Abstract
Background and Purpose:
Stenting for symptomatic carotid stenosis (CAS) carries a higher risk of procedural stroke or death than endarterectomy (CEA). It is unclear whether this extra risk is present both on the day of procedure and within 1–30 days thereafter and whether clinical risk factors differ between these periods.
Methods:
We analyzed the risk of stroke or death occurring on the day of procedure (immediate procedural events) and within 1–30 days thereafter (delayed procedural events) in 4597 individual patients with symptomatic carotid stenosis who underwent CAS (n=2326) or CEA (n=2271) in four randomized trials.
Results:
Compared with CEA, patients treated with CAS were at greater risk for immediate procedural events (110 versus 42, 4.7% versus 1.9%; OR 2.6, 95% CI 1.9–3.8), but not for delayed procedural events (59 versus 46, 2.5% versus 2.0%, OR 1.3, 0.9–1.9; interaction p=0.006). In patients treated with CAS, age increased the risk for both immediate and delayed events, while qualifying event severity only increased the risk of delayed events. In patients treated with CEA, we found no risk factors for immediate events, while a higher level of disability at baseline and known history of hypertension were associated with delayed procedural events.
Conclusions:
The increased procedural stroke or death risk associated with CAS compared with CEA was caused by an excess of events occurring on the day of procedure. This finding demonstrates the need to enhance the procedural safety of CAS by technical improvements of the procedure and increased operator skill. Higher age increased the risk for both immediate and delayed procedural events in CAS, mechanisms of which remain to be elucidated.
Keywords: carotid artery stenting, risk, endarterectomy, revascularization, carotid stenosis
Introduction
In patients with recently symptomatic carotid stenosis, carotid artery stenting (CAS) is associated with a higher risk of stroke or death in the procedural period (defined as within 30 days of treatment) than endarterectomy (CEA).1 This extra risk associated with stenting is mostly attributed to an increase in minor or non-disabling strokes occurring in patients older than 70 years.1, 2 Beyond the procedural period, stenting seems to be as effective as endarterectomy in preventing recurrent stroke.3–5 Within the 30-day procedural period, it has been unclear whether the extra risk associated with stenting is present both on the day of procedure and within 30 days thereafter.
The Carotid Stenosis Trialists’ Collaboration (CSTC) pooled data of individual patients with symptomatic carotid stenosis enrolled in the Endarterectomy Versus Angioplasty in patients with Symptomatic Severe Carotid Stenosis trial (EVA-3S), the Stent-Protected Angioplasty versus Carotid Endarterectomy trial (SPACE), the International Carotid Stenting Study (ICSS) and the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). All trials have completed their long-term follow-up and the results have been published.3–6
In the present analysis, we compared the risk of stroke or death occurring on the day of procedure and within 1–30 days following both treatments. In addition, we investigated if clinical risk factors for stroke or death differed between these periods.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. This meta-analysis includes individual patient data from EVA-3S (NCT 00190398), SPACE (ISRCTN 57874028), CREST (NCT00004732) and ICSS (ISRCTN 25337470). Patients were recruited from 2000–2005 in EVA-3S, from 2001–2006 in SPACE, from 2001–2008 in ICSS, and from 2000–2008 in CREST. Ethics approval for the contributing trials was obtained at the competent institutional review boards and all patients provided written informed consent. The pooled analysis of individual patient data was agreed upon at the design stage of these trials.7 All four trials were randomized clinical trials with blinded outcome adjudication. Detailed inclusion and exclusion criteria of all trials have been reported previously.8–11 In summary, all trials included patients with symptomatic moderate to severe carotid stenosis (≥50% reduction of lumen diameter measured according to the method used in the North American Symptomatic Carotid Endarterectomy Trial [NASCET]), who were equally suitable for either procedure. CREST additionally included patients with asymptomatic carotid stenosis, but only data from symptomatic patients were included in the present analysis. Patients were randomly allocated in equal proportions to CAS or CEA.9, 11–13
In EVA-3S, SPACE and ICSS all stents had to be CE (Communauté Européenne) marked. In CREST, the protocol specified the use of the RX Acculink stent. In EVA-3S, the use of distal filter protection devices became mandatory early in the trial, after the risk of stroke within the procedural period was found to be unacceptably high in patients treated with unprotected CAS. In CREST, the use of the RX Accunet embolic protection device was recommended whenever feasible. In ICSS and SPACE, the use of protection devices remained optional throughout the trials. Surgeons could perform standard or eversion endarterectomy under local or general anesthesia, with or without the use of shunts or patches. CAS or CEA was deemed initiated if the patient had been given general or local anesthetic in preparation for the intervention.
The primary outcome event for this analysis was stroke or death occurring either on the day of treatment (immediate procedural event) or within 1–30 days thereafter (delayed procedural event). Stroke was defined as an acute deficit of focal neurological function which led to symptoms lasting longer than 24 hours, resulting from intracranial vascular disturbance (ischemia or hemorrhage). For the present analysis, only the first event was considered, because we assumed that second events (e.g. death or another stroke occurring after a first stroke) would rarely be independent of the first event. In addition, second events of the same type occurring in the peri-procedural may not have been reported separately in the source trials.
Statistical analysis
The primary analysis population included all patients in whom the randomly allocated treatment was initiated (per-protocol analysis).1 Patients crossing over to the other treatment, those who did not receive either treatment, and those who died before treatment were excluded. A per-protocol analysis rather than intention-to-treat analysis was chosen because the main difference between the two analysis populations consisted of patients who did not receive either treatment (Figure 1). In addition, the primary aim of our research question was to investigate whether the risk of stroke or death differed between CAS and CEA in two distinct time periods (day of treatment and 1–30 days after treatment).
Figure 1 – flow chart.
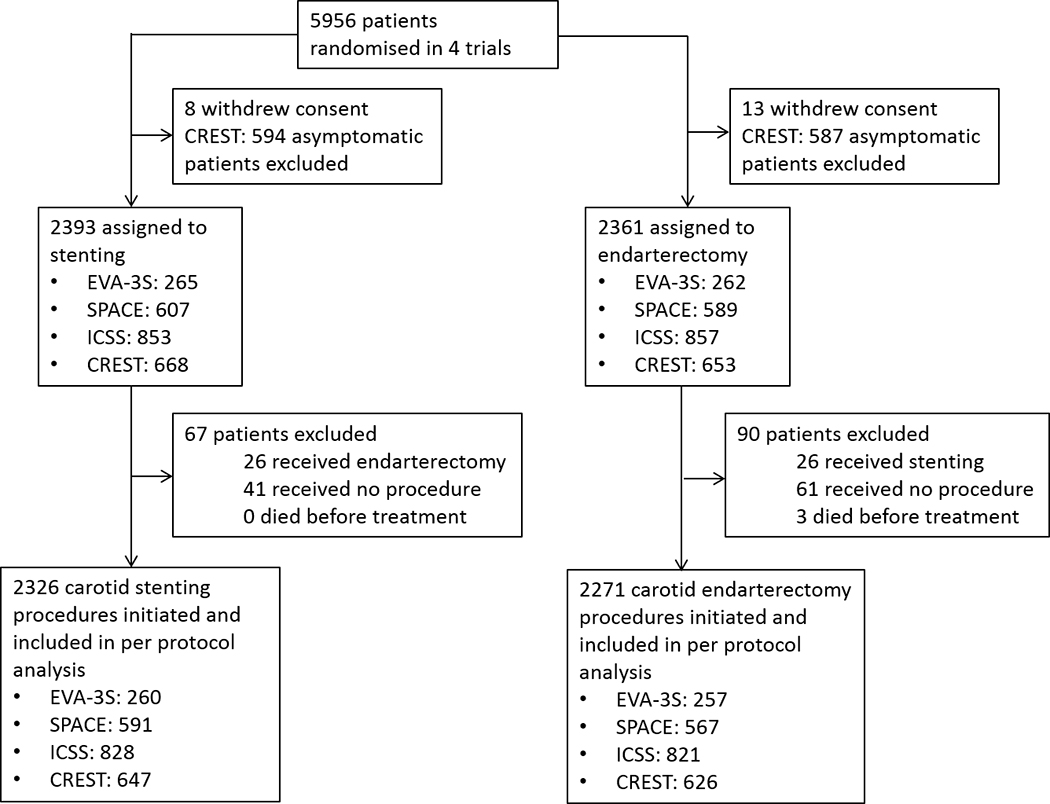
Study flow chart depicting all patients in the source trials included in this meta-analysis as well as events that precluded them from analysis.
Data were analyzed with generalized linear mixed-effects models (GLMM) with binomial error and logit link function, with a random intercept for each source trial. The CAS versus CEA treatment effect was expressed as an odds ratio (OR) with 95% confidence interval (CI), both for immediate procedural events and for delayed procedural events, with CEA as the reference treatment. To investigate whether the CAS versus CEA treatment effect differed between the immediate and delayed procedural period, we reshaped the analysis set to include two observations (rows) per patient, one for immediate procedural events and one for delayed procedural events, and included a random intercept for each source trial and patient. We chose this approach to be able to investigate whether the odds ratio for the primary outcome differed between the immediate and the delayed procedural period by formal testing of statistical interaction. We did this by including treatment (CAS versus CEA), time of event (immediate versus delayed) and an interaction term between treatment and time in the model. We performed a sensitivity analysis excluding patients who had the primary outcome event in the immediate procedural period from the population at risk in the delayed procedural period.
We investigated if the following baseline patient characteristics were associated with stroke or death in the immediate (day 0), the delayed (day 1–30) and the full procedural period (day 0–30) by a forward variable selection approach based on the Akaike information criterion (AIC), in each treatment group separately: patient age and sex, systolic blood pressure at baseline, previous diagnosis of hypertension, diabetes mellitus, hyperlipidemia or coronary heart disease, any history of smoking (current or past), modified Rankin Scale at baseline, degree of ipsilateral stenosis measured according to NASCET criteria14 dichotomized into moderate (50–69%) or severe (70–99%), presence of contralateral stenosis (>/=70%) according to NASCET criteria14 or occlusion, and severity of the qualifying event (analyzed by trend: hemispheric ischemic stroke > transient ischemic attack [TIA] > ocular ischemia [including amaurosis fugax or retinal infarction]). Qualifying event severity was analyzed by trend because patients with previous ocular events have a lower risk of future ischemic stroke compared to patients who had a TIA, and patients with a TIA have a lower risk than patients who had a hemispheric stroke.15
To investigate if associations between patient characteristics and procedural events differed between the immediate and the delayed procedural period, we again used the data structure with two observations per patient (using the subset with CAS or CEA) and included baseline patient characteristics which were associated with immediate or delayed procedural events and their interactions with time.
In addition, we fitted a GLMM for the immediate, the delayed and the full procedural period each, adjusted for any variables identified as significant predictors of the primary outcome event in any of these periods by the forward selection approach described above. We used these models to display the risk factor associations for the different periods in a forest plot.
A p-value of <0.10 for interaction terms was considered statistically significant. For all other statistical analyses, a p-value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed as complete case analyses (no imputation of missing values), using the statistical software environment R (Version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
In total, 5956 patients were enrolled in the 4 contributing trials. The pooled per-protocol analysis set included 4,597 patients, 2,271 of whom received CEA and 2,326 CAS. Reasons for exclusion of patients from the per-protocol analysis are provided in Figure 1. Baseline characteristics were well balanced between the stenting and the endarterectomy group (Table 1). In ICSS, SPACE and EVA-3S closed-cell stents were used in 61.8%, while in CREST the use of the open-cell RX Acculink stent device was mandatory. In CREST 96.1% of patients were treated with the RX Accunet embolic protection device, while in ICSS, SPACE and EVA-3S 61% of patients were treated with various embolic protection devices (Supplemental table I).
Table 1.
Patient characteristics at baseline
| CEA (n = 2271) |
CAS (n = 2326) |
|
|---|---|---|
| Male n (%) | 1593/2271 (70.1%) | 1615/2326 (69.4%) |
| Age years (mean, SD) | 69.4 ± 9.2 | 69.2 ± 9.2 |
| Systolic blood pressure mmHg (mean, SD) | 142.8 ± 21 | 143.9 ± 21 |
| Hypertension n (%) | 1718/2264 (75.9%) | 1743/2264 (75.3%) |
| Diabetes n (%) | 576/2270 (25.4%) | 575/2325 (24.7%) |
| Hyperlipidemia* or LLT n (%) | 1471/2271 (64.7%) | 1462/2326 (62.9%) |
| LLT | 1443/2271 (63.5%) | 1439/2326 (61.9%) |
| Smoking (current or past) n (%) | 1472/2254 (65.3%) | 1489/2308 (64.5%) |
| Coronary artery disease n (%) | 630/2218 (28.4%) | 626/2276 (27.5%) |
| mRS at baseline n (%) | ||
| 0 n (%) | 1133/2252 (50.3%) | 1167/2305 (50.6%) |
| 1 n (%) | 587/2252 (26.1%) | 622/2305 (27.0%) |
| 2 n (%) | 365/2252 (16.2%) | 358/2305 (15.5%) |
| >2 n (%) | 167/2252 (7.4%) | 158/2305 (6.9%) |
| Degree of ipsilateral carotid stenosis | ||
| Moderate (50–69%) n (%) | 443/2271 (19.5%) | 441/2326 (19%) |
| Severe (70–99%) n (%) | 1828/2271 (80.5%) | 1885/2326 (81.0%) |
| Contralateral stenosis or occlusion | 301/2037 (14.8%) | 308/2326 (14.8%) |
| Qualifying event type | ||
| Ocular ischemia n (%) | 388/2256 (17.2%) | 394/2312 (17.0%) |
| Transient ischemic attack n (%) | 835/2256 (37.0%) | 847/2312 (36.6%) |
| Hemispheric stroke n (%) | 1033/2256 (45.8%) | 1071/2312 (46.3%) |
| Days from QE to treatment median (IQR)† | 29.0 (13.0, 67.0) | 26.0 (11.0, 61.0) |
| Treatment within 7 days of QE n (%) † | 214/1907 (11.2%) | 277/1926 (14.4%) |
Baseline data of patients in the stenting and endarterectomy group. Percentages exclude missing data. CEA = carotid endarterectomy; CAS = carotid artery stenting; LLT = lipid lowering therapy: EVA-3S recorded LLT use at baseline but patients were only considered to be taking LLT if started >3months prior to randomization. SPACE and CREST collected data on LLT use at randomization. ICSS did not collect information on LLT use at baseline but did collect these data at the one-month follow-up, which were included in the table. QE = qualifying event; SD = standard deviation.
Data were not collected in SPACE.
Date of the qualifying event before randomization was not collected in the SPACE trial initially, but for the pooled analysis, these dates were gathered where available.
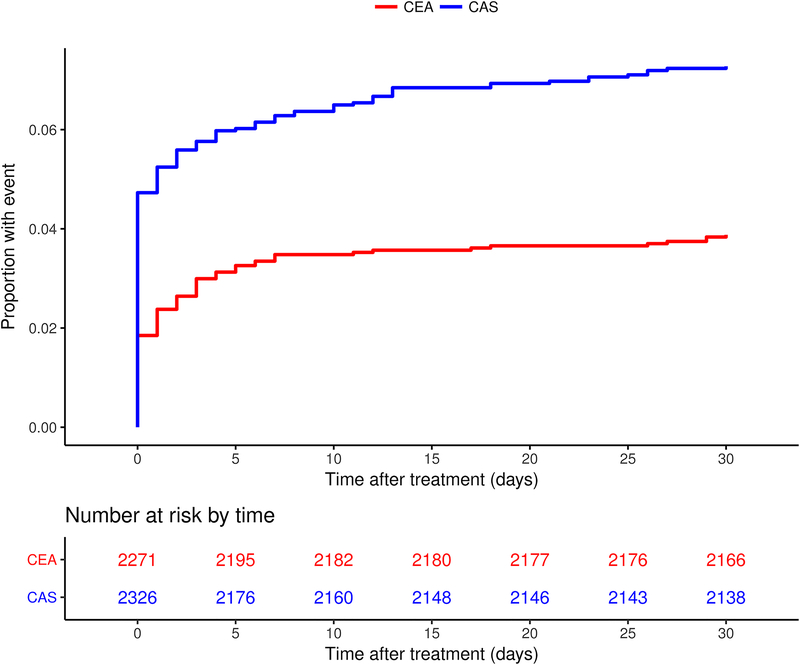
A total of 257 patients had a stroke or died during the full 30-day procedural period, 169 in the CAS group (7.3% risk) and 88 in the CEA group (3.9% risk; OR 1.94, 95% CI 1.48–2.54). Compared with CEA, patients treated with CAS more often had a stroke or died on the day of procedure (110 versus 42 patients, 4.7% versus 1.9% risk; OR 2.64, 95% CI 1.50–2.53), but not between 1 and 30 days thereafter (59 versus 46 patients, 2.5% versus 2.0% risk, OR 1.26, 0.86–1.87; Figure 2). The treatment effect ORs differed significantly between the time periods (interaction p=0.006). We performed a post-hoc sensitivity analysis excluding all patients who experienced the outcome measure on the day of procedure (n=152) from the population at risk for an event between day 1–30. This yielded very similar results compared to our original model and we again found no significant difference in the occurrence of the outcome measure between CAS and CEA between day 1–30 (OR 1.30, 95% CI 0.87–1.91). Only two patients who had a stroke on day 0, had another stroke between day 1–30.
Figure 2 – Kaplan-Meier curve.
Kaplan-Meier curve of the cumulative incidence of periprocedural stroke or death within 30 days after treatment in the stenting and endarterectomy group seperately. Number of events: 169 events in the CAS group, 88 events in the CEA group. The cumulative incidence of stroke or death was 7.3% in the CAS group and 3.9% in the CEA group.
Details of outcome events are provided in Table 2. In both treatment groups, the large majority of strokes, both occurring on the day of procedure and between day 1 and 30 after procedure, were located in the territory supplied by the treated carotid artery. In both treatment groups combined, 2 of 151 strokes (1%) occurring on the day of procedure and 15 of 95 strokes (16%) occurring between day 1 and 30 after the procedure were of hemorrhagic type.
Table 2.
Outcome events occurring on the day of procedure vs. day 1–30 thereafter
| CAS (n=2326) |
CEA (n=2271) |
Total (n=4597) |
||||
|---|---|---|---|---|---|---|
| Day of procedure | Day 1–30 | Day of procedure | Day 1–30 | Day of procedure | Day 1–30 | |
| Any stroke n(%) | 109 | 52 | 42 | 43 | 151 | 95 |
| Ipsilateral | 100 (92%) | 47 (90%) | 42 (100%) | 37 (86%) | 142 (94%) | 84 (88%) |
| Non-ipsilateral | 9 (8%) | 5 (10%) | 0 (0%) | 6 (14%) | 9 (6%) | 11 (12%) |
| Ischemic stroke n (%) | 108 (99%) | 48 (92%) | 41 (98%) | 32 (74%) | 149 (99%) | 80 (84%) |
| Hemorrhagic stroke n (%) | 1 (1%) | 4 (8%) | 1 (2%) | 11 (26%) | 2 (1%) | 15 (16%) |
| Non-stroke death | 1 | 7 | 0 | 3 | 1 | 10 |
Data are numbers and percentages of patients who experienced an outcome event. CAS = carotid artery stenting; CEA = carotid endarterectomy.
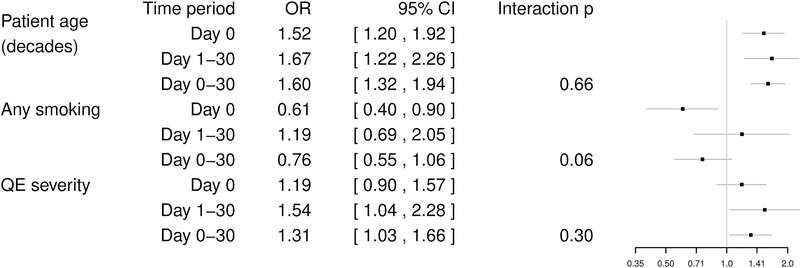
In the stenting group, the forward selection model identified age and smoking as independent predictors of immediate procedural events: age was positively (OR 1.54 per decade, 95% CI 1.21–1.95) and smoking was negatively (OR 0.61, 95% CI 0.40–0.91) associated with stroke or death occurring on the day of treatment. For the delayed procedural period (day 1–30 after treatment) age (OR 1.63 per decade, 95% CI 1.21–2.21) and qualifying event (QE) severity (stroke > TIA > ocular ischemia, analyzed by trend; OR 1.54, 95% CI 1.03–2.31) were found to be significant predictors for stroke or death. There were no significant differences in the strength of the associations between these risk factors and events between the immediate and delayed procedural period with the exception of smoking (interaction p=0.061; Figure 3). For the entire procedural period both age (OR 1.63 per decade; 95% CI 1.37–1.95) and QE severity (analyzed by trend; OR 1.31, 95% CI 1.04–1.64) remained significant predictors for stroke or death occurring between day 0–30 after treatment.
Figure 3 – Effects of baseline variables on the risk of stroke or death in patients treated with carotid artery stenting.
Forest plot showing the odds ratios for the effects of the three baseline variables “patient age”, any “history of smoking”, and “qualifying event severity” on the incidence of stroke or death on the day of treatment (day 0), between day 1 and 30, or within 30 days in patients treated with stenting. ORs were estimated by three separate GLMMs (one for the day of treatment, one for day 1–30, and one for the full procedural period), each containing age, any history of smoking, and QE severity (stroke > TIA > ocular ischemia). CI=Confidence Interval; OR=Odds Ratio; QE=Qualifying event; GLMM=general linear mixed model.
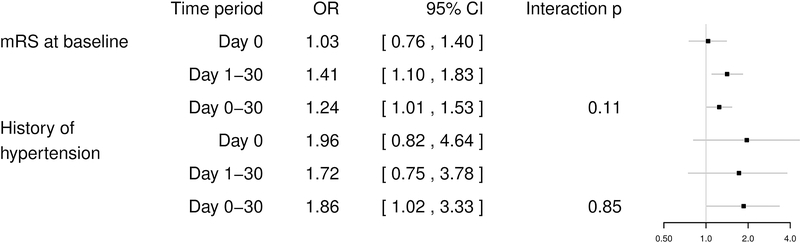
In the endarterectomy group, we found no significant predictors for immediate stroke or death. However, a higher level of disability at baseline, assessed with the modified Rankin Scale, was a significant predictor for delayed stroke or death (OR 1.42, 95% CI 1.10–1.84). The association between this risk factor and procedural stroke or death did not differ significantly between the immediate and delayed procedural period (Figure 4). Higher level of disability at baseline and history of hypertension were found to be significant predictors for stroke or death in the full procedural period (OR 1.24, 95% CI 1.01–1.51, and OR 1.84, 95% CI 1.04–3.33, respectively).
Figure 4 – Effects of baseline variables on the risk of stroke or death in patients treated with carotid endarterectomy.
Forest plot showing the odds ratios for the effects of the baseline variable “mRS at baseline”, and “history of hypertension” on the incidence of stroke or death on the day of treatment (day 0), between day 1 and 30, or within 30 days in patients treated with endarterectomy. ORs were estimated by three separate GLMMs (one for the day of treatment, one for day 1–30, and one for the full procedural period). OR=Odds ratio; CI=confidence interval; GLMM=general linear mixed model; mRS=modified Rankin Scale
Discussion
In this pooled analysis of individual patient data from four randomized clinical trials, we found that the excess occurrence of procedural stroke or death associated with CAS compared with CEA was limited to the day of treatment. For the remainder of the procedural period, there was no difference in the risk of stroke or death between the two treatments. Age was a risk factor for stroke or death in the CAS group, notably both in the immediate (day of procedure) and the delayed procedural period (day 1–30).
Procedure-related stroke or death in carotid revascularization is commonly defined as all events occurring within 30 days after the procedure. However, from experience, most such events occur on the day of treatment. In our analysis, we were able to confirm this assumption. We found that about two thirds (110 out of 169) of all procedural stroke or death outcomes in patients receiving CAS and about half (42 out of 88) of the events in patients treated with CEA occurred on the day of procedure. On the day of procedure, the risk of procedural stroke or death was significantly higher in CAS than in CEA, but between 1 and 30 days thereafter the risk was similar in both treatment groups.
Stroke or death events occurring on the day of procedure might differ in pathogenesis and associated risk factors from events occurring later in the 30-day period. However, as far as clinical risk factors are concerned, we found no significant differences in the observed associations between the immediate and delayed procedural period. Most importantly, increasing age among patients treated with CAS was significantly associated with procedural stroke or death in both the immediate (day of procedure) and delayed procedural (day 1–30) period. It has been hypothesized that higher age is associated with vessel elongation and therefore more pronounced angulation of the vasculature, and that the resulting, more complex anatomy of the supra-aortic arteries could lead to increased technical difficulty of the procedure and hence to the higher risk associated with CAS in older patients.16, 17 Our finding that age is associated with an increase in both immediate and delayed procedural events argues against vascular anatomy as the sole mediating factor. Older patients might have more unstable atheromatous lesions than younger patients, which may cause thromboembolic strokes not only on the day of procedure, but also during the following days.18, 19 However, the mechanisms mediating the association between age and procedural risk of CAS remains poorly understood; our finding that older patients are also at risk for delayed procedural events adds to the complexity of this matter.
Interestingly, we found that history of smoking decreased the risk for stroke or death on the day of procedure in the CAS group. A similar association with stroke or death in the full procedural period was already described in ICSS.20 One possible explanation for this rather surprising relationship might have been that smokers were younger than non-smokers and hence at lower risk. Indeed, the mean age of smokers in our study population was 67.5 years, while the mean age of non-smokers was 72.5 years. However, the effect of smoking was adjusted for age in our analysis indicating that the inverse association with stroke or death is not confounded by age. Nevertheless, we cannot rule out that this unexpected finding was due to residual confounding by patient characteristics not measured in the trials.
The fact that the excess of procedural stroke or death occurring in the stenting group is limited to the day of procedure suggests that these events might potentially be avoided by improving operator skill or technical aspects of the procedure itself. Whether the use of intraluminal protection devices reduces the risk of embolic stroke is a matter of ongoing controversy. In EVA-3S the use of distal filter protection devices became mandatory early in the trial, after the risk of stroke within the procedural period was found to be about three times higher in patients treated with unprotected CAS. In CREST the use of cerebral protection devices was recommended whenever feasible. In ICSS and SPACE the use of protection devices remained optional throughout the trials. The ICSS-MRI substudy showed that the use of distal filter devices, which was the type of protection device predominately used in all four contributing trials, was associated with an increased risk of new ischemic brain lesions after the procedure.21 Two small randomized studies comparing stenting with embolic filter protection to unprotected stenting confirmed these results.22, 23 In light of these findings, considerable uncertainty remains, whether distal filter devices truly increase the safety of CAS.
Irrespective of this question, one must acknowledge the fact that the trials contributing to the present analysis largely enrolled their patients in the 2000s and that considerable technical advance of CAS has occurred since. For example, alternative methods of cerebral protection such as systems exerting a reversal of blood flow before the lesion is crossed with the catheter have been introduced and appear to lower the risk of thromboembolism.24 However, not all patients tolerate flow reversal in the carotid artery and to date insufficient data exist to justify a general recommendation for the use of such devices, although the available data seem promising.25, 26 The ARMOUR study investigated the safety and effectiveness of proximal embolic protection with the Mo.MA device and showed a very low 30-day stroke rate of 0.9%.26 Another possible source of thromboembolism to the brain during stenting is the aortic arch and the access vasculature. To avoid the necessity of navigating the aortic arch with potentially difficult anatomy, alternative access routes such as direct carotid access have been proposed.27 The ROADSTER study investigating CAS with direct transcarotid access and proximal embolic protection showed a very low 30-day stroke rate of 1.4%.28 Although the ARMOUR and the ROADSTER studies enrolled patients with both symptomatic and asymptomatic carotid stenosis, which renders a direct comparison with our results difficult, these low stroke rates are remarkable.
In the endarterectomy group, the only clinical risk factors found to influence the risk of stroke or death were a higher level of disability at baseline and known history of hypertension. Hypertension increased the risk for stroke or death over the entire procedural period of 30 days, a finding which is consistent with previous reports.15
This analysis has important limitations. First, we did not collect information as to the mechanism of stroke across all four contributing trials (e.g. embolic, hemodynamic, stent thrombosis). Second, with regard to events occurring on the day of treatment, we do not know if the events occurred during or after the procedure, as the exact timing of stroke or death was not recorded. Third, all participating trials recruited patients between 2000 and 2008. Since that time, there has been substantial progress in the development of new stent designs, the introduction of cerebral protection devices and new access routes, all of which may help reduce the risk of immediate procedural complications. Thus, the peri-procedural stroke rate among patients with symptomatic carotid stenosis using current generation CAS technologies and cerebral protection devices may be lower than demonstrated in these 4 trials. Fourth, in order to be able to investigate whether the CAS versus CEA treatment effect differed between the immediate and delayed procedural period by formal tests of statistical interaction, we included all patients in the population at risk for an event between day 1–30, even those who experienced an event on the day of procedure (n=152). However, in a post-hoc analysis excluding these patients from the population at risk for an event between day 1–30, the results remained essentially unchanged.
The fact that the increased risk of stroke or death in the stenting group is limited to the day of procedure demonstrates the need to improve the procedural safety of carotid artery stenting. This may potentially be achieved by technical advances (route of access, stent design and new protection devices) and increased operator skill. However, more data from randomized trials to evaluate these new devices and access routes are needed. Our finding that age is associated with both immediate and delayed procedural events in the stenting group argues against vascular anatomy as the sole mediating factor. Other, currently unknown factors are likely to contribute to this effect and remain to be elucidated.
Supplementary Material
Acknowledgements
J-L. Mas, P.A. Ringleb, and L.H. Bonati extracted individual patient data from the contributing trials. S. von Felten planned and performed the data analysis. S. von Felten, M. D. Müller and L. H. Bonati interpreted the data. M. D. Müller wrote the first version of the manuscript. All authors made substantial contributions to conception and design of the study, acquisition of data, or analysis and interpretation of data, and were involved in revision of the manuscript. All authors gave final approval to submit for publication.
Funding Sources
L.H. Bonati was supported by grants from the Swiss National Science Foundation (PBBSB-116873 and 32003B-156658), the Swiss Heart Foundation, the University of Basel, Switzerland, and The Stroke Association. M.M. Brown’s Chair in Stroke Medicine at University College London is supported by the Reta Lila Weston Trust for Medical Research. A. Halliday’s research is funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Center. G. Howard is funded by the National Institute of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS). Detailed information about the Carotid Stenosis Trialists’ Collaboration and Acknowledgments including funding of the contributing studies are provided in the online-only Data Supplement.
Footnotes
Disclosures
LHB has received an unrestricted research grant from AstraZeneca, as well as consultancy and advisory board fees from Amgen, Bayer, Bristol-Myers Squibb, and Claret Medical. PR has received lecture fees and advisory board fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, and Pfizer. OJ received speaker fees from Medtronic, Stryker and Philips. All other authors declare no competing interests.
Clinical Trial Registration:
EVA-3S - URL: https://clinicaltrials.gov/ct2/show/NCT00190398; Unique identifier: NCT00190398.
SPACE - URL: http://www.isrctn.com/ISRCTN57874028; Unique identifier: ISRCTN57874028.
ICSS - URL: http://www.isrctn.com/ISRCTN25337470; Unique identifier: ISRCTN25337470.
CREST- URL:https://clinicaltrials.gov/ct2/show/NCT00004732; Unique identifier: NCT00004732.
References
- 1.Carotid Stenting Trialists Collaboration, Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: A preplanned meta-analysis of individual patient data. Lancet. 2010;376:1062–1073 [DOI] [PubMed] [Google Scholar]
- 2.Howard G, Roubin GS, Jansen O, Hendrikse J, Halliday A, Fraedrich G, et al. Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: A meta-analysis of pooled patient data from four randomised trials. Lancet. 2016;387:1305–1311 [DOI] [PubMed] [Google Scholar]
- 3.Bonati LH, Dobson J, Featherstone RL, Ederle J, Van Der Worp HB, De Borst GJ, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: The international carotid stenting study (icss) randomised trial. Lancet. 2015;385:529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mas JL, Arquizan C, Calvet D, Viguier A, Albucher JF, Piquet P, et al. Long-term follow-up study of endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis trial. Stroke. 2014;45:2750–2756 [DOI] [PubMed] [Google Scholar]
- 5.Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. The New England journal of medicine. 2016;374:1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the stent-protected angioplasty versus carotid endarterectomy (space) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902 [DOI] [PubMed] [Google Scholar]
- 7.Brown MM, Hacke W. Carotid artery stenting:The need for randomised trials. Cerebrovasc Dis. 2004;18:57–61 [DOI] [PubMed] [Google Scholar]
- 8.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia JF, et al. Design of the carotid revascularization endarterectomy vs. Stenting trial (crest). International journal of stroke. 2010;5:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Featherstone RL, Brown MM, Coward LJ. International carotid stenting study: Protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis. 2004;18:69–74 [DOI] [PubMed] [Google Scholar]
- 10.EVA-3S Investigators. Endarterectomy vs. Angioplasty in patients with symptomatic severe carotid stenosis (eva-3s) trial. Cerebrovasc Dis. 2004;18:62–65 [DOI] [PubMed] [Google Scholar]
- 11.Ringleb PA, Kunze A, Allenberg JR, Hennerici MG, Jansen O, Maurer PC, et al. The stent-supported percutaneous angioplasty of the carotid artery vs. Endarterectomy trial. Cerebrovasc Dis. 2004;18:66–68 [DOI] [PubMed] [Google Scholar]
- 12.Mas JL, Chatellier G, Beyssen B, Investigators E-S. Carotid angioplasty and stenting with and without cerebral protection: Clinical alert from the endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (eva-3s) trial. Stroke. 2004;35:e18–20 [DOI] [PubMed] [Google Scholar]
- 13.Brott TG, Brown RD, Jr., Meyer FB, Miller DA, Cloft HJ, Sullivan TM. Carotid revascularization for prevention of stroke: Carotid endarterectomy and carotid artery stenting. Mayo Clin Proc. 2004;79:1197–1208 [DOI] [PubMed] [Google Scholar]
- 14.Committee NASCET. North american symptomatic carotid endarterectomy trial. Methods, patient characteristics, and progress. Stroke. 1991;22:711–720 [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ, Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924 [DOI] [PubMed] [Google Scholar]
- 16.Lin SC, Trocciola SM, Rhee J, Dayal R, Chaer R, Morrissey NJ, et al. Analysis of anatomic factors and age in patients undergoing carotid angioplasty and stenting. Annals of vascular surgery. 2005;19:798–804 [DOI] [PubMed] [Google Scholar]
- 17.Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. Journal of vascular surgery. 2007;45:875–880 [DOI] [PubMed] [Google Scholar]
- 18.Redgrave JN, Lovett JK, Rothwell PM. Histological features of symptomatic carotid plaques in relation to age and smoking: The oxford plaque study. Stroke. 2010;41:2288–2294 [DOI] [PubMed] [Google Scholar]
- 19.Grufman H, Schiopu A, Edsfeldt A, Bjorkbacka H, Nitulescu M, Nilsson M, et al. Evidence for altered inflammatory and repair responses in symptomatic carotid plaques from elderly patients. Atherosclerosis. 2014;237:177–182 [DOI] [PubMed] [Google Scholar]
- 20.Doig D, Turner EL, Dobson J, Featherstone RL, Lo RT, Gaines PA, et al. Predictors of stroke, myocardial infarction or death within 30 days of carotid artery stenting: Results from the international carotid stenting study. Eur J Vasc Endovasc Surg. 2016;51:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, et al. New ischaemic brain lesions on mri after stenting or endarterectomy for symptomatic carotid stenosis: A substudy of the international carotid stenting study (icss). Lancet Neurology. 2010;9:353–362 [DOI] [PubMed] [Google Scholar]
- 22.MacDonald S, Evans DH, Griffiths PD, McKevitt FM, Venables GS, Cleveland TJ, et al. Filter-protected versus unprotected carotid artery stenting: A randomised trial. Cerebrovascular Diseases. 2010;29:282–289 [DOI] [PubMed] [Google Scholar]
- 23.Barbato JE, Dillavou E, Horowitz MB, Jovin TG, Kanal E, David S, et al. A randomized trial of carotid artery stenting with and without cerebral protection. Journal of vascular surgery. 2008;47:760–765 [DOI] [PubMed] [Google Scholar]
- 24.Mokin M, Dumont TM, Chi JM, Mangan CJ, Kass-Hout T, Sorkin GC, et al. Proximal versus distal protection during carotid artery stenting: Analysis of the two treatment approaches and associated clinical outcomes. World Neurosurg. 2014;81:543–548 [DOI] [PubMed] [Google Scholar]
- 25.Bijuklic K, Wandler A, Hazizi F, Schofer J. The profi study (prevention of cerebral embolization by proximal balloon occlusion compared to filter protection during carotid artery stenting): A prospective randomized trial. Journal of the American College of Cardiology. 2012;59:1383–1389 [DOI] [PubMed] [Google Scholar]
- 26.Ansel GM, Hopkins LN, Jaff MR, Rubino P, Bacharach JM, Scheinert D, et al. Safety and effectiveness of the invatec mo.Ma proximal cerebral protection device during carotid artery stenting: Results from the armour pivotal trial. Catheterization and Cardiovascular Interventions. 2010;76:1–8 [DOI] [PubMed] [Google Scholar]
- 27.Palombo G, Stella N, Faraglia V, Rizzo L, Fantozzi C, Bozzao A, et al. Cervical access for filter-protected carotid artery stenting: A useful tool to reduce cerebral embolisation. European Journal of Vascular and Endovascular Surgery. 2010;39:252–257 [DOI] [PubMed] [Google Scholar]
- 28.Kwolek CJ, Jaff MR, Leal JI, Hopkins LN, Shah RM, Hanover TM, et al. Results of the roadster multicenter trial of transcarotid stenting with dynamic flow reversal. Journal of vascular surgery. 2015;62:1227–1234 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.