Abstract
Objective:
To evaluate the impact of background parenchymal enhancement (BPE) on breast MRI and pathological tumor responses to neoadjuvant chemotherapy (NAC) in breast cancer patients.
Methods:
A panel of 372 MRI from 186 pathologically confirmed breast cancer patients who underwent breast MRI before and after NAC were selected. BPE was classified into four categories before and after NAC. The association between BPE and the pathological tumor response to NAC, recurrence-free survival (RFS) and molecular subtypes were analyzed. We also evaluated the associations between the baseline BPE before NAC and menopausal status or mammographic parenchymal density.
Results:
Baseline BPE did not differ significantly according to the pathological tumor response to NAC (p = 0.2019). However, changes in BPE after NAC were significantly greater in the pathological complete remission (pCR) group than in the non-pCR group (p = 0.0008). There was no statistically significant association between BPE and RFS or molecular subtypes. The baseline BPE of pre-menopausal females (2.77 ± 0.86) were greater than those of post-menopausal females (2.05 ± 0.69), with statistical significance (p < 0.0001). Baseline BPE showed no significant difference according to mammographic parenchymal density.
Conclusion:
The degree of BPE reduction in breast MRI correlates with the pathological tumor response to NAC in breast cancer patients. No significant difference in BPE was observed according to RFS or molecular subtypes of tumors.
Advances in knowledge:
This study suggests that the change in BPE may have potential as a biomarker of tumor response in breast cancer patients receiving NAC.
Introduction
Neoadjuvant chemotherapy (NAC) is increasingly used for the treatment of patients with locally advanced breast cancer, although a wide spectrum of systemic treatments, including target agents, are available.1–4 NAC enables large tumors to be downstaged and allows more patients to undergo breast-conserving surgery in place of mastectomy.5, 6 In vivo assessments of the efficacy of systemic treatment are also expected. However, only 10–20% of patients achieve a complete pathological response to NAC,7, 8 which can only be determined after the surgical removal of the tumor. Introducing a biomarker that is predictive of the tumor response to NAC prior to surgery may promote personalized treatment and improve patient outcomes.
Normal fibroglandular tissues of the female breast can show contrast enhancement on breast MRI, in particular, background parenchymal enhancement (BPE).9 BPE categories are classified as minimal, mild, moderate, or marked according to the Breast Imaging-Reporting and Data System (BI-RADS®) lexicon.10 The significance of the amount of BPE and its influence on diagnosis and treatment are still being studied. Park et al has shown that a strong (marked or moderate) BPE is a significant predictor for a positive resection margin as well as positive extensive intraductal component.11 In the study, cases with strong BPE were younger and showed a higher percentage of progesterone receptor-positive cancers than those with weak (mild or minimal) BPE. Other studies have demonstrated that higher BPE may be another factor of poor prognosis in patients with breast cancer.12
Few studies have investigated the influence of breast cancer treatment on the degree of BPE. The quantitatively measured reduction of BPE by NAC in the contralateral breast has been reported.13 An almost complete suppression of BPE due to taxane-containing NAC was observed in another study.14 It remains unclear whether BPE in breast MRI are associated with the breast tumor response to NAC. Thus, we analyzed the effects of NAC on BPE in a breast cancer cohort, as classified according to the BI-RADS® 2013 categories, and the relationship between changes in the BPE and the pathological tumor response.
Methods and Materials
Patient selection
This retrospective study was approved by the institutional review board of Asan Medical Center and the requirement to obtain informed consent was waived. Between January and December 2010, 261 consecutive patients with biopsy-proven invasive breast cancer received NAC before surgery at our institution. We assessed BPE in breast cancer patients who underwent breast MRI both before and after NAC. In total, 75 patients were excluded, because a breast MRI was not available from before (n = 10), after (n = 58), or both before and after (n = 7) NAC. Finally, a series of 372 MR images of 186 pathologically confirmed invasive breast cancer patients who underwent breast MRI before and after NAC was selected for this study. The mean age of these study cases at the time of their breast cancer diagnoses was 45 years (range, 25–81 years). To avoid any delays in treatment, breast MRIs were performed without regard to the menstrual cycle.
The NAC regimens of the 186 patients were as follows: adriamycin with cyclophosphamide plus docetaxel (n = 126); human epidermal growth factor receptor 2 (HER2) targeted agent-based regimens (n = 22); adriamycin with cyclophosphamide (n = 20); and 5-fluorouracil, epirubicin and cyclophosphamide (FEC) (n = 18). No patient received antihormonal or radiation therapy during NAC. Post-NAC MRI was performed after completion of four, six, or eight cycles of NAC.
Breast MRI protocol
Bilateral breast MRI was performed with the patients in the prone position using a 1.5 T (Magnetom Avanto; Siemens Healthcare, Erlangen, Germany) commercially available system with a dedicated four channel phased-array breast surface coil. MRI was performed with the following imaging protocols: an axial T2 weighted sequence (repetition time [TR]/echo time [TE], 1100/131 msec; flip angle, 125°; inversion time, 130 msec; matrix, 118 × 196; field of view [FOV], 341 × 210 mm2; matrix size, 256 × 416; acquisition time, 134 s; slice thickness, 1.5 mm without an interslice gap), axial T1 weighted STIR sequence (TR/TE, 4400/74 msec; inversion time, 130 msec; matrix, 118 × 196; FOV, 340 × 340 mm2; matrix size, 224 × 448; acquisition time, 134 s; slice thickness, 5 mm without an interslice gap) and a three-dimensional (3D) T1 weighted fast low-angle shot dynamic gradient-echo sequence with fat suppression (TR/TE, 5.0/2.4 msec; flip angle, 10°; slice thickness, 0.9 mm without an interslice gap; isotropic voxel, 0.9 × 0.9 × 0.9 mm3; one unenhanced and five contrast-enhanced image acquisitions with a temporal resolution of 61 s). An i.v. bolus injection of 0.1 mmol kg–1 gadoterate meglumine (Dotarem, Guerbet) or 0.2 ml kg–1 gadopentetate dimeglumine (Magnevist, Bayer Schering Pharma, Berlin, Germany) was administered using an MRI-compatible power injector (Spectris; Medrad, Pittsburgh, PA) with a flow rate of 2 ml s–1 followed by a 20 ml saline solution flush. An axial 3D delayed contrast-enhanced turbo spin-echo pulse sequence (TR/TE, 767/12 msec; FOV, 350 × 350 mm2; matrix size, 250 × 384; section thickness, 5 mm) was used to evaluate the axillary lymph nodes. Post-processing included standard subtraction (enhanced images minus non-enhanced images) for all of the dynamic phases and 3D maximum-intensity-projection images.
Clinicopathological data review
Clinicopathological data for the patients were reviewed, including the menopausal status, histological tumor type and molecular subtypes based on the immunohistochemical profile. The molecular subtypes of breast cancer were categorized into the following four groups: hormone receptor-positive and HER2-negative (luminal A subtype), hormone receptor-positive and HER2-positive (luminal B subtype), hormone receptor-negative and HER2-positive (HER2 subtype) and hormone receptor-negative and HER2-negative (triple-negative subtype).
The pathological tumor response to NAC was recorded for each patient. The pathological complete remission (pCR) following NAC was defined as the absence of residual invasive cancer cells in the breast and ipsilateral lymph nodes (ductal carcinoma in situ may have been present) (ypT0/is, ypN0). We also reviewed recurrence-free survival (RFS). Breast cancer recurrence included loco-regional recurrence (limited to the ipsilateral breast or chest wall and/or axillary, infraclavicular, or supraclavicular lymph nodes), contralateral breast recurrence and distant metastasis to other parts of the body. RFS was defined as the time from the date of curative surgery to the date of the first breast cancer recurrence. Patients without evidence of a recurrence were censored on the date of the most recent follow-up. The mean follow-up time was 56 months (range, 5–75 months).
Image analysis
All pre- and post-NAC MR images from the 186 patients included were retrospectively reviewed by two radiologists with 5 and 10 years of clinical experience. When there was a discrepancy between two radiologists, the final decision was made by consensus after discussion. BPE assessment was based on both the volume and intensity of enhancement by using a combination of first post-contrast sequences and maximum-intensity-projection images, which were created by subtracting the fat-suppressed pre-contrast T1 weighted image from the contrast-enhanced image. It was consistent across all the subjects. BPE was evaluated in the opposite breast with breast cancer. Qualitative assessments of BPE were recorded after reaching consensus. BPE patterns were classified into four categories (1 = minimal, 2 = mild, 3 = moderate, 4 = marked), according to the BI-RADS lexicon for breast MRI;10 these categories were assessed for pre-NAC and post-NAC MRI, respectively. Changes in BPE between baseline and post-NAC BPE were also recorded. The readers were blinded to the clinicopathological information, including the patient’s age, menopausal status, phase of the menstrual cycle, the pathological tumor response to NAC and pathological information, although they were aware that the patients each had a diagnosis of breast cancer and had received NAC. Mammographic parenchymal density of patients were also recorded by the same radiologists. The visually estimated mammographic breast density was determined for each patient based on the four categories of breast composition as described by the American College of Radiology BI-RADS.15
Statistical analysis
All statistical analyses were performed using SPSS v. 19.0 (IBM Corp., New York, NY) and p < 0.05 was considered to indicate statistical significance. The association between BPE and clinicopathological data including the pathological tumor response to NAC, recurrence and the molecular subtype of the tumor were analyzed using analysis of variance. We also evaluated the association among baseline BPE before NAC, menopausal status and mammographic parenchymal density. The data are expressed as means ± standard deviations. The log-rank test was used to compare the RFS of low (minimal and mild) vs high (moderate and marked) baseline BPE groups. Patients without recurrence were censored at the date of the most recent follow-up.
Results
Patients and tumor characteristics
Among the 186 breast cancer patients included in this study, the pathological tumor response after NAC was a pCR in 38 patients (20.4%) and non-pCR in 148 patients (79.6%). In all, 128 patients were pre-menopausal and the remaining 58 were post-menopausal. The mammographic parenchymal densities matched pattern a in 4 patients (2.2%), pattern b in 44 patients (23.7%), pattern c in 72 patients (38.7%) and pattern d in 66 patients (35.5%). According to immunohistochemical results, 82 cases were luminal A, 24 cases were luminal B, 32 cases were HER2-positive and 48 cases were triple-negative. There were 49 patients (26.3%) with recurrences during the follow-up period (range, 5–67 months), which included 19 loco-regional recurrences, 4 contralateral breast recurrences and 26 distant recurrences.
Association between BPE and clinicopathological data
The distribution of BPE at baseline and post-NAC MRI according to tumor response is indicated in Table 1. The baseline BPE did not differ significantly according to tumor response to NAC in terms of pCR and non-pCR groups (p = 0.2019). However, the post-NAC BPE was lower in the pCR group (1.21 ± 0.47) than in the non-pCR group (1.59 ± 0.62), with statistical significance (p = 0.0004, Table 1). The average decrease in BPE during NAC was 1.5 ± 1.13 in the pCR group (Figure 1) and 0.91 ± 0.9 in the non-pCR group (Figure 2), and the difference between groups was also significant (p = 0.0008).
Table 1.
Association between BPE and pathological tumor responses to NAC
| pCR (n = 38) |
Non-pCR (n = 148) | p value | |
| Baseline BPE | 2.71 ± 1.04 | 2.51 ± 0.83 | 0.2019 |
| Post-NAC BPE | 1.21 ± 0.14 | 1.59 ± 0.62 | 0.0004 |
| Change in BPE | 1.50 ± 1.13 | 0.91 ± 0.9 | 0.0008 |
BPE, background parenchymal enhancement; NAC, neoadjuvant chemotherapy; pCR, pathological complete remission.
Data are presented as a mean ± standard deviation.
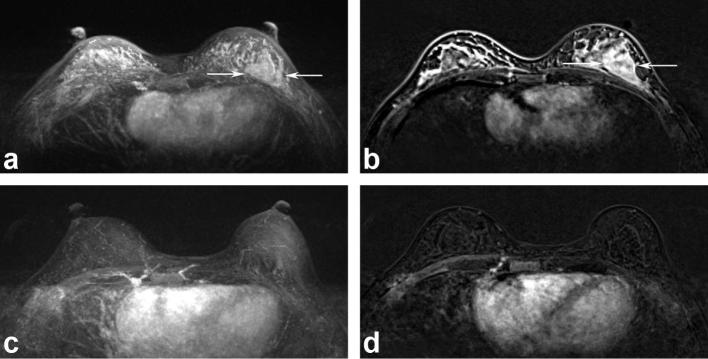
Figure 1.
MR images of a 38-year-old female with marked BPE before (a and b) and minimal BPE after (c and d) NAC. A tumor involving the left breast (arrows) had disappeared after NAC, and the BPE was significantly reduced. The histopathological tumor response was a complete remission. No recurrence was detected in a recent follow-up. BPE, background parenchymal enhancement; NAC, neoadjuvant chemotherapy.
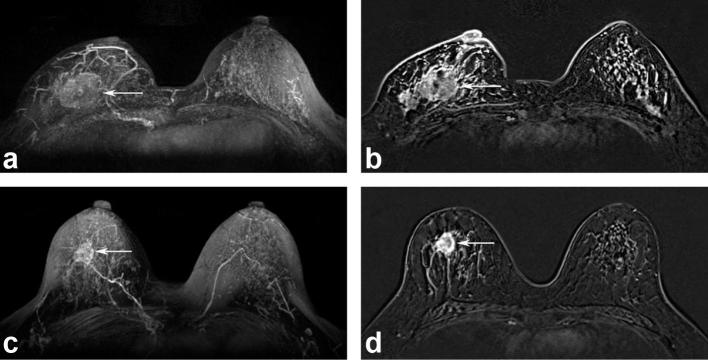
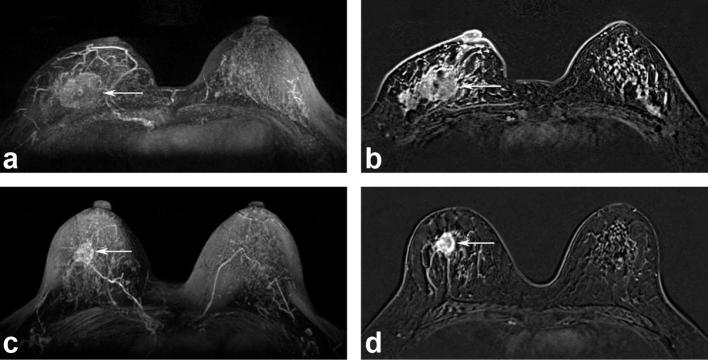
Figure 2.
MR images of a 46-year-old female with moderate BPE before (a and b) and after (c and d) NAC. A tumor involving the right breast (arrows) decreased in size; however, the BPE was almost unchanged after NAC. The histopathological tumor response was a partial response. After 28 months, locoregional recurrence occurred in the ipsilateral lymph nodes. BPE, background parenchymal enhancement; NAC, neoadjuvant chemotherapy.
Table 2 and Figure 3 show the relationship between BPE and recurrence or RFS. The mean baseline and post-NAC BPE and changes in BPE did not significantly differ in the recurrence-free and recurrence groups (Table 2). Patients with high (moderate and marked) baseline BPE had a poorer RFS than patients with low (minimal and mild) BPE, but these differences were not statistically significant (Figure 3a). There was no significant association between BPE and the molecular subtype of breast cancer (Table 3).
Table 2.
Association between BPE and recurrence
| Recurrence-free (n = 138) |
Recurrence (n = 49) |
p value | |
| Baseline BPE | 2.53 ± 0.87 | 2.59 ± 0.89 | 0.6871 |
| Post-NAC BPE | 1.49 ± 0.64 | 1.59 ± 0.57 | 0.3113 |
| Change in BPE | 1.04 ± 1.01 | 1 ± 0.89 | 0.7881 |
BPE, background parenchymal enhancement; NAC, neoadjuvant chemotherapy.
Figure 3.
Recurrence-free survival curves in patients who received NAC according to baseline (a) and post-NAC (b) BPE on breast MRI. The p values for baseline and post-NAC BPEs were 0.6788 and 0.5786, respectively, determined by the log-rank test [the blue line represents low (minimal and mild) BPE, and the red line represents high (moderate and marked) BPE]. BPE, background parenchymal enhancement; NAC, neoadjuvant chemotherapy.
Table 3.
Association between BPE and molecular subtypes
| Luminal A (n = 82) | Luminal B (n = 24) | HER2 (n = 32) | Triple-negative (n = 48) | p value | |
| Baseline BPE | 2.55 ± 0.85 | 2.42 ± 0.97 | 2.47 ± 0.92 | 2.67 ± 0.86 | 0.6445 |
| Post-NAC BPE | 1.55 ± 0.63 | 1.58 ± 0.5 | 1.41 ± 0.61 | 1.5 ± 0.62 | 0.6611 |
| Change in BPE | 1 ± 0.98 | 0.83 ± 1.02 | 1.06 ± 0.98 | 1.17 ± 0.93 | 0.5691 |
BPE, background parenchymal enhancement; NAC, neoadjuvant chemotherapy.
Table 4 presents the association among the baseline BPE, menopausal status and mammographic parenchymal density. The baseline BPE of pre-menopausal females (2.77 ± 0.86) was greater than those of post-menopausal females (2.05 ± 0.69), and the difference was significant (p < 0.0001). Mammographic parenchymal density did not show a significant association with baseline BPE (p = 0.0551).
Table 4.
Baseline BPE and menopausal status/mammographic densities
| Baseline BPEs | p value | ||
| Menopausal status | Pre | 2.77 ± 0.86 | <0.0001 |
| Post | 2.05 ± .069 | ||
| Mammographic density |
a | 2.25 ± 0.5 | 0.0551 |
| b | 2.25 ± 0.65 | ||
| c | 2.67 ± 0.95 | ||
| d | 2.64 ± 0.91 | ||
BPEs, background parenchymal enhancements.
Discussion
Similar to mammographic breast density, the level of BPE in MR imaging after contrast medium administration is a feature of normal breast tissue.9 This fibroglandular enhancement could affect the evaluation of breast lesions. Most previous studies have been concerned with breast BPE in diagnostic settings. One such study reported that the accuracy of MRI for evaluating the extent of a tumor was significantly better in cases with minimal or mild BPE (84%) than those with moderate or marked BPE (52%, p = 0.002).16
BPE and their relation to NAC outside the diagnostic setting have not been investigated in many studies to date. In our present study, we evaluated the relationship between BPE on a breast MRI and the pathological tumor response to NAC in breast cancer patients. We found that post-NAC BPE and average change in BPE after NAC were correlated with this pathological tumor response. Although the baseline BPE did not differ significantly according to the pathological tumor response (p = 0.2019), the post-NAC BPE was significantly lower in the pCR group than in the non-pCR group (p = 0.0004). The change in BPE after NAC was also significantly greater in the pCR group (1.5 ± 1.13) than in the non-pCR group (0.91 ± 0.9, p = 0.0008). Recognizing the pathological tumor response after NAC is important because it can predict long-term outcomes in breast cancer patients and therefore is a surrogate marker for survival. Our current study findings have suggested that BPE is associated with breast pathological tumor responses following NAC, as did a previous study by Predibsch et al17 and that the post-NAC BPE and the change in BPE may have potential as predictors of these pathological tumor responses.
It has been noted that high stromal signal enhancement on MRI is related to lower local recurrence and longer disease-free survival.18 This study was based on the concept that the normal-appearing breast stroma outside the tumor may have a role in tumor pathogenesis and treatment response. It was postulated that a high stromal signal enhancement may reflect high microvessel density resulting in greater delivery of the chemotherapeutic agents to the tumor, which is related to a good treatment response and a better clinical outcome.18 In contrast, Kim et al19 found that a higher parenchymal signal enhancement ratio on pre-operative MRI was a significant predictor for ipsilateral breast tumor recurrence in breast cancer patients following breast-conserving surgery. Choi et al20 also found that high BPE on pre-NAC MRI were significantly associated with a poorer RFS in an NAC setting. There is no agreement on local recurrence and RFS. In our current study also, there was no statistically significant association found between BPE and RFS or even the molecular subtypes of breast cancer.
In our present analysis, the baseline BPE was greater in pre-menopausal females than in post-menopausal females, with statistical significance (p < 0.0001). This result is consistent with that of Preibsch et al.17 Our current results suggested that ovarian function may be related to the BPE. Several studies looked at the association between BPE and menopausal status of women.10, 12 Significant difference in BPE was noted between post-menopausal and pre-menopausal females, and the menstrual cycle at the time of MRI examination can affect BPE pattern.21, 22 For females with strong hormone effects, normal fibroglandular enhancement can be fast and strong.12
Our study had some limitations of note. First, this was a retrospective study with a relatively small sample size and was conducted at a single institute. Therefore, further analyses might be needed in larger study population. Larger scale studies are also warranted to validate the applicability of our study results. Second, all of the patients we included were diagnosed with breast cancer and underwent NAC, and the timing of the MR examination could not be adjusted to the menstrual cycle, because of the risk of delay in treatment. Third, the assessment of the degree of BPE is subjective, and it might depend on the reader’s experience. Observer variability may be an issue, but we could not evaluate the intra-observer and inter-observer reproducibility because we made a decision by consensus. If a quantitative analysis were possible, it would be more objective.
In conclusion, the degree of BPE reduction on breast MRI correlates with the pathological tumor response to NAC in breast cancer patients. However, baseline BPE does not serve as a predictor of this pathological tumor response. Our current findings suggest that the change in BPE may have potential as a biomarker for pathological tumor response in breast cancer patients treated with NAC. Further research is needed to obtain concordant results on the relationship between BPE and survival in patients receiving NAC.
Contributor Information
Seon Jeong Oh, Email: hanna5sj@naver.com.
Eun Young Chae, Email: chaeey@amc.seoul.kr.
Joo Hee Cha, Email: jhcha@amc.seoul.kr.
Hee Jung Shin, Email: docshin@amc.seoul.kr.
Woo Jung Choi, Email: friendship02@hanmail.net.
Hak Hee Kim, Email: hhkim@amc.seoul.kr.
REFERENCES
- 1.Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, et al. . Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 2008; 26: 814–9. doi: 10.1200/JCO.2007.15.3510 [DOI] [PubMed] [Google Scholar]
- 2.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001; 2001: 96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469 [DOI] [PubMed] [Google Scholar]
- 3.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001; 19: 4224–37. doi: 10.1200/JCO.2001.19.22.4224 [DOI] [PubMed] [Google Scholar]
- 4.Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. . The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2003; 21: 4165–74. doi: 10.1200/JCO.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 5.Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol 2012; 30: 1747–9. doi: 10.1200/JCO.2011.41.3161 [DOI] [PubMed] [Google Scholar]
- 6.Ikeda T, Jinno H, Matsu A, Masamura S, Kitajima M. The role of neoadjuvant chemotherapy for breast cancer treatment. Breast Cancer 2002; 9: 8–14. doi: 10.1007/BF02967540 [DOI] [PubMed] [Google Scholar]
- 7.Giordano SH. Update on locally advanced breast cancer. Oncologist 2003; 8: 521–30. doi: 10.1634/theoncologist.8-6-521 [DOI] [PubMed] [Google Scholar]
- 8.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. . Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–804. doi: 10.1200/JCO.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 9.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011; 260: 50–60. doi: 10.1148/radiol.11102156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris EA, Comstock CE, Lee CH. ACR BI-RADS magnetic resonance imaging : ACR BI-RADS atlas, breast imaging reporting and data system. Reston, VA: The British Institute of Radiology.; 2013. [Google Scholar]
- 11.Park SY, Kang DK, Kim TH. Does background parenchymal enhancement on MRI affect the rate of positive resection margin in breast cancer patients? Br J Radiol 2015; 88: 20140638. doi: 10.1259/bjr.20140638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JY, Kim SH, Kim YJ, Kang BJ, An YY, Lee AW, et al. . Enhancement parameters on dynamic contrast enhanced breast MRI: do they correlate with prognostic factors and subtypes of breast cancers? Magn Reson Imaging 2015; 33: 72–80. doi: 10.1016/j.mri.2014.08.034 [DOI] [PubMed] [Google Scholar]
- 13.Chen JH, Yu HJ, Hsu C, Mehta RS, Carpenter PM, Su MY. Background parenchymal enhancement of the contralateral normal breast: association with tumor response in breast cancer patients receiving neoadjuvant chemotherapy. Transl Oncol 2015; 8: 204–9. doi: 10.1016/j.tranon.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrading S, Kuhl CK. Breast cancer: influence of taxanes on response assessment with dynamic contrast-enhanced MR imaging. Radiology 2015; 277: 687–96. doi: 10.1148/radiol.2015150006 [DOI] [PubMed] [Google Scholar]
- 15.Sickles EA, D’Orsi CJ, Bassett LW. ACR BI-RADS mammography : ACR BI-RADS atlas, breast imaging reporting and data system. Reston, VA: The British Institute of Radiology.; 2013. [Google Scholar]
- 16.Uematsu T, Kasami M, Watanabe J. Does the degree of background enhancement in breast MRI affect the detection and staging of breast cancer? Eur Radiol 2011; 21: 2261–7. doi: 10.1007/s00330-011-2175-6 [DOI] [PubMed] [Google Scholar]
- 17.Preibsch H, Wanner L, Bahrs SD, Wietek BM, Siegmann-Luz KC, Oberlecher E, et al. . Background parenchymal enhancement in breast MRI before and after neoadjuvant chemotherapy: correlation with tumour response. Eur Radiol 2016; 26: 1590–6. doi: 10.1007/s00330-015-4011-x [DOI] [PubMed] [Google Scholar]
- 18.Hattangadi J, Park C, Rembert J, Klifa C, Hwang J, Gibbs J, et al. . Breast stromal enhancement on MRI is associated with response to neoadjuvant chemotherapy. AJR Am J Roentgenol 2008; 190: 1630–6. doi: 10.2214/AJR.07.2533 [DOI] [PubMed] [Google Scholar]
- 19.Kim MY, Cho N, Koo HR, Yun BL, Bae MS, Chie EK, et al. . Predicting local recurrence following breast-conserving treatment: parenchymal signal enhancement ratio (SER) around the tumor on preoperative MRI. Acta Radiol 2013; 54: 731–8. doi: 10.1177/0284185113483676 [DOI] [PubMed] [Google Scholar]
- 20.Choi JS, Ko ES, Ko EY, Han BK, Nam SJ. Background parenchymal enhancement on preoperative magnetic resonance imaging: association with recurrence-free survival in breast cancer patients treated with neoadjuvant chemotherapy. Medicine 2016; 95: e3000. doi: 10.1097/MD.0000000000003000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baltzer PA, Dietzel M, Vag T, Burmeister H, Gajda M, Camara O, et al. . Clinical MR mammography: impact of hormonal status on background enhancement and diagnostic accuracy. Rofo 2011; 183: 441–7. doi: 10.1055/s-0029-1246072 [DOI] [PubMed] [Google Scholar]
- 22.Uematsu T, Kasami M, Watanabe J. Should breast MRI be performed with adjustment for the phase in patients’ menstrual cycle? Correlation between mammographic density, age, and background enhancement on breast MRI without adjusting for the phase in patients' menstrual cycle. Eur J Radiol 2012; 81: 1539–42. doi: 10.1016/j.ejrad.2011.04.059 [DOI] [PubMed] [Google Scholar]