Introduction
Hypertrophic cardiomyopathy (HCM) is diagnosed in the presence of left ventricular hypertrophy of ≥15 mm in adult index cases, or ≥13 mm in relatives of known affected patients, that is not solely explained by abnormal loading conditions1, 2. In children, the left ventricle (LV) wall thickness should be more than two standard deviations above the predicted population mean1. The typical anatomo-pathological findings include myocyte hypertrophy, disarray, interstitial fibrosis and small-vessel disease3. Although all the myocytes are supposed to be affected, pathological alterations are not uniformly distributed throughout the myocardium4. Asymmetrical hypertrophy of the interventricular septum is the most commonly observed phenotype, but any pattern of hypertrophy is consistent with the diagnosis5.
Aetiology
HCM is mainly caused by mutations in genes encoding for sarcomeric proteins, which account for almost 60% of the cases6. It has been estimated that up to 10% of patients with HCM present with other genetic or non-genetic phenocopies7, 8, but up to 30% of HCM cases remain unexplained6.
Common diagnostic dilemmas include the differentiation of HCM from physiological hypertrophy in athletes9–11 and conditions such as systemic hypertension or valvular heart disease. People of sub-Saharan African ancestry may be more prone to developing myocardial hypertrophy, even in the absence of severe predisposing factors12. It is important to correctly distinguish between these phenocopies and HCM to prevent fatal outcomes, not only for the index cases, but also for affected family members
Rare, but important, causes of HCM include infiltrative disorders like amyloidosis, lysosome and glycogen storage diseases, and mitochondrial diseases13–17. In children, neuromuscular disorders, inborn errors of metabolism, RASopathies or malformation syndromes are more prevalent18, 19. Many of these disorders display disease-specific clinical features that, together with the age of presentation, can assist in the diagnostic work-up13. Early identification of these conditions facilitates a tailored approach to disease management, which in some cases, may lead to significant modifications of prognosis and quality of life1, 13.
Clinical manifestations and therapeutic implications
Clinical manifestations in HCM are highly variable20. Classical symptoms in HCM are usually related to left ventricular outflow tract (LVOT) obstruction, mitral regurgitation, myocardial ischemia, diastolic dysfunction, abnormal vascular responses, and supraventricular and ventricular arrhythmias20, 21.
Dyspnoea
Reduced functional capacity is common in HCM. Orthopnea and paroxysmal nocturnal dyspnoea are rare, but bendopnoea and postprandial breathlessness are common symptoms. LVOT obstruction is a common cause of these clinical manifestations, but exertional dyspnoea is also caused by diastolic and systolic LV impairment and atrial arrhythmia20.
The first approach to LVOT obstruction is based on medical treatment optimization1, 2. It is important to ensure adequate ventricular filling and relaxation and to avoid vasodilators and inotropic agents20, 22. Selective beta-blocking agents are considered the gold standard therapy. Verapamil and diltiazem are considered less effective, although they can be used in patients who are intolerant or have contraindications to beta-blockers20, 22. It is important to monitor ECG changes when using disopyramide to avoid QT prolongation, and although generally tolerated, patients should be warned of cholinergic side-effects. Verapamil or diltiazem are also considered options for the treatment of LV obstruction, and can be used in conjunction with beta-blockers under close monitoring20, 22. For drug-refractory patients, septal reduction (with surgery or alcohol septal ablation) may be considered20. In experienced centers, septal reduction shows marked symptom improvement without significant intraoperative morbidity and mortality23–26. The use of atrio-ventricular sequential pacing can also be considered but is generally less effective27, 28.
Mavacamten© (MYK-461, Myokardia Inc., San Francisco, California, United States) is a small molecule that reduces the steady-state ATPase activity by inhibiting the rate of phosphate release of β-cardiac myosin52. This results in diminished hypercontractility in the sarcomere and a consistent reduction in LV obstruction and symptoms, which is expected to persist in mid-term follow-up53. The phase 2 PIONEER-HCM (A Phase 2 study of Mavacamten (formerly MYK-461) in Symptomatic Obstructive Hypertrophic Cardiomyopathy Patients) clinical trial has completed without significant safety concerns. The PIONEER-HCM, the EXPLORER-HCM and the MAVERIK-HF trials, will evaluate the effect in different phenotypes and larger cohorts.
Many patients can have severe symptoms in the absence of significant LV outflow tract obstruction29. Exercise echocardiography should be considered in such patients to rule out provocable LVOT obstruction, mitral regurgitation or diastolic impairment with pulmonary hypertension20. The principle mechanism of symptoms in non-obstructive patients is diastolic dysfunction and much less commonly mitral regurgitation caused by intrinsic mitral valve abnormalities. It is important to perform a comprehensive study to determine the exact mechanisms of symptoms and define the best treatment strategy. Left atrial enlargement and pulmonary hypertension are usually related to the degree of diastolic impairment in HCM patients29. In the absence of concomitant obstructive haemodynamics, the addition of diuretics can help in some situations20. To date, several trials have tried to modify the disease course via blockade of the renin-angiotensin-aldosterone axis with little effect.
Chest pain
Angina in HCM is most commonly caused by obstruction and microvascular coronary abnormalities30. Nonetheless, it is important to rule out significant epicardial coronary artery disease, particularly in older populations and patients with cardiovascular risk factors1, 2 using coronary angiography (either by CT coronary angiography or cardiac catheterization)20. In addition, cardiac catheterization can provide relevant information on coronary artery bridging and the anatomy of the septal arteries that can be useful in refractory patients who are candidates for alcohol septal ablation. Verapamil and diltiazem are the preferred drugs to treat chest pain, in the absence of systolic dysfunction, as they are thought to improve microvascular blood flow20. Nitrates can be used with caution in the absence of an LVOTO1.
A number of drugs that alter myocardial energy metabolism have been evaluated in HCM. The RESTYLE-HCM (ranolazine in patients with symptomatic hypertrophic cardiomyopathy) trial, failed to demonstrate benefit in exercise performance or quality of life despite showing a reduction in ventricular premature complexes31. Perhexiline, a carnitine palmitoyltransferase (CPT)-1 inhibitor that improves myocardial performance by creating a switch from fatty acid to glucose metabolism32, has been shown to improve HCM symptoms by improving myocardial energetics, but safety issues regarding hepatitis and neuropathy has limited its widespread use33.
Syncope and arrhythmias
Symptoms related to brief loss of consciousness may appear in one of every five HCM patients, but it is hard to identify the precise mechanisms in most of cases, in spite of a detailed comprehensive study34. Typical mechanisms include left ventricular outflow tract obstruction, abnormal vascular responses and arrhythmias34.
The incidence of atrial fibrillation (AF) is estimated to be around 3% in HCM, and is considered the most common sustained arrhythmia in HCM35. AF is most commonly paroxysmal and the life-long prevalence is estimated to be around 30%36. The incidence increases in the presence of other concomitant risk factors like hypertension, dyslipidemia, diabetes mellitus or sleep apnoea35, 37. The development of AF is related to atrial electrical and structural remodeling, which in turn relates to the severity of ventricular hypertrophy and left atrial size38. AF in HCM is often accompanied by clinical deterioration and is related to adverse cardiovascular events35. The hemodynamic deterioration is presumed to be due to rapid ventricular rate and loss of atrial contribution, causing inadequate filling and exacerbating LVOT obstruction. The implementation of early and aggressive rhythm control strategies using antiarrhythmic drugs and in selected cases radiofrequency AF ablation is essential39. Long-term anticoagulation is recommended for all patients with HCM patients who present with AF, irrespective of predictive risk scores such as CHA2DS2-VASc score35, 40. Vitamin-K antagonists are considered the first choice in this setting35, although direct anticoagulants are suitable alternatives for thromboembolic prevention, and might be preferred in selected individuals41.
Non-sustained ventricular tachycardia occurs in almost one third of patients during the follow-up and is considered a major risk factor for adverse events, particularly sudden cardiac death (SCD)42, 43. The intrinsic mechanisms involved in the development of arrhythmias include hypertrophy, ischemia, fibrosis, myocyte disarray and electric uncoupling43, 44. Certain mutations may be associated with SCD43, and consequently, genotyping may be considered as part of the risk stratification in some specific cases43.
Antiarrhythmic drugs have no role in the primary prevention of SCD but are useful in the rare patient with symptomatic ventricular tachycardia. Catheter ablation for VT is also feasible in drug-refractory patients, although the best results are achieved by using a combined endo and epicardial approach44. ICDs should be considered in all patients with symptomatic VT20, 45.
Clinical phenotypes in hypertrophic cardiomyopathy
The classical HCM phenotype includes asymmetric hypertrophy, that predominantly affects the interventricular septum46, 47 (Figure 1). Typical ECG findings include LV hypertrophy, deep and wide Q waves, and negative T waves. A normal ECG is present in around 5% of patients. Pathogenic sarcomeric gene mutations are most common in patients with this classical phenotype48–50.
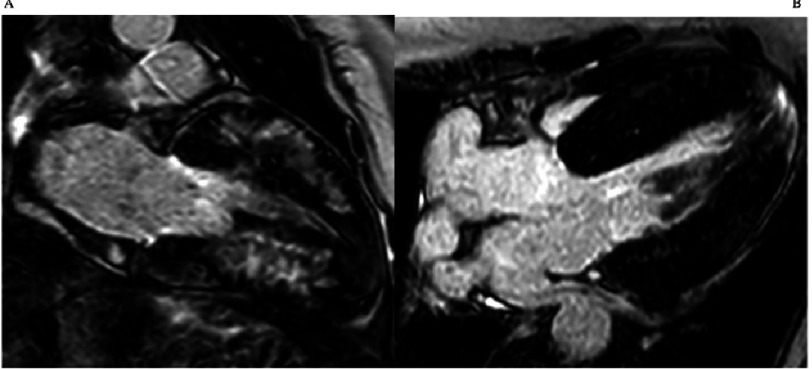
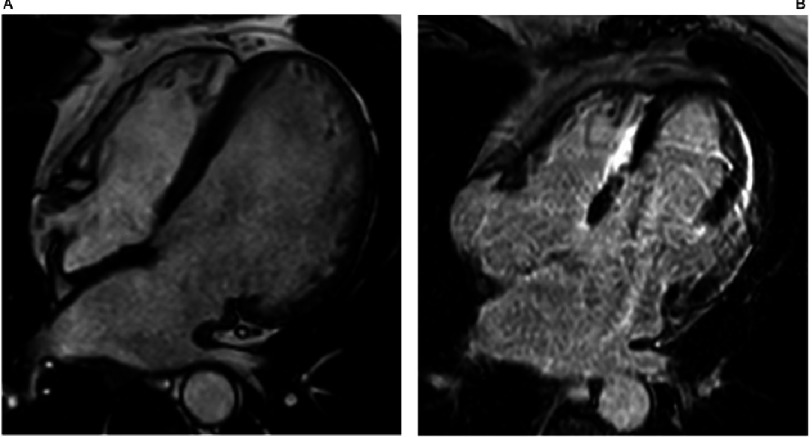
Figure 1. Cardiac Magnetic Resonance (CMR) late gadolinium enhancement images: Classical hypertrophic cardiomyopathy in an individual who suffered a sudden cardiac death.
MYBPC3 c.2149-1G>A. A) 2 chamber view with severe hypertrophy in the segments of maximum hypertrophy. B) 4 chamber view showing biventricular hypertrophy, apical insertion of the papillary muscles and slight late gadolinium enhancement in the apical segment.
Patients with the classical phenotype are more predisposed to have dynamic subaortic obstruction caused by systolic anterior movement (SAM) of the mitral valve (Figure 2)20. The mitral valve the subvalvular apparatus may be abnormal and contributes the development of LVOT obstruction and mitral regurgitation51. In childhood, LVOT obstruction is common, and in some cohorts it has been reported to occur in around 50% of the patients. Interestingly, it occurs in the presence of greater degrees of hypertrophy and smaller left ventricular volumes than in the adult cohorts18. LVOTO is associated with morbidity and mortality in terms of disease progression, heart failure symptoms and cardiovascular events20.
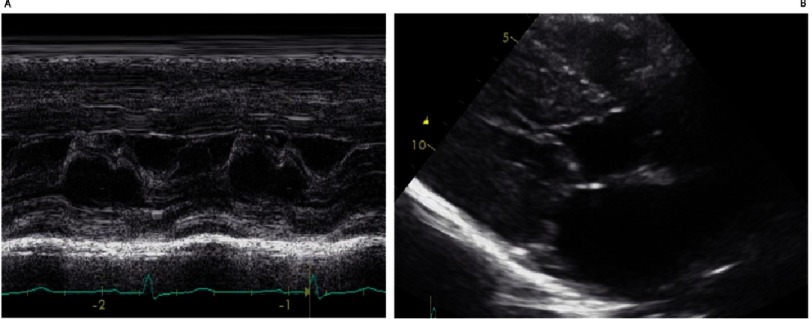
Figure 2. A) M-Mode echocardiography: Classical systolic anterior movement (SAM) of the mitral valve causing obstruction.
B) B-Mode Echocardiography: Paraesternal longitudinal axis showing contact between interventricular septum and the anterior leaflet of the mitral valve in the beginning of systole.
Non-classic HCM phenotypes
Hypertrophy may involve any location of the ventricular wall48. Although some of these less typical phenotypes have been related to mutations in the thin-filaments, casual mutations may involve any the sarcomere genes6.
Mid-ventricular hypertrophy in conjunction with abnormal distribution of the papillary muscles and hypercontractility of the lateral ventricular wall generating a mid-cavity obstructive gradient occurs in less than 10 % of cases54, but is particularly important as a cause of symptoms55. Mid-cavity obstruction may correlate with end-stage disease progression and development of an apical aneurysm. Stroke from thrombus formation and ventricular arrhythmias can occur in these patients, and the phenotype may be associated with cardiovascular death20. (Figure 3)
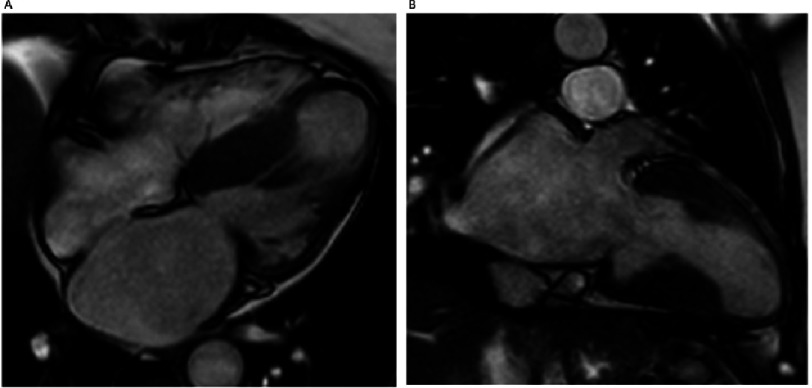
Figure 3. Cardiac Magnetic Resonance (CMR) cine apical 4 and 2 chamber view showing apical aneurism and mid-cavity hypertrophy.
TNNI 3 p.Ala157Val.
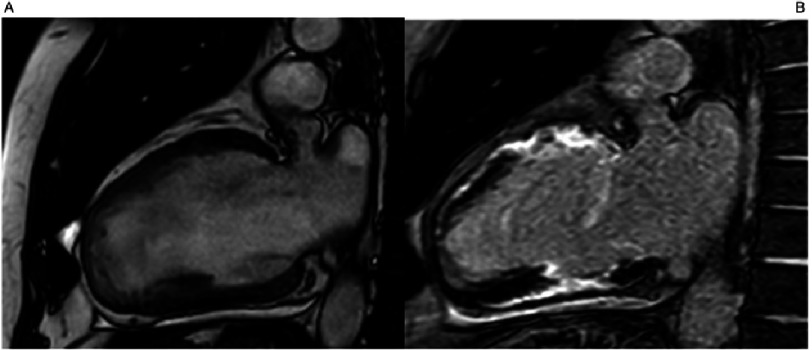
The apical hypertrophic phenotype is associated with deep negative T waves in precordial leads, and the “ace of spades” appearance on ventriculography56. It is often overlooked because of poor apical visualization on standard echocardiographt and may require echo contrast agents or CMR for identification48. Patients with apical hypertrophy have been considered to have a benevolent course, but the evidence is contradictory57. Indeed diastolic impairment, left atrial enlargement and impaired excercise capacity, might be frequent on apical forms58, 59. (Figure 4)
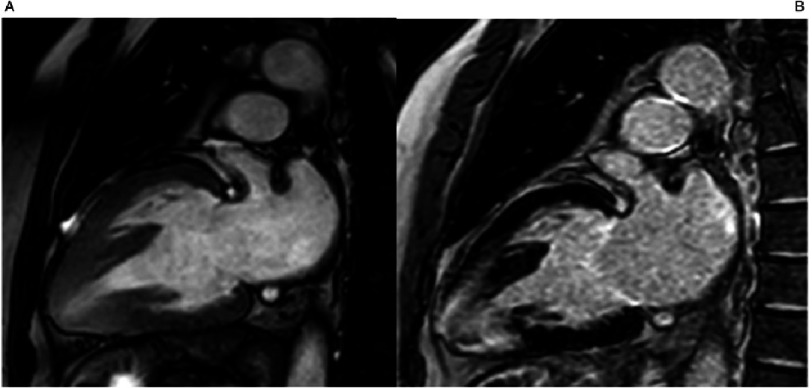
Figure 4. Cardiac Magnetic Resonance (CMR) Apical 2CH view: A) Cine: Apical hypertrophic cardiomyopathy with the classical “ace of spades” shape.
B) Late gadolinium enhancement images: severe fibrosis in the segments of maximum hypertrophy.
Genotype positive and phenotype negative HCM
Next generation sequencing and family screening have resulted in the identification of a large number of relatives with mild or subclinical disease. Electrocardiographic markers and minor echocardiographic changes may be helpful in diagnosing such individuals when they are relatives of clearly affected HCM patients60. Minor abnormalities seen in relatives include low end-systolic volumes, mild contractile and diastolic alterations, increased myocardial trabeculation, limited late gadolinium enhancement and an increase in the extracellular volume fraction20.
In a recent study, 30% of evaluated relatives were identified as having HCM at first screening. Moreover, 16% developed HCM during 7 years of repeated evaluation. Therefore, genotype positive patients need prolonged surveillance although adverse events were extremely infrequent in relatives without a clinically evident phenotype in the presence of a positive genotype61. Current recommendations are to perform screening at yearly intervals in children and adolescents until their mid twenties and at 3–5 year intervals in older individuals20.
End-stage HCM
Progressive systolic dysfunction, often accompanied by ventricular dilatation and left ventricular wall thinning occurs in 2.5% to 15% of patients62. End-stage HCM with LV dilation and systolic dysfunction is the most common clinical profile of patients undergoing heart transplantation63. In these advanced stages of the disease, patients usually present with mixed phenotypes displaying different degrees of remodelling, restrictive filling patterns and systolic dysfunction20. Furthermore, left ventricular trabeculae and non-compaction is not rare, further complicating the clinical diagnosis (Figures 5 and 6). Rarely, patients may be indistinguishable from dilated cardiomyopathy in advanced cases (Figure 7). Severe diffuse fibrosis is one of the hallmarks of end-stage disease62. Many factors have been proposed to account for end-stage disease including gene mutation, environmental and epigenetic factors20.
Figure 5. Cardiac Magnetic Resonance (CMR) Apical 4CH view: A) Cine and B) Late gadolinium enhancement images.
End-stage HCM with progressive remodelling and sever systolic dysfunction, atrial fibrillation and ventricular tachycardia. MYH7 p.Arg453Cys.
Figure 6. Cardiac Magnetic Resonance (CMR) Apical 2CH view: A) Cine and B) Late gadolinium enhancement images.
End-stage HCM with progressive remodelling and sever systolic dysfunction, atrial fibrillation and ventricular tachycardia. MYH7 p.Arg453Cys.
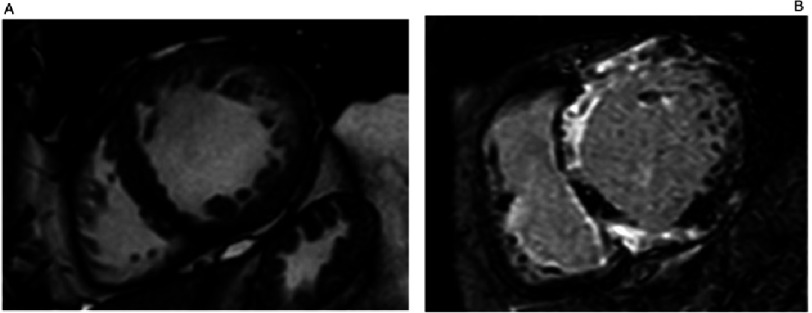
Figure 7. Cardiac Magnetic Resonance (CMR) Apical short axis view: A) Cine and B) Late gadolinium enhancement images.
End-stage HCM with progressive remodelling and severe systolic dysfunction, atrial fibrillation and ventricular tachycardia. MYH7 p.Arg453Cys.
Treatment of HCM patients who develop LV systolic dysfunction should be according to the standard heart failure guidelines with a few provisos64. Patients should be referred early for ICD implantation for SCD prevention because the rate of ventricular arrhythmias has been shown to be consistently higher in this population62. Cardiac resynchronization therapy has not consistently shown to improve left ventricular systolic function or survival in these patients65. Long-term mechanical circulatory support in selected cases with refractory heart failure as a bridge to transplant strategy is feasible1. In this regard, the use of continuous flow devices might be restricted to severely dilated ventricles to allow satisfactory support and avoid device complications.
Finally, patients with cardiomyopathies tend to be younger than common HF patients, and are able to tolerate advanced stages of the disease with apparent good exercise performance. Accordingly, it is important to routinely refer these patients to experienced centres, in order to perform a comprehensive heart failure risk stratification.
Conclusions
In the last 50 years, the knowledge of HCM pathophysiological features and its clinical perspective has increased enormously. It is possible to make an aetiological diagnosis in more than two-thirds of patients, with implications for clinical management, familial screening and preclinical investigation. Early disease detection facilitates early implementation of treatment strategies to prevent disease complications.
Acknowledgments
We would like to thank Dr. José González-Costello for his contribution to this review.
References
- 1.Elliott PM, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 2.Gersh BL, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(24):2761–96. doi: 10.1161/CIR.0b013e318223e230. [DOI] [PubMed] [Google Scholar]
- 3.Varnava A, Elliot P, Sharma S, McKenna W, et al. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84(5):476–482. doi: 10.1136/heart.84.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner B, Seebohm B, Tripathi S, Montag J, et al. Familial hypertrophic cardiomyopathy: functional variance among individual cardiomyocytes as a trigger of FHC-phenotype development. Front. Physiol. 2014;5:392. doi: 10.3389/fphys.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64(1):83–99. doi: 10.1016/j.jacc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60:705–715. doi: 10.1016/j.jacc.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 7.McLeod CJ, Bos JM, Theis JL, et al. Histologic characterization of hypertrophic cardiomyopathy with and without myofilament mutations. Am Heart J. 2009;158:799–805. doi: 10.1016/j.ahj.2009.09.006. [PMC free article] [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey N, Luedde M, Katus HA. Mechanisms of disease: hypertrophic cardiomyopathy. Nat Rev Cardiol. 2011;9:91–100. doi: 10.1038/nrcardio.2011.159. [DOI] [PubMed] [Google Scholar]
- 9.Corrado D, Schmied C, Basso C, et al. Risk of sports: do we need a pre-participation screening for competitive and leisure athletes? Eur Heart J. 2011;32:934–944. doi: 10.1093/eurheartj/ehq482. [DOI] [PubMed] [Google Scholar]
- 10.La Gerche A, Burns AT, Mooney DJ, et al. Exercise induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 11.Nijenkamp LL, Güclü A, Appelman Y, van der Velden J, Kuster DW. Sex-dependent pathophysiological mechanisms in hypertrophic cardiomyopathy: Implications for rhythm disorders. Heart Rhythm. 2015;12:433–439. doi: 10.1016/j.hrthm.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Geske JB, Ong KC, Siontis KC, Hebl VB, et al. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart H. 2017;38(46):3434–3440. doi: 10.1093/eurheartj/ehx527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes LR, Elliott PM. New approaches to the clinical diagnosis of inherited heart muscle disease. Heart. 2013 doi: 10.1136/heartjnl-2012-301995. [DOI] [PubMed] [Google Scholar]
- 14.Charron P, Villard E, Sébillon P, et al. Danon’s disease as a cause of hypertrophic cardiomyopathy: a systematic survey. Heart. 2004;90:842–6. doi: 10.1136/hrt.2003.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy RT, Mogensen J, McGarry K, Bahl A, Evans A, Osman E, Syrris P, Gorman G, Farrell M, Holton JL, Hanna MG, Hughes S, Elliott PM, MacRae CA, McKenna WJ. Adenosine monophosphate-activated protein kinase disease mimicks hypertrophic cardiomyopathy and Wolff-Parkinson-White syndrome: natural history. J Am Coll Cardiol. 2005;45:922–930. doi: 10.1016/j.jacc.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 16.Linhart A, Elliott PM. The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart. 2007;93:528–35. doi: 10.1136/hrt.2005.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermeer AMC, Janssen A, Boorsma PC, Mannens MMAM. Transthyretin amyloidosis: a phenocopy of hypertrophic cardiomyopathy. Amyloid. 2017;24(2):87–91. doi: 10.1080/13506129.2017.1322573. [DOI] [PubMed] [Google Scholar]
- 18.Nugent AW, Daubeney PEF, Chondros P, et al. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation. 2005;112:1332–8. doi: 10.1161/CIRCULATIONAHA.104.530303. [DOI] [PubMed] [Google Scholar]
- 19.Alday LE, Moreyra E. Hypertrophic cardiomyopathy in infants and children. In: Veselka J, editor. Cardiomyopathies - from basic research to clinical management. InTech; New York: 2012. [Google Scholar]
- 20.Sen-Chowdhry S, Jacoby D, Moon JC, McKenna WJ. Update on hypertrophic cardiomyopathy and guide to the guidelines. Nat Rev Cardiol. 2016;13(11):651–675. doi: 10.1038/nrcardio.2016.140. [DOI] [PubMed] [Google Scholar]
- 21.Maron MS, Olivotto I, Zenovich AG, Link MS, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 22.Ammirati E, Contri R, Coppini R, Cecchi F, et al. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur. J. Heart Fail. 2016;18(9):1106–18. doi: 10.1002/ejhf.541. [DOI] [PubMed] [Google Scholar]
- 23.Kim L, et al. Hospital volume outcomes after septal myectomy and alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy: US Nationwide Inpatient Database, 2003–2011. JAMA Cardiol. 2016;1:324–332. doi: 10.1001/jamacardio.2016.0252. [DOI] [PubMed] [Google Scholar]
- 24.Rastegar H, Boll G, Rowin EJ, Dolan N, et al. Results of surgical septal myectomy for obstructive hypertrophic cardiomyopathy: the Tufts experience. Ann Cardiothorac Surg. 2017;6(4):353–363. doi: 10.21037/acs.2017.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen MK, Prinz C, Horstkotte D, van Buuren F, Bitter T, Faber L, Bundgaard H. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile. Heart. 2013;99 doi: 10.1136/heartjnl-2012-303339. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic obstructive cardiomyopathy: Surgical myectomy and septal ablation. Circ Res. 2017;121(7):771–783. doi: 10.1161/CIRCRESAHA.116.309348. [DOI] [PubMed] [Google Scholar]
- 27.Maron BJ, Nishimura RA, McKenna WJ, Rakowski H, Josephson ME, Kieval RS. Assessment of permanent dual-chamber pacing as a treatment for drug-refractory symptomatic patients with obstructive hypertrophic cardiomyopathy. A randomized, double-blind, crossover study (M-PATHY) Circulation. 1999;99 doi: 10.1161/01.cir.99.22.2927. [DOI] [PubMed] [Google Scholar]
- 28.Kappenberger LJ, Linde C, Jeanrenaud X, Daubert C, McKenna W, Meisel E, Sadoul N, Chojnowska L, Guize L, Gras D, et al. Clinical progress after randomized on/off pacemaker treatment for hypertrophic obstructive cardiomyopathy. Pacing in Cardiomyopathy (PIC) Study Group. Europace. 1999;1:77–84. doi: 10.1053/eupc.1998.0024. [DOI] [PubMed] [Google Scholar]
- 29.Covella M, Rowin EJ, Hill NS, Preston IR, et al. Mechanism of progressive heart failure and significance of pulmonary hypertension in obstructive hypertrophic cardiomyopathy. Circ Heart Fail. 2017;10(4):e003689. doi: 10.1161/CIRCHEARTFAILURE.116.003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olivotto I, Girolami F, Sciagra R, Ackerman MJ, Sotgia B, Bos JM, Nistri S, Sgalambro A, Grifoni C, Torricelli F, Camici PG, Cecchi F. Microvascular function is selectively impaired in patients with hypertrophic cardiomyopathy and sarcomere myofilament gene mutations. J Am Coll Cardiol. 2011;58:839–848. doi: 10.1016/j.jacc.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Olivotto I, Camici PG, Merlini PA, Rapezzi C. Efficacy of ranolazine in patients with symptomatic hypertrophic cardiomyopathy: The RESTYLE-HCM randomized, double-blind, placebo-controlled study. Circ Heart Fail. 2018;11(1):e004124. doi: 10.1161/CIRCHEARTFAILURE.117.004124. [DOI] [PubMed] [Google Scholar]
- 32.Yin X, Dwyer J, Langley SR, Mayr U. Effects of perhexiline-induced fuel switch on the cardiac proteome and metabolome. J Mol Cell Cardiol. 2013;55:27–30. doi: 10.1016/j.yjmcc.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abozguia K1, Elliott P, McKenna W, Phan TT. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 2010;122(16):1562–9. doi: 10.1161/CIRCULATIONAHA.109.934059. [DOI] [PubMed] [Google Scholar]
- 34.Williams L, Frenneaux M. Syncope in hypertrophic cardiomyopathy: mechanisms and consequences for treatment. Europace. 2007;9(9):817–22. doi: 10.1093/europace/eum093. [DOI] [PubMed] [Google Scholar]
- 35.Rowin EJ1, Hausvater A2, Link MS2, Abt P. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation. 2017;136(25):2420–2436. doi: 10.1161/CIRCULATIONAHA.117.029267. [DOI] [PubMed] [Google Scholar]
- 36.Guttmann OP, Rahman MS, O’Mahony C, Anastasakis A, et al. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart. 2014;100:465–472. doi: 10.1136/heartjnl-2013-304276. [DOI] [PubMed] [Google Scholar]
- 37.Camm CF, Camm AJ. Atrial fibrillation and anticoagulation in hypertrophic cardiomyopathy. Arrhythm Electrophysiol Rev. 2017;6(2):63–68. doi: 10.15420/aer.2017.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maron BJ, et al. Left atrial remodeling in hypertrophic cardiomyopathy and susceptibility markers for atrial fibrillation identified by cardiovascular magnetic resonance. Am. J. Cardiol. 2014;113:1394–1400. doi: 10.1016/j.amjcard.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 39.Vaidya K, Semsarian C, Chan KH. Atrial fibrillation in hypertrophic cardiomyopathy. Heart Lung Circ. 2017;26(9):975–982. doi: 10.1016/j.hlc.2017.05.116. [DOI] [PubMed] [Google Scholar]
- 40.Guttmann OP, Rahman S, O’Mahony C, Anastasakis A, Elliott P. Systematic review of atrial fibrillation and stroke in patients with hypertrophic cardiomyopathy. Eur Heart J. 2013;34(S1):2965. doi: 10.1136/heartjnl-2013-304276. [DOI] [PubMed] [Google Scholar]
- 41.Dominguez F, Climent V, Zorio E, Ripoll-Vera T. Direct oral anticoagulants in patients with hypertrophic cardiomyopathy and atrial fibrillation. Int J Cardiol. 2017;248:232–238. doi: 10.1016/j.ijcard.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Monserrat L, Elliott PM, Gimeno JR, Sharma S, et al. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42(5):873–9. doi: 10.1016/s0735-1097(03)00827-1. [DOI] [PubMed] [Google Scholar]
- 43.Liu O, Li D, Berger AE, Johns RA, Gao L. Survival and prognostic factors in hypertrophic cardiomyopathy: a meta-analysis. Sci Rep. 2017;7:11957. doi: 10.1038/s41598-017-12289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santangeli P, Di Biase L, Lakkireddy D, Burkhardt JD, et al. Radiofrequency catheter ablation of ventricular arrhythmias in patients with hypertrophic cardiomyopathy: safety and feasibility. Heart Rhythm. 2010;7(8):1036–42. doi: 10.1016/j.hrthm.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 45.O’Mahony C, Jichi F, Pavlou M, Monserrat L, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD) Eur Heart J. 2014;35(30):2010–20. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- 46.Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J. 1958;20:1–8. doi: 10.1136/hrt.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 48.Maron MS, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2009;54:220–228. doi: 10.1016/j.jacc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Binder J, Ommen SR, Gersh BJ, et al. Echocardiography-guided genetic testing in hypertrophic cardiomyopathy: septal morphological features predict the presence of myofilament mutations. Mayo Clin Proc. 2006;81:459–467. doi: 10.4065/81.4.459. [DOI] [PubMed] [Google Scholar]
- 50.Maron BJ, Niimura H, Casey SA, Soper MK. Development of left ventricular hypertrophy in adults in hypertrophic cardiomyopathy caused by cardiac myosin-binding protein C gene mutations. J Am Coll Cardiol. 2001;38(2):315–21. doi: 10.1016/s0735-1097(01)01386-9. [DOI] [PubMed] [Google Scholar]
- 51.Harrigan CJ, Appelbaum E, Maron BJ, Buros JL, et al. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. 2008;101:668–6. doi: 10.1016/j.amjcard.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 52.Kawas RF, Anderson RL, Ingle SRB, Song Y, et al. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem. 2017;292(40):16571–16577. doi: 10.1074/jbc.M117.776815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green EM, Wakimoto H, Anderson RL, Evanchik MJ, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351(6273):617–621. doi: 10.1126/science.aad3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falicov RE, Resnekov L. Mid ventricular obstruction in hypertrophic obstructive cardiomyopathy. New diagnostic and therapeutic challenge. Br Heart J. 1977;39:701–5. doi: 10.1136/hrt.39.7.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minami Y, Kajimoto K, Terajima Y, et al. Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2011;57:2346–55. doi: 10.1016/j.jacc.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi H, Ishimura T, Nishiyama S, et al. Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): ventriculographic and echocardiographic features in 30 patients. Am J Cardiol. 1979;44:401–12. doi: 10.1016/0002-9149(79)90388-6. [DOI] [PubMed] [Google Scholar]
- 57.Eriksson MJ, Sonnenberg B, Woo A, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;39:638–45. doi: 10.1016/s0735-1097(01)01778-8. [DOI] [PubMed] [Google Scholar]
- 58.Moon J, Shim CY, Ha JW, et al. Clinical and echocardiographic predictors of outcomes in patients with apical hypertrophic cardiomyopathy. Am J Cardiol. 2011;108:1614–9. doi: 10.1016/j.amjcard.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Maron MS, Finley JJ, Bos JM, et al. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation. 2008;118:1541–9. doi: 10.1161/CIRCULATIONAHA.108.781401. [DOI] [PubMed] [Google Scholar]
- 60.McKenna W, Spirito P, Desnos M, Dubourg O, Komajda M. Experience from clinical genetics in hypertrophic cardiomyopathy: proposal for new diagnostic criteria in adult members of affected families. Heart. 1997;77(2):130–2. doi: 10.1136/hrt.77.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Velzen HG, Schinkel AFL Baart, SJ, Oldenburg RA, et al. Outcomes of contemporary family screening in hypertrophic cardiomyopathy. Circ Genom Precis Med. 2018(4):e001896. doi: 10.1161/CIRCGEN.117.001896. [DOI] [PubMed] [Google Scholar]
- 62.Biagini E, Coccolo F, Ferlito M, Perugini E, et al. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J Am Coll Cardiol. 2005;46(8):1543–50. doi: 10.1016/j.jacc.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 63.Biagini E, Spirito P, Leone O, Picchio FM, et al. Heart transplantation in hypertrophic cardiomyopathy. Am J Cardiol. 2008;101:387–392. doi: 10.1016/j.amjcard.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 64.Ponikowski P, Voors AA, Anker SD, Bueno H, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 65.Killu AM, Park JY, Sara JD, Hodge DO, et al. Cardiac resynchronization therapy in patients with end-stage hypertrophic cardiomyopathy. Europace. 2018;20(1):82–88. doi: 10.1093/europace/euw327. [DOI] [PubMed] [Google Scholar]