Abstract
Hypertrophic cardiomyopathy (HCM) is most commonly transmitted as an autosomal dominant trait, caused by mutations in genes encoding cardiac sarcomere proteins1–3. Other inheritable causes of the disease include mutations in genes coding for proteins important in calcium handling or that form part of the cytoskeleton4–6. At present, the primary clinical role of genetic testing in HCM is to facilitate familial screening to allow the identification of individuals at risk of developing the disease7,8. It is also used to diagnose genocopies, such as lysosomal9–11 and glycogen storage disease which have different treatment strategies, rates of disease progression and prognosis12–14. The role of genetic testing in predicting prognosis is limited at present, but emerging data suggest that knowledge of the genetic basis of disease will assume an important role in disease stratification15–17 and offer potential targets for disease-modifying therapy in the near future18.
Genetic architecture of HCM
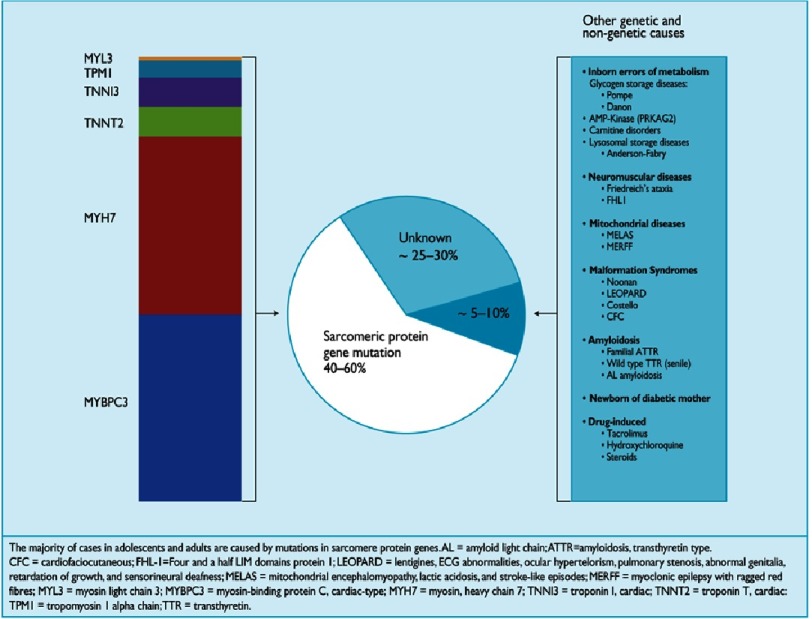
Familial HCM is characterized by locus and allelic heterogeneity, with a high frequency of novel individual mutations7,19. The first genetic mutation to be identified was a single base substitution in the MYH7 gene encoding ß-myosin heavy chain, a key component of the cardiac sarcomere20. Since then, many different mutations in MYH7 and other genes of the cardiac sarcomere have been identified. In 5–10% of cases, HCM is caused by mutations in genes that cause metabolic disorders21–23, neuromuscular disease24–26 or inherited genetic syndromes including Noonan syndrome27–29 (Figure 1).
Figure 1. Representation of the percentage of hypertrophic cardiomyopathy cases accounted for by pathogenic mutations in sarcomeric and non-sarcomere genes.
Elliott PM, et al; 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC), European Heart Journal 2014; 35 (39): 2733–2779, doi:10.1093/eurheartj/ehu284. Reproduced by permission of Oxford University Press on behalf of the European Society of Cardiology. © European Society of Cardiology 2014. All rights reserved. For permissions please email journals.permissions@oup.com. This figure is not included under the Open Access license of this publication.
The cardiac sarcomere
Every cardiomyocyte is composed of myofibrils that run longitudinally along the cell and are transversely subdivided into contractile units called sarcomeres30,31. The sarcomere constitutes the fundamental motor unit of the cardiomyocyte and is composed of two principal components – the thick filament composed of around 300 molecules of myosin, each made up of 2 protein units of β- or α-myosin heavy chain and 4 myosin light chain molecules, and the thin filament composed of repeating actin molecules, closely associated with the regulatory troponin complex (troponin T (TnT), troponin I (TnI) and troponin C (TnC)) and α-tropomyosin31–33. An additional protein, cardiac myosin-binding protein C, contributes to the regulation of actin–myosin interaction and cross-bridge kinetics31 (Figure 2).
Figure 2. Representation of the cardiac sarcomere with associated proteins and interactions.
Reproduced from Ref. [31]. Abbreviations: TnI, Troponin I; TnC, Troponin C; TnT, Troponin T; Tm, Tropomyosin; c-MYBP-C, cardiac myosin binding protein-c; ELC, Essential Light Chain; RLC, Regulatory Chain; LMM, Light meromyosin. The domains of cMyBP-C are numbered from C0–C10; m is the regulatory motif between domains C1 and C2 and contains the PKA phosphorylation site; PA represents a proline / alanine-rich linker sequence between domains C0 and C1.
Muscle contraction results from the interaction between myosin and actin in which the globular head of the myosin molecule bends towards and then binds to actin, contracts, releases actin, and then initiates a new cycle. The links between the myosin head and actin are called cross-bridges; the contraction of the myosin S1 region which requires the hydrolysis of ATP to release energy is called the power stroke31,34.
Electrical activation of the heart and cardiomyocyte contraction are coupled through the intracellular movement of calcium. Depolarisation of the cardiomyocyte cell membrane activates the L-type voltage-dependent calcium channels in the T tubule causing an influx of calcium into the cell that triggers opening of ryanodine-receptor channels in the adjacent sarcoplasmic reticulum with a rapid increase in cytosolic calcium31. Calcium binds to TnC, inducing an allosteric conformational change in TnI and TnT that is transmitted to tropomyosin and exposes the myosin-binding sites of actin allowing the cross-bridges to form33. The myosin heavy chain head, with ADP and inorganic phosphate bound to its nucleotide-binding pocket, then interacts with the exposed actin-binding sites followed by the release of ADP and inorganic phosphate, which occurs simultaneously with the power stroke. ATP then binds to the nucleotide-binding pocket of the myosin heavy chain head, which detaches from actin and myosin then hydrolyses ATP into ADP and inorganic phosphate restarting the contraction cycle31.
Sarcomere mutations in HCM
Mutations in ßMYH7 and MYBPC3 account for 60–70% of HCM patients with pathogenic variants1,19,32,35. Mutations in other sarcomere and associated protein genes are listed in Table 1. Mutations in MYH7 are predominantly missense, with single nucleotide-base substitutions resulting in a non-synonymous single amino acid substitution20,36. In contrast, the majority of mutations in MYBPC3 are nonsense mutations due to insertion/deletions, splice-site variants or frameshifts causing a premature stop codon that results in a truncated protein transcript1,32,37.
Table 1. List of genes in which pathogenic mutations are associated with hypertrophic cardiomyopathy.
The chromosome location and the proportion of HCM cases attributed to mutations in these specific genes are included.
| Protein | Gene | Chromosome location | Proportion of HCM caused by mutations |
|---|---|---|---|
| SARCOMERIC PROTEINS | |||
| B-Myosin Heavy Chain 7 | MYH7 | 14q12 | 40–44% |
| Myosin-Binding Protein C 3 | MYBPC3 | 11p11 | 35–40% |
| Troponin T | TNNT2 | 1q32 | 5–15% |
| Troponin I | TNNI3 | 19q13 | 5% |
| Tropomyosin alpha-1 chain | TPM1 | 15q22 | 3% |
| Regulatory Myosin Light Chain | MYL2 | 12q24 | 1–2% |
| Essential Myosin Light Chain | MYL3 | 3p21 | 1% |
| Actin | ACTC1 | 15q14 | 1% |
| Troponin C | TNNC1 | 3p21 | <1% |
| Z-DISK PROTEINS | |||
| ZASP –LIM binding domain 3 | LBD3 | 10q22 | 1–5% |
| Alpha-Actinin-2 | ACTN2 | 1q42 | <1% |
| Ankyrin repeat domain containing protein –1 | ANKRD1 | 10q23 | <1% |
| Muscle LIM Protein | CSRP3 | 11p15 | <1% |
| Myozenin-2 | MYOZ2 | 4q26 | <1% |
| Telethonin | TCAP | 17q12 | <1% |
| Vinculin | VCL | 10q22 | <1% |
| Nexilin | NEXN | 1p31 | <1% |
| Filamin C | FLNC | 7q32 | <1% |
| SARCOMERE-ASSOCIATED PROTEINS | |||
| Desmin | DES | 2q35 | <1% |
| Four and a Half Lim Domain Protein –1 | FHL-1 | Xq26 | <1% |
| CALCIUM-HANDLING PROTEINS | |||
| Phospholamban | PLN | 6q22 | <1% |
| Calreticulin 3 | CALR3 | 19p13 | <1% |
| Calsequestrin 2 | CASQ2 | 1p13 | <1% |
| Junctophilin 2 | JPH2 | 20q13 | <1% |
| HCM PHENOCOPIES (METABOLIC & LYSOSOMAL STORAGE DISORDERS) | |||
| AMP-gamma2 subunit | PRKAG2 | 7q36 | Together with other HCM phenocopies account for 5–10% of HCM cases |
| Glucosidase A (Pompe’s disease) | GAA | 17q25 | |
| Alpha-Galactosidase A (Anderson-Fabry Disease) | GLA | Xq22 | |
| Lysosomal-associated membrane protein 2 (Danon’s Syndrome) | LAMP2 | Xq24 | |
| HCM PHENOCOPIES (Ras-MAPK) | |||
| Noonan Syndrome | KRAS | 12p12 | Together with other HCM phenocopies account for 5–10% of HCM cases |
| SOS1 | 2p22 | ||
| PTPN11 | 12q24 | ||
| RAF1 | 3p25 | ||
| LEOPARD syndrome | PTPN11 | 12q24 | |
| RAF1 | 3p25 | ||
| HCM PHENOCOPY (NEUROMUSCULAR DISORDERS) | |||
| Friedreich’s Ataxia | GAA expansion in FXN | 9q13 | Together with other HCM phenocopies account for 5–10% of HCM cases |
The majority of missense mutations are believed to have a dominant negative effect in which the mutant protein is incorporated into the sarcomere, but its interaction with the normal wild-type protein disrupts normal sarcomeric assembly and function (poison polypeptide hypothesis)31,38. Individual missense mutations may change an amino acid in a highly-conserved residue, alter important kinase domains (affecting ligand interaction) or change surface-exposed portion of a molecule altering protein-protein interaction. Missense mutations can also cause protein misfolding and accelerated degradation by ubiquitin-proteasomal surveillance pathways.
In truncating mutations, haploinsufficiency is thought to be the major disease mechanism39,40. The reported absence of detectable truncated myosin-binding protein C in western-blot analysis of myectomy specimens of patients with this mutation may be due to nonsense mediated mRNA decay of abnormal transcripts or ubiquitin-mediated proteasomal degradation of aberrant truncated protein40–43.
Allelic heterogeneity can be partly explained by the effect of different mutations on the structure and function of the complete peptide. ß-myosin heavy chain, for example, consists of a globular head, an α-helical rod and a hinge region. The globular head contains binding sites for ATPase and actin as well as interaction sites for regulatory and essential light chains in the head-rod region31. The majority of disease-causing ß-myosin heavy chain mutations are found in one of four locations: the actin binding site, the nucleotide binding pocket, the hinge region adjacent to the binding site for two reactive thiols and in the α-helix close to the essential light chain interaction site44. Thus, different effects on protein function might be expected depending on the position of the mutation.
It has been speculated that the HCM disease phenotype results from reduced contractile function caused by altered actin-myosin interactions, and consequent inappropriate compensatory hypertrophic remodelling45,46. However, some MYH7 mutations are associated with increased cardiomyocyte mechanical contractile forces in vitro and show an increase in calcium sensitivity, leading to increases in tension generation and ATPase activity. Animal and cell studies have also confirmed altered calcium homeostasis as a key contributor to the pathophysiological processes that lead to the development of LV hypertrophy47,48.
Troponin T mutations account for less than 5% of all cases of HCM but often show a particular phenotype. TNNT2 mouse models show varying degrees of myocyte disarray and fibrosis with minimal LVH, in common with TNNT2 disease expression in humans49,50. Troponin-mutated mice exhibit severely impaired myocardial relaxation, independent of the degree of fibrosis, and consistent with the finding of increased calcium sensitivity51–53. Some studies suggest that some TNNT2 mutations are associated with a high incidence of sudden death which may also relate to calcium loading in cardiomyocytes54,55.
Findings from murine models of HCM suggest that the increased contractility seen with some mutations is at the expense of inefficient ATP utilization. Furthermore, animal and human studies suggest that HCM is associated with depleted energy stores and abnormal ATP/ADP ratios56–59. Inefficient utilization of ATP is also seen in metabolic disorders or mitochondrial cytopathies, which can produce a pattern of LV hypertrophy similar to that in sarcomeric HCM.
Recently, mutations in genes encoding z-disc proteins including myozenin (MYOZ2), telethonin (TCAP), alpha-actinin-2 (ACTN2), muscle LIM protein (CRP3) and nexilin (NEXN) have been implicated in HCM31. Some mutations in Z-disc proteins have a pleotropic effect, causing an HCM phenotype in certain individuals and a DCM phenotype in others within the same family60.
Childhood-onset HCM
The importance of sarcomeric protein gene mutations in childhood hypertrophic cardiomyopathy is unknown. The observation that the development of left ventricular hypertrophy in individuals with familial disease often occurs during the period of somatic growth in adolescence has led to the suggestion that sarcomeric protein disease in very young children is rare61,62. However, studies of children with HCM have shown that, as in adults, sarcomeric protein gene mutations account for approximately 50% of cases of idiopathic HCM, even in infants and young children63,64.
Phenotypic variability
There is a substantial variation in the expression of identical mutations indicating that other genetic and possibly environmental factors influence disease expression. The effect of age is perhaps the best characterized factor, most patients developing ECG and echocardiographic manifestations of the disease after puberty and before the age of thirty65,66. Sex also appears to influence disease expression in sarcomere protein disease67,68. Other potential modifying factors include renin-angiotensin-aldosterone system gene polymorphism69–71, and the occurrence of homozygosity and compound heterozygosity72–74.
Genetic advancements
The impact of more rapid, cost-effective gene sequencing methods, together with improved cellular models including induced pluripotent stem cells, gene-editing technology, larger sequenced control cohorts and deep clinical phenotyping in cases means that we are at an exciting crossroads in the genetics of HCM. This provides researchers with significant opportunity to identify novel candidate causative genes and to potentially allow clearer genotype-phenotype correlations to be made, thereby laying the foundation for more personalised patient care.
References
- 1.Richard P, et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107(17):2227–32. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 2.Lopes LR, Rahman MS, Elliott PM. A systematic review and meta-analysis of genotype-phenotype associations in patients with hypertrophic cardiomyopathy caused by sarcomeric protein mutations. Heart. 2013;99(24):1800–11. doi: 10.1136/heartjnl-2013-303939. [DOI] [PubMed] [Google Scholar]
- 3.Erdmann J, et al. Mutation spectrum in a large cohort of unrelated consecutive patients with hypertrophic cardiomyopathy. Clin Genet. 2003;64(4):339–49. doi: 10.1034/j.1399-0004.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 4.Landstrom AP, et al. PLN-encoded phospholamban mutation in a large cohort of hypertrophic cardiomyopathy cases: summary of the literature and implications for genetic testing. Am Heart J. 2011;161(1):165–71. doi: 10.1016/j.ahj.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landstrom AP, et al. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol. 2007;42(6):1026–35. doi: 10.1016/j.yjmcc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landstrom AP, Ackerman MJ. Beyond the cardiac myofilament: hypertrophic cardiomyopathy-associated mutations in genes that encode calcium-handling proteins. Curr Mol Med. 2012;12(5):507–18. doi: 10.2174/156652412800620020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charron P, et al. Genetic counselling and testing in cardiomyopathies: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2010;31(22):2715–26. doi: 10.1093/eurheartj/ehq271. [DOI] [PubMed] [Google Scholar]
- 8.Authors/Task Force, m et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(39):2733–79. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 9.Elliott P, et al. Prevalence of Anderson-Fabry disease in patients with hypertrophic cardiomyopathy: the European Anderson-Fabry Disease survey. Heart. 2011;97(23):1957–60. doi: 10.1136/heartjnl-2011-300364. [DOI] [PubMed] [Google Scholar]
- 10.Ommen SR, Nishimura RA, Edwards WD. Fabry disease: a mimic for obstructive hypertrophic cardiomyopathy? Heart. 2003;89(8):929–30. doi: 10.1136/heart.89.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachdev B, et al. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105(12):1407–11. doi: 10.1161/01.cir.0000012626.81324.38. [DOI] [PubMed] [Google Scholar]
- 12.Charron P, et al. Danon’s disease as a cause of hypertrophic cardiomyopathy: a systematic survey. Heart. 2004;90(8):842–6. doi: 10.1136/hrt.2003.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DH, et al. Hypertrophic cardiomyopathy in pompe disease is not limited to the classic infantile-onset phenotype. JIMD Rep. 2014;17:71–5. doi: 10.1007/8904_2014_339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosmini S, et al. Relationship between aetiology and left ventricular systolic dysfunction in hypertrophic cardiomyopathy. Heart. 2017;103(4):300–306. doi: 10.1136/heartjnl-2016-310138. [DOI] [PubMed] [Google Scholar]
- 15.Lopes LR, et al. Novel genotype-phenotype associations demonstrated by high-throughput sequencing in patients with hypertrophic cardiomyopathy. Heart. 2015;101(4):294–301. doi: 10.1136/heartjnl-2014-306387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivotto I, et al. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc. 2008;83(6):630–8. doi: 10.4065/83.6.630. [DOI] [PubMed] [Google Scholar]
- 17.van Velzen HG, et al. Value of Genetic Testing for the Prediction of Long-Term Outcome in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2016;118(6):881–887. doi: 10.1016/j.amjcard.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 18.Kawas RF, et al. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem. 2017;292(40):16571–16577. doi: 10.1074/jbc.M117.776815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60(8):705–15. doi: 10.1016/j.jacc.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 20.Geisterfer-Lowrance AA, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62(5):999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 21.Murphy RT, et al. Adenosine monophosphate-activated protein kinase disease mimicks hypertrophic cardiomyopathy and Wolff-Parkinson-White syndrome: natural history. J Am Coll Cardiol. 2005;45(6):922–30. doi: 10.1016/j.jacc.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 22.MacRae CA, et al. Familial Hypertrophic cardiomyopathy with Wolff-Parkinson-White syndrome maps to a locus on chromosome 7q3. J Clin Invest. 1995;96(3):1216–20. doi: 10.1172/JCI118154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gollob MH, et al. PRKAG2 cardiac syndrome: familial ventricular preexcitation, conduction system disease, and cardiac hypertrophy. Curr Opin Cardiol. 2002;17(3):229–34. doi: 10.1097/00001573-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Filla A, et al. The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am J Hum Genet. 1996;59(3):554–60. [PMC free article] [PubMed] [Google Scholar]
- 25.Weidemann F, et al. The cardiomyopathy in Friedreich’s ataxia - New biomarker for staging cardiac involvement. Int J Cardiol. 2015;194:50–7. doi: 10.1016/j.ijcard.2015.05.074. [DOI] [PubMed] [Google Scholar]
- 26.Weidemann F, et al. The heart in Friedreich ataxia: definition of cardiomyopathy, disease severity, and correlation with neurological symptoms. Circulation. 2012;125(13):1626–34. doi: 10.1161/CIRCULATIONAHA.111.059477. [DOI] [PubMed] [Google Scholar]
- 27.Hickey EJ, et al. Survival implications: hypertrophic cardiomyopathy in Noonan syndrome. Congenit Heart Dis. 2011;6(1):41–7. doi: 10.1111/j.1747-0803.2010.00465.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson JD, et al. Outcomes in children with Noonan syndrome and hypertrophic cardiomyopathy: a study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2012;164(3):442–8. doi: 10.1016/j.ahj.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Colquitt JL, Noonan JA. Cardiac findings in Noonan syndrome on long-term follow-up. Congenit Heart Dis. 2014;9(2):144–50. doi: 10.1111/chd.12102. [DOI] [PubMed] [Google Scholar]
- 30.Sequeira V, et al. The physiological role of cardiac cytoskeleton and its alterations in heart failure. Biochim Biophys Acta. 2014;1838(2):700–22. doi: 10.1016/j.bbamem.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Lopes LR, Elliott PM. A straightforward guide to the sarcomeric basis of cardiomyopathies. Heart. 2014;100(24):1916–23. doi: 10.1136/heartjnl-2014-305645. [DOI] [PubMed] [Google Scholar]
- 32.Harris SP, Lyons RG, Bezold KL. In the thick of it: HCM-causing mutations in myosin binding proteins of the thick filament. Circ Res. 2011;108(6):751–64. doi: 10.1161/CIRCRESAHA.110.231670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tardiff JC. Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res. 2011;108(6):765–82. doi: 10.1161/CIRCRESAHA.110.224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore JR, Leinwand L, Warshaw DM. Understanding cardiomyopathy phenotypes based on the functional impact of mutations in the myosin motor. Circ Res. 2012;111(3):375–85. doi: 10.1161/CIRCRESAHA.110.223842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morita H, et al. Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med. 2008;358(18):1899–908. doi: 10.1056/NEJMoa075463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watkins H, et al. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med. 1992;326(17):1108–14. doi: 10.1056/NEJM199204233261703. [DOI] [PubMed] [Google Scholar]
- 37.Carrier L, et al. Organization and sequence of human cardiac myosin binding protein C gene (MYBPC3) and identification of mutations predicted to produce truncated proteins in familial hypertrophic cardiomyopathy. Circ Res. 1997;80(3):427–34. [PubMed] [Google Scholar]
- 38.Keren A, Syrris P, McKenna WJ. Hypertrophic cardiomyopathy: the genetic determinants of clinical disease expression. Nat Clin Pract Cardiovasc Med. 2008;5(3):158–68. doi: 10.1038/ncpcardio1110. [DOI] [PubMed] [Google Scholar]
- 39.Barefield D, et al. Haploinsufficiency of MYBPC3 exacerbates the development of hypertrophic cardiomyopathy in heterozygous mice. J Mol Cell Cardiol. 2015;79:234–43. doi: 10.1016/j.yjmcc.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marston S, et al. Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ Res. 2009;105(3):219–22. doi: 10.1161/CIRCRESAHA.109.202440. [DOI] [PubMed] [Google Scholar]
- 41.Carrier L, et al. The ubiquitin-proteasome system and nonsense-mediated mRNA decay in hypertrophic cardiomyopathy. Cardiovasc Res. 2010;85(2):330–8. doi: 10.1093/cvr/cvp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarikas A, et al. Impairment of the ubiquitin-proteasome system by truncated cardiac myosin binding protein C mutants. Cardiovasc Res. 2005;66(1):33–44. doi: 10.1016/j.cardiores.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 43.van Dijk SJ, et al. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 2009;119(11):1473–83. doi: 10.1161/CIRCULATIONAHA.108.838672. [DOI] [PubMed] [Google Scholar]
- 44.Woo A, et al. Mutations of the beta myosin heavy chain gene in hypertrophic cardiomyopathy: critical functional sites determine prognosis. Heart. 2003;89(10):1179–85. doi: 10.1136/heart.89.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Q, et al. In vivo modeling of myosin binding protein C familial hypertrophic cardiomyopathy. Circ Res. 1999;85(9):841–7. doi: 10.1161/01.res.85.9.841. [DOI] [PubMed] [Google Scholar]
- 46.Razumova MV, et al. Effects of the N-terminal domains of myosin binding protein-C in an in vitro motility assay: Evidence for long-lived cross-bridges. J Biol Chem. 2006;281(47):35846–54. doi: 10.1074/jbc.M606949200. [DOI] [PubMed] [Google Scholar]
- 47.Lan F, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12(1):101–13. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guinto PJ, et al. Temporal and mutation-specific alterations in Ca2+ homeostasis differentially determine the progression of cTnT-related cardiomyopathies in murine models. Am J Physiol Heart Circ Physiol. 2009;297(2):H614–26. doi: 10.1152/ajpheart.01143.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasquale F, et al. Long-term outcomes in hypertrophic cardiomyopathy caused by mutations in the cardiac troponin T gene. Circ Cardiovasc Genet. 2012;5(1):10–7. doi: 10.1161/CIRCGENETICS.111.959973. [DOI] [PubMed] [Google Scholar]
- 50.Ferrantini C, et al. Pathogenesis of Hypertrophic Cardiomyopathy is Mutation Rather Than Disease Specific: A Comparison of the Cardiac Troponin T E163R and R92Q Mouse Models. J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.116.005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lombardi R, et al. Differential interactions of thin filament proteins in two cardiac troponin T mouse models of hypertrophic and dilated cardiomyopathies. Cardiovasc Res. 2008;79(1):109–17. doi: 10.1093/cvr/cvn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maass AH, et al. Hypertrophy, fibrosis, and sudden cardiac death in response to pathological stimuli in mice with mutations in cardiac troponin T. Circulation. 2004;110(15):2102–9. doi: 10.1161/01.CIR.0000144460.84795.E3. [DOI] [PubMed] [Google Scholar]
- 53.Tardiff JC, et al. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest. 1999;104(4):469–81. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watkins H, et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332(16):1058–64. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 55.Gimeno JR, et al. Hypertrophic cardiomyopathy. A study of the troponin-T gene in 127 Spanish families. Rev Esp Cardiol. 2009;62(12):1473–7. [PubMed] [Google Scholar]
- 56.Palmer BM, et al. Differential cross-bridge kinetics of FHC myosin mutations R403Q and R453C in heterozygous mouse myocardium. Am J Physiol Heart Circ Physiol. 2004;287(1):H91–9. doi: 10.1152/ajpheart.01015.2003. [DOI] [PubMed] [Google Scholar]
- 57.Redwood CS, Moolman-Smook JC, Watkins H. Properties of mutant contractile proteins that cause hypertrophic cardiomyopathy. Cardiovasc Res. 1999;44(1):20–36. doi: 10.1016/s0008-6363(99)00213-8. [DOI] [PubMed] [Google Scholar]
- 58.Spindler M, et al. Diastolic dysfunction and altered energetics in the alphaMHC403/+ mouse model of familial hypertrophic cardiomyopathy. J Clin Invest. 1998;101(8):1775–83. doi: 10.1172/JCI1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tyska MJ, et al. Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ Res. 2000;86(7):737–44. doi: 10.1161/01.res.86.7.737. [DOI] [PubMed] [Google Scholar]
- 60.Bos JM, Ackerman MJ. Z-disc genes in hypertrophic cardiomyopathy: stretching the cardiomyopathies? J Am Coll Cardiol. 2010;55(11):1136–8. doi: 10.1016/j.jacc.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Colan SD, et al. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115(6):773–81. doi: 10.1161/CIRCULATIONAHA.106.621185. [DOI] [PubMed] [Google Scholar]
- 62.Nugent AW, et al. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation. 2005;112(9):1332–8. doi: 10.1161/CIRCULATIONAHA.104.530303. [DOI] [PubMed] [Google Scholar]
- 63.Rupp S, et al. Genetic basis of hypertrophic cardiomyopathy in children. Clin Res Cardiol. 2018 doi: 10.1007/s00392-018-1354-8. [DOI] [PubMed] [Google Scholar]
- 64.Kaski JP, et al. Prevalence of sarcomere protein gene mutations in preadolescent children with hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2009;2(5):436–41. doi: 10.1161/CIRCGENETICS.108.821314. [DOI] [PubMed] [Google Scholar]
- 65.Michels M, et al. Disease penetrance and risk stratification for sudden cardiac death in asymptomatic hypertrophic cardiomyopathy mutation carriers. Eur Heart J. 2009;30(21):2593–8. doi: 10.1093/eurheartj/ehp306. [DOI] [PubMed] [Google Scholar]
- 66.Charron P, et al. Penetrance of familial hypertrophic cardiomyopathy. Genet Couns. 1997;8(2):107–14. [PubMed] [Google Scholar]
- 67.Olivotto I, et al. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46(3):480–7. doi: 10.1016/j.jacc.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 68.Geske JB, et al. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J. 2017;38(46):3434–3440. doi: 10.1093/eurheartj/ehx527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaufman BD, et al. RAAS gene polymorphisms influence progression of pediatric hypertrophic cardiomyopathy. Hum Genet. 2007;122(5):515–23. doi: 10.1007/s00439-007-0429-9. [DOI] [PubMed] [Google Scholar]
- 70.Perkins MJ, et al. Gene-specific modifying effects of pro-LVH polymorphisms involving the renin-angiotensin-aldosterone system among 389 unrelated patients with hypertrophic cardiomyopathy. Eur Heart J. 2005;26(22):2457–62. doi: 10.1093/eurheartj/ehi438. [DOI] [PubMed] [Google Scholar]
- 71.Ortlepp JR, et al. Genetic polymorphisms in the renin-angiotensin-aldosterone system associated with expression of left ventricular hypertrophy in hypertrophic cardiomyopathy: a study of five polymorphic genes in a family with a disease causing mutation in the myosin binding protein C gene. Heart. 2002;87(3):270–5. doi: 10.1136/heart.87.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou N, et al. Whole-exome sequencing identifies rare compound heterozygous mutations in the MYBPC3 gene associated with severe familial hypertrophic cardiomyopathy. Eur J Med Genet. 2018 doi: 10.1016/j.ejmg.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Marziliano N, et al. A case of compound mutations in the MYBPC3 gene associated with biventricular hypertrophy and neonatal death. Neonatology. 2012;102(4):254–8. doi: 10.1159/000339847. [DOI] [PubMed] [Google Scholar]
- 74.Maron BJ, Maron MS, Semsarian C. Double or compound sarcomere mutations in hypertrophic cardiomyopathy: a potential link to sudden death in the absence of conventional risk factors. Heart Rhythm. 2012;9(1):57–63. doi: 10.1016/j.hrthm.2011.08.009. [DOI] [PubMed] [Google Scholar]