Abstract
Objective:
To evaluate whether diagnostic accuracy and complications of CT-guided core needle biopsy (CNB) differ for solid and part-solid lung lesions
Methods:
This retrospective study included 354 consecutive patients from April 2012 to July 2016 who underwent CT-guided CNB of lung lesions by a radiologist. Patient demographics, lung lesions’ characteristics; solid or part-solid, underlying pulmonary disease, distance of path, procedure time, complications (hemorrhage or pneumothorax), histopathological results of biopsy specimens and final diagnosis were reviewed. The diagnostic yields, biopsy-related factors and complications were compared for patients with solid lesions and patients with part-solid lesions. Factors related to true diagnoses and complications were analyzed statistically.
Results:
The biopsies of part-solid lesions take more time (p = 0.021). Non-diagnostic biopsies were not statistically different between solid and part-solid lesions (p = 0.804). There was no significant difference in the diagnostic yields including sensitivity, specificity, accuracy, positive predictive value and negative predictive value for solid and part-solid lesions statistically. The occurrence of hemorrhage on postbiopy follow-up CT was significantly higher (p = 0.016) for part-solid lesions. The occurrence of symptomatic major hemorrhage (p = 0.859) and pneumothorax (p = 0.106) was not significantly different between solid and part-solid lesions.
Conclusion:
The diagnostic accuracy of CT-guided CNB for diagnosing malignancy was comparable for solid and part-solid lesions. The frequency of hemorrhage on the follow up CT was higher in patients with part-solid lesions, but there were no significant differences in major hemorrhage and pneumothorax for solid and part-solid lesions.
Advances in knowledge:
The diagnostic yield of CT-guided CNB for diagnosing malignancy is comparable for solid and part-solid lesions. Although the post procedural hemorrhage occurs more frequently in part-solid lesions, the occurrence of symptomatic major hemorrhage is not significantly different. Therefore, CT-guided CNB should be considered for histopathological confirmation of intrapulmonary lesions regardless of the presence of ground-glass opacity portion.
INTRODUCTION
CT-guided core needle biopsy (CNB) is a minimally invasive procedure that is an indispensable tool in the diagnosis of thoracic lesions. With recent developments in CT screening for lung nodules, detection of ground-glass nodule (GGN) has increased.1 GGN is a non-specific finding that can indicate inflammatory disease, focal fibrosis, atypical adenomatous hyperplasia, adenocarcinoma in situ, and adenocarcinoma. The clinical significance of part-solid nodules is the higher incidence of malignancy compared with solid nodules.2, 3
The previously reported diagnostic accuracy of CT-guided CNB is 62% to 97% for overall solid and part-solid lesions and 64.6% to 93.0% for part-solid lesions.4–15 A difference in diagnostic accuracy according to the proportion of GGN is reported in several studies, but is not significant in all studies.7, 10,12,13 A difference in diagnostic accuracy of CT-guided aspiration biopsy between the solid and part-solid nodule is reported.12 However, to our knowledge, the difference with CNB has not been reported.
Previous studies proposed that if suspicion of malignancy for a part-solid nodule is high on CT or if any increase in the size of whole lesions or solid portions of part-solid nodules is observed, CT-guided biopsy can be omitted and surgical biopsy should be considered for pathological diagnosis.16 The omission is associated with the possibility of lower diagnostic accuracy, higher likelihood of grade underestimation, and higher frequency of hemorrhage after biopsy than solid lesions. However, to the best of our knowledge, we do not currently have a definite conclusion on whether the diagnostic yields and occurrence of complications of CT-guided CNB are different between solid and part-solid nodules.
Thus, the purpose of this study was to evaluate if diagnostic accuracy and complications of CT-guided CNB differ by lesion characteristics between solid and part-solid lung lesions.
methods and MATERIALS
Study population
Under approval from the institutional review board, we retrospectively reviewed medical records of 673 consecutive patients who underwent percutaneous needle biopsy of the thorax in single center from April 2012 to July 2016. We excluded 319 patients who underwent fluoroscopy (n = 191), USG guided needle biopsy (n = 8), fine-needle aspiration (FNA, n = 26), second biopsy (n = 16), or biopsy of mediastinal (n = 30), chest wall (n = 21), or pleural lesions (n = 27). We classified the patients with prolonged prothrombin time (above 15.0 s), thrombocytopenia and/or pulmonary hypertension as the contraindication of the CNB. Anticoagulant or antiaggregant medication was discontinued at least 1 week before the procedure. A total of 354 patients who underwent CT-guided CNB of intrapulmonary lesions were included. We retrospectively reviewed medical records. Patient demographics including age, sex, and body mass index (BMI) were reviewed. All patients had neither history nor evidence of malignancy in other organs.
Preprocedure CT acquisition and assessment
Preprocedural CT scans were performed within a month of the date of CNB. CT scans were acquired using a multidetector CT system (Somatom Sensation 64 or dual-source Flash 128 multi-detector CT system, Siemens Medical Solutions, Erlangen, Germany). Scanning was performed after intravenous administration of contrast medium (140 ml Iopamidol, Pamiray 300, Dongkook Pharm., Seoul, Korea) with a power injector (Mallinckrodt, Tyco and Vistron CT, Medrad, Arrendale, PA) at an injection rate of 2.5 ml s−1 if there was no contraindication. Scanning parameters were 120 kVp, 90–150 mA, 0.5 s tube rotation time, 1.2 pitch. Three radiologists with 2–10 years of experience in chest CT interpretation retrospectively reviewed preprocedural CT images by consensus, to classify the types of nodules as solid and part-solid nodules. Images were displayed with lung window setting of level 600 HU and width 1500 HU. One-dimensional measurements of whole primary lung cancer lesions were performed for size measurement on axial images. We divided lung lesions into solid and part-solid lesions according to Fleischner 2017 guideline. Solid lesions were defined as lesions with homogenous soft-tissue attenuation and part-solid lesions as lesions with both ground-glass and solid soft-tissue attenuation components. We classified the pure GGN as the part-solid lesion. Other characteristics including the presence or absence of cavity, air-bronchogram, and underlying lung disease of emphysema or fibrosis were also reviewed by consensus. Cavity was defined as a gas-filled space, seen as lucency or low-attenuation area, within lung lesions. Air-bronchogram was defined as a pattern of air-filled bronchi on a background of a high-attenuation airless lesion. We regarded pulmonary emphysema as focal areas or regions of low attenuation, usually without visible walls in whole lung parenchyma or around the target lesion. When diffuse reticular and ground glass opacities with or without honeycombing were seen in subpleural or peribronchial area, these were classified as underlying pulmonary fibrosis regardless of specific diagnosis of fibrosis.
CT-guided CNB and investigated parameters
Biopsies were performed by a chest radiologist with 9 years of experience in thoracic biopsy. Written informed consent was obtained from each patient before each biopsy. All biopsies were performed under CT guidance using a multidetector CT system (Somatom Sensation 64 multi-detector CT system, Siemens Medical Solutions, Erlangen, Germany). At the time of biopsy, selected images were obtained of the area of interest with 2 mm contiguous transverse CT sections. Biopsies were planned to avoid bony structures and to cross the fewest pleural surfaces. Procedures were performed with patients in the prone, supine, or lateral decubitus position, depending on lesion location. Local anesthesia was done with subcutaneous injection of 2% lidocaine (Lidocaine HCl, Huons, Korea). Core biopsy specimens were obtained with detachable 18-gauge and 16-inch non-coaxial needles (ACN Automatic Cutting Needle, Medical Device Technologies, Gainesville, FL). Using intermittent CT scans to evaluate needle trajectory, needle was inserted through the pleura and advanced close to the target lesion during a single breath-hold. After the needle tip position was confirmed on CT, the operator fitted a spring-activated automated gun (Manan Pro-Mag 2.2, Manan Medical Products, Northbrook, IL) with a core needle and shot the needle. The cannula and stylet were released in rapid sequence and the core specimen was acquired and held in the sampling notch of the stylet. After removing the needle from the patient, immediate follow-up CT was performed. If the first sample was judged satisfactory, no other biopsy was performed. We evaluated procedure-related factors including distance of the biopsy needle path and procedure time. The distance of needle path was measured from the skin to the lesion on CT scans obtained during biopsy.
Pathological results
Benign or malignant pathological results from biopsy specimens were evaluated. Biopsied specimens were fixed overnight in buffered 10% neutral formalin. Then a paraffin-embedded and hematoxylin and eosin-stained section was examined under a light microscope. When we were unable to determine the diagnosis from biopsy specimens with inappropriate cells or the lesion has a non-specific benign result, biopsies were classified as non-diagnostic. Final diagnoses of biopsied lesions were investigated and confirmed by independent surgical pathology (n = 93), rebiopsy or other biopsy (n = 25) or clinical follow-up (n = 219). Clinical proof of benign lesions was accepted when any of the following conditions were satisfied: (1) spontaneous resolution, (2) resolution after appropriated management such as antibiotics or corticosteroid treatment, and (3) benign morphology with no change on the serial follow-up CT.13, 17 All pathological results excluding non-diagnostic biopsy specimens were classified as true or false diagnoses by whether biopsy specimen diagnoses agreed or disagreed with final diagnoses. The non-diagnostic biopsy specimens were classified as false diagnoses.
Complications
Postprocedural complications including pneumothorax and hemorrhage were evaluated by immediate follow-up CT. Pneumothorax involving more than 10% of the hemithorax or requiring additional treatment including chest tube was defined as major pneumothorax. Our institution usually performed chest tube insertion for patients with significant pneumothorax or persistent or increasing amount, or patients who complained of dyspnea due to pneumothorax. Major hemorrhage was defined as hemorrhage presenting with hemoptysis as assessed by operator’s procedural records and review of patients’ medical charts, regardless of the amount of blood.
Statistical analysis
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and postprocedural complication rates were calculated for overall, solid and part-solid lesions. Lesion characteristics were compared between true and false diagnoses. Comparisons used independent-sample t-test for continuous variables or Pearson’s χ2 test for categorical variables. Logistic regression analysis was used to identify factors related to true diagnosis and occurrence of complications. Statistical analysis used SPSS software (SPSS® v. 19.0; IBM Corp., Armonk, NY; formerly SPSS Inc., Chicago, IL).
RESULTS
The study included 354 patients (225 males, 129 females; age range: 19–88 years; mean age: 65 years). Pathological results from CT-guided CNB specimens were malignant in 211 and benign in 107 cases. 36 lesions were non-diagnostic results. Final diagnoses were 253 malignant and 101 benign lesions. 281 biopsy specimen results agreed with final diagnoses and 37 specimen results disagreed.
Overall lesion characteristics and procedure-related factors are in Table 1. Mean lesion diameter was 36 mm (range: 8–115 mm). There were 300 solid lesions and 54 part-solid lesions. In addition, 36 lesions had cavities and 102 lesions had air-bronchograms. There were underlying emphysema in 61 patients and pulmonary fibrosis in 12 patients. For biopsy procedures, mean distance of the total needle path was 55 mm (range: 16–119 mm) and mean procedure time was 18.9 min (range: 9.7–44.7 min).
Table 1.
Lesion characteristics and procedure-related factors
| Size (mm) | Mean | Range |
| 36 | 8–115 | |
| Character | Number of patients | |
| Solid | 300 | |
| Part-solid | 54 | |
| Cavity | 36 | |
| Air-bronchogram | 102 | |
| Underlying lung disease | ||
| Emphysema | 61 | |
| Fibrosis | 12 | |
| Distance (mm) | Mean | Range |
| Total needle path | 55 | 16–119 |
| Skin to pleura | 36 | 12–74 |
| Pleura to lesion | 19 | 0–64 |
| Procedure time (min) | 18.9 | 9.7–44.7 |
Comparing solid and part-solid lesions (Table 2), the lesion size was larger in solid lesions (p < 0.001) and air-bronchogram was found more frequently in part-solid lesions (p < 0.001). Underlying pulmonary emphysema was more frequent in patients with solid lesions (p = 0.011). BMI was significantly higher in patients with part-solid lesions (p < 0.001). However, no significant difference was seen for path distance between solid and part-solid lesions. Procedure time is longer in patients with part-solid lesions than solid lesions (p = 0.021). Mean procedure time was 18.6 min in patients with solid lesions and 20.6 min in patients with part-solid lesions. Malignant results of biopsy specimens (p = 0.003) and final diagnoses of malignancy (p = 0.006) were significantly more frequent in part-solid lesions. Non-diagnostic biopsies were not statistically different between solid and part-solid lesions (p = 0.804).
Table 2.
Comparison of solid and part-solid nodules on CT and biopsy specimen
| Factors | Solid nodules (n = 300) |
Part-solid nodules (n = 54) |
p-value |
| Age (years) | 65.2 | 64.7 | 0.796 |
| Sex | 0.102 | ||
| Male | 196 | 29 | |
| Female | 104 | 25 | |
| BMI | 22.2 | 24.1 | <0.001 |
| Hospital stay (days) | 11.9 | 12.0 | 0.973 |
| Size (mm) | 37.7 | 29.2 | <0.001 |
| Cavity | 33 | 3 | 0.327 |
| Air-bronchogram | 57 | 45 | <0.001 |
| Underlying emphysema | 58 | 3 | 0.011 |
| Underlying fibrosis | 11 | 1 | 0.701 |
| Distance of path (mm) | 54.1 | 58.6 | 0.120 |
| Pleura to lesion (mm) | 18.55 | 21.0 | 0.268 |
| Skin to pleura (mm) | 35.7 | 37.6 | 0.228 |
| Procedure time (min) | 18.6 | 20.6 | 0.021 |
| Pathologic result | 0.003 | ||
| Malignant | 169 | 42 | |
| Benign | 101 | 6 | |
| Non-diagnostic result | 30 | 6 | 0.804 |
| Final diagnosis | 0.006 | ||
| Malignant | 206 | 47 | |
| Benign | 94 | 7 |
BMI, body mass index
Data are presented as number of studies or means. Means are weighted by procedures.
For CT-guided CNB for diagnosing malignancy, overall sensitivity was 83.0%, specificity was 99.0%, accuracy was 87.6%, PPV was 99.5%, and NPV was 69.9% (Table 3). There was no statistically significant difference in the diagnostic yields including sensitivity, specificity, accuracy, PPV and NPV between solid and part-solid lesions. The NPV and sensitivity in part-solid lesions were lower than solid lesions but not statistically significant (Figure 1).
Table 3.
Diagnostic confidence and accuracy of CT-guided CNB based on lesion character of solid and part-solid
| Variable (%) | All | Solid nodule | Part-solid nodule | p-value |
| Sensitivity | 83.0 (210/253) | 81.5 (168/206) | 89.3 (42/47) | 0.200 |
| Specificity | 99.0 (100/101) | 98.9 (93/94) | 100.0 (7/7) | 0.781 |
| Accuracy | 87.6 (310/354) | 87.0 (261/300) | 90.7 (49/54) | 0.448 |
| PPV | 99.5 (210/211) | 99.4 (168/169) | 100.0 (42/42) | 0.615 |
| NPV | 69.9 (100/143) | 70.9 (93/131) | 58.3 (7/12) | 0.364 |
CNB, core needle biopsy; PPV, positive predictive value; NPV, negative predictive value.
Data in parenthesis are presented as number of procedures.
Figure 1.
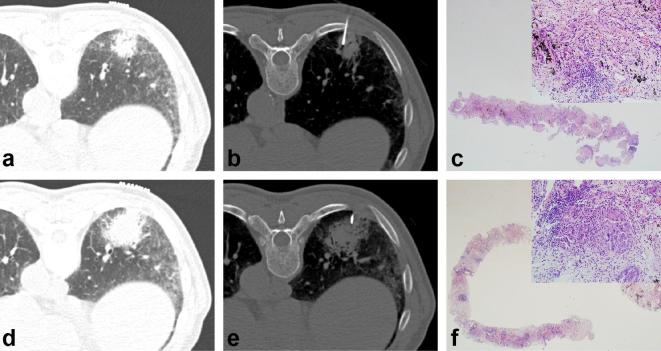
Acinar-type adenocarcinoma in a 73-year-old male that was diagnosed on the second CT-guided CNB. (a) Preprocedural CT scan shows a 36 mm part-solid nodule with air-bronchogram in the right lower lobe. Reticulation and GGO is seen in the subpleural area of adjacent lung parenchyma, consistent with underlying pulmonary fibrosis. (b) CT scan obtained during the biopsy shows the needle tip targeting the solid portion of the part-solid nodule. (c) Low and high magnification of the first core biopsy specimen shows only fibrotic septal thickening with infiltration of lymphoplasma cells (hematoxylin–eosin stain, 12.5x; insert 100x). (d) 80 days after the first CT-guided CNB, the second biopsy was performed. Preprocedural CT scan shows the part-solid nodule which is increased in size compared with prior study. (e) CT scan obtained during the second biopsy shows the needle targeting the solid portion of the part-solid nodule. (f) Low and high magnification of the second core biopsy specimen shows several foci of abortive glandular structures against a background of fibrotic lung parenchyma with infiltration of lymphoplasma cells and histiocytes. Tumor cells are characterized by hyperchromatic nuclei with prominent nucleoli and an increased nuclear-to-cytoplasmic ratio (hematoxylin–eosin stain, 12.5x; insert 100x). CNB, core needle biopsy; GGO, ground-glass opacity.
Comparing true and false diagnoses, there was no significant difference of lesion characteristics (Table 4). By logistic regression analysis, histopathological malignancy was the only factor significantly related to true diagnosis (p < 0.001, odd ratio (OR) = 106.479 [95% confidence interval (CI):14.337–790.828]). The characteristic of solid or part-solid lesion was not significantly related to false diagnosis of CT-guided CNB specimen.
Table 4.
Comparison between true and false diagnosis group on CT and biopsy specimen
| Factors | True diagnosis (n = 281) |
False diagnosis (n = 37) |
p-value |
| Age (years) | 65.0 | 67.7 | 0.181 |
| Sex | 0.505 | ||
| Male | 174 | 25 | |
| Female | 107 | 12 | |
| BMI | 23.0 | 23.5 | 0.415 |
| Hospital stay (days) | 12.2 | 14.1 | 0.536 |
| Size (mm) | 36.1 | 41.5 | 0.168 |
| Solid vs part-solid | 0.439 | ||
| Solid | 237 | 33 | |
| Part-solid | 44 | 4 | |
| Cavity | 25 | 2 | 0.753 |
| Air-bronchogram | 84 | 10 | 0.719 |
| Underlying emphysema | 51 | 5 | 0.486 |
| Underlying fibrosis | 10 | 2 | 0.638 |
| Distance of path (mm) | 55.4 | 50.7 | 0.168 |
| Pleura to lesion (mm) | 19.3 | 15.8 | 0.197 |
| Skin to pleura (mm) | 36.1 | 34.9 | 0.542 |
| Procedure time (min) | 18.7 | 19.6 | 0.360 |
| Pathologic result | <0.001 | ||
| Malignant | 210 | 36 | |
| Benign | 71 | 1 | |
| Final diagnosis | 0.002 | ||
| Malignant | 210 | 36 | |
| Benign | 71 | 1 |
BMI, body mass index
Data are presented as number of studies or means. Means are weighted by procedures.
For biopsy-induced complications, hemorrhage was the most frequent complication (Table 5). It appeared on CT images performed immediately after biopsy in 144 patients (41%). Among these patients, 62 (18%) had major hemorrhage. In 93 (26%) patients, pneumothorax was found by immediate follow-up CT scan. Among these patients, 18 (5%) had major pneumothorax. When comparing between solid and part-solid lesion, the hemorrhage on postprocedural follow-up CT was frequently observed in part-solid lesions (p = 0.016) (Figure 2). The occurrence of major hemorrhage (p = 0.859), pneumothorax (p = 0.106) and major pneumothorax (p = 0.240) was not significantly different.
Table 5.
Complication rates of CT-guided CNB in solid and part-solid and overall lesions and comparison of complication rates of CT-guided CNB between solid and part-solid lesions group
| Compli cations |
Overall (%) | Solid (%) | Part-solid (%) | p-value |
| Hemorrhage | 144 (41) | 114 (38) | 30 (56) | 0.016 |
| Major hemorrhage | 62 (18) | 53 (18) | 9 (17) | 0.859 |
| Pneumothorax | 93 (26) | 74 (25) | 19 (35) | 0.106 |
| Major pneumothorax | 18 (5) | 17 (6) | 1 (2) | 0.240 |
Figure 2.
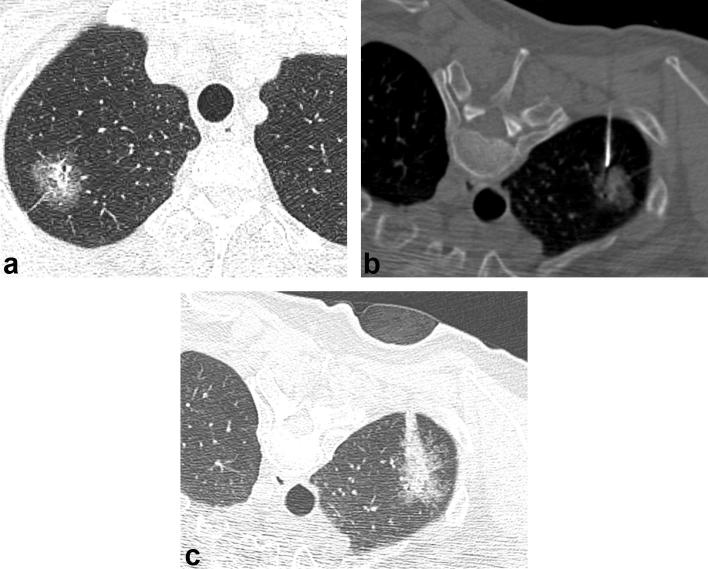
CT-guided CNB in a 60-year-old male with a part-solid nodule in the right upper lobe. (a) Axial CT scan obtained 8 days before CT-guided CNB shows a 25 mm part-solid nodule in the right upper lobe. (b) CT scan obtained during the biopsy shows the needle tip targeting the solid portion of the part-solid nodule. (c) CT scan obtained after the biopsy shows consolidation and GGO developed along the needle insertion pathway, consistent with hemorrhage. Patient had no symptoms and did not require any treatment. The result of CNB was adenocarcinoma with undetermined invasiveness. The specimen obtained through the surgical resection revealed minimally invasive adenocarcinoma, pT1 (not shown here). CNB, core needle biopsy; GGO, ground-glass opacity.
When potential factors related to hemorrhage were evaluated, part-solid lesions were significantly related to postprocedural hemorrhage [p = 0.017, OR = 2.039 95% CI (1.136–3.662)] by univariate logistic regression. In multivariate logistic regression analysis, path distance [p = 0.001, OR = 1.021 95% CI (1.009–1.034)], lesion size [p = 0.029, OR = 0.985 95% CI (0.972–0.998)], and patient BMI [p = 0.045, OR = 1.054 95% CI (1.001–1.109)] were significant factors related to postprocedural hemorrhage. There was no significant factor related to the major hemorrhage.
By univariate logistic regression analysis, lesion size (p = 0.018) and underlying pulmonary emphysema (p = 0.012) were significantly related to pneumothorax. The factor related to major pneumothorax was the underlying pulmonary emphysema [p = 0.001, OR = 5.462 95% CI (2.070–14.408)].
DISCUSSION
CT-guided CNB is a widely accepted technique and is one of the procedures of choice for the evaluation of pulmonary lesions including part-solid nodules, which have a high incidence of malignancy.
The previously reported diagnostic accuracy of CT-guided CNB was 62–93% for malignant lesions in general. The overall sensitivity (83.0%), specificity (99.0%), and accuracy (87.6%) of CT-guided CNB in this study were similar to those in earlier studies.4–9 Several studies have focused on differences in the diagnostic accuracy of CT-guided needle biopsy according to ground-glass opacity (GGO) portion. According to Shimizu et al., the diagnostic yields of CT-guided aspiration biopsy are significantly lower for GGO-dominant lesions than solid lesions.12 Meanwhile, Hur et al. also reported that diagnostic accuracy was significantly influenced by GGO component.10 However, these results might be related to the use of an aspiration needle, not a cutting needle. The difference in accuracy might be due to the low cellularity of aspirates from part-solid lesions.18–20 Thus, the results of studies using FNA biopsy might differ from studies using CNB, which can obtain adequate core specimens. Yamagami et al. reported that the diagnostic accuracy of malignancy differs significantly according to GGO component, even with CNB.13 However, Kim et al. reported conflicting results, specifically that the diagnostic accuracy of CNB is not influenced by GGO component proportion.7 These two CNB studies included only patients with part-solid lesions and analyzed differences according to GGO component proportion.
In this study, we compared the diagnostic accuracy between solid and part-solid lesions, which was not significantly different. The occurrence of non-diagnostic results were also not significantly different for solid (n = 30, 10.0%) and part-solid lesions (n = 6, 11.1%, p = 0.804). CT-guided biopsies targeted the solid portion of both solid and part-solid lesions, which often represents areas of invasive adenocarcinoma in otherwise in situ neoplasms.21, 22 The GGO extent of part-solid nodules correlates with the extent of tumor cell lepidic growth.16, 23,24 Thus, we assumed that if the GGO portion of part-solid lesions comprised neoplastic cells with a lepidic pattern, differentiation between malignant and benign lesions would be possible based on pathological results from only the GGO portions of the biopsy specimens, although the invasiveness of whole tumors was difficult to evaluate.
The sensitivity and NPV were lower for part-solid lesions than solid lesions, but this difference was not statistically significant. Part-solid lesions were more often malignant than solid lesions,2, 3 and lower NPV is thought to be influenced by the higher prevalence of malignancy of part-solid lesions than solid lesions.
The reported incidence of postbiopsy pneumothorax, which is the most common complication of needle aspiration or biopsy of lung lesions, is 17.9–54.3%. And the chest tube insertion rate is 1–14.2%.17,25–29 Pulmonary hemorrhage is the second most common complication of needle biopsy, with a reported incidence of 2–7%.5, 8,17,25 The pneumothorax rate (26%) in this study was similar to reported rates, but the prevalence of pulmonary hemorrhage (41%) was above the reported range. The high hemorrhage rate was likely because all patients with perilesional opacity on follow-up CT scan were accounted for, regardless of the presence of hemoptysis, which previous studies used as the definition of postprocedural hemorrhage. The rate of major hemorrhage (18%), defined as hemorrhage presenting with hemoptysis, was within the reported range.
Choi et al. reported that postbiopsy hemoptysis is more common for part-solid nodules than solid tumors.30 In agreement with their study, this result indicated that the part-solid characteristic was significantly related to postprocedural hemorrhage (p = 0.017). We attributed this to the patent airways and blood vessels in part-solid nodules. The extent of GGO in a lung nodule on CT is correlated with the extent of lepidic tumor growth on histopathology, indicating growth along the alveolar wall without destroying the underlying architecture.16, 23,24 Thus, patent airways and blood vessels within part-solid nodules might be more closely related to a higher risk of postbiopsy hemorrhage compared to solid nodules. In addition, during biopsy of part-solid nodules, needle passage through the aerated portion, which exhibits less compression than solid portions, may be inevitable. Choi et al. suggested that the relative looseness of part-solid nodules may lead to lack of effective tamponade of injured structures in tissue adjacent to biopsies.30 Targeting to avoid violating aerated lungs is an important aspect of preventing hemorrhage in part-solid nodules.31
Small tumor size was reportedly associated with an increased risk of pneumothorax.2, 13,28,32 In our study, the small size was associated with pneumothorax on univariate logistic regression. However, because the nodules of this study were relatively large, its statistical significance was unclear on multivariate logistic regression.
CT-guided CNB took a little longer for part-solid lesions than for solid lesions. Mean procedure time was 18.6 min in solid lesions and 20.6 min in part-solid lesions. Although the difference was not that great, there was a need to plan for the part-solid lesions carefully which were smaller than solid lesions and the non-solid portion was avoided when obtaining tissue.
This study had several limitations. First, a relatively small number of patients with part-solid lesions (54 of 354) were included. Thus, diagnostic yields and postbiopsy complication rates for part-solid lesions require further validation with a larger study population. Second, this study did not include very small lung nodules (<8 mm). Thus, we cannot confidently state that our results apply to very small lung nodules. When we assessed the histopathological results of CNB specimens, we decided only agreements by dividing results into malignant, benign and non-diagnostic categories without considering specific pathological differences. And, we did not quantitatively correlate the GGO portions with pathological features.
In conclusion, the accuracy of CT-guided CNB for diagnosing malignancy was comparable for solid and part-solid lesions. Although postprocedural hemorrhage occurred more frequently in part-solid lesions, the frequency of major hemorrhage was not significantly different. Therefore, CT-guided CNB can be considered for histopathological confirmation of intrapulmonary lesions regardless the presence of GGO portion.
Contributor Information
Sam Yun, Email: ysam88@naver.com.
Hee Kang, Email: kanghi81@gmail.com.
Sekyoung Park, Email: yks1ok@naver.com.
Beom Su Kim, Email: zxczxc@gmail.com.
Jung Gu Park, Email: cibertim@naver.com.
Min Jung Jung, Email: mj2smile@hanmail.net.
REFERENCES
- 1.Park CM, Goo JM, Lee HJ, Lee CH, Kim HC, Chung DH, et al. CT findings of atypical adenomatous hyperplasia in the lung. Korean J Radiol 2006; 7: 80–6. doi: 10.3348/kjr.2006.7.2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li F, Sone S, Abe H, Macmahon H, Doi K. Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings. Radiology 2004; 233: 793–8. doi: 10.1148/radiol.2333031018 [DOI] [PubMed] [Google Scholar]
- 3.Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS. ELCAP Group CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002; 178: 1053–7. doi: 10.2214/ajr.178.5.1781053 [DOI] [PubMed] [Google Scholar]
- 4.Klein JS, Salomon G, Stewart EA. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology 1996; 198: 715–20. doi: 10.1148/radiology.198.3.8628859 [DOI] [PubMed] [Google Scholar]
- 5.Lucidarme O, Howarth N, Finet JF, Grenier PA. Intrapulmonary lesions: percutaneous automated biopsy with a detachable, 18-gauge, coaxial cutting needle. Radiology 1998; 207: 759–65. doi: 10.1148/radiology.207.3.9609901 [DOI] [PubMed] [Google Scholar]
- 6.Tsukada H, Satou T, Iwashima A, Souma T. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol 2000; 175: 239–43. doi: 10.2214/ajr.175.1.1750239 [DOI] [PubMed] [Google Scholar]
- 7.Kim TJ, Lee JH, Lee CT, Jheon SH, Sung SW, Chung JH, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2008; 190: 234–9. doi: 10.2214/AJR.07.2441 [DOI] [PubMed] [Google Scholar]
- 8.Haramati LB. CT-guided automated needle biopsy of the chest. AJR Am J Roentgenol 1995; 165: 53–5. doi: 10.2214/ajr.165.1.7785631 [DOI] [PubMed] [Google Scholar]
- 9.Hayashi N, Sakai T, Kitagawa M, Kimoto T, Inagaki R, Ishii Y, et al. CT-guided biopsy of pulmonary nodules less than 3 cm: usefulness of the spring-operated core biopsy needle and frozen-section pathologic diagnosis. AJR Am J Roentgenol 1998; 170: 329–31. doi: 10.2214/ajr.170.2.9456939 [DOI] [PubMed] [Google Scholar]
- 10.Hur J, Lee HJ, Nam JE, Kim YJ, Kim TH, Choe KO, et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2009; 192: 629–34. doi: 10.2214/AJR.08.1366 [DOI] [PubMed] [Google Scholar]
- 11.Lu CH, Hsiao CH, Chang YC, Lee JM, Shih JY, Wu LA, et al. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol 2012; 7: 143–50. doi: 10.1097/JTO.0b013e318233d7dd [DOI] [PubMed] [Google Scholar]
- 12.Shimizu K, Ikeda N, Tsuboi M, Hirano T, Kato H. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006; 51: 173–9. doi: 10.1016/j.lungcan.2005.10.019 [DOI] [PubMed] [Google Scholar]
- 13.Yamagami T, Yoshimatsu R, Miura H, Yamada K, Takahata A, Matsumoto T, et al. Diagnostic performance of percutaneous lung biopsy using automated biopsy needles under CT-fluoroscopic guidance for ground-glass opacity lesions. Br J Radiol 2013; 86: 20120447. doi: 10.1259/bjr.20120447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li GC, Fu YF, Cao W, Shi YB, Wang T. Computed tomography-guided percutaneous cutting needle biopsy for small (≤ 20 mm) lung nodules. Medicine 2017; 96: e8703. doi: 10.1097/MD.0000000000008703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SM, Park CM, Lee KH, Bahn YE, Kim JI, Goo JM. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: clinical experience in 1108 patients. Radiology 2014; 271: 291–300. doi: 10.1148/radiol.13131265 [DOI] [PubMed] [Google Scholar]
- 16.Goo JM, Park CM, Lee HJ. Ground-glass nodules on chest CT as imaging biomarkers in the management of lung adenocarcinoma. AJR Am J Roentgenol 2011; 196: 533–43. doi: 10.2214/AJR.10.5813 [DOI] [PubMed] [Google Scholar]
- 17.Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW, Cheung YC, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004; 126: 748–54. doi: 10.1378/chest.126.3.748 [DOI] [PubMed] [Google Scholar]
- 18.Wang SE, Nieberg RK. Fine needle aspiration cytology of sclerosing hemangioma of the lung, a mimicker of bronchioloalveolar carcinoma. Acta Cytol 1986; 30: 51–4. [PubMed] [Google Scholar]
- 19.Greif J, Marmur S, Schwarz Y, Man A, Staroselsky AN. Percutaneous core cutting needle biopsy compared with fine-needle aspiration in the diagnosis of peripheral lung malignant lesions: results in 156 patients. Cancer 1998; 84: 144–7. [DOI] [PubMed] [Google Scholar]
- 20.Idowu MO, Powers CN. Lung cancer cytology: potential pitfalls and mimics - a review. Int J Clin Exp Pathol 2010; 3: 367–85. [PMC free article] [PubMed] [Google Scholar]
- 21.Sawada S, Komori E, Nogami N, Segawa Y, Shinkai T, Yamashita M. Evaluation of lesions corresponding to ground-glass opacities that were resected after computed tomography follow-up examination. Lung Cancer 2009; 65: 176–9. doi: 10.1016/j.lungcan.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 22.Vazquez M, Carter D, Brambilla E, Gazdar A, Noguchi M, Travis WD, et al. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 2009; 64: 148–54. doi: 10.1016/j.lungcan.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HY, Lee KS. Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imaging 2011; 26: 106–18. doi: 10.1097/RTI.0b013e3181fbaa64 [DOI] [PubMed] [Google Scholar]
- 24.Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013; 31: 992–1001. doi: 10.1200/JCO.2012.46.9270 [DOI] [PubMed] [Google Scholar]
- 25.Khan MF, Straub R, Moghaddam SR, Maataoui A, Gurung J, Wagner TO, et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol 2008; 18: 1356–63. doi: 10.1007/s00330-008-0893-1 [DOI] [PubMed] [Google Scholar]
- 26.Covey AM, Gandhi R, Brody LA, Getrajdman G, Thaler HT, Brown KT. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol 2004; 15: 479–83. doi: 10.1097/01.RVI.0000124951.24134.50 [DOI] [PubMed] [Google Scholar]
- 27.Saji H, Nakamura H, Tsuchida T, Tsuboi M, Kawate N, Konaka C, et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: the angle of the needle trajectory is a novel predictor. Chest 2002; 121: 1521–6. [DOI] [PubMed] [Google Scholar]
- 28.Laurent F, Michel P, Latrabe V, Tunon de Lara M, Marthan R. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR Am J Roentgenol 1999; 172: 1049–53. doi: 10.2214/ajr.172.4.10587145 [DOI] [PubMed] [Google Scholar]
- 29.Laurent F, Latrabe V, Vergier B, Michel P. Percutaneous CT-guided biopsy of the lung: comparison between aspiration and automated cutting needles using a coaxial technique. Cardiovasc Intervent Radiol 2000; 23: 266–72. doi: 10.1007/s002700010067 [DOI] [PubMed] [Google Scholar]
- 30.Choi JW, Park CM, Goo JM, Park YK, Sung W, Lee HJ, et al. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of small (≤ 20 mm) lung nodules: diagnostic accuracy and complications in 161 patients. AJR Am J Roentgenol 2012; 199: W322–30. doi: 10.2214/AJR.11.7576 [DOI] [PubMed] [Google Scholar]
- 31.Moore EH. Technical aspects of needle aspiration lung biopsy: a personal perspective. Radiology 1998; 208: 303–18. doi: 10.1148/radiology.208.2.9680552 [DOI] [PubMed] [Google Scholar]
- 32.Heyer CM, Reichelt S, Peters SA, Walther JW, Müller KM, Nicolas V. Computed tomography-navigated transthoracic core biopsy of pulmonary lesions: which factors affect diagnostic yield and complication rates? Acad Radiol 2008; 15: 1017–26. doi: 10.1016/j.acra.2008.02.018 [DOI] [PubMed] [Google Scholar]