0.00) and 15 ± 6% in CON (p = 0.00); the difference being trivial (p = 0.94). Peak isokinetic torque at 90°.s−1 increased by 30 ± 25% (HOT; p = 0.00) and 34 ± 33% (CON; p = 0.01), with no difference (p = 0.84) between limbs. Rate of torque development increased ∼40%, with no difference between limbs (p = 0.73). The increase in 3-RM strength was also similar in HOT (75 ± 16%) and CON (71 ± 14%; p = 0.80 for difference). No differences in mass or strength changes were evident between sexes. In conclusion, supplemental heating of active muscle during and after each bout of resistance training showed no clear positive (or negative) effect on training-induced hypertrophy or function.
KEYWORDS: DXA, force, torque, isokinetic, females, quadriceps
Introduction
The myriad benefits of resistance training are well established and relevant across the spectrum of human performance, from clinical to elite athletic populations. Skeletal muscles adapt architecturally and functionally to repeated loading, becoming larger, stronger, faster, and more efficient.1 Maintaining or improving muscle mass and strength throughout life can play a significant role in improving functional capabilities, and preventing or delaying chronic disease,2 thereby promoting quality of life and healthy aging.
In contrast to the known efficacy of resistance training, the potential adaptive value of heat – an intrinsic stressor of exercise – is not as well understood and is of growing interest. Temperature can affect muscle physiology acutely and chronically in ways that could impact structural or functional outcomes of resistance training. Acutely, muscle temperature affects passive and active components of contractility and force transmission,3,4 and metabolic rate and substrate partitioning.5,6 Chronically, heat has the potential to protect against atrophy and even produce hypertrophy.7–9 Such effects could be useful for facilitating the repair and regeneration of trauma-damaged muscle, whereas icing can retard such recovery.10 Multiple underlying mechanisms, such as the heat shock response,11–13 may be responsible. Heat shock proteins (HSP) – a family of heat-inducible cellular chaperones involved in repairing proteins – may facilitate muscle protein synthesis.14,15 Alternatively, heat-induced hyperemia might increase delivery of oxygen and nutrients to damaged cells.16 However, incorporating supplemental heat into resistance training heat might cause counter-productive effects, as elevated HSP may inhibit hypertrophy by pre-protecting muscle from the mildly-damaging effects of mechanical stess17,18 encountered in resistance training.
Only one previous study has, to our knowledge, reported the chronic effects of a resistance training programme with supplemental heating of muscle. Goto et al.8 heated one biceps brachii in each of nine untrained men for 60 minutes (initiated 30 minutes prior to exercise bouts) across 10 weeks of resistance training. Training comprised of 40 sessions of low-load contractions, i.e., 30 repetitions at <50% of 1 repetition maximum (1RM: the maximum force that can be exerted in a single effort). They observed an increase in cross-sectional area (CSA +8%) and the torque produced during a maximal voluntary contraction (MVC +18%) after. No change was observed in the non-heated, identically-trained arm. The purpose of the present study was to add to the findings of the Goto et al. study, and extend them by examining effects of heat stress combined with resistance training on lower-limb hypertrophy and strength, i.e., to a muscle group that is larger and of different fibre composition. We hypothesized that muscle mass and strength changes would be greater when a resistance training programme was performed with heating of the active muscle mass compared to traditional training (i.e., without supplemental heat).
Methods
Experimental overview
A contralateral limb-control design was used to investigate the effects of local muscle heating on physiological and functional adaptations to a 12-week resistance training protocol. The protocol involved participants performing 30 supervised resistance training sessions across 12 weeks, with a minimum of 48 h between sessions. Each participant had one limb randomly allocated to the HOT training condition prior to the first training session, with the same limb heated to ∼38 °C in all sessions. Seated knee extension exercise was used to train each limbs' quadriceps femoris muscles identically, i.e., load matched relative to non-heated 1RM, as explained below. Participants were not informed of the hypothesis until the end of the training programme, but were informed that heat has potentially adaptive or maladaptive effects on muscle.
Participants
Ten healthy participants (5 female) completed the 12-week training programme. Participants were physically active (2 ± 1 h aerobic exercise/week;) but had not performed resistance training within the previous six months. Means ± SD of the participants' characteristics are as follows: age, 21 ± 3 y (range: 19 – 27); height, 172 ± 4 cm (168 – 177); body mass, 69 ± 9 kg (55 – 81); body fat, 28 ± 11% (12 – 46). Sex-specific body mass and adiposity data were, for males, 69 ± 9 kg (56 – 79) and 20 ± 8% (12 – 30), and for females, 70 ± 10 kg (56 – 81), and 35 ± 8% (25 – 45). Participants were asked to arrive at training sessions in a fed and euhydrated state, and instructed not to perform aerobic exercise on training days. They were screened to exclude (reported) use of anti-inflammatory medications or vitamin supplements, heat intolerance and heat training or recent heat exposure (e.g., sauna bathing). Participants had no known or suspected heart disease or hypertension, or musculoskeletal injury that could be aggravated during resistance exercise. The study was approved by the University of Otago Ethics Committee (H15/065). Written informed consent was obtained from all participants.
Resistance training protocol
Participants attended the exercise clinic 2–3 days/week for 12 weeks (30 sessions total). Time of day was standardised within participants, and sessions were separated by at least 48 h to allow for sufficient recovery and adaptation. Participants performed a 10-min cycling warm up at a light intensity (70–80 rpm; RPE ∼9–11). A rheostatically-controlled heat pad (∼40 °C; Sunbeam EP5000, Sydney, NSW, Australia) was then wrapped around the HOT thigh with a custom-made neoprene wrap insulating the limb and heat pad to enhance heat transfer into the thigh. Pilot research (n = 5) confirmed that the described method elevated muscle temperatures to ∼38 °C (unheated limb: 37 °C) during and following exercise, matching the temperatures elicited in the Goto et al.8 protocol. Specifically, temperatures were measured at depths of 1, 2, and 3 cm in the vastus lateralis muscle belly, and when averaged across depths, were 35.2 ± 1.1 °C at baseline, 38.2 ± 0.9 °C immediately post, 38.7 ± 0.7 °C at 20-min post exercise in HOT, and 35.6 ± 0.9, 36.6 ± 0.9 and 37.1 ± 0.8 °C in CON.
Resistance training consisted of four sets of eight knee eccentric and concentric contractions of the knee extensors at 70% of 1RM, alternating legs between each set. Training volume was therefore 32 repetitions per leg per session. Exercise was performed at a moderately slow tempo (3-s eccentric and 1-s concentric contraction, with 1-s pause between each) to control muscle time under tension. After the final (64th) repetition, the participant remained seated for 20 min with the heat pad on. During this rest period participants were provided with a drink containing 24 g protein (NZ Protein, Auckland, NZ) to aid muscle protein synthesis. Training loads were incremented at four-week intervals to maintain 70% of 1RM loading, based off CON leg, as described below.
Measurements
Tissue mass: Total body DXA (software version 16.0, Lunar Prodigy, GE Healthcare, Chicago, IL, USA) scans were performed before and after the 12-week intervention to determine regional lean tissue mass changes in the lower limbs. One researcher conducted and analysed all scans. Thigh muscle mass was determined by redefining the region of interest (ROI; Figure 1) using the manufacturer's body composition analysis software and to match those described previously by Segal et al.19 Briefly, the superior and inferior perimeter of the ROI was a line through the femoral neck parallel and proximal to the greater trochanter and the knee joint. The lateral border was outside of the adipose shadow, and the medial border was between the thighs.
Figure 1.
DXA thigh region of interest (ROI), showing 1) left thigh, and 2) right thigh borders used to determine muscle mass from total body scan analyses.
Strength was measured using 3RM test and isokinetic dynamometry at baseline and every four weeks. Mean and peak voluntary concentric torque, and rate of torque development were measured using an isokinetic dynamometer (Biodex II, model 880–125, Shirley, NY, USA), recorded via a separate A-D converter and software (LabChart 7, ADI Instruments, Dunedin, NZ). Participants were seated in the dynamometer and performed eight repetitions of maximal-effort concentric knee extensions for each leg at a standardised velocity (90°.s−1). Muscle quality was derived as peak torque divided by DXA-measured thigh lean mass. Dynamic strength was measured using a 3RM test, which was also used to adjust training load across the 12 wks.
At the conclusion of the 12-wk resistance training programme, participants were asked to rate the perceived effect (positive, neutral, or negative) of muscle heating during exercise on comfort, strain, and recovery compared to the non-heated limb. Participants were also permitted to provide general comments about training with and without muscle heating. Qualitative perceptions during exercise and recovery were described both within and between sessions.
Data analysis
Data reduction: All torque-related measures were calculated offline within the LabChart software. Peak torque was taken as the maximum height of the torque curve prior to full extension of the knee. Peak rate of torque development was measured from the steepest positive slope of the torque curve, and calculated as the change in torque over change in time.
Statistical analyses: All data were analysed using a two-way repeated measures analysis of variance (condition × time) with Sidak post-hoc correction. Strength-related data (peak torque, mean torque, rate of torque development, 3RM) were analysed across conditions (CON versus HOT) and time (0, 4, 8, and 12 weeks). Muscle-related data (muscle mass and quality) were analysed across conditions and time (pre versus post). Sex differences were analysed as covariates within each variable. Statistical significance was set at an α of p < 0.05. Statistical analyses were performed using SPSS (version 19.0, IBM, IL, USA). Figures were produced using Prism (version 7, GraphPad, CA, USA) and are presented as means with 95% CI. Individual responses of each participant are displayed with a unique colour marker consistent across all figures to allow for visualisation of inter-individual variability in each measure. All in-text variability data are SD and effect sizes are described according to Hopkins20 interpretation of the magnitudes of mean differences.
Results
Training compliance and completion
All participants completed all 30 supervised training sessions, with each session separated by 67 ± 30 hours. All training and testing sessions were completed under supervision at the prescribed intensity, and same time of day within participants.
Muscle mass
Quadriceps lean mass increased significantly across the 12-week training programme, by 752 ± 304 g (p = 0.00) and 761 ± 280 g (p = 0.00) in the CON and HOT limbs, respectively (Figure 1). The respective relative increases were 15 ± 7 and 15 ± 6% in the HOT and CON limbs, with the between-limb difference being trivial 0 ± 6% (p = 0.94).
Isokinetic dynamometry
Peak concentric torque of the knee extensors increased significantly across 12 weeks, by 32 ± 28 Nm in CON (p = 0.01) and 31 ± 25 Nm in HOT (p = 0.00), representing an increase of 34 ± 33 and 30 ± 25%, respectively (Figure 2A). The increase was similar between conditions (right panel of Figure 2A; p = 0.84).
Figure 2.
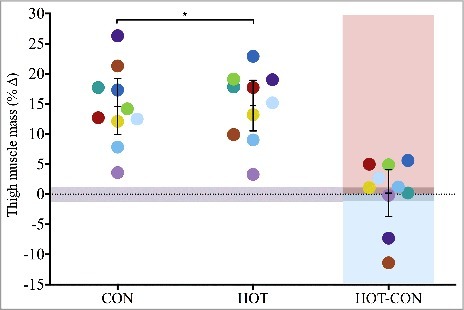
Mean (±95% CI) and individual (n = 10) percent change in thigh muscle mass of control (CON) and heated (HOT) limbs when measured after 12-week resistance training programme; participants' between-condition (HOT-CON) differences are highlighted with red and blue shaded areas indicating greater HOT and CON response, respectively; grey shaded area indicates zone of trivial effect; *significantly different (p < 0.01) to baseline.
Mean concentric torque increased significantly across 12 weeks, by 26 ± 28 Nm in CON (p = 0.02) and 29 ± 31 Nm in HOT (p = 0.02), representing an increase of 33 ± 38 and 34 ± 37%, respectively (Figure 2B). A small, 3 ± 14 Nm benefit in HOT, relative to CON, was not significant (p = 0.82).
Peak rate of torque development (Figure 2C) increased significantly, by 273 ± 242 Nm.s−1 (p = 0.02) for CON and 320 ± 355 Nm.s−1 (p = 0.04) for HOT, representing an increase of 37 ± 34% and 42 ± 47%, respectively. No significant difference was evident between conditions (5 ± 44%; p = 0.73).
Dynamic strength, measured by 3-RM knee extension, increased significantly from baseline for both CON (23 ± 7 kg; p = 0.00) and HOT (24 ± 8 kg; p = 0.00), representing an increase of 71 ± 14 and 75 ± 16%, respectively (Figure 3). The 4 ± 5% benefit in HOT relative to CON represented a trivial difference (p = 0.80).
Figure 3.
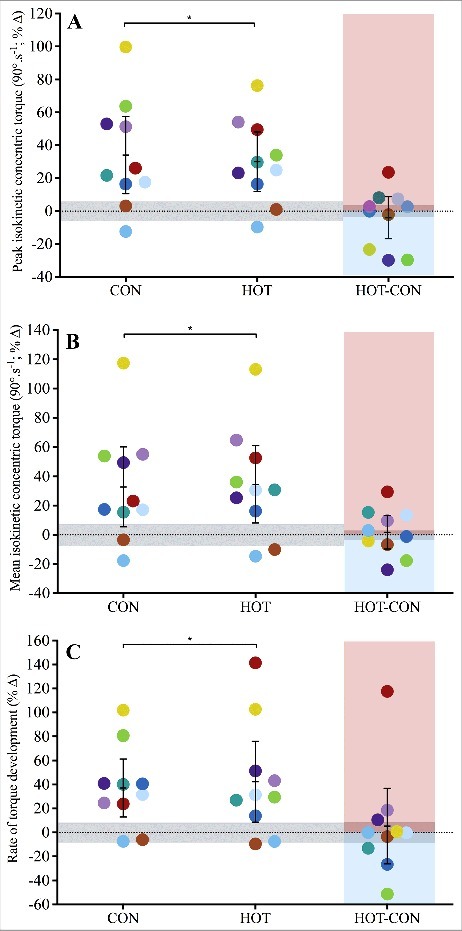
Mean (±95% CI) and individual (n = 10) percent change in A) peak isokinetic concentric torque, B) mean isokinetic concentric torque, and C) peak rate of torque development of control (CON) and heated (HOT) limbs when measured after 12-week resistance training; participants' between-condition (HOT- CON) differences are highlighted with red and blue shaded areas indicating greater HOT and CON response, respectively; grey shaded areas indicate zone of trivial effect; *significantly different (p < 0.05) to baseline.
Muscle quality
Muscle quality, derived as peak torque per kilogram of thigh muscle mass, was not significantly different between conditions at baseline (p = 0.85). The increase in quality across 12 wk was not different between CON (2 ± 5 Nm.kg−1; p = 0.21) and HOT (2 ± 5 Nm.kg−1; p = 0.18). Relative increases were 16 ± 35 and 18 ± 34%, respectively.
Perceptions
Eight participants (80%) reported the HOT limb as feeling more comfortable than the non-heated limb during training. One reported the increased sweat rate – exacerbated by the heat pad preventing sweat evaporation/skin cooling – as being less comfortable. Eight reported reduced feelings of ‘strain’ in the heated limb when performing knee extensions. Six described the heated limb as feeling “easier”, or the non-heated limb as “harder” to perform exercise on, especially in later sets and repetitions when nearing task failure. (The other four provided no comment). Nine reported improved recovery in the heated limb compared to the non-heated limb. Participants described the heated limbs as fatiguing later in sets and the overall training sessions, often delaying onset of task failure during sets.
Discussion
In the current study, we investigated the effect of localised muscle heating on physical and functional adaptations when applied to one thigh during, and for 20 minutes after, each of 30 resistance training bouts over 12 weeks. Muscle heating had no clear benefit (or detriment) on hypertrophy (Figure 2) or strength (Figures 3–4) gains achieved during the 12-week resistance training programme compared to the gains in the non-heated contralateral limb.
Figure 4.
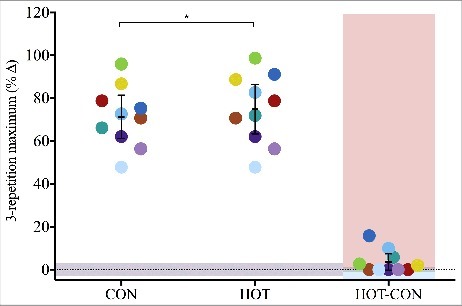
Mean (±95% CI) and individual (n = 10) percent change in knee extensor 3-repetition maximum (3RM) strength of control (CON) and heated (HOT) limbs when measured after 12-week resistance training; participants' between-condition (HOT-CON) differences are highlighted with red and blue shaded areas indicating greater HOT and CON response, respectively; grey shaded areas indicate zone of trivial effect; *significantly different (p < 0.05) from baseline.
Large inter-individual variability was observed in participants' hypertrophic adaptation (Figure 2) and strength gains (Figures 3–4), whereas muscle quality became markedly more uniform between participants. Most participants showed substantive (>5%) hypertrophy with little difference between heated and control limbs. Similarly, most participants (80%) showed substantive (>10%) gains in strength (peak torque, mean torque, 3RM), but indistinct effects of localised heating. Our results are in line with the typically-observed large and highly variable gains in strength, with generally trivial effects of localised heating applied over the muscles being trained. Specifically, mean functional and structural effects of heating across 12 wk were <10% of the mean training-induced gains over this period (see Figures 2–5).
Figure 5.
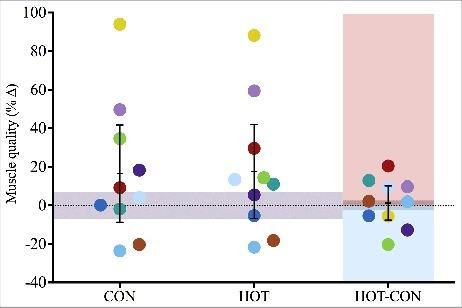
Mean (±95% CI) and individual (n = 10) percent change in knee extensor muscle quality of control (CON) and heated (HOT) limbs when measured after 12-week resistance training; participants' between-condition (HOT-CON) differences are highlighted with red and blue shaded areas indicating greater HOT and CON response, respectively; grey shaded areas indicate zone of trivial effect.
Notwithstanding the present results, heat stress alone, or with additional stressors, has previously been found to exert acute and chronic effects on muscle physiology. Some studies have detailed the potential role of heat in muscle regeneration and repair,8,9,11–13,21 through the primary signalling pathways activated by resistance training: tensile- and leukocyte-mediated mechanisms that upregulate apoptosis and muscle protein synthesis.14,15 Various studies have also detailed the ability of heat stress to attenuate atrophy7,14,22,23 and muscle soreness,18 typically related to unloading or eccentric-loading damage, respectively. Active muscle heating of the biceps brachii prior to and during low-intensity resistance exercise has been shown to elicit a superior increase in CSA and MVC over 10 weeks.8 Upregulated expression of HSP 7224 and intracellular signalling of protein synthesis14,15 via heat stress have been postulated as the main factors contributing to greater hypertrophy and strength. However, the expression of HSPs via heat stress has been shown to precondition muscle to mechanical stress for ∼72 hours, which could impair chronic adaptation to resistance training;17,18 an effect that cannot be discounted in offsetting other beneficial effects, within the present study. Other beneficial effects might include increased blood flow16 delivering nutrients and anabolic hormones to the muscle and improving clearance of metabolites during and after exercise; heat stress increasing phosphorylation of signalling kinases (Akt, mTOR, p70 S6K) that regulate muscle protein synthesis;15,25 and, hastening neuromuscular fatigue,3 which could result in higher-threshold motor unit activation, thereby stimulating a larger dose-response.
It is unclear why the results of the present study differed from those of Goto et al.8, who observed increased CSA and isometric strength in the heated upper limb only, following a similar intervention to that of the present study. One possible explanation relates to the effect of exercise intensity on HSP expression.26,27 Given that resistance training was performed at a higher relative intensity in the present study (70 vs <50% 1RM), it is possible that the participants had higher HSP accumulation within the heated limb, thereby conferring a larger protective effect and reducing the exercise-induced damage incurred within the muscle. Alternatively, HSP 72 has been shown to increase in the biceps brachii but not in the vastus lateralis 48 hours after eccentric-loading exercise,28 so if HSPs were responsible for the heat-induced benefit observed by Goto et al., the difference would appear to be limb specific. The lower limb has a larger muscle mass and greater proportion of slow-twitch fibres,29 potentially making it less amenable to supplemental heat. Neither Goto et al.8 nor the present study included sampling of muscle to examine effects of heating on heat shock proteins (e.g., HSP 25/72) or expression of kinases (e.g., PI3-K, Akt, mTOR, p70S6K), so reasons for differences remain unknown.
Studies on the effects of heat stress on muscle growth and repair in animal and humans report muscle temperatures in the range of 37–42 °C. Goto et al.8 reported a mean biceps brachii muscle temperature of 38 °C during the training protocol, which appears to have been similar to those in the vastus lateralis within the present study. Pilot research showed the unheated limb muscle temperature was approximately 37 °C, which may have conferred some benefit but no more than would be gained from resistance training alone. Timing and duration of heating also differed between this and other studies, and seemingly minor differences in such parameters may also affect the activation or suppression of signaling factors, as has been indicated for AMPK.25 Other human studies elicited increases in intracellular signalling,14 HSP expression,30 and/or physical and functional adaptations9,31 from heating prior to and during exercise.
Heating did, however, appear beneficial for perceptions of performing resistance exercise. A majority (8–9/10) of participants reported improved comfort and recovery and reduced strain during exercise with local heating. In almost every training session, participants reported the heated limb feeling “better”, “easier”, “stronger”, or “less fatigued” among other positive responses, in comparison to the non-heated contralateral limb, despite being neutralized to the hypothesis prior to the study, which indicates that it is a genuine response. Discomfort is a common issue associated with physical activity,32 including sweating, muscle soreness and fatigue. Such perceptions can act as barriers to participation or adherence.33 From an exercise perspective, the beneficial effect of heating improving comfort and/or; reducing pain and reducing the perceived work being performed could potentially improve adherence and possibly allow individuals to push themselves harder, but must also be balanced against the notably increased time and other resource usage involved with limb heating before, during or after exercise. If it takes >20 minutes to elicit an increase in muscle temperature of this magnitude for only a slight benefit, then it is arguable how worthwhile muscle heating modalities are, adjunct to resistance training. However, this could be considered as an alternative to increasing volume or intensity of exercise, especially in clinical, rehabilitating, or athletic populations.
In conclusion, heat stress during and after training did not improve or impair hypertrophy or strength gains arising from lower-limb resistance training, which differs from previous findings observed from upper-limb training. A limitation of the present and previous study was the lack of muscle and blood sampling to explain potential mechanisms. Future studies should investigate the intracellular signalling and HSP responses in different muscles to elucidate the effects of muscle heating supplementary to resistance training, as well as effects of training parameters (e.g., relative intensity/load;, task failure, volume). Relevant populations for future research include those with barriers to physical activity, such as the elderly and those in pre/rehabilitation; or chronic clinical care, as has been suggested by others.9,34
Biography
AS and JC conceptualised and developed the project. All authors contributed to finalising the design and experimental details. AS undertook all data collection, including recruitment and supervising all training sessions, except that KMJ undertook DXA and analyses thereof. AS analysed all other data, and drafted the manuscript. All authors provided critical input into the manuscript.
Funding Statement
The project was supported by internal funding from the School of Physical Education, Sport and Exercise Sciences, University of Otago.
Abbreviations
- CSA
Cross-sectional area
- DXA
Dual-energy x-ray absorptiometry
- HSP
Heat shock proteins
- MVC
Maximal voluntary contraction
- ROI
Region of interest
- 1 / 3RM
1-/3-repetition maximum
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to: Participants for their time and effort; Austina Clark and Tim Jowett, Dept Mathematics and Statistics, University of Otago for their statistical advice; and Nigel Barrett School of Physical Education, Sport and Exercise Sciences, University of Otago, for his technical assistance.
References
- 1.Marini M, Veicsteinas A. The exercised skeletal muscle: a review. European J Transl Myol. 2010;20(3):105–120. 10.4081/bam;.2010.3.105 [DOI] [Google Scholar]
- 2.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–482. [DOI] [PubMed] [Google Scholar]
- 3.Ball D, Burrows C, Sargeant AJ. Human power output during repeated sprint cycle exercise: the influence of thermal stress. Eur J Appl Physiol Occup Physiol. 1999;79(4):360–366. 10.1007/s004210050521 [DOI] [PubMed] [Google Scholar]
- 4.Girard O, Brocherie F, Bishop DJ. Sprint performance under heat stress: A review. Scand J Med Sci Sports. 2015;25 Suppl 1:79–89. 10.1111/sms.12437 [DOI] [PubMed] [Google Scholar]
- 5.Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey MF. Effect of heat stress on muscle energy metabolism during exercise. J Appl Physiol. 1994;77(6):2827–2831. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson RA, Ball D, Sargeant AJ. Effect of muscle temperature on rate of oxygen uptake during exercise in humans at different contraction frequencies. J Exper Biol. 2002;205:981–987. [DOI] [PubMed] [Google Scholar]
- 7.Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol. 2000;88(1):359–363. [DOI] [PubMed] [Google Scholar]
- 8.Goto K, Oda H, Morioka S, Naito T, Akema T, Kato H, Fujiya H, Nakajima Y, Sugiura T, Ohira Y. Skeletal muscle hypertrophy induced by low-intensity exercise with heat-stress in healthy human subjects. Jpn J Aerosp Environ Med. 2007;44:13–18. [Google Scholar]
- 9.Goto K, Oda H, Kondo H, Igaki M, Suzuki A, Tsuchiya S, Murase T, Hase T, Fujiya H, Matsumoto I, et al.. Responses of muscle mass, strength and gene transcripts to long-term heat stress in healthy human subjects. Eur J Appl Physiol. 2011;111(1):17–27. 10.1007/s00421-010-1617-1 [DOI] [PubMed] [Google Scholar]
- 10.Takagi R, Fujita N, Arakawa T, Kawada S, Ishii N, Miki A. Influence of icing on muscle regeneration after crush injury to skeletal muscles in rats. J Appl Physiol. 2011;110(2):382–388. 10.1152/japplphysiol.01187.2010 [DOI] [PubMed] [Google Scholar]
- 11.Goto K, Okuyama R, Sugiyama H, Honda M, Kobayashi T, Uehara K, Akema T, Sugiura T, Yamada S, Ohira Y, et al.. Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. Pflugers Arch. 2003;447(2):247–253. 10.1007/s00424-003-1177-x [DOI] [PubMed] [Google Scholar]
- 12.Uehara K, Goto K, Kobayashi T, Kojima A, Akema T, Sugiura T, Yamada S, Ohira Y, Yoshioka T, Aoki H. Heat-stress enhances proliferative potential in rat soleus muscle. Jpn J Physiol. 2004;54(3):263–271. 10.2170/jjphysiol.54.263 [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Goto K, Kojima A, Akema T, Uehara K, Aoki H, Sugiura T, Ohira Y, Yoshioka T. Possible role of calcineurin in heating-related increase of rat muscle mass. Biochem Biophys Res Commun. 2005;331(4):1301–1309. 10.1016/j.bbrc.2005.04.096 [DOI] [PubMed] [Google Scholar]
- 14.Kakigi R, Naito H, Ogura Y, Kobayashi H, Saga N, Ichinoseki-Sekine N, Yoshihara T, Katamoto S. Heat stress enhances mTOR signaling after resistance exercise in human skeletal muscle. J Physiol Sci. 2011;61(2):131–140. 10.1007/s12576-010-0130-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshihara T, Naito H, Kakigi R, Ichinoseki-Sekine N, Ogura Y, Sugiura T, Katamoto S. Heat stress activates the Akt/mTOR; signalling pathway in rat skeletal muscle. Acta Physiol. 2013;207(2):416–426. 10.1111/apha.12040 [DOI] [PubMed] [Google Scholar]
- 16.Giombini A, Giovannini V, Di Cesare A, Pacetti P, Ichinoseki-Sekine N, Shiraishi M, Naito H, Maffulli N. Hyperthermia induced by microwave diathermy in the management of muscle and tendon injuries. Br Med Bull. 2007;83(1):379–396. 10.1093/bmb/ldm020 [DOI] [PubMed] [Google Scholar]
- 17.Frier BC, Locke M. Heat stress inhibits skeletal muscle hypertrophy. Cell Stress Chaperones. 2007;12(2):132–141. 10.1379/CSC-233R.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosaka K, Muthalib M, Lavender A, Laursen PB. Attenuation of muscle damage by preconditioning with muscle hyperthermia 1-day prior to eccentric exercise. Eur J Appl Physiol. 2007;99(2):183–192. 10.1007/s00421-006-0331-5 [DOI] [PubMed] [Google Scholar]
- 19.Segal NA, Glass NA, Baker JL, Torner JC. Correcting for fat mass improves DXA quantification of quadriceps specific strength in obese adults aged 50–59 years. J Clin Densitom. 2009;12(3):299–305. 10.1016/j;.jocd.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins W. A new view of statistics: A scale of magnitudes for effect sizes. Retrieved January 2006;9:2014. [Google Scholar]
- 21.Takeuchi K, Hatade T, Wakamiya S, Fujita N, Arakawa T, Miki A. Heat stress promotes skeletal muscle regeneration after crush injury in rats. Acta Histochem. 2014;116(2):327–334. 10.1016/j.acthis.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 22.Goto K, Honda M, Kobayashi T, Uehara K, Kojima A, Akema T, Sugiura T, Yamada S, Ohira Y, Yoshioka T. Heat stress facilitates the recovery of atrophied soleus muscle in rat. Jpn J Physiol. 2004;54(3):285–293. 10.2170/jjphysiol.54.285 [DOI] [PubMed] [Google Scholar]
- 23.Yoshida N, Morimoto Y, Kataoka H, Sakamoto J, Nakano J, Okita M. Effects of combination therapy of heat stress and muscle contraction exercise induced by neuromuscular electrical stimulation on disuse atrophy in the rat gastrocnemius. J Phys Ther Sci. 2013;25(2):201–206. 10.1589/jpts.25.201 [DOI] [Google Scholar]
- 24.Naito H, Yoshihara T, Kakigi R, Ichinoseki-Sekine N, Tsuzuki T. Heat stress-induced changes in skeletal muscle: Heat shock proteins and cell signaling transduction. J Phys Fitness Sports Med. 2012;1(1):125–131. 10.7600/jpfsm.1.125 [DOI] [Google Scholar]
- 25.Tamura Y, Hatta H. Heat stress induces mitochondrial adaptations in skeletal muscle. J Phys Fitness Sports Med. 2017;6(3):151–158. 10.7600/jpfsm.6.151 [DOI] [Google Scholar]
- 26.Liu Y, Mayr S, Opitz-Gress A, Zeller C, Lormes W, Baur S, Lehmann M, Steinacker JM. Human skeletal muscle HSP70 response to training in highly trained rowers. J Appl Physiol. 1999;86(1):101–104. [DOI] [PubMed] [Google Scholar]
- 27.Vogt M, Puntschart A, Geiser J, Zuleger C, Billeter R, Hoppeler H. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J Appl Physiol. 2001;91(1):173–182. [DOI] [PubMed] [Google Scholar]
- 28.Thompson HS, Maynard EB, Morales ER, Scordilis SP. Exercise-induced HSP27, HSP70 and MAPK responses in human skeletal muscle. Acta Physiol Scand. 2003;178(1):61–72. 10.1046/j.1365-201X.2003.01112.x [DOI] [PubMed] [Google Scholar]
- 29.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18(1):111–129. 10.1016/0022-510X(73)90023-3 [DOI] [PubMed] [Google Scholar]
- 30.Castellani JW, Zambraski EJ, Sawka MN, Urso ML. Does high muscle temperature accentuate skeletal muscle injury from eccentric exercise? Physiol Rep. 2016;4(9):e12777 https://doi.org/10.14814/phy2.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casadio JR, Storey AG, Merien F, Kilding AE, Cotter JD, Laursen PB. Acute effects of heated resistance exercise in female and male power athletes. Eur J Appl Physiol. 2017. 10.1007/s00421-017-3671-4 [DOI] [PubMed] [Google Scholar]
- 32.Wilson PM, Sabiston CM, Mack DE, Blanchard CM. On the nature and function of scoring protocols used in exercise motivation research: An empirical study of the behavioral regulation in exercise questionnaire. Psychol Sport Exerc. 2012;13(5):614–622. 10.1016/j.psychsport.2012.03.009 [DOI] [Google Scholar]
- 33.Bouma AJ, van Wilgen P, Dijkstra A. The barrier-belief approach in the counseling of physical activity. Patient Educ Couns. 2015;98(2):129–136. 10.1016/j.pec.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 34.Racinais S, Wilson MG, Periard JD. Passive heat acclimation improves skeletal muscle contractility in humans. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R101–R107. 10.1152/ajpregu.00431.2016 [DOI] [PubMed] [Google Scholar]


