Abstract
Objective:
To investigate metabolic parameters as predictive of local response after stereotactic body radiation therapy (SBRT) for liver-oligometastases.
Methods:
Inclusion criteria of the present retrospective study were: (a) liver oligometastases with controlled primary tumor; (b) absence of progressive disease ≥6 months; (c) metastases ≤ 3; (d) evaluation of SBRT-response by means of 18-fludeoxyglucose-PET/CT for at least two subsequent evaluations; (e) Karnofsky performance status >80; (f) life-expectancy >6 months. The following metabolic parameters were defined semi-quantitatively for each metastases: (1) standardized uptake value (SUVmax; (2) SUV-mean; (3) metabolic tumor volume (MTV), tumor volume with a SUV ≥3, threshold 40%; (4) total lesion glycolysis (TLG), i.e. the product of SUV-mean and MTV. Local control was defined as absence of recurrence in the field of irradiation.
Results:
41 liver metastases were analyzed. Pre-SBRT, median SUV-max was 8.7 (range, 4.5–23.59), median SUV-mean was 4.6 (range, 3–7.5), median MTV was 5.7 cc (range, 0.9–80.6) and median total lesion glycolysis was 24.1 (range, 3.6–601.5). At statistical analysis, metastases with SUV-mean >5 (p 0.04; odds ratio 4.75, sensitivity = 50%, specificity = 82.6%, area under the curve 0.66) and SUV-max >12 (p 0.02; odds ratio 5.03, sensitivity = 69%, specificity = 70%, area under the curve = 0.69) showed higher rates of infield-failure compared to the remaining lesions.
Conclusion:
According to current findings, pre-SBRT SUV-max and SUV-mean could be predictable of local response in liver oligometastases.
Advances in knowledge:
Present findings could support the hypothesis that fludeoxyglucose-PET/CT may be a powerful tool to predict tumor control. Specifically, current results might be helpful for clinicians in the decision-making process regarding liver oligometastatic patient selection as well as the individual therapy stratification distinguishing between slowly local progressing patients and rapidly progressing patients.
INTRODUCTION
In oligometastatic patients, the adoption of stereotactic body radiation therapy (SBRT) is worldwide increasing. In this setting of disease, the increased interest regarding SBRT has been substantially related to two main factors: (i) the hypothesis postulated by Weichselbaum and Hellman according to which during the metastatic phase an intermediate state exists; it is defined “oligometastatic” and it is characterized by a limited number of metastases for which local treatment could be curative; (ii) the reported efficacy and safety profile following metastases-directed SBRT.1–4 During these last years, the role of SBRT has been explored in several oligometastatic series, including liver oligometastatic patients, with different results in terms of midterm local control.5–8 In many cases, an individualized approach was advocated8 as well as a dose–response relationship has been was documented to guarantee high-rates of local control.3–6 Apart from the dose delivered, the heterogeneity reported in literature in terms of local efficacy could be attributable to other factors, such as the patient selection as well as the intrinsic metastases-responsiveness to high-dose per fraction. To date, it remains an investigational issue the identification of predictive factors of SBRT effectiveness in oligometastatic patients. Possible parameters of SBRT efficacy could help clinicians to complete the ongoing process of precision medicine in oligometastatic patients.
In the present analysis, we explored the role of 18F-fluorodeoxiglucose (FDG) metabolic parameters as predictive of LC response after SBRT for liver-oligometastases.
methods and MATERIALS
Study design
Inclusion criteria of the present retrospective study were: (a) liver oligometastases with controlled primary tumor; (b) absence of progressive disease ≥6 months; (c) number of metastases treated by SBRT ≤ 3; (d) staging by FDG-positron emission tomography integrated with CT (FDG-PET/CT) carried out at our Cancer Care Center; (e) evaluation of SBRT-response by means of FDG-PET/CT for at least two subsequent evaluations; (f) Karnofsky performance status >80; (g) life-expectancy >6 months.
Definition of the metabolic parameters
Pre-SBRT, FDG-PET/CT three-dimensional (3D) scans (i.e. without gating) were performed with thepatient within the same fixation devices to be used for treatment, whereas in the post-SBRT PET/CT 3D-scans no fixation device was adopted. The scans were performed with a Siemens Biograph mCT-S (64) system (Siemens, Knoxville, TN). Tomographic images were reconstructed by using the TrueX point spread function plus time of flight iterative reconstruction algorithm (3 iterations, 21 subsets, and a 5 mm full-width at half-maximum Gaussian filter) and analyzed with the Siemens SyngoTrueD 3D VOI isocontour tool (Siemens). PET acquisitions were started 60 min after administration of 2.96 MBq kg–1 of 18F; patients were enrolled if their blood glucose level was lower than 140 mg dl−1.
For the intent of the present study, the following FDG-metabolic parameters were retrospectively defined: (1) SUV-max [i.e. the highest uptake value over all pixels within the region of interest (ROI)]; (2) SUV-mean (i.e. the mean uptake value within the ROI); (3) metabolic tumor volume (MTV) (i.e. the total volume with an SUV of 3 or greater, threshold at 40%); (4) total lesion glycolysis (TLG) as an estimate of tumor metabolic rate (i.e. the product of SUV-mean and MTV). Both pre- and post-SBRT FDG-PET/CT data sets were analyzed semi-quantitatively with Syngo Multimodality Workplace software (Siemens AG, Erlangen, Germany) by two nuclear physicians who were blinded to all imaging studies and clinical and pathological results. For each metastasis, the irregular isocontour ROI was determined on the basis of a fixed threshold for the FDG-SUV (e.g. SUV >3).9 PET-CT SUV values were standardized according to the European Association for Nuclear Medicine procedure guidelines for tumor imaging, v. 2.0.10
SBRT procedures
Before SBRT, patients were staged with contrast CT-scan and PET/CT. A planning CT scan was acquired for all patients, who were positioned supine with their arms above the head; patients were immobilized by means of a thermoplastic body mask including a Styrofoam block for abdominal compression to minimize internal organ motion. Contrast-free and 3-phases contrast-enhanced were acquired in free-breathing mode at 3 mm slice thickness. Planning CT images were co-registeredwith three-phases contrast-enhanced CT and PET/CT to identify of the gross tumor volume (GTV). The clinical target volumewas defined as equal to the GTV. The planning target volume (PTV) was generated from clinical target volume by adding an overall margin of 7–10 mm in the craniocaudal axis and 4–6 mm in the anteroposterior and lateral axes.3
The median prescribed dose was 45 Gy (range, 30–60 Gy) delivered in a median of six fractions (range, 3–10) on consecutive working days. The normal liver dose constraint was set at more than 700 cm3 of normal liver receiving less than 15 Gy. Image-guided SBRT was performed by means of a megavoltage Cone beam CT before each daily session to minimize set up uncertainties. All plans were performed by Rapid Arc, v. 10.0.28 (Varian Inc., Palo Alto, CA) volumetric modulated arc therapy.
Evaluation of tumor response
Evaluation of tumor response was assessed by means of FDG-PET/CT11 and abdomen CT-scan or MRI both with contrast medium, within 3 months after SBRT and every 3 months, thereafter. Local failure was scored when there was evidence of tumor viability by either the SUV-max >6 or by the response evaluation criteria in solid tumour criteria of expansion of a solid mass with discrete borders within the treated planning target volume by 20% in the longest dimension relative to the most recent prior CT-scan.9
Statistical analysis
To summarize the most relevant features of the clinical variables, descriptive statistics were performed. All the categorical variables were analyzed with contingency tables with Fisher’s exact test or Pearson’s Χ2 test, whereas the continuous variables were analyzed by one-way analysis of variance, t-tests (with equal or unequal variance), or non-parametric Wilcoxon (Mann–Whitney) and Kruskal–Wallis tests. LC) was defined as the absence of local recurrence in field (in the prior irradiation field).
Logistic regression models were used to assess: (1) the impact of each clinical variables [biologically effective dose (BED), dose per fraction, type of primary tumor, tumor volume or GTV, maximum diameter of the metastasis] with LC and (2) pre-SBRT metabolic parameters (SUV-max, SUV-mean, MTV, and TLG considering pre- and post-SBRT values) with LC.
After each period of observation during follow up, metastases locally or distantly progressed were excluded from the statistical analysis due to the subsequently administration of systemic drugs influencing the endpoints of the present study.
The receiver operating characteristic (ROC) curves were used to assess the sensitivity and specificity of pre-SBRT metabolic parameters in correlation with the complete response of metastases after SBRT. The area under the curve (AUC) was used to verify the accuracy.In the case of a moderately accurate test (AUC ≥0.7), the product of maximum sensitivity and specificity was chosen as the cut-off value.
A p-value of 0.05 or less was considered statistically significant. Statistical analysis was performed with SAS, v. sion 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
According to inclusion criteria, 41 liver metastases for 30 patients were selected from our Institutional database and analyzed.
Post-SBRT, the median follow up was 16 months (range, 13–25months). The median interval between pre-SBRT PET/CT and the first fraction of SBRT was 3 days (range, 2–5 days). Pre-SBRT, median SUV-max was 8.74 (range, 4.49–23.59), median SUV-mean was 4.6 (range, 3–7.46), median MTV was 5.74 cm3 (range, 0.9–80.64 cm3) and median TLG was 24.1 (range, 3.6–601.5). The median prescribed dose was 45 Gy (range, 30–60 Gy) with median six fractions (range, 3–10). The median dose per fraction was 7.5 Gy (range, 5–20 Gy). The median BED estimated was 78.75 Gy (range, 48–180 Gy), where an α/β ratio equal to 10 was assumed for all the metastatic lesions. In Table 1, are summarized the metastases’ characteristics.
Table 1.
Summary of lesions’ characteristics treated with stereotactic body radiation therapy
| Lesions’ number | 41 |
| Primitive cancer (lesions’ number) | |
| Colorectal | 16 |
| Esophagus | 9 |
| Lung | 8 |
| Pancreas | 4 |
| Ovary | 3 |
| Breast | 1 |
| Mean diameter lesions (range) (cm) | 2 (1–6) |
| Mean volume lesions (range) (cm3) | |
| Clinical target volume | 6 (1–71) |
| Planning target volume | 25 (6–147) |
| Liver outside the planning target volumes receiving less than 15 Gy (range) (cm3) | 1117 (812–2129) |
Post-SBRT, the mean values of SUV-max varied as follows for all the lesions here are analyzed: at 3 months (in comparison with baseline) −48% of reduction (25th percentile −68%; 75th percentile −44%); at 6 months (in comparison with 3 months) −23% of reduction (25th percentile −22%; 75th percentile +38%); at 9 months (in comparison with 6 months) −10% of reduction (25th percentile −26%; 75th percentile 0%); at 12 months (in comparison with 9 months) −1.5% of reduction (25th percentile 0%; 75th percentile 0%).
The mean values of SUV-mean varied as follows: at 3 months (in comparison with baseline) −31% of reduction (25th percentile −44%; 75th percentile −21%); at 6 months (in comparison with 3 months) +15% (25th percentile 0%; 75th percentile +34%); at 9 months (in comparison with 6 months) −5% of reduction (25th percentile −3%; 75th percentile 0%); at 12 months (in comparison with 9 months) −1.6% of reduction (25th percentile 0%; 75th percentile 0%). In Table 2, the metastases’ metabolic responses during the period of observation are detailed. Lesions progressive in PET imaging also failed according to morphological measurements by abdomen CT-scan or MRI.
Table 2.
Metastases’ metabolic responses during follow up after stereotactic body radiotherapy
| Response evaluated by PERCIST criteria | 3 months (No. of metastases analyzed) | 6 months (No. of metastases analyzed) | 9 months (No. of metastases analyzed) | 12 months (No. of metastases analyzed) | 18 months (No. of metastases analyzed) |
| Complete response | 25 | 19 | 16 | 13 | 9 |
| Partial response | 12 | / | 2 | / | / |
| Stable disease | 3 | 13 | 12 | 4 | / |
| Progression of disease | 1 | 8 | 2 | 1 | / |
| Total metastases analyzed | 41 | 40 | 30 | 18 | 9 |
PERCIST, positron emission tomography response criteria in solid tumors.
Clinical-pathologic parameters and local control
No correlation was observed between pre-SBRT clinical-pathologic parameters and LC, as follow: maximum diameter [odds ratio (OR): 3.05; 95% CI: 0.8–11.1; p-value: 0.09)], GTV (OR: 1.85; 95% CI: 0.5–6.6; p-value: 0.33)], BED (OR: 0.39; 95% CI: 0.1–1.4; p-value: 0.15), type of primary tumor (OR: 0.35; 95% CI: 0.09–1.3; p-value: 0.12), dose per fraction (OR: 7.07; 95% CI: 0.7–70.1; p-value: 0.09).
Metabolic parameters predictive of local control
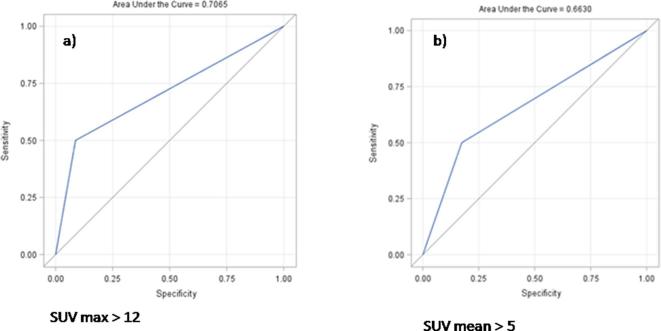
At univariate logistic regression analysis, only high values of SUV-max and SUV-mean pre-SBRT revealed to be statistically related to local failure. In fact, a statistical significance was observed for increasing values by SUV-max >9. A moderately accurate test (i.e. AUC ≥0.7) was recorded only in case of SUV-max >12 (p 0.008; OR10.5; sensitivity = 50%, specificity = 91%, AUC = 0.7). Regarding SUV-mean, a statistical significance was found for SUV-mean >5 (p 0.04; OR 4.7; sensitivity = 50%, specificity = 82.6%). The pre-planned accuracy of the test (i.e. AUC ≥ 0.7) was not reached for SUV-mean >5 (AUC = 0.66). In Figure 1, receiver operating characteristic curves considering SUV-max >12 and SUV-mean >5 are showed. Local disease free survival curves separated by SUV-max >12 and SUV-mean >5 are depicted in Figures 2 and 3.
Figure 1.
ROC curves considering SUV-max >12 (a) and SUV-mean >5 (b). ROC, receiver operating characteristic; SUV, standardized uptake value.
Figure 2.
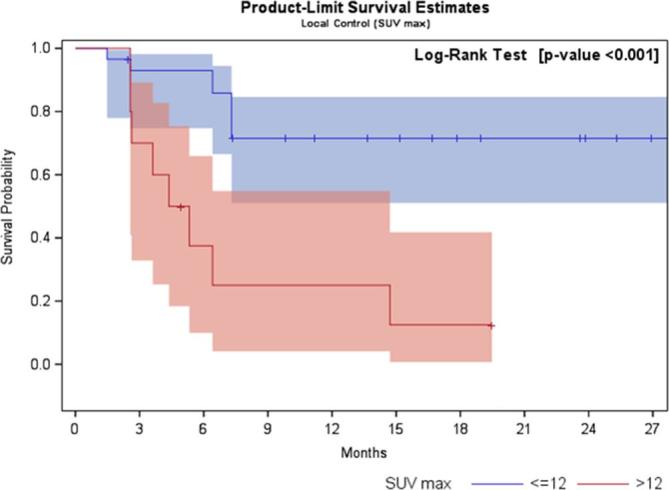
Local disease-free survival curve separated by SUV-max >12 and ≤12. SUV, standardized uptake value.
Figure 3.
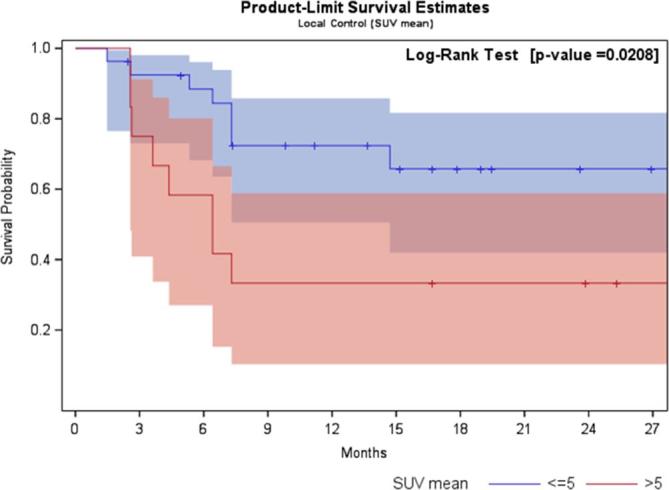
Local disease free survival curve separated by SUV-mean >5 and ≤5. SUV, standardized uptake value.
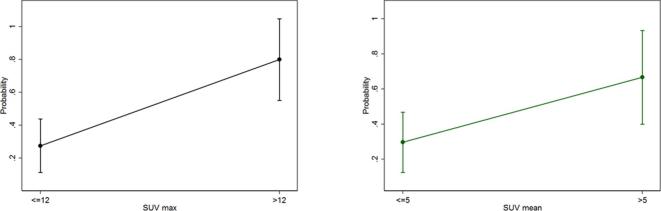
The estimated probabilities of local recurrence were 80% for SUV-max >12 and 66% for SUV-mean >5 (Figure 4). No statistical correlation was found between pre-SBRT, TLG and MTV values and LC.
Figure 4.
Predicted probability model of local recurrence for SUV-max >12 and ≤12 (curve on the left-side) and SUV-mean >5 and ≤5 (curve on the right-side). SUV, standardized uptake value.
Analyzing the rates of local failures during follow up, the higher local progression was registered at 6 months after SBRT (Table 2). Statistical analysis regarding metabolic parameters was furtherly made examining lesions response at this last time point (6 months post-SBRT). Findings regarding the impact of SUV-max and SUV-mean with local recurrence was similar to the previous analysis conducted considering all the population of study during the entire period of observation post-SBRT. In particular, a statistical significance and test’s accuracy was obtained for SUV-max >11 (p 0.03; OR 7.5; sensitivity = 71.4%, specificity = 75%, AUC = 0.73) and SUV-mean >5 (p 0.04; OR 4.7; sensitivity = 50%, specificity = 82.6%, AUC >0.73). Of contrast, evaluating liver metastases affected by local failure at 6 months post-SBRT, a more accurate test was recorded comparing to all the population of study during the entire period of follow up (AUC = 0.66 vs AUC = 0.73, respectively).
DISCUSSION
In the present study, we explored the role of semi-quantitative metabolic parameters to evaluate the SBRT-efficacy in liver oligometastases. Four semi-quantitative parameters by means of FDG-PET/CT were here detected; these variables were, therefore, analyzed with the intent to found a possible correlation with local response post-SBRT. The role of FDG-PET/CT is confined to disease staging, treatment planning of radiotherapy and response evaluation. During the last years, there has been a growing interest in regard to the semi-quantitative parameters by FDG-PET/CT for predicting prognosis and therapeutic response in several oncologic scenario, including oligometastases.12, 13
The major criticism is related to the possible confounding factor related to the inflammation following SBRT that could affect the reliability of the diagnostic performances, specifically in lung SBRT.14 Of contrast, in the setting of SBRT for liver lesions, evaluation of response could be limited using only response evaluation criteria in solid tumour criteria. Instead, functional imaging modality could allow a better evaluation of the liver treated by SBRT.15 Concerning the attitude of SUV changes after SBRT for liver metastases, Stinauer and colleagues found that SUV-max of controlled lesions decreased until a “nadir” value of 3.1. Of note, this last cut-off value is considered the median SUV-max of normal liver whereas a SUV-max ≥6 is consistent with local recurrence after SBRT for liver metastases.16 Thus, in the present population of study, we decided to adopt these last values to consider a metabolic response rather than a local failure post-SBRT. Interestingly, in our experience, high values of pre-SBRT SUV-max and SUV-mean revealed to be statistically related to local failure, irrespectively to BED, dose per fraction, type of primary tumor and tumor volume irradiated. These results were confirmed for all the population of study and for a specific subgroup of lesions presenting higher local failure at 6 months after SBRT comparing to the remaining metastases. Concerning the hypothetical impact of BED in tumor control, our results are in contrast to recent and far larger publications on SBRT in liver metastases.17, 18
These current findings could support the hypothesis that FDG-PET/CT may be a powerful tool to predict tumor control. Undoubtedly, present findings could be affected by several limitations including the retrospective nature, the limited sample size and the heterogeneity of the population of study that do not allow to draw conclusions.
Biologically, it is recognized that FDG-uptake represents the degree of glucose metabolism, which often refers to the aggressiveness of the cancer cells.19 Likewise, some authors tried to identify metabolic indicators to predict tumor control for patients affected by liver tumors. In the study by Huang et al20 PET-scores were significantly correlated with tumor control probability in patients affected by hepatocellular carcinoma treated by SBRT.
If standardized, present findings might be helpful for clinicians in the decision-making process regarding liver oligometastatic patient selection as well as the individual therapy stratification distinguishing between slowly local progressing patients and rapidly progressing patients. The exact therapeutic implication for intervention remains to be determined, and a possible suggestion could be the adoption as primary use of the systemic therapy or the metastasectomy in patients with high PET-SUVs. However, the possibility to alter the treatment-strategy in liver oligometastases based predominantly on PET-SUV values remains only an intriguing hypothesis that deserve further validations.
Footnotes
Conflicts of interest: We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no financial support for this work that could have influenced its outcome.
Contributor Information
Rosario Mazzola, Email: rosariomazzola@hotmail.it.
Sergio Fersino, Email: sefersin@sacrocuore.it.
Pierpaolo Alongi, Email: palong@libero.it.
Gioacchino Di Paola, Email: jacky@yahoo.it.
Fabiana Gregucci, Email: gregucc@yahoo.it.
Dario Aiello, Email: aiell@libero.it.
Umberto Tebano, Email: teban@libero.it.
Stefano Pasetto, Email: pasett@hotmail.it.
Ruggero Ruggieri, Email: ruggeror@libero.it.
Matteo Salgarello, Email: salgarello@sacrocuore.it.
Filippo Alongi, Email: filippoalongi@gmail.com.
REFERENCES
- 1.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011; 8: 378–82. doi: 10.1038/nrclinonc.2011.44 [DOI] [PubMed] [Google Scholar]
- 2.Rusthoven KE, Kavanagh BD, Burri SH, Chen C, Cardenes H, Chidel MA, et al. . Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009; 27: 1579–84. doi: 10.1200/JCO.2008.19.6386 [DOI] [PubMed] [Google Scholar]
- 3.Scorsetti M, Arcangeli S, Tozzi A, Comito T, Alongi F, Navarria P, et al. . Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys 2013; 86: 336–42. doi: 10.1016/j.ijrobp.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 4.Triggiani L, Alongi F, Buglione M, Detti B, Santoni R, Bruni A, et al. . Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer 2017; 116: 1520–5. doi: 10.1038/bjc.2017.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, et al. . Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 2010; 78: 486–93. doi: 10.1016/j.ijrobp.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 6.Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. . Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009; 27: 1572–8. doi: 10.1200/JCO.2008.19.6329 [DOI] [PubMed] [Google Scholar]
- 7.Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H, et al. . Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006; 45: 823–30. doi: 10.1080/02841860600904854 [DOI] [PubMed] [Google Scholar]
- 8.Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R, et al. . Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009; 27: 1585–91. doi: 10.1200/JCO.2008.20.0600 [DOI] [PubMed] [Google Scholar]
- 9.Stinauer MA, Diot Q, Westerly DC, Schefter TE, Kavanagh BD, et al. . Fluorodeoxyglucose positron emission tomography response and normal tissue regeneration after stereotactic body radiotherapy to liver metastases. Int J Radiat Oncol Biol Phys 2012; 83: e613–e618. doi: 10.1016/j.ijrobp.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 10.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. . FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015; 42: 328–54. doi: 10.1007/s00259-014-2961-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 Suppl 1: 122S–50. doi: 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. . Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: 441–6. doi: 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzola R, Fiorentino A, Di Paola G, Giaj Levra N, Ricchetti F, Fersino S, et al. . Stereotactic ablative radiation therapy for lung oligometastases: predictive parameters of early response by 18FDG-PET/CT. J Thorac Oncol 2017; 12: 547–55. doi: 10.1016/j.jtho.2016.11.2234 [DOI] [PubMed] [Google Scholar]
- 14.Huang K, Dahele M, Senan S, Guckenberger M, Rodrigues GB, Ward A, et al. . Radiographic changes after lung stereotactic ablative radiotherapy (SABR) can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012; 102: 335–42. doi: 10.1016/j.radonc.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 15.Tétreau R, Llacer C, Riou O, Deshayes E. Evaluation of response after SBRT for liver tumors. Rep Pract Oncol Radiother 2017; 22: 170–5. doi: 10.1016/j.rpor.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stinauer MA, Diot Q, Westerly DC, Schefter TE, Kavanagh BD. Fluorodeoxyglucose positron emission tomography response and normal tissue regeneration after stereotactic body radiotherapy to liver metastases. Int J Radiat Oncol Biol Phys 2012; 83: e613–e618. doi: 10.1016/j.ijrobp.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 17.Mahadevan A, Blanck O, Lanciano R, Peddada A, Sundararaman S, D’Ambrosio D, et al. . Stereotactic Body Radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch® Patient Registry. Radiat Oncol 2018; 13: 26. doi: 10.1186/s13014-018-0969-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andratschke NH, Nieder C, Heppt F, Molls M, Zimmermann F. Stereotactic radiation therapy for liver metastases: factors affecting local control and survival. Radiat Oncol 2015; 10: 69. doi: 10.1186/s13014-015-0369-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci 2016; 41: 211–8. doi: 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang WY, Kao CH, Huang WS, Chen CM, Chang LP, Lee MS, et al. . 18F-FDG PET and combined 18F-FDG-contrast CT parameters as predictors of tumor control for hepatocellular carcinoma after stereotactic ablative radiotherapy. J Nucl Med 2013; 54: 1710–6. doi: 10.2967/jnumed.112.119370 [DOI] [PubMed] [Google Scholar]