Abstract
Objective:
Studies examining radiation-induced heart toxicity in breast cancer patients are inconclusive. The aim of this study was to prospectively and quantitatively asses myocardial blood flow (MBF) with, for the first time, 15O-H2O PET/CT as a marker of heart damage in irradiated breast cancer patients.
Methods:
15 breast cancer patients receiving intact breast or chest wall irradiation were included in the analysis (six with right-sided and nine with left-sided breast cancer). They underwent 15O-H2O PET/CT before radiotherapy (RT) and 2 and 8 months after RT. MBF was quantitatively assessed at rest and under stress conditions in 17 heart segments distinguished according to the American Ultrasound Association classification. Regional MBF values were derived in each of the coronary artery territories.
Results:
MBF decreased in 53% and increased in 33% of cases 2 months after RT in both left-sided and right-sided breast cancer patients. Stress testing was more sensitive than at-rest testing, demonstrating decreased perfusion in the segments supplied by the left anterior descending coronary artery (LAD) [5.41 ± 1.74 vs 4.52 ± 1.82 ml (g*min)−1; p = 0.018], which persisted at 6 months [5.41 ± 1.74 vs 4.40 ± 1.38 ml (g*min)−1; p = 0.032] and a decrease in global heart perfusion [5.14 ± 1.49 vs 4.46 ± 1.73 ml (g*min)−1; p = 0.036]. A minimal radiation dose applied to the LAD correlated with MBF changes observed 2 months after RT (r = −0.57; p = 0.032). Radiological findings were not correlated with clinical symptoms of heart toxicity.
Conclusion:
15O-H2O PET/CT is safe and effective for the early detection and quantitative analysis of subclinical post-RT changes in heart perfusion in breast cancer patients. The LV segments supplied by the LAD are the main site of MBF changes. A minimum radiation dose deposited in the LAD may be a predictor of radiation-induced heart toxicity.
Advances in knowledge:
This is the first time that 15O-H2O PET/CT has been used to assess MBF after RT and the first granular description of the distribution of blood flow changes after breast cancer RT.
Introduction
Early observations from the 1960s on irradiated Hodgkin’s disease patients1, 2 and later on Hiroshima and Nagasaki A-bomb survivors3–5 showed that the heart is radiosensitive and that both high- and low-dose radiation are cardiotoxic. The clinical consequences of radiation-induced heart damage are varied and include pericardial and valvular disease, myocardial infarction, conduction defects, congestive heart failure, and rheumatic and hypertensive heart disease.5–7 However, studies examining heart toxicity in breast cancer patients receiving low- and intermediate-dose radiation to the breast or chest wall do not unequivocally confirm previous observations, with some indicating a significant increase in cardiovascular mortality8–12 and others suggesting the opposite4,13–19 or cardiotoxicity only in left-sided breast cancer patients.9–20
There is also debate as to whether radiation-induced toxicity results from damage to the microvasculature (injury to the myocardial endothelial cells) or macrovasculature (injury to coronary vessels). Both of these mechanisms could disturb organ perfusion21 and ultimately tissue function. With this putative mechanism in mind, quantification of heart perfusion rates might be a suitable and informative approach to investigate the pathogenesis of radiation-induced cardiovascular disease. Several functional heart imaging methods including single-photon emission CT (SPECT) and cardiac magnetic resonance have been used to directly analyze heart perfusion in radiation-exposed patients, and several prospective studies describe myocardial perfusion deficits in irradiated breast cancer patients.21–29 However, these modalities are not considered the best tools for heart perfusion testing by specialist cardiologists, with 15O-H2O PET/CT the established gold standard for quantitative myocardial blood flow (MBF) imaging in vivo. 15O-H2O PET/CT is characterized by both high specificity and significantly higher diagnostic accuracy than SPECT.30 Additionally, 15O possesses the characteristics of an ideal tracer for quantifying MBF due to its short half-life (about 2 min), low absorbed dose (around 1 mSv) per examination, free diffusion, and metabolic inertia.31 Perhaps surprisingly, 15O-H2O PET/CT has never been used to estimate radiation-induced heart perfusion disturbances. We, therefore, conducted the pilot study of MBF in irradiated breast cancer patients using for the first time 15O-H2O PET/CT.
Aims
The primary aim of this study was to prospectively assess MBF in irradiated breast cancer patients with 15O-H2O PET/CT before radiotherapy (RT) and 2 and 8 months after completion of RT. The secondary aim was to analyze the location of MBF disturbances within the heart and correlations with individual radiation dose distribution.
Methods and materials
A pilot group of 15 females [mean age 50.5 years; range 32–68 years; (Table 1)] were included in the analysis. Six had right-sided breast cancer and nine left-sided breast cancer. All patients received three-dimensional tangential photon RT with 6/15 MV X-rays to the breast (n = 10) or chest wall (n = 5) to standard total doses of 42.5 or 45.0 Gy at 2.5 or 2.25 Gy per fraction, respectively. Patients with intact breasts (n = 10) received an additional boost of 11.25 or 10 Gy at 2.25 or 2.5 Gy per fraction to the tumor bed, respectively (Table 1). When treated, the internal mammary nodes were included in the tangent fields.
Table 1.
Patient characteristics
| Characteristics | Total (n = 15) | Right side (n = 6) | Left side (n = 9) | p-value |
| Age: mean (SD) | 50.5 (11.5) | 52.83 (12.7) | 49.2 (11.2) | 0.56 |
| Radiation dose (%) | ||||
| 45 Gy | 5 (33) | 2 (33) | 3 (33) | 0.89 |
| 45 + 11.25 Gy | 4 (27) | 2 (33) | 2 (22) | 0.58 |
| 42.5 + 10 Gy | 6 (50) | 2 (33) | 4 (45) | 0.54 |
| Pre-RT CHTH (%) | ||||
| Yes | 8 (56) | 3 (50) | 5 (55) | 0.72 |
| No | 7 (47) | 3 (50) | 4 (45) | |
| Post-RT HTH (%) | ||||
| Yes | 11 (73) | 5 (83) | 6 (67) | 0.58 |
| No | 4 (27) | 1 (17) | 3 (33) | |
| Co-morbidities | 4 (27) | 1 (17) | 3 (33) | 0.58 |
CHTH, chemotherapy; HTH, hormonal therapy; RT, radiotherapy; SD, standard deviation.
8 out of 15 (53%) females received pre-RT cyclophosphamide, doxorubicin and paclitaxel-based chemotherapy and 11 out of 15 (73%) received post-RT hormonal therapy (Table 1). None of the patients received Her2-inhibitors. 4 out of 15 (27%) females had co-morbidities: diabetes (n = 1) or hypertension (n = 3). None of the patients had a history of cardiovascular diseases including atherosclerosis, angina pectoris or myocardial infarction that might have influenced the study outcome. None of them has ever used cardioprotective drugs.
Ethical statement
The study was performed according to the principles of the Helsinki Declaration and was approved by the Ethics Committee of Nicolaus Copernicus University Collegium Medicum (KB 558/2012). All patients gave written informed consent before 15O-H2O PET/CT imaging.
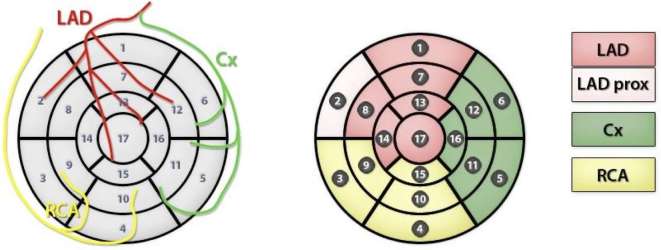
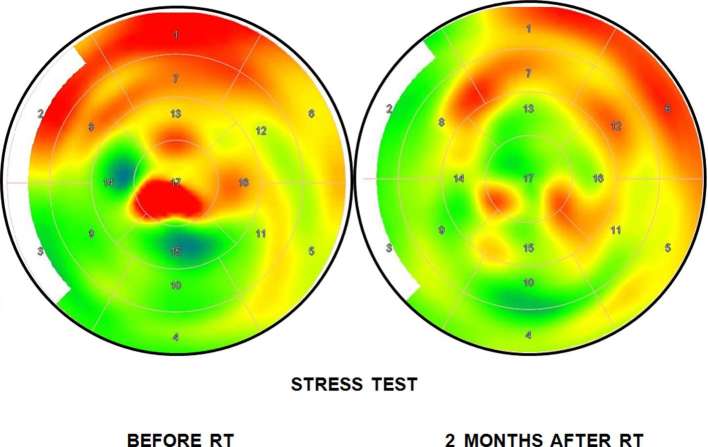
Heart perfusion studies were carried out prior to RT and 2 and 8 months after completion of RT. Each patient underwent resting and stressed 15O-H2O PET imaging, the latter conducted after adenosine-induced vasodilation (Biograph mCT 128 scanner, Siemens, Germany) according to the previously described standard protocol.32 MBF was quantified and expressed in milliliters per minute per gram (ml min–1 g–1) of perfusable tissue and analyzed on a per-segment basis according to the 17-segment left ventricle (LV) myocardium model of the American Heart Association. This model presents the LV as a surface divided into basal, midcavity, and apical segments localized with reference to the long axis of the LV. Using this model, the anterior, posterior, lateral, and septal walls of the LV can be distinguished. Regional MBF values were derived in each of the coronary artery territories: the left anterior descending coronary artery (LAD), the left circumflex artery (Cx), and the right coronary artery (Figure 1). A typical image of PET results before and after radiation therapy is presented in Figure 2.
Figure 1.
AHA. A 17–segment model of the left ventricular myocardial surface. The individual coronary artery territories are distinguished. Abbreviations: AHA, American Heart Association; Cx, left circumflex artery; LAD, left anterior descending coronary artery; RCA, right coronary artery.
Figure 2.
Myocardial blood flow images at PET stress test before and 2 months after radiotherapy. RT, radiotherapy.
In each patient, CT for RT planning was performed without contrast prior to first PET study. In order to calculate radiation doses in the heart and coronary vessels, we delineated these structures on the 2 mm thick CT slices according to the published heart atlas proposed by Feng et al.33
Statistical analysis
All statistical analyses were performed using STATISTICA (v. 10.0, StatSoft Polska, Poland). The Shapiro–Wilk test was used to evaluate normality of individual parameters, and, due to normality, results were presented as the arithmetic mean (X) and standard deviation. The difference between individual values of a given parameter was estimated using the Student’s t-test. Correlations between parameters were tested with Pearson’s coefficients. The incidence of perfusion defects with respect to each patient-related factor was assessed using both univariate and multivariate logistic regression. A p-value less than 0.05 was considered statistically significant.
Results
Radiation-induced MBF changes were observed in both left- and right-sided breast cancer patients. 2 months after RT MBF decreased in 8/15 (53%) of females: 5/9 (55%) females with left-sided breast cancer and 3/6 (50%) with right-sided breast cancer. Interestingly, 5/15 (33%) females had an increase in MBF: 3/5 (60%) females with left-sided breast cancer and 2/5 (40%) with right-sided breast cancer. 8 months after RT, MBF decreased in 10/15 (66%) of females: 7/9 (77%) females with left-sided breast cancer and 3/6 (50%) with right-sided breast cancer. An increased MBF was noted in 5/15 (33%) females: 2/9 (33%) females with left-sided breast cancer and 3/6 (50%) with right-sided breast cancer (Figure 3). Although the measurable MBF changes were statistically significant, they did not achieve a threshold [2.3 ml (g*min)−1 for stress perfusion] considered predictive for hemodynamically significant coronary artery disease.32
Figure 3.
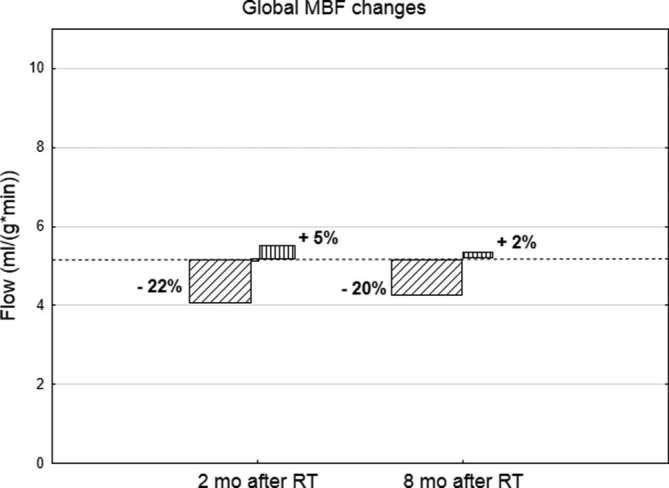
MBF changes (increases and decreases) 2 and 8 months after radiotherapy. Dotted line illustrates the mean MBF before RT. MBF, myocardial blood flow.
At-rest testing
In the at-rest testing case, only the seventh myocardial segment (anterior midcavity wall supplied by the LAD) showed decreased perfusion after both 2 (1.23 ± 0.30 vs 1.10 ± 0.27; p = 0.009) and 8 months (1.23 ± 0.30 vs 1.09 ± 0.24; p = 0.026) after RT completion (Table 2).
Table 2.
At-rest testing
| Area | MBF [ml/(g*min)]before RT | MBF [ml/(g*min)]2 months after RT | MBF [ml/(g*min)]8 months after RT | p-value |
| Global | 1.15 ± 0.33 | 1.14 ± 0.35 | 1.15 ± 0.25 | 1 vs 2 p = 0.841 1 vs 3 p = 0.960 |
| LAD | 1.20 ± 0.35 | 1.16 ± 0.36 | 1.11 ± 0.27 | 1 vs 2 p = 0.472 1 vs 3 p = 0.197 |
| Cx | 1.30 ± 0.42 | 1.23 ± 0.33 | 1.27 ± 0.28 | 1 vs 2 p = 0.230 1 vs 3 p = 0.735 |
| RCA | 0.99 ± 0.30 | 1.08 ± 0.45 | 1.36 ± 0.94 | 1 vs 2 p = 0.411 1 vs 3 p = 0.204 |
| Seg 1 | 1.03 ± 0.28 | 0.94 ± 0.30 | 0.93 ± 0.18 | 1 vs 2 p = 0.058 1 vs 3 p = 0.053 |
| Seg 2 | 0.81 ± 0.16 | 0.79 ± 0.21 | 0.79 ± 0.12 | 1 vs 2 p = 0.574 1 vs 3 p = 0.728 |
| Seg 3 | 0.85 ± 0.23 | 0.84 ± 0.29 | 0.89 ± 0.27 | 1 vs 2 p = 0.851 1 vs 3 p = 0.526 |
| Seg 4 | 1.01 ± 0.30 | 1.06 ± 0.42 | 1.80 ± 2.60 | 1 vs 2 p = 0.578 1 vs 3 p = 0.319 |
| Seg 5 | 1.15 ± 0.39 | 1.10 ± 0.36 | 1.15 ± 0.23 | 1 vs 2 p = 0.204 1 vs 3 p = 0.995 |
| Seg 6 | 1.25 ± 0.29 | 1.17 ± 0.33 | 1.19 ± 0.31 | 1 vs 2 p = 0.072 1 vs 3 p = 0.334 |
| Seg 7 | 1.23 ± 0.30 | 1.10 ± 0.27 | 1.09 ± 0.24 |
1 vs 2 p = 0.009 1 vs 3 p = 0.026 |
| Seg 8 | 0.88 ± 0.20 | 0.87 ± 0.24 | 0.86 ± 0.17 | 1 vs 2 p = 0.759 1 vs 3 p = 0.752 |
| Seg 9 | 0.88 ± 0.24 | 0.89 ± 0.28 | 0.98 ± 0.28 | 1 vs 2 p = 0.882 1 vs 3 p = 0.291 |
| Seg 10 | 1.07 ± 0.37 | 1.16 ± 0.54 | 1.91 ± 2.36 | 1 vs 2 p = 0.451 1 vs 3 p = 0.225 |
| Seg 11 | 1.27 ± 0.44 | 1.25 ± 0.40 | 1.36 ± 0.27 | 1 vs 2 p = 0.862 1 vs 3 p = 0.385 |
| Seg 12 | 1.40 ± 0.47 | 1.27 ± 0.31 | 1.28 ± 0.31 | 1 vs 2 p = 0.173 1 vs 3 p = 0.252 |
| Seg 13 | 1.28 ± 0.39 | 1.22 ± 0.34 | 1.13 ± 0.34 | 1 vs 2 p = 0.170 1 vs 3 p = 0.054 |
| Seg 14 | 0.99 ± 0.30 | 1.01 ± 0.35 | 1.03 ± 0.29 | 1 vs 2 p = 0.762 1 vs 3 p = 0.679 |
| Seg 15 | 1.13 ± 0.40 | 1.42 ± 0.80 | 2.07 ± 2.33 | 1 vs 2 p = 0.199 1 vs 3 p = 0.175 |
| Seg 16 | 1.44 ± 0.51 | 1.32 ± 0.31 | 1.41 ± 0.45 | 1 vs 2 p = 0.226 1 vs 3 p = 0.722 |
| Seg 17 | 1.53 ± 0.63 | 1.44 ± 0.51 | 1.38 ± 0.50 | 1 vs 2 p = 0.505 1 vs 3 p = 0.181 |
Cx, left circumflex artery; LAD, left anterior descending artery; MBF, myocardial blood flow; RCA, right coronary artery; RT, radiotherapy.
Significant MBF decreases after RT were only observed in one segment localized in the anterior mid-cavity wall supplied by the LAD. Significant differences are highlighted in bold.
Stress testing
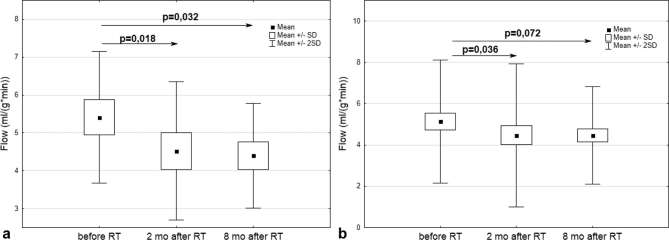
2 months after RT, stress testing demonstrated decreased perfusion in the segments supplied by the LAD (5.41 ± 1.74 vs 4.52 ± 1.82; p = 0.018) (Figure 4a), two segments (11th and 16th) supplied by the Cx (5.12 ± 1.39 vs 4.49 ± 1.76; p = 0.048 and 5.58 ± 1.60 vs 4.66 ± 2.01; p = 0.015, respectively), and a global decrease in heart perfusion (5.14 ± 1.49 vs 4.46 ± 1.73; p = 0.036) (Figure 4b). In the anterior apical and midcavity wall areas supplied by the LAD, the perfusion decrease persisted for the next 6 months (Table 3 and Figure 4a). Although a significant global MBF decrease was observed 2 months after RT, this did not persist to 8 months although there was a trend to this effect (Figure 4b).
Figure 4.
(a) MBF changes in the myocardial segments supplied by the LAD during stress testing: before RT and 2 and 8 months after its completion. (b) Global MBF changes during stress testing: before RT and 2 and 8 months after its completion. LAD, left anterior descending artery; MBF, myocardial blood flow; RT, radiotherapy; SD, standard deviation.
Table 3.
Stress testing results
| Area | MBF [ml/(g*min)] Before RT | MBF [ml/(g*min)] 2 mo after RT | MBF [ml/(g*min)] 8 mo after RT | p-value |
| Global | 5.14 ± 1.49 | 4.46 ± 1.73 | 4.47 ± 1.18 |
1 vs 2 p = 0.036 1 vs 3 p = 0.207 |
| LAD | 5.41 ± 1.74 | 4.52 ± 1.82 | 4.40 ± 1.38 |
1 vs 2 p = 0.018 1 vs 3 p = 0.032 |
| Cx | 5.04 ± 1.36 | 4.48 ± 1.81 | 4.80 ± 1.22 | 1 vs 2 p = 0.069 1 vs 3 p = 0.937 |
| RCA | 4.72 ± 1.45 | 4.44 ± 1.80 | 4.41 ± 1.21 | 1 vs 2 p = 0.375 1 vs 3 p = 0.527 |
| Seg 1 | 4.73 ± 1.68 | 4.10 ± 1.85 | 4.37 ± 1.26 | 1 vs 2 p = 0.069 1 vs 3 p = 0.897 |
| Seg 2 | 3.37 ± 1.07 | 3.24 ± 1.49 | 3.37 ± 1.14 | 1 vs 2 p = 0.073 1 vs 3 p = 0.993 |
| Seg 3 | 3.94 ± 1.50 | 3.81 ± 1.79 | 3.62 ± 1.37 | 1 vs 2 p = 0.689 1 vs 3 p = 0.756 |
| Seg 4 | 4.82 ± 1.46 | 4.20 ± 1.71 | 4.45 ± 1.20 | 1 vs 2 p = 0.086 1 vs 3 p = 0.414 |
| Seg 5 | 4.58 ± 1.27 | 4.63 ± 2.09 | 4.53 ± 1.02 | 1 vs 2 p = 0.925 1 vs 3 p = 0.884 |
| Seg 6 | 4.85 ± 1.20 | 4.52 ± 1.59 | 4.69 ± 1.15 | 1 vs 2 p = 0.29 1 vs 3 p = 0.566 |
| Seg 7 | 5.23 ± 1.46 | 4.32 ± 1.85 | 4.49 ± 1.26 |
1 vs 2 p = 0.009 1 vs 3 p = 0.05 |
| Seg 8 | 4.21 ± 1.41 | 3.66 ± 1.64 | 3.83 ± 1.21 |
1 vs 2 p = 0.038 1 vs 3 p = 0.276 |
| Seg 9 | 3.98 ± 1.24 | 4.10 ± 1.92 | 3.91 ± 1.19 | 1 vs 2 p = 0.718 1 vs 3 p = 0.818 |
| Seg 10 | 4.89 ± 1.43 | 4.55 ± 1.76 | 4.68 ± 1.00 | 1 vs 2 p = 0.335 1 vs 3 p = 0.518 |
| Seg 11 | 5.12 ± 1.39 | 4.49 ± 1.76 | 4.95 ± 1.12 |
1 vs 2 p = 0.048 1 vs 3 p = 0.657 |
| Seg 12 | 5.25 ± 1.47 | 4.57 ± 1.86 | 4.84 ± 1.22 | 1 vs 2 p = 0.056 1 vs 3 p = 0.218 |
| Seg 13 | 5.59 ± 1.93 | 4.42 ± 1.83 | 4.34 ± 1.47 |
1 vs 2 p = 0.008 1 vs 3 p = 0.011 |
| Seg 14 | 4.95 ± 1.84 | 4.41 ± 1.80 | 4.12 ± 1.33 | 1 vs 2 p = 0.146 1 vs 3 p = 0.089 |
| Seg 15 | 5.58 ± 1.78 | 5.22 ± 1.96 | 5.04 ± 1.26 | 1 vs 2 p = 0.385 1 vs 3 p = 0.212 |
| Seg 16 | 5.58 ± 1.60 | 4.66 ± 2.01 | 4.95 ± 1.37 |
1 vs 2 p = 0.015 1 vs 3 p = 0.181 |
| Seg 17 | 6.24 ± 2.23 | 5.14 ± 2.17 | 4.81 ± 1.87 |
1 vs 2 p = 0.035 1 vs 3 p = 0.029 |
Cx, left circumflex artery; LAD, left anterior descending artery; MBF, myocardial blood flow; RCA, right coronary artery; RT, radiotherapy.
Mean doses applied to the coronary arteries.
The effects of RT on perfusion seen in the area supplied by the LAD may be a function of the radiation dose, which was highest for the LAD compared to the other coronary arteries as assessed by the RT planning system (Table 4).
Table 4.
Mean doses applied to the coronary arteries
| Heart | LAD | Cx | RCA | |
| D min (Gy) | 0.77 ± 0.5 | 3.4 ± 3.9 | 1.5 ± 1.2 | 1.5 ± 0.3 |
| D max (Gy) | 31.72 ± 19.8 | 29.9 ± 21.9 | 1.8 ± 1.4 | 3.4 ± 2.0 |
| D mean (Gy) | 5.09 ± 3.98 | 25.5 ± 19.4 | 1.7 ± 1.3 | 2.2 ± 0.7 |
Cx, left circumflex artery. LAD, left anterior descending artery; RCA, right coronary artery.
The effects of RT on perfusion seen in the area supplied by the LAD may be a function of the radiation dose, which was highest for the LAD compared to the other coronary arteries as assessed by the RT planning system (Table 4).
Only the minimum radiation dose absorbed by the LAD was correlated with MBF changes observed 2 months after RT (Table 5). Only LAD doses above 2 Gy caused a decrease in heart perfusion compared to baseline (Figure 5).
Table 5.
Correlations between doses applied to the coronary arteries as assessed by the RT planning system and MBF changes 2 and 8 months after RT
| MBF changes 2 months after RT | MBF changes 8 months after RT | |||
| r | p-value | r | p-value | |
| Heart Dmin | −0.22 | 0.455 | −0.08 | 0.781 |
| Heart Dmax | −0.01 | 0.983 | −0.28 | 0.336 |
| Heart Dmean | −0.35 | 0.226 | 0.07 | 0.808 |
| LAD Dmin | −0.57 | 0.032 | 0.41 | 0.141 |
| LAD Dmax | −0.02 | 0.937 | −0.26 | 0.365 |
| LAD Dmean | −0.03 | 0.912 | −0.29 | 0.316 |
| Cx Dmin | −0.45 | 0.105 | 0.30 | 0.306 |
| Cx Dmax | −0.42 | 0.137 | 0.24 | 0.400 |
| Cx Dmean | −0.44 | 0.116 | 0.28 | 0.332 |
| RCA Dmin | −0.07 | 0.824 | 0.12 | 0.691 |
| RCA Dmax | −0.28 | 0.335 | 0.42 | 0.133 |
| RCA Dmean | 0.06 | 0.833 | 0.09 | 0.765 |
Cx, left circumflex artery; LAD, left anterior descending artery; MBF, myocardial blood flow; RCA, right coronary artery; RT, radiotherapy.
Figure 5.
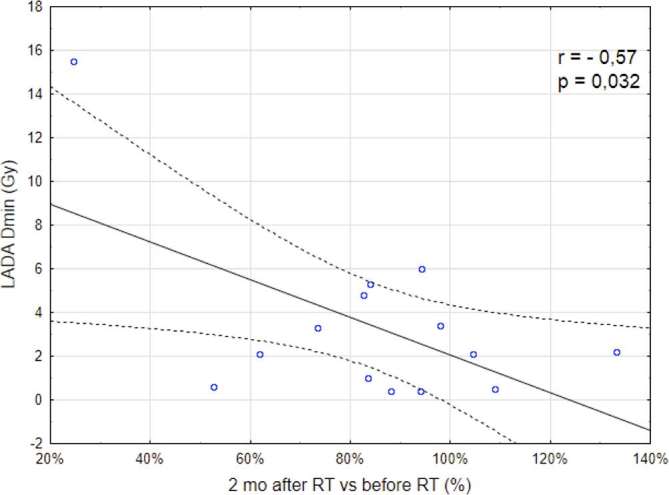
Correlation between a minimal radiation dose absorbed by the LAD as assessed by the RT planning system and MBF changes observed 2 months after RT. LAD, left anterior descending artery; MBF, myocardial blood flow; RT, radiotherapy.
Other potential factors influencing MBF defects
The incidence of perfusion defects with respect to each patient-specific and treatment-specific factor was assessed using both univariate and multivariate logistic regression. Ad hoc methods to detect colinearity were applied. For this analysis, perfusion defects were scored as either present or absent, irrespective of severity. Of the analyzed variables, age was considered a continuous variable and all others were considered categorical (Table 6). There were no associations between MBF defects and other clinicopathological factors including: age, left-sided cancer, the presence of heart risk factors (hypertension, diabetes), or additional treatment modalities (chemotherapy, hormonal therapy).
Table 6.
Correlations between MBF defects and patient-specific/treatment-specific factors
|
Variable |
Univariable | Multivariable | ||
| OR (95% CI) | p-valuea | OR (95% CI) | p-valuea | |
| Age | 1.049 (0.955–1.153) | 0.318 | 1.140 (0.930–1.397) | 0.208 |
| Left side (yes vs no) | 0.667 (0.076–5.878) | 0.715 | 2.119 (0.081–55.291) | 0.652 |
| Chemotherapy (yes vs no) | 0.240 (0.027–2.116) | 0.198 | 0.096 (0.003–3.481) | 0.201 |
| HTH (yes vs no) | 2.800 (0.196–40.059) | 0.448 | 0.387 (0.008–19.527) | 0.635 |
| Heart risk factors (yes vs no) | 0.876 (0.084–8.240) | 0.876 | 0.028 (0.000–5.699) | 0.188 |
CI, confidence interval; HTH, hormonal therapy; MBF, myocardial blood flow; OR, odds ratio.
Logistic regression Wald X2 statistic probability.
Discussion
Here, we show that 15O-H2O PET/CT is valuable for detecting early heart perfusion changes in irradiated patients. This is the first study to assess the utility of PET for monitoring MBF after RT. Previous analyses of heart perfusion in oncology patients used SPECT performed under resting and stressed conditions, similar to the 15O-H2O PET protocol used here. In most previous studies, the evaluation of MBF changes was qualitative with the presence or absence of MBF disturbances in individual heart areas estimated by a nuclear medicine specialist, although in some cases a semi-quantitative MBF scoring scale was applied.22, 28 For the first time, our study applies a quantitative assessment of MBF changes presented as the difference in their absolute values measured in ml per min per g in each individual heart region before and after RT. Our method eliminates the risk of error resulting from the subjective evaluation of heart perfusion. Furthermore, these quantitative data enable other researchers to directly compare their results with our own.
Of note, we detected both MBF decreases and increases in 53 and 33% of females, respectively, which were independent of the side of the chest wall irradiated. Radiation-induced MBF changes occurred as early as 2 months after RT; previous studies examining post-radiation myocardial perfusion disturbances by SPECT have reported MBF deficits in up to 60% of left-sided breast cancer patients as early as 6 months after RT.21,24–26,28 Using SPECT in a case-control study, Sioka et al showed that myocardial perfusion abnormalities also occur in right-sided breast cancer patients compared to controls without significant differences according to the irradiated side,22 which is consistent with our results. However, the group size was small and the authors did note a statistical trend toward greater perfusion disturbances in left-sided breast cancer patients.22 There were no associations between MBF defects and other clinicopathological factors including age, the presence of heart risk factors (hypertension, diabetes), and additional treatment modalities (chemotherapy, hormonal therapy); however, a larger cohort of patients would be useful to further examine these associations.
MBF disturbances are usually presented as collective or global results for the entire heart.22, 28 Here, we also assessed perfusion in individual heart segments, the results of which suggested a non-uniform distribution of MBF changes and enabled us to establish which areas had the highest MBF disturbances. We also, for the first time, considered the heart vasculature and doses applied to individual arteries.
The phenomenon of very early MBF disturbances at two months might suggest—contrary to a well-established opinion—that the heart is the organ early reacting to irradiation. The increased perfusion observed as early in 33% of patients might suggest the presence of an acute inflammatory response and, if confirmed, could be a therapeutic target to limit the late cardiovascular toxicity. The increased MBF did not usually persist to 6 months and actually decreased to baseline or even lower, it did persist in one female. One could speculate that MBF decrease commonly considered a direct risk factor for radiation induced heart diseases is a consequence of a former inflammatory process. The probable individual dynamics of the phenomenon might be the reason why MBF changes were stable or progressive in different patients. Marks et al showed that the incidence of perfusion decrease was mounting in time and 24 months after RT it attained the highest value.26
We observed correlation between MBF disturbances and the minimum radiation dose to the LAD, which, in breast cancer patients, usually receives the highest radiation doses. This minimum dose may reflect a threshold over which an inflammatory response is triggered. Our future studies will include measuring MBF earlier than 2 months after completion of RT.
Previous studies did not analyze the relationship between heart perfusion changes and the radiation dose applied to specific heart structures, with the mean heart dose usually used as a predictor of heart damage. Based on a population of 108 patients receiving radiation to the left side of the chest, Evans et al reported that the left ventricular volume was a significant predictor of heart perfusion disturbances.34 Further, population studies have estimated that a mean heart dose of 10 Gy increases a risk of cardiovascular events by 3.2%, 30 years after RT.35
In our study and previous reports, the observed heart perfusion disturbances were subclinical and there are currently no convincing data to suggest that they predict future, clinically relevant heart toxicities. Thus, implementing cardioprotection in this group of patients is currently not advised.
The pilot study we conducted has some limitations, mainly the small study group and a short time of observation, although this did allow us to assess early changes. Nevertheless, we demonstrated that 15O-H2O PET/CT is a valuable tool for evaluating radiation-induced heart perfusion changes. Our study shows a relationship between the irradiated heart area and its perfusion and suggests that the radiation dose applied to the LAD might be a potential predictor of MBF disturbances. Further, larger studies are required to confirm and develop our findings.
Conclusions
15O-H2O PET/CT is safe and effective for the early detection and quantitative analysis of subclinical post-RT changes of heart perfusion in breast cancer patients. The LV segments supplied by the LAD are the main site of MBF changes. A minimum radiation dose deposited in the LAD may be a predictor of radiation-induced heart toxicity.
Contributor Information
Agnieszka Żyromska, Email: agnieszka.zyromska@gmail.com.
Bogdan Małkowski, Email: malkowskib@co.bydgoszcz.pl.
Tomasz Wiśniewski, Email: wisniewskitomasz9@gmail.com.
Karolina Majewska, Email: majewskak@co.bydgoszcz.pl.
Joanna Reszke, Email: reszkej@co.bydgoszcz.pl.
Roman Makarewicz, Email: makarewiczr@co.bydgoszcz.pl.
REFERENCES
- 1.Fajardo LF, Stewart JR, Cohn KE. Morphology of radiation-induced heart disease. Arch Pathol 1968; 86: 512–9. [PubMed] [Google Scholar]
- 2.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, Van’t Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol 2003; 21: 3431–9. doi: 10.1200/JCO.2003.07.131 [DOI] [PubMed] [Google Scholar]
- 3.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950-1997. Radiat Res 2003; 160: 381–407. doi: 10.1667/RR3049 [DOI] [PubMed] [Google Scholar]
- 4.Gustavsson A, Bendahl PO, Cwikiel M, Eskilsson J, Thapper KL, Pahlm O. No serious late cardiac effects after adjuvant radiotherapy following mastectomy in premenopausal women with early breast cancer. Int J Radiat Oncol Biol Phys 1999; 43: 745–54. doi: 10.1016/S0360-3016(98)00454-4 [DOI] [PubMed] [Google Scholar]
- 5.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res 2012; 177: 229–43. doi: 10.1667/RR2629.1 [DOI] [PubMed] [Google Scholar]
- 6.Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003; 13: 346–56. doi: 10.1016/S1053-4296(03)00026-2 [DOI] [PubMed] [Google Scholar]
- 7.Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 2006; 24: 4100–6. doi: 10.1200/JCO.2005.05.1037 [DOI] [PubMed] [Google Scholar]
- 8. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early breast cancer trialists’ collaborative group. Lancet 2000; 355: 1757–70. [PubMed] [Google Scholar]
- 9.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005; 6: 557–65. doi: 10.1016/S1470-2045(05)70251-5 [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, Redmond C, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 1994; 12: 447–53. doi: 10.1200/JCO.1994.12.3.447 [DOI] [PubMed] [Google Scholar]
- 11.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99: 365–75. doi: 10.1093/jnci/djk064 [DOI] [PubMed] [Google Scholar]
- 12.Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, Møller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer 2007; 7: 9. doi: 10.1186/1471-2407-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Højris I, Overgaard M, Christensen JJ, Overgaard J. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomised trials. Radiotherapy committee of the Danish breast cancer cooperative group. Lancet 1999; 354: 1425–30. doi: 10.1016/S0140-6736(99)02245-X [DOI] [PubMed] [Google Scholar]
- 14.Vallis KA, Pintilie M, Chong N, Holowaty E, Douglas PS, Kirkbride P, et al. Assessment of coronary heart disease morbidity and mortality after radiation therapy for early breast cancer. J Clin Oncol 2002; 20: 1036–42. doi: 10.1200/JCO.2002.20.4.1036 [DOI] [PubMed] [Google Scholar]
- 15.Woodward WA, Strom EA, McNeese MD, Perkins GH, Outlaw EL, Hortobagyi GN, et al. Cardiovascular death and second non-breast cancer malignancy after postmastectomy radiation and doxorubicin-based chemotherapy. Int J Radiat Oncol Biol Phys 2003; 57: 327–35. doi: 10.1016/S0360-3016(03)00594-7 [DOI] [PubMed] [Google Scholar]
- 16.Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 2005; 97: 419–24. doi: 10.1093/jnci/dji067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patt DA, Goodwin JS, Kuo YF, Freeman JL, Zhang DD, Buchholz TA, et al. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol 2005; 23: 7475–82. doi: 10.1200/JCO.2005.13.755 [DOI] [PubMed] [Google Scholar]
- 18.Rutqvist LE, Liedberg A, Hammar N, Dalberg K. Myocardial infarction among women with early-stage breast cancer treated with conservative surgery and breast irradiation. Int J Radiat Oncol Biol Phys 1998; 40: 359–63. doi: 10.1016/S0360-3016(97)00765-7 [DOI] [PubMed] [Google Scholar]
- 19.Nixon AJ, Manola J, Gelman R, Bornstein B, Abner A, Hetelekidis S, et al. No long-term increase in cardiac-related mortality after breast-conserving surgery and radiation therapy using modern techniques. J Clin Oncol 1998; 16: 1374–9. doi: 10.1200/JCO.1998.16.4.1374 [DOI] [PubMed] [Google Scholar]
- 20.Paszat LF, Mackillop WJ, Groome PA, Schulze K, Holowaty E. Mortality from myocardial infarction following postlumpectomy radiotherapy for breast cancer: a population-based study in Ontario, Canada. Int J Radiat Oncol Biol Phys 1999; 43: 755–62. doi: 10.1016/S0360-3016(98)00412-X [DOI] [PubMed] [Google Scholar]
- 21.Seddon B, Cook A, Gothard L, Salmon E, Latus K, Underwood SR, et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol 2002; 64: 53–63. doi: 10.1016/S0167-8140(02)00133-0 [DOI] [PubMed] [Google Scholar]
- 22.Sioka C, Exarchopoulos T, Tasiou I, Tzima E, Fotou N, Capizzello A, et al. Myocardial perfusion imaging with 99 mTc-tetrofosmin SPECT in breast cancer patients that received postoperative radiotherapy: a case-control study. Radiat Oncol 2011; 6: 151. doi: 10.1186/1748-717X-6-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyenes G, Fornander T, Carlens P, Glas U, Rutqvist LE. Detection of radiation-induced myocardial damage by technetium-99m sestamibi scintigraphy. Eur J Nucl Med 1997; 24: 286–92. [DOI] [PubMed] [Google Scholar]
- 24.Hardenbergh PH, Munley MT, Bentel GC, Kedem R, Borges-Neto S, Hollis D, et al. Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results. Int J Radiat Oncol Biol Phys 2001; 49: 1023–8. doi: 10.1016/S0360-3016(00)01531-5 [DOI] [PubMed] [Google Scholar]
- 25.Lind PA, Pagnanelli R, Marks LB, Borges-Neto S, Hu C, Zhou SM, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys 2003; 55: 914–20. doi: 10.1016/S0360-3016(02)04156-1 [DOI] [PubMed] [Google Scholar]
- 26.Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, Blazing M, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 2005; 63: 214–23. doi: 10.1016/j.ijrobp.2005.01.029 [DOI] [PubMed] [Google Scholar]
- 27.Tzonevska A, Tzvetkov K, Parvanova V, Dimitrova M. 99mTc-MIBI myocardial perfusion scintigraphy for assessment of myocardial damage after radiotherapy in patients with breast cancer. J Buon 2006; 11: 505–9. [PubMed] [Google Scholar]
- 28.Prosnitz RG, Hubbs JL, Evans ES, Zhou SM, Yu X, Blazing MA, et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer 2007; 110: 1840–50. doi: 10.1002/cncr.22965 [DOI] [PubMed] [Google Scholar]
- 29.HŁjris I, Sand NP, Andersen J, Rehling M, Overgaard M. Myocardial perfusion imaging in breast cancer patients treated with or without post-mastectomy radiotherapy. Radiother Oncol 2000; 55: 163–72. doi: 10.1016/S0167-8140(00)00170-5 [DOI] [PubMed] [Google Scholar]
- 30.Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol 2012; 59: 1719–28. doi: 10.1016/j.jacc.2011.12.040 [DOI] [PubMed] [Google Scholar]
- 31.Knaapen P, de Haan S, Hoekstra OS, Halbmeijer R, Appelman YE, Groothuis JG, et al. Cardiac PET-CT: advanced hybrid imaging for the detection of coronary artery disease. Neth Heart J 2010; 18: 90–8. doi: 10.1007/BF03091744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danad I, Uusitalo V, Kero T, Saraste A, Raijmakers PG, Lammertsma AA, et al. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease: cutoff values and diagnostic accuracy of quantitative [15O]H2O PET imaging. J Am Coll Cardiol 2014; 64: 1464–75. doi: 10.1016/j.jacc.2014.05.069 [DOI] [PubMed] [Google Scholar]
- 33.Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011; 79: 10–18. doi: 10.1016/j.ijrobp.2009.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans ES, Prosnitz RG, Yu X, Zhou SM, Hollis DR, Wong TZ, et al. Impact of patient-specific factors, irradiated left ventricular volume, and treatment set-up errors on the development of myocardial perfusion defects after radiation therapy for left-sided breast cancer. Int J Radiat Oncol Biol Phys 2006; 66: 1125–34. doi: 10.1016/j.ijrobp.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 35.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368: 987–98. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]