Abstract
Objective:
The purpose of this preliminary retrospective study was to analyse if cone beam CT (CBCT) is able to identify more bleeding sites and corresponding feeding arteries in patients with haemorrhage of uncertain origin.
Methods:
In 18 vascular territories (16 patients), pre-interventional CT angiography (CTA) and selective angiograms resulted in discordant information regarding the suspected bleeding site and hence received CBCT. Image data of CTA and selective angiograms in comparison to CBCT were independently reviewed by two interventional radiologists. Image quality, diagnostic confidence, number of bleeding sites and involved vascular territories were investigated. Additionally, the correlation between number of bleeding sites and involved vascular territories with a clinical gold-standard (super-selective angiographic findings and definitive clinical outcomes) was analysed.
Results:
Overall, subjective image quality did not significantly differ between investigated imaging modalities. However, CBCT significantly improved diagnostic confidence in both readers in detecting bleeding vessel (s) (p = 0.0024/0.0005; Reader 1/Reader2). High correlation coefficients regarding the number of bleeding sites (r = 0.9163/0.7692) in contrast to the number of involved vascular territories (r = 0.2888/0.0105) were observed for CTA in comparison to clinical gold-standard. In this context, CBCT demonstrated a very strong correlation for both parameters, the number of bleeding vessels (r = 0.9720/0.9721) and the number involved vascular territories (r = 0.9441/0.9441).
Conclusion:
In complex cases of suspected haemorrhage, CBCT images can aid the interventionalist in detecting bleeding sites as well as narrowing down the number of involved vascular territories and thereby identifying feeding arteries of the bleeding source.
Advances in knowledge:
(1) CBCT showed no improvement in image quality. However, in complex bleeding cases CBCT information might aid in treatment planning. (2) CBCT improves visualization of bleeding vessels and involved feeding arteries. (3) Particularly, less experienced interventionalists might benefit from the three-dimensional information gathered by CBCT.
INTRODUCTION
Since the first embolization of a bleeding gastric ulcer with an autologous blood clot in 1972, emergency transarterial embolization has become a pivotal treatment option in the setting of acute arterial bleeding.1, 2 In the last decades, considerable improvements of catheters as well as embolization agents made transarterial embolization of bleeding sites in virtually every vascular territory of the human body technically feasible.3–10 Immediate identification of the injured or affected vessel in a bleeding patient is crucial for fast control and effective treatment of the haemorrhagic source. Even with high quality pre-interventional CT angiography (CTA) images complex arterial branching territories often requires several angiographic acquisitions at different angles. These acquisitions are necessary to identify the injured artery and enable effective treatment decisions.11–13 In the context of haemorrhage of uncertain origin, defined as a bleeding site where the feeding artery cannot be exactly localized for selective catheterization, cone beam CT (CBCT) may be an effective diagnostic alternative. CBCT is capable of peri-interventional three-dimensional (3D) imaging in combination with high vascular contrast and high spatial resolution.14–17 In recent studies, CBCT demonstrated high diagnostic accuracy for the detection of tumour lesions and small feeding arteries to hepatic neoplasms for the guidance of transarterial chemoembolization (TACE).18, 19 CBCT might also be of help in treatment planning of patients with haemorrhage of uncertain origin. However, objective data assessing the benefit of using CBCT images in this setting are lacking.
Therefore, the aim of this study was to assess the clinical applicability, accuracy and image quality of CBCT-based cross-sectional images acquired during the work-up of patients with haemorrhage of uncertain origin, where CTA and selective regional angiography were unable to identify the exact source of haemorrhage.
methods and MATERIALS
Patient population
This retrospective HIPAA compliant study was approved by the local institutional ethics committee.
During a period of 6 months (Oct 2016 to Mar 2017), a total of 50 patients with acute and symptomatic bleeding were referred to interventional radiology for embolization. Inclusion criteria for the study were as follows:
Patients who are 18 years or older.
Major acute bleeding according to International Society on Thrombosis and Haemostasis criteria defined as symptomatic bleeding and/or bleeding causing a decrease in the haemoglobin level of at least 2 mg dl−1.20 In these patients conservative management has failed.
Decision to pursue endovascular embolization was made by a multidisciplinary team (including the referring clinician, interventional radiologist and anaesthesiologist)
Active bleeding on CTA was defined as uncontained contrast extravasation as opposed to pseudoaneurysm which was defined as focus of contained contrast extravasation or endoscopic/bronchoscopic proven active bleeding.
Maximum time interval of 1 h between CTA-examination and selective regional angiographic evaluation of the patient. This limited time interval increases the likelihood of an active bleeding detection. CBCT images were acquired immediately after the selective angiograms.
Due to radiation constraints and lack of clinical necessity, CBCT was not performed in every bleeding patient. CBCT images were only acquired in selected cases with the following criteria (Figure 1).
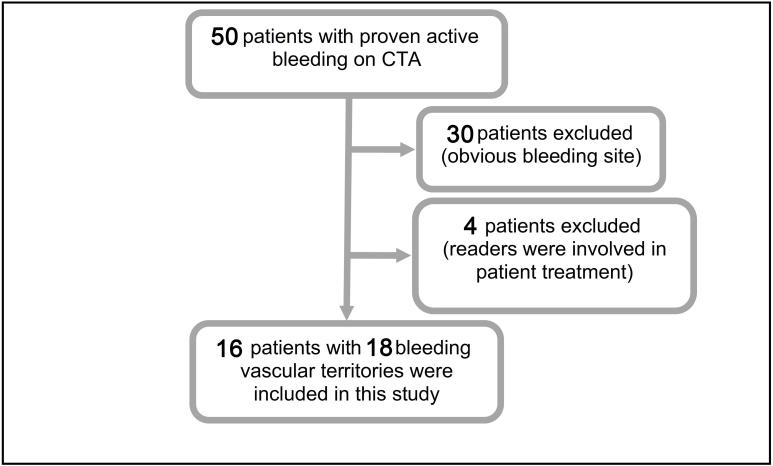
Figure 1.
During a 6-month time period, a total of 50 adult patients were angiographically examined. 34 patients were excluded: (I) straightforward embolization of bleeding source without need for CBCT; (II) treatment involvement of the study readers preventing to blind the readers. 16 patients with 18 bleeding vascular territories were included in this retrospective analysis. CBCT, cone beam CT; CTA, CT angiography.
Pre-interventional CTA imaging showed contrast medium extravasation and selective angiograms could not detect the suspected bleeding site, or
ases in which both imaging modalities were unable to delineate the localization of the feeding artery despite detection of contrast medium extravasation.
Hence, the patients undergoing CBCT were a selected group of challenging bleeding cases based on a realistic clinical scenario. In all cases, the decision whether or not to perform CBCT was finally made by the interventional radiologist in charge of the case. 30 patients were excluded due to identification of the bleeding site on CTA and selective angiographic series. 4 of the 50 subjects (8%) had to be excluded since the primary readers of this study (UG and GG) were directly involved in their care. Therefore, the final study cohort consisted of 16 patients with 18 involved vascular territories.
CT protocol
All patients included in this study underwent CTA examinations using the standard institutional blunt trauma protocol. Axial and coronal images were acquired using a third-generation CT system (Somatom Force, Siemens Medical Solutions, Forchheim, Germany) or a second-generation dual source CT scanner (Somatom Definition Flash, Siemens Medical Solutions, Forchheim, Germany). Enteric contrast material was not administered to ensure visualization of extravasation. Native (real native or virtual native in the case of dual source CT), arterial and portal-venous or late venous phase images were acquired. Body weight adjusted iodinated contrast material was administered (iodine concentration of 400 mg ml−1, Imeron 400®, Bracco, Milan, Italy) via a dual-syringe power injector (Medrad, Bayer, Germany). Scan delay for acquisition of arterial phase images was obtained by means of bolus tracking with positioning a region of interest at the level of the proximal abdominal aorta. Image acquisition was triggered at an attenuation threshold of 150 Hounsfield Unit. Scan delay for starting the portal-venous and late venous phase was 90and 180 s post-contrast injection.
Angiography and embolization procedure
Diagnostic angiography and endovascular intervention were performed using the robotic digital subtraction angiography system (Artis Zeego Q, VE 40 A, Siemens, Forchheim, Germany). Percutaneous common femoral artery access was achieved using a 19 G needle under local anaesthesia. This was followed by placement of a 4 Fr sheath (Terumo, Leuven, Belgium). Selective angiograms (N = 18; 2 frames per second) were performed using 4 Fr IMA, C2 or SIM1 configured catheters. 4 Fr pigtail catheters were used for aortography (N = 5; 3 frames per second). Depending on the selected arterial territory and the expected arterial blood flow 60% diluted contrast medium (Ultravist 370; Bayer Schering, Leverkusen, Germany) was injected by an automated power injector (Accutron-HP-D, Medtron, Saarbrücken, Germany). This was followed by a saline flush. The flow rates were set between 4 and 15 ml s−1. A total amount of up to 40 ml contrast medium was injected.
Whenever possible, the arms of the patients were placed above their head for CBCT acquisition to minimize streak artefacts over the area of interest. Depending on the selected arterial territory CBCT, images were acquired 1.5–2 s after the start of the administration of 50% diluted contrast medium. For selective angiography between 9 and 40 ml (depending on the blood flow of the selected territory) of Ultravist 370 contrast were administered at a rate of 2–10 ml s−1 for 8–9 s. The rotation time for CBCT (Siemens Dyna CT) was 6 s with the detector moving at 60° s−1. CBCT was acquired with breathholding technique. The source power was 90 kVp and the field of view was 48 cm with a voxel matrix of 512 × 512. The applied dose–area product (DAP) of the selective angiograms and the CBCT scan were noted.
In this context, it is important to note the definitions used in this paper:
A vascular territory was defined as an anatomic region supplied by a first-order arterial branch of the aorta, e.g. all arterial branches of the superior mesenteric artery.
All angiographic images acquired at different positions in the first-order arterial branch of the aorta were defined as selective (regional) angiography, for example selection of the superior mesenteric artery.
Angiographic images acquired in second- or third-order arterial branches were defined as super-selective angiography, e.g. jejunal arteries as branches of the superior mesenteric artery or proper hepatic artery as a branch of the celiac trunk.
Please note that in one patient case (Patient #1 demonstrated in Figure 2), an exception from this definition was made and angiography of the proximal gastroduodenal artery was defined as “selective angiography”. Angiography of the anterior/posterior pancreaticoduodenal arcade and right gastroepiploic artery were defined as “super-selective angiography” in this case.
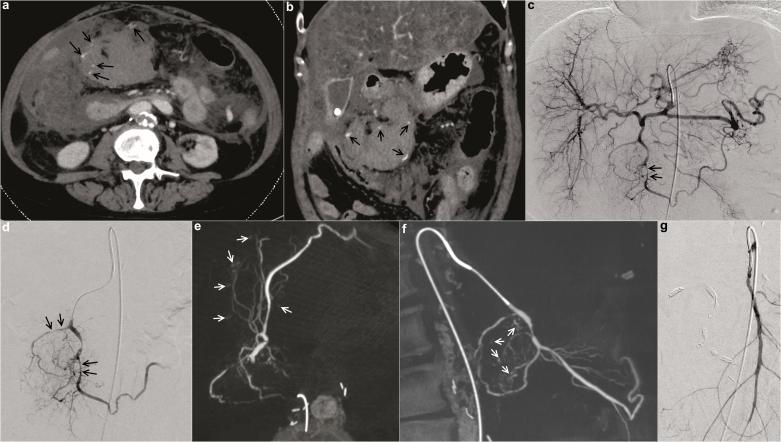
Figure 2.
Images of a hemodynamically unstable 63-year-old female patient with history of liver cirrhosis who presented with acute pancreatitis, hepatic failure and clinical concern for acute GI bleeding. In the emergency department, the patient had a decrease in haemoglobin of 2.4 mg dl−1 within 150 min. Pre-interventional CTA (a, b) demonstrated multiple bleeding sites (marked with arrows) suspected to originate from different feeding vessels of the celiac trunk and superior mesenteric artery. Selective angiograms [of the celiac trunk (c) and the proximal gastroduodenal artery (d)] could not clearly delineate the bleeding source. Gross vasospasm of gastroduodenal artery branches (marked with arrows) was seen. CBCT from the same catheter position as in (d) was performed. CBCT revealed all sites of active haemorrhage of the gastroduodenal artery branches precisely and involvement of the superior mesenteric artery could be excluded [axial (e) and sagittal MIP reconstructions (f); bleeding sites marked by arrows]. Selective and super-selective angiography of the superior mesenteric artery confirmed lack of involvement (g). The patient was treated successfully with super-selective pushable coil embolization of the major bleeding sites and coil embolization of the proximal gastroduodenal artery. CBCT, cone beam CT; CTA, CT angiography; GI, gastrointestinal.
Super-selective angiography of the bleeding source vessel was performed using a coaxially passed 2.7 Fr microcatheter (Progreat, Terumo, Leuven, Belgium). Embolic agents consisted of pushable coils (N = 12) or combinations of different agents (N = 3). These different agents included pushable coils, polyvinyl alcohol particles (size 500–700 µm) and n-Butyl cyanoacrylate (histoacryl) mixed with lipiodol. Selection of the type of embolic agent in the patients was dependent on bleeding rate, vessel size and preference of the interventional radiologist in charge of the case.
Image data set evaluation
The aim of the subjective image evaluation on a dedicated picture archiving andcommunication system workstation (Centricity® Radiology RA1000, GE Healthcare, Barrington, IL, USA) was to assess image quality, diagnostic confidence, number of detected bleeding sites and sum of involved vascular territories. An ordinal 5-point Likert scale was used to grade overall subjective image quality and diagnostic confidence. With regard to image quality, readers were asked to evaluate artefacts, vessel contrast and delineation of the bleeding site with the following scale: 1 = excellent; 2 = good; 3 = fair; 4 = below average; 5 = poor. With regard to diagnostic confidence, the scale was as follows: 1 = very high confidence to localize the feeding artery of the detected contrast medium extravasation; 2 = high confidence; 3 = moderate confidence; 4 = low confidence; 5 = very low confidence.21
In a first reading session, two board-certified interventional radiologists independently and retrospectively reviewed CT images and selective angiograms. The readers were blinded to the patient cases. All deidentified images were assessed independently. One reader (GG) was a board-certified interventional radiologist with more than 5/3 years of experience in cross-sectional imaging/interventional radiology. The other reader (UG) was a board-certified interventional radiologist with more than 5/1 year(s) of experience in cross-sectional imaging/interventional radiology. To prevent recall bias in a second reading session 4 weeks after the first reading session, CBCT images in axial and coronal reconstructions were independently reviewed. “Additional findings” in addition to the above-mentioned criteria were recorded. Please note that in the above-mentioned four cases with aortograms and first-order selective angiograms, CBCT images were acquired in the aortography position.
Due to the possibility of intermittent bleeding, both readers critically reviewed clinical outcomes as well. Clinical outcome was assessed based on haemodynamic stabilization of the patient in the following 14 days post-procedure and based on follow-up CTA before discharge. Consensus reading of all acquired images and particularly the super-selective angiograms was done with two additional experienced board-certified interventional radiologists (RS and DK) who were primarily involved in the treatment of all included patients in this study. This was done to achieve the highest possible accurate interpretation of the acquired super-selective angiograms, which served in combination with clinical/operative outcomes as a “gold standard” endpoint.
Statistical analysis
JMP 9.0.0 (SAS Institute Inc., Vary, NC) as well as MedCalc (v. 13.0, MedCalc Software, Mariakerke, Belgium) was used for statistical analysis. Median values and interquartile range were calculated for image quality and diagnostic confidence. Image quality and diagnostic confidence of the 18 vascular territories were investigated based on modality (CTA, selective angiograms and CBCT) using the non-parametric Friedman test, followed by Bonferroni–Dunn's post hoc test. Due to the use of the Friedman test all 18 involved vascular territories were separately analysed. Mean cumulative DAPs of selective angiograms and CBCT data was tested for a significant difference using a non-parametric Wilcoxon signed-rank test. Due to multiple testing, Bonferroni corrected p-values are listed below and a corrected p-value of less than 0.05 was considered statistically significant.
Weighted κ was used to assess inter-reader agreement of image quality and diagnostic confidence scoring between the two readers.
Number of bleeding sites as well as number of involved vascular territories detected in CTA, angiographic and CBCT images were correlated with the “clinical gold standard” (as described above) using Spearman rank correlation coefficients. Spearman correlations are interpreted as follows: |r| > 0.90= very strong correlation; 0.6< |r| < 0.9= strong correlation; 0.4 < |r| < 0.6= moderate correlation; |r| < 0.4= weak correlation.
RESULTS
During a period of 6 months (10/2016 to 03/2017), a total of 50 patients with acute bleeding were referred to the interventional radiology service. 30 cases (60 %) presented with obvious bleeding sites on CTA images and selective angiograms. These patients were not in need of a CBCT study. In addition, four patients who received a CBCT examination were excluded due to the direct involvement of the two study readers (UG and GG) in their clinical care. Therefore, the patient cohort of this study consisted of 16 patients (32% of all patients; 6 female patients; age 61.6 ± 12.8 years; BMI 23.6 ± 4.0 kg m−2) with investigation of a total of 18 vascular territories applying CBCT.
Image quality did not differ significantly between CTA and CBCT (p = 0.1699 (Reader 1), p = 0.5001 (Reader 2). It is worth noting CTA image quality was rated relatively high compared to selective angiograms or CBCT data sets. In three cases, both readers noted a severe deterioration of image quality of the selective angiograms (even with pixel-shift and remasking as digital subtraction angiography post-processing techniques) and CBCT images due to motion artefacts. Two of these three cases were related to bowel movement artefacts and the third case revealed breathing artefacts. Inter-reader agreement on image quality was good for CTA (weighted κ= 0.804 ± 0.101) and moderate for selective angiography (weighted κ = 0.627 ± 0.127) as well as for CBCT (weighted κ = 0.491 ± 0.171).
Diagnostic confidence in detecting the precise location of bleeding vessels was significantly improved for CBCT in comparison to selective angiogram and/or CTA. (Table 1; p = 0.0024 for Reader 1 and p = 0.0005 for Reader 2).
Table 1.
Evaluation for statistical significant differences (N = 18 vascular territories) regarding image quality (using an ordinal 5-point Likert scale with 1 being “excellent image quality”) and diagnostic confidence (using an ordinal 5-point Likert scale with 1 being “very high confidence of detecting the bleeding site”) for both readers were analysed with the non-parametric Friedman test (Median values with interquartile range in brackets as well as Bonferroni corrected p-values are stated and significant values are marked with an asterisk)
| Reader 1 | Reader 2 | |||||
| CTA | Selective regional angiogram | CBCT | CTA | Selective regional angiogram | CBCT | |
| Image quality | 1.0 (1.0–2.0) | 2.0 (1.8–4.0) | 2.0 (1.8–2.0) | 1.0 (1.0–2.0) | 2.0 (2.0–3.3) | 2.0 (2.0–3.0) |
| p = 0.1699 | p = 0.5001 | |||||
| Diagnostic confidence | 3.0 (3.0–4.3) | 4.0 (2.8–5.0) | 1.5 (1.0–3.3) | 3.0 (2.0–3.0) | 5.0 (3.5–5.0) | 2.0 (1.8–3.0) |
| p = 0.0024* | p = 0.0005* | |||||
CBCT, cone beam CT; CTA, CT angiography.
For the less experienced Reader 1, pairwise post hoc tests revealed a statistically significant increase of diagnostic confidence for CBCT compared to CTA (p = 0.034) and for CBCT compared to selective regional angiograms (p = 0.033) . There was no statistical significant difference for CTA vs selective angiograms in Reader 1 (p = 1.000). For the more experienced Reader 2, post hoc analyses found a statistical significant difference for CBCT compared to selective angiograms (p = 0.006) but not for CBCT compared to CTA (p = 1.000) and CTA vs selective angiograms (p = 0.095). Inter-reader agreement of diagnostic confidence was moderate for CTA (weighted κ = 0.400±0.132), moderate for selective angiography (weighted κ = 0.513 ± 0.138) and good for CBCT (weighted κ = 0.632 ± 0.143).
The sum of all bleeding sites and involved vascular territories assessed by CTA, selective angiography and CBCT for each patient is listed in Tables 2 and 3. Regarding the number of bleeding sites, Spearman rank correlation analysis demonstrates a strong to very strong relationship between the clinical gold standard and CTA (r = 0.7692 in Reader 1 and r = 0.9163 in Reader 2) as well as between the clinical gold-standard and CBCT (r = 0.9720 in Reader 1 and r = 0.9721 in Reader 2). Only a weak to moderate correlation could be found between the clinical gold-standard and selective angiograms (r = 0.3623 in Reader 1 and r = 0.4932 in Reader 2). In four cases (Reviewer 1 and 2), CBCT images enabled a visualization of an active bleeding source despite prior negative selective angiograms, which was later confirmed with super-selective angiography.
Table 2.
Bleeding site localization, aetiology (including referring department) and bleeding site characteristics (number of bleeding sites and involved vascular territories; if no bleeding sites could be detected vascular territories are marked with a dash) with CTA, selective regional angiograms, CBCT as well as super-selective angiograms (defined as “gold-standard” in conjunction with clinical outcome) per patient for the less experienced Reader 1and more experienced Reader 2 (separated by a forward slash) are given please note that the bleeding in Patient 8 was self-limited (no imaging modality detected a contrast medium extravasation) but multiple vessels with aneurysms could be detected by using CBCT. These pathological vessels could be confirmed intraoperatively 3 days after imaging
| Bleeding site characteristics (less experienced Reader 1/more experienced reader 2) | ||||||||||
| Patient | Bleeding location | Aetiology (referring department) |
CTA | Selective regional angiograms | CBCT | Super-selective angiograms and clinical/operative outcome | ||||
| Bleeding sites | Vascular territories | Bleeding sites | Vascular territories | Bleeding sites | Vascular territories | Bleeding sites | Vascular territories | |||
| 1 | Upper abdomen | Spontaneous (emergency) | 6/5 | 2/2 | 0/0 | –/– | 5/4 | 1/1 | 7 | 1 |
| 2 | Kidney | Iatrogenic—reperfusion (internal medicine) | 1/2 | 1/2 | 1/1 | 1/1 | 2/2 | 2/2 | 3 | 3 |
| 3 | Liver | Spontaneous (emergency) | 3/2 | 1/1 | 0/0 | –/– | 2/2 | 1/1 | 3 | 1 |
| 4 | Jejunum (first intervention) | Tumour (internal medicine) | 1/1 | 1/1 | 0/0 | –/– | 0/0 | -/- | 0 | – |
| Jejunum (second intervention) | Tumour (internal medicine) | 1/1 | 1/1 | 0/0 | –/– | 1/1 | 1/1 | 1 | 1 | |
| 5 | Duodenum | Tumour (internal medicine) | 0/0 | –/– | 0/0 | –/– | 0/0 | -/- | 0 | – |
| 6 | Pelvis (left) |
Iatrogenic—post-operative (urology) | 1/1 | 1/3 | 1/0 | 1/– | 2/2 | 1/1 | 2 | 2 |
| Pelvis (right) | Iatrogenic—post-operative (urology) | 1/2 | 1/3 | 1/2 | 1/1 | 2/3 | 2/2 | 3 | 2 | |
| 7 | Upper abdomen | Spontaneous (emergency) | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1 | 1 |
| 8 | Thorax | Post-infectious (emergency) | 0/0 | –/– | 0/0 | –/– | 0/0 | 10/13 | 0 | Approx. 50 (intraoperative confirmed) |
| 9 | Thorax | Tumour (emergency) | 3/2 | 1/2 | 1/1 | 1/1 | 5/4 | 4/3 | 6 | 3 |
| 10 | Pelvis | Iatrogenic—post-operative (urology) | 1/2 | 2/2 | 1/1 | 1/1 | 2/2 | 1/1 | 3 | 1 |
| 11 | Pelvis | Iatrogenic—post-operative (urology | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1 | 1 |
| 12 | Pelvis | Spontaneous (orthopaedics) | 1/1 | 1/1 | 0/1 | –/1 | 1/1 | 1/1 | 1 | 1 |
| 13 | Upper abdomen | Spontaneous (internal medicine) | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1 | 1 |
| 14 | Upper abdomen | Spontaneous (surgery) | 1/1 | 1/1 | 1/1 | 1/1 | 2/2 | 2/2 | 2 | 2 |
| 15 | Upper abdomen | Spontaneous (surgery) | 1/1 | 1/1 | 1/2 | 1/2 | 2/2 | 2/2 | 2 | 2 |
| 16 | Kidney | Traumatic (emergency) | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1 | 1 |
CTA, CT angiography; CBCT, cone beam CT.
Please note that the bleeding in Patient 8 was self-limited (no imaging modality detected a contrast medium extravasation) but multiple vessels with aneurysms could be detected by using CBCT. These pathological vessels could be confirmed intraoperatively 3 days after imaging.
Table 3.
Patient characteristics (sex, age and BMI, region of suspected bleeding, amount of angiographic series as well as catheter positions of sequential acquired selective regional angiograms (number of acquisitions per catheter position are given in brackets) and cumulative DAPs for selective regional angiograms (including amount of acquired series) are shown
| Selective regional angiograms | CBCT | |||||||
| Patient | Sex. age (year) | BMI (kg m−²) | Region | Amount of angiographic series | Catheter position during contrast media injection | Cumulative dose–area product (µGym²) | Catheter position during contrast media injection | Cumulative dose–area product (µGym²) |
| 1 | W.63 | 31.1 | CT/SMA | 3 | CT, AGD (2x) | 4309.6 | AGD | 5549.0 |
| 2 | W.47 | 19.6 | RA | 1 | RA | 897.68 | RA | 2975.7 |
| 3 | W.80 | 21.6 | CT | 1 | CT | 715.93 | CT | 5174.4 |
| 4 | M.73 | 22.7 | CT/SMA | 4 | CT, SMA (3x) | 5241.5 | SMA | 2135.3 |
| M.73 | 22.7 | CT/SMA | 5 | SMA (5x) | 5026.3 | SMA | 2733.2 | |
| 5 | M.57 | 25.3 | CT/SMA | 2 | CT (2x) | 2232.2 | CT | 6594.6 |
| 6 | M.59 | 19.0 | HA (left) | 1 | HA | 1818.3 | HA (left) | 4343.7 |
| M.59 | 19.0 | HA (right) | 3 | HA | 2044.1 | HA (right) | 3786.2 | |
| 7 | W.69 | 27.3 | CT/SMA | 5 | SMA (2x), CT (3x) | 12422.9 | CT | 7215.3 |
| 8 | M.31 | 28.3 | TA & IA | 2 | TA, IA | 4332.3 | TA | 4779.7 |
| 9 | M.59 | 25.0 | TA & IA | 2 | TA, IA | 4646.3 | TA | 3549.8 |
| 10 | M.73 | 17.6 | AA & HA | 4 | AA, HA (3x) | 4336.1 | AA | 4131.5 |
| 11 | M.80 | 22.2 | AA & HA | 5 | AA, HA (4x) | 2750,26 | AA | 3049.7 |
| 12 | M.62 | 30.0 | AA & HA | 3 | AA, HA (2x) | 2135.9 | AA | 7448.9 |
| 13 | W.57 | 19.5 | CT/SMA | 3 | SMA, CT (2x) | 1064.0 | CT | 2856.4 |
| 14 | W.64 | 27.7 | CT/SMA | 1 | CT | 2079.0 | CT | 5297.4 |
| 15 | M.63 | 24.7 | CT/SMA | 2 | SMA, CT | 1802.0 | CT | 5307.0 |
| 16 | M.41 | 21.6 | RA | 2 | RA (2x) | 9122.0 | RA | 7226.0 |
AA, abdominal aortography; BMI, body mass index; CBCT, cone beam CT; CT, coeliac trunk; CTA, CTangiography; DAP, dose–area product ; SMA, superior mesenteric artery; RA, renal artery; AGD, gastroduodenal artery; HA, hypogastric artery; TA, thoracic aortography; IA, intercostal artery.
Evaluating the number of involved vascular territories, Spearman rank correlation analysis demonstrated only a weak correlation between the clinical gold-standard and CTA (r = 0.0105 in Reader 1 and r = 0.2888 in Reader 2) as well as between the clinical gold-standard and selective angiograms (r = 0.4415 in Reader 1 and r = 0.2521 in Reader 2). CBCT and the clinical gold-standard showed a very strong correlation (r = 0.9441 in Reader 1 and r = 0.9441 in Reader 2).
The investigated imaging modalities did not differ in their ability to characterize bleeding sites (pseudoaneurysm or active extravasation). The mean radiation dose for CBCT was higher than that of selective angiograms (4675 vs 3721 µGym²), albeit not being statistically significant (p-value = 0.2321).
DISCUSSION
Depending on the vascular territory, its arterial branching pattern and the extent of vascular injury, angiographic detection of bleeding vessels can be time-consuming and challenging.22 Sequential selective regional angiographic image acquisitions from different angles are frequently necessary to delineate the culprit bleeding vessel. Within this context, CBCT images are supposed to aid in treatment planning due to 3D real-time imaging capabilities, high spatial resolution and strong vascular contrast (exemplary shown in Figure 3). It needs to be emphasized that CBCT is not necessary in every patient with haemorrhage undergoing angiography. CBCT may be beneficial for a subset of patients with contradicting or inconclusive pre-interventional CTA and selective angiography. In these cases, CBCT may help to identify and confirm the bleeding vessel, thereby turning empiric into targeted embolization.
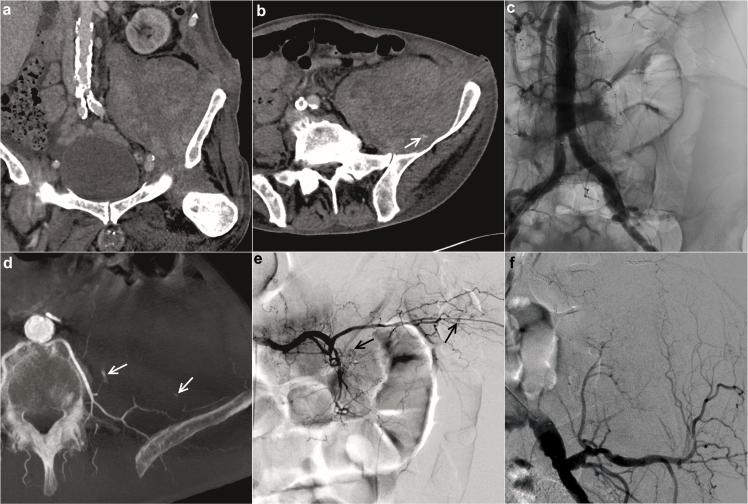
Figure 3.
In another representative case, a 73-year-old male patient with chronic myelomonocytic leukaemia presented with bleeding complications after bone marrow biopsy. A decrease in haemoglobin of 2.0 mg dl−1 within 120 min was detected. Pre-interventional CTA (a. b) demonstrated a huge haematoma near the left psoas muscle and barely detectable bleeding source (arrow in b). The bleeding source appeared to originate from an uncertain vascular territory, potentially lumbar or iliolumbar artery. Aortography (c) and selective internal iliac angiography could not delineate signs of haemorrhage. CBCT acquired from the aortography position revealed two sites of active haemorrhage (arrows in d) from the left L5 lumbar artery without involvement of the iliolumbar artery. These findings with an additional third minor bleeding site could be confirmed by super-selective angiography of the left L5 lumbar artery (e; bleeding sites marked by arrows) and the left iliolumbar artery (f). The patient was treated successfully with super-selective pushable coil embolization of the left L5 lumbar artery. CBCT, cone beam CT; CTA, CT angiography.
In interventional transarterial liver tumour therapy, CBCT imaging has been increasingly utilized. The technique has been shown to facilitate the localization of smaller tumour lesions and the identification of small arterial feeders.18 In the setting of acute bleedings, limited data exists for evaluating intraprocedural feasibility and value of CBCT imaging. A neurointerventional case report described a ruptured anterior communicating artery aneurysm in which the exact site of rupture was identified by CBCT.23 In accordance with this paper, Patil et al reported a case of a 67-year-old female with an angiographically occult bleeding of a splenic pseudoaneurysm. The pseudoaneurysm could only be detected and successfully embolized based on intraprocedural CBCT.24 Both case report papers concluded that CBCT angiography has the ability to characterize vascular pathology precisely, thereby serving as a problem-solving tool in complex cases of suspected bleeding. CBCT may be particularly beneficial when the standard options of CTA and selective angiography are inconclusive. New software developments using 3D CBCT data enable automatic detection of tumour feeding arteries in the liver. These new solutions are under investigation and may facilitate angiographic catheter navigation in feeding vessels.25, 26 Regarding the use of this advanced software techniques during the embolization of injured vessels, Iwazawa et al published their experience with CBCT in combination with vessel detection software during the endovascular treatment of visceral arterial bleeding in five patients. The authors concluded that their technique can be used safely in the hands of a trained operator.27 Carrafiello et al recently investigated the utility of an automatic vessel detection software using CBCT data sets in 20 patients with arterial bleeding and reported a very high detection accuracy of the used software (95% technical success).28 Miyayama et al embolized ruptured pancreaticoduodenal artery aneurysms of two patients with an automatic vessel detection software using CBCT data sets. In this paper, it was concluded that this novel software based technology significantly reduces procedure time.29
In the presented study, both readers could not detect a statistical significant difference in image quality between the investigated imaging modalities. Even though in three cases, severe deterioration of image quality was observed due to motion artefacts (bowel movement and breathing) affecting angiography and CBCT, but not CTA. In a critically ill patient cohort, this is not an unexpected finding since a fast examination, such as CTA is less likely affected by motion artefacts compared to dynamic angiography and CBCT. The relatively high image quality and decreased artefact-vulnerability of the imaging modality CTA are likely both responsible for the good inter-reader agreement compared to a moderate inter-reader agreement of angiography and CBCT. Regardless of image quality, CBCT significantly improved the diagnostic confidence of both readers in detecting bleeding vessel(s). Good inter-reader agreement could only be found for CBCT data sets. It is worth noting that no statistically significant difference between CTA and CBCT images regarding the diagnostic confidence of the more experienced Reader 2 could be found. Particular less experienced interventional radiologists may benefit from the information provided by the 3D CBCT images based on the findings in this study.
With regard to the number of bleeding sites, a very strong correlation between clinical gold standard and CBCT images could be found. The number of involved feeding arteries of a bleeding site has a major impact on the outcome of an embolization treatment. CBCT images and the clinical gold standard demonstrated a strong correlation evaluating the involved vascular territories. Both information (number of bleeding sites and involved vascular territories) most likely aid the interventionalist in selected challenging cases, especially if CTA and selective angiography provide insufficient information regarding the source of haemorrhage.
The results of this study indicate that CBCT imaging is able to correctly narrow down the number of involved vascular territories compared to selective angiograms and compared to CTA examinations. Application of intraprocedural CBCT might provide the potential of contrast medium and radiation exposure reduction. For example, if multiple injured vessels are suspected on a pre-interventional CTA, one selective angiogram in combination with CBCT can be performed instead of multiple angiographic acquisitions at different angles. At this point, the potential to save contrast medium and radiation exposure is speculative and beyond the scope of this study. This aspect should be assessed in larger prospective clinical trials.
Results of the correlation analysis (Table 2) indicate that CBCT imaging correctly identified more injured vessels compared to selective angiograms and even compared to CTA examinations. CBCT has the benefits of a 3D information, high spatial resolution and strong vascular contrast. Furthermore, due to the relatively long acquisition time in combination with a more selective contrast medium injection, CBCT enables superior visualization of bleeding sites compared to CTA. However, it is worth noting that CBCT acquisition bears a higher total radiation exposure and therefore, should be reserved for use in selected complex cases. As stated in Table 3 and supported by no notable statistically significant difference between the DAPs of the selective angiograms and CBCT examinations, acquisition of multiple selective regional angiograms in complex cases may result in a similar or even higher radiation exposure.
The study has several limitations. First, the study is retrospective in design and the size of the patient cohort is very limited. However, as stated above, CBCT images were only acquired in complex selected bleeding cases which represented roughly one-third of patients referred for embolization of suspected bleeding in a 6 months period. In this context, the subjective reader-based evaluation of the acquired images is another major limitation worth noting. Therefore, our results should be regarded with care and re-evaluated in a prospective clinical trial which investigates patients with a bleeding of uncertain origin in a pre-determined body region.
In addition, different bleeding locations (thorax, abdomen and pelvis) were included in this study. Therefore, in five cases regional angiography consisted of an aortogram (two cases with thoracic and three cases with abdominal-pelvic aortography) in combination with first-order selective angiograms. In these cases, both angiographic acquisitions, aortogram and first-order selective angiograms were compared to CBCT datasets. In a comparative evaluation, this is not expected to represent a beneficial factor for the CBCT method. Third, certain clinical parameters, such as precise procedure time (without time loss due to required aesthetic interventions), loss of time due to repositioning of the arms for CBCT image acquisitions as well as exact amount/injection rate of used contrast medium were not consistently recorded. Cumulative DAPs depend on numerous factors and therefore, presented results of this analysis should be verified in a prospective study.
Forth, intermittent and self-limited bleeding is an additional potential confounding factor in such kind of studies and might have impacted our results.
CONCLUSIONS
In complex cases of suspected bleeding, CBCT images show sufficient image quality to aid the interventionalist in narrowing down the number of involved vascular territories and hence, the number of involved feeding arteries of a bleeding source. An increased number of injured vessels can be detected with CBCT in comparison to pre-interventional CTA and intraprocedural selective regional angiography. Therefore, CBCT might be a reasonable alternative to several angiographic acquisitions at different angles. CBCT may be of particular benefit for less experienced interventionalists.
REFERENCES
- 1.Rösch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology 1972; 102: 303–6. doi: 10.1148/102.2.303 [DOI] [PubMed] [Google Scholar]
- 2.Angle JF, Siddiqi NH, Wallace MJ, Kundu S, Stokes L, Wojak JC, et al. Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 2010; 21: 1479–86. doi: 10.1016/j.jvir.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 3.Papakostidis C, Kanakaris N, Dimitriou R, Giannoudis PV. The role of arterial embolization in controlling pelvic fracture haemorrhage: a systematic review of the literature. Eur J Radiol 2012; 81: 897–904. doi: 10.1016/j.ejrad.2011.02.049 [DOI] [PubMed] [Google Scholar]
- 4.Waldenberger P, Chemelli A, Hennerbichler A, Wick M, Freund MC, Jaschke W, et al. Transarterial embolization for the management of hemarthrosis of the knee. Eur J Radiol 2012; 81: 2737–40. doi: 10.1016/j.ejrad.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 5.Bellemann N, Sommer CM, Mokry T, Kortes N, Gnutzmann D, Gockner T, et al. Hepatic artery stent-grafts for the emergency treatment of acute bleeding. Eur J Radiol 2014; 83: 1799–803. doi: 10.1016/j.ejrad.2014.06.030 [DOI] [PubMed] [Google Scholar]
- 6.Liang HL, Chiang CL, Chen MC, Lin YH, Huang JS, Pan HB. Pharmaco-induced vasospasm therapy for acute lower gastrointestinal bleeding: a preliminary report. Eur J Radiol 2014; 83: 1811–5. doi: 10.1016/j.ejrad.2014.06.032 [DOI] [PubMed] [Google Scholar]
- 7.Ierardi AM, Duka E, Lucchina N, Floridi C, De Martino A, Donat D, et al. The role of interventional radiology in abdominopelvic trauma. Br J Radiol 2016; 89: 20150866. doi: 10.1259/bjr.20150866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ittrich H, Klose H, Adam G. Radiologic management of haemoptysis: diagnostic and interventional bronchial arterial embolisation. Rofo 2015; 187: 248–59. doi: 10.1055/s-0034-1385457 [DOI] [PubMed] [Google Scholar]
- 9.Ruhnke H, Kröncke TJ, Aneurysms VA. Visceral artery aneurysms and pseudoaneurysms: retrospective analysis of interventional endovascular therapy of 43 aneurysms. Rofo 2017; 189: 632-639. doi: 10.1055/s-0043-107239 [DOI] [PubMed] [Google Scholar]
- 10.Syha R, Benz T, Hetzel J, Spengler W, Kohlhäufl MJ, Gatidis S, et al. Bronchial artery embolization in hemoptysis: 10-Year survival and recurrence-free survival in benign and malignant etiologies - a retrospective study. Rofo 2016; 188: 1061–6. doi: 10.1055/s-0042-112227 [DOI] [PubMed] [Google Scholar]
- 11.Chang WC, Tsai SH, Chang WK, Liu CH, Tung HJ, Hsieh CB, et al. The value of multidetector-row computed tomography for localization of obscure acute gastrointestinal bleeding. Eur J Radiol 2011; 80: 229–35. doi: 10.1016/j.ejrad.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 12.Chalumeau-Lemoine L, Khalil A, Prigent H, Carette MF, Fartoukh M, Parrot A. Impact of multidetector CT-angiography on the emergency management of severe hemoptysis. Eur J Radiol 2013; 82: e742–e747. doi: 10.1016/j.ejrad.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 13.Kessing R. Gastrointestinal bleeding - accurate localization and detection by dual source CT scan. Rofo 2013; 185: 922. [PubMed] [Google Scholar]
- 14.Ogo T, Fukuda T, Tsuji A, Fukui S, Ueda J, Sanda Y, et al. Efficacy and safety of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension guided by cone-beam computed tomography and electrocardiogram-gated area detector computed tomography. Eur J Radiol 2017; 89: 270–6. doi: 10.1016/j.ejrad.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 15.Plank C, Wolf F, Langenberger H, Loewe C, Schoder M, Lammer J. Adrenal venous sampling using dyna-CT—a practical guide. Eur J Radiol 2012; 81: 2304–7. doi: 10.1016/j.ejrad.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 16.Petersen TO, Reinhardt M, Fuchs J, Gosch D, Surov A, Stumpp P, et al. Analysis of patients' X-ray exposure in 146 percutaneous radiologic gastrostomies. Rofo 2017; 189: 820–7. doi: 10.1055/s-0043-109690 [DOI] [PubMed] [Google Scholar]
- 17.Blendl C, Fiebich M, Voigt JM, Selbach M, Uphoff C. Investigation on the 3 D geometric accuracy and on the image quality (MTF, SNR and NPS) of volume tomography units (CT, CBCT and DVT). Rofo 2012; 184: 24–31. doi: 10.1055/s-0031-1281818 [DOI] [PubMed] [Google Scholar]
- 18.Syha R, Grözinger G, Grosse U, Maurer M, Zender L, Horger M, et al. C-arm computed tomography parenchymal blood volume measurement in evaluation of hepatocellular carcinoma before transarterial chemoembolization with drug eluting beads. Cancer Imaging 2015; 15: 22. doi: 10.1186/s40644-015-0057-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tacher V, Radaelli A, Lin M, Geschwind J-F. How I do it: Cone-beam CT during transarterial chemoembolization for liver cancer. Radiology 2015; 274: 320–34. doi: 10.1148/radiol.14131925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692–4. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 21.Lissitz RW, Green SB. Effect of the number of scale points on reliability: a Monte Carlo approach. J Appl Psychol 1975; 60: 10–13. doi: 10.1037/h0076268 [DOI] [Google Scholar]
- 22.Wildgruber M, Wrede CE, Zorger N, Müller-Wille R, Hamer OW, Zeman F, et al. Computed tomography versus digital subtraction angiography for the diagnosis of obscure gastrointestinal bleeding. Eur J Radiol 2017; 88: 8–14. doi: 10.1016/j.ejrad.2016.12.029 [DOI] [PubMed] [Google Scholar]
- 23.Lauric A, Heller RS, Schimansky S, Malek AM. Benefit of cone-beam CT angiography in visualizing aneurysm shape and identification of exact rupture site. J Neuroimaging 2015; 25: 56–61. doi: 10.1111/jon.12120 [DOI] [PubMed] [Google Scholar]
- 24.Patil VV, Fischman AM, Patel RS, Kim E, Lookstein RA, Tabori NE, et al. GI hemorrhage arising from splenic artery: intraprocedure cone-beam CT as problem-solving tool to aide in safe catheterization of offending vessel. Clin Imaging 2015; 39: 928–30. doi: 10.1016/j.clinimag.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 25.Miyayama S, Yamashiro M, Hashimoto M, Hashimoto N, Ikuno M, Okumura K, et al. Identification of small hepatocellular carcinoma and tumor-feeding branches with cone-beam CT guidance technology during transcatheter arterial chemoembolization. J Vasc Interv Radiol 2013; 24: 501–8. doi: 10.1016/j.jvir.2012.12.022 [DOI] [PubMed] [Google Scholar]
- 26.Tacher V, Radaelli A, Lin M, Geschwind JF. How I do it: Cone-beam CT during transarterial chemoembolization for liver cancer. Radiology 2015; 274: 320–34. doi: 10.1148/radiol.14131925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwazawa J, Ohueo S, Hashimoto N, Mitani T. Feasibility of using vessel-detection software for the endovascular treatment of visceral arterial bleeding. Diagn Interv Radiol 2014; 20: 160. doi: 10.5152/dir.2013.13267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrafiello G, Ierardi AM, Duka E, Radaelli A, Floridi C, Bacuzzi A, et al. Usefulness of cone-beam computed tomography and automatic vessel detection software in emergency transarterial embolization. Cardiovasc Intervent Radiol 2016; 39: 530–7. doi: 10.1007/s00270-015-1213-1 [DOI] [PubMed] [Google Scholar]
- 29.Miyayama S, Yamashiro M, Ogi T, Kayahashi M, Kawamura K, Yoshida M, et al. Usefulness of automated feeder-detection software for identification of access routes to small pancreaticoduodenal artery aneurysms during embolotherapy. Vascular 2015; 23: 663–7. doi: 10.1177/1708538114567186 [DOI] [PubMed] [Google Scholar]