Abstract
Individuals with 22q11.2 deletion syndrome (22q11.2DS) are at elevated risk of developing treatable psychiatric and neurological disorders, including anxiety disorders, schizophrenia, seizures, and movement disorders, often beginning in adolescence or early to mid adulthood. Here, we provide an overview of neuropsychiatric features associated with 22q11.2DS in adulthood. Results of a new case series of 13 individuals with 22q11.2DS and catatonic features together with 5 previously reported cases support a potential association of this serious psychomotor phenotype with the 22q11.2 deletion. As in the general population, catatonic features in 22q11.2DS occurred in individuals with schizophrenia, other psychotic and non-psychotic psychiatric disorders, and neurological disorders like Parkinson’s disease. We place the results in the context of an updated review of catatonia in genetic conditions. The complex neuropsychiatric expression and risk profile of 22q11.2DS highlights the need to consider co-morbid factors and provide care tailored to the individual patient. The results reinforce the need for periodic monitoring for the emergence of psychiatric and neurological features, including catatonic features. Pending further research, enhanced recognition and informed anticipatory care promise to facilitate the early diagnosis that allows for timely implementation and optimization of effective treatments.
The phenotypic expression of 22q11.2 deletion syndrome (22q11.2DS) is variable between individuals and across the lifespan [Bassett et al., 2011; Fung et al., 2015]. Survival to adulthood is the norm, related in part to advances in pediatric care, e.g., of associated features such as congenital cardiac and other anomalies [Bassett et al., 2009; McDonald-McGinn et al., 2015]. Thus there is an urgent need to better understand and manage the persisting and later onset features of the 22q11.2DS phenotype. Major neuropsychiatric manifestations that may affect the adult 22q11.2DS population include intellectual disability, anxiety disorders, schizophrenia, seizures/epilepsy, and Parkinson’s disease [Bassett et al., 2017; Butcher et al., 2013; Fung et al., 2015; Wither et al., 2017]. Psychiatric disorders comprise the most common group of later onset manifestations associated with 22q11.2DS [Bassett et al., 2005; Fung et al., 2010; Philip and Bassett 2011]. The one in four risk of developing schizophrenia in 22q11.2DS is of particular concern to patients and their families given its potential seriousness, associated stigma, and impact on functioning [Butcher et al., 2012; Hercher and Bruenner 2008; Karas et al., 2014]. Parkinson’s disease and other neuromotor features have also emerged as important aspects of the adult expression of 22q11.2DS [Boot et al., 2015; Butcher et al., 2013; Butcher et al., 2017; Mok et al., 2016].
Here, we provide an overview of the psychiatric, neurological and co-morbid features associated with 22q11.2DS in adulthood. To illustrate the complex neuropsychiatric expression and multiple confounding factors often associated with the syndrome, we present a new case series of 13 patients with 22q11.2DS who demonstrated features of catatonia, a complex psychomotor phenotype that may be another manifestation of 22q11.2DS. In this context, we also review 5 previously reported cases and the literature on other genetic conditions with catatonia.
Neuropsychiatric manifestations of 22q11.2DS
Schizophrenia and other psychotic disorders
Schizophrenia associated with 22q11.2DS is indistinguishable from other forms of schizophrenia with respect to prodrome, age at onset, the core signs and symptoms, and cognitive profile, with the exception of overall lower average IQ and the absence of sex differences [Bassett et al., 2003; Chow et al., 2006; Fung et al., 2015; Karayiorgou et al., 2010; van Amelsvoort et al., 2004]. Schizophrenia and related psychotic disorders, such as schizoaffective disorder, are highly enriched in 22q11.2DS [Bassett et al., 2017; McDonald-McGinn et al., 2015]. Symptoms include delusions, hallucinations, disorganized thinking, disorganized emotional expression and/or behavior, as well as blunted affect, reduced speech, social withdrawal, and motor disturbances [Association ; Walther and Strik 2012]. While mood disorders with psychotic features are reported, there is no evidence that prevalence is greater than general population expectations [Fung et al., 2015].
Non-psychotic disorders
Other psychiatric disorders that do not involve psychosis also occur in adults with 22q11.2DS at elevated rates [Bassett et al., 2017]. Specifically, anxiety disorders are reported in approximately 30% of adolescents and adults with 22q11.2DS, most often as generalized anxiety disorder, social phobia, and/or panic disorder [Fung et al., 2010; Schneider et al., 2014]. In contrast, rates of depression do not appear to be elevated [Fung et al., 2010]. Anxiety disorders may emerge during adulthood or persist from childhood. Attention deficit hyperactivity disorder (ADHD) persists to adulthood in as many as 15% of adults with 22q11.2DS, most commonly manifesting as the inattentive subtype [Schneider et al., 2014]. Notably, the presence of childhood autism spectrum disorders (ASD) in 22q11.2DS appears to be unrelated to the later appearance of schizophrenia [Fiksinski et al., 2017; Vorstman et al., 2013]. Even though the symptoms of ASD usually persist throughout life, to date there are no reports of ASD in cohorts of adult patients with 22q11DS, probably because systematic diagnostic screening for ASD is usually not part of standard assessments of adults.
Seizure disorders
All types of seizures have been associated with 22q11.2DS (Table 1) and may emerge at any age [Fung et al., 2015; Kao et al., 2004; Wither et al., 2017]. Seizures in patients with 22q11.DS may be unprovoked, or related to factors such as hypocalcemia, psychiatric medication use, neurodevelopmental cortical malformations, fever, hypoxia, ischemia, or surgery. The associated seizures can be of generalized or focal onset. A recent study found that 4% of adults with 22q11.2DS met diagnostic criteria for epilepsy [Wither et al., 2017], higher than general population estimates (0.5–1%). Seizures may involve abnormal and/or repetitive movements such as twitching, and abnormal postures and importantly, may be difficult to distinguish from other causes of such presenting symptoms [Benbadis 2009].
Table 1.
Co-morbidity and neuropsychiatric expression in adults with 22q11.2 deletion syndrome.a
| Feature (estimated prevalence) | Clinical implications |
|---|---|
| Endocrine | |
| Hypocalcemia (>60%)/hypoparathyroidism | Monitoring of calcium/PTH levels; vitamin D and calcium supplementation |
| Hypomagnesemia (15%) | Monitoring of magnesium levels; magnesium supplementation |
| Thyroid dysfunction and/or treatment-related | Annual monitoring of thyroid function; standard treatment for the disorder |
| Hypothyroidism (20%) | Initiate or adjust treatment |
| Hyperthyroidism (5%) | Initiate or adjust treatment |
| Hypoxia, e.g., obstructive sleep apnea, asthma, COPD | Standard management |
| Musculoskeletal | |
| Craniocervical spine anomalies with spinal cord compression | MRI; cervical traction therapy, neurosurgery, as required |
| Patellar dislocation (10%) | As applicable |
| Scoliosis (30%) | Standard assessment; surgery, bracing, physical therapy, as necessary |
| Neuropsychiatric and/or treatment-related | |
| Psychotic disorders (25%); considerations for antipsychotics include lowered seizure threshold, propensity to obesity, vulnerability to motor side-effects | Consider medication choice based on efficacy and seriousness of side effects; prophylactic use of an anticonvulsant with clozapine treatment |
| Anxiety disorders | Standard management |
| Medication-induced neurologic/psychiatric abnormalities, e.g., antipsychotics and parkinsonism; L-dopa and psychosis | Adjust dosage, reconsider medication choice, “start low, go slow” dosing approach; consider medication to treat the side effect |
| Motor abnormalities associated with co-morbid psychiatric features, e.g., schizophrenia, anxiety | Treatment of the underlying psychiatric disorder |
| Parkinsonism of unknown etiology | Manage symptoms |
| Parkinson’s disease, especially early-onset (>5%) | Dopaminergic therapy; careful balancing with antipsychotics, as applicable |
| Psychogenic/functional neurological disorder | Standard management |
| Seizures (40%) | |
| Epilepsy (<5%) | EEG, neuroimaging (CT and/or MRI), anticonvulsant medications |
| Hypocalcemic seizures | As above for hypocalcemia |
| Other, e.g., fever, surgery | Manage as applicable, consider differential diagnoses/contributing factors |
| Catatonic features | Standard management; treatment of underlying disorder |
| Stroke, long term sequelae | Manage symptoms |
| Substance use disorder. e.g., drug, alcohol | Standard management |
| Caffeine-related anxiety, agitation, and/or tremor | Enquire about, encourage decrease/discontinuation, e.g., cola /energy drinks |
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography; EEG, electroencephalography; MRI, magnetic resonance imaging; PTH, parathyroid hormone; y, year.
Adapted from Boot et al., 2015. Monitoring and treatment recommendations for 22q11.2DS are described in detail elsewhere [Bassett et al., 2011; Fung et al., 2015]
Estimates of lifetime prevalence of features for 22q11.2DS based on available data [Bassett et al., 2009; Bassett et al., 2005; Bassett et al., 2011; Fung et al., 2015; McDonald-McGinn et al., 2015]. Estimates may vary depending on age of the patient and patient ascertainment sources, or change with increasing knowledge of 22q11.2DS. Onset may occur from pediatric through adult years as applicable, see text for details.
Parkinson’s disease and neurodegenerative features
Recently confirmed to be associated with 22q11.2DS [Butcher et al., 2013; Mok et al., 2016], Parkinson’s disease is a progressive neurodegenerative disorder characterized by motor symptoms including bradykinesia, resting tremor, rigidity, and postural instability [Hughes et al., 1992], and the loss of nigrostriatal dopaminergic neurons. Initial evidence suggests that the 22q11.2 deletion may account for approximately 0.5% of early-onset Parkinson’s disease cases in the general population [Mok et al., 2016] and that Parkinson’s disease may affect approximately 6% of individuals aged 36 to 64 years with 22q11.2DS [Butcher et al., 2013]. Individuals with the 22q11.2DS form of Parkinson’s disease appear to show similar dopaminergic cell loss, clinical symptoms, disease course, and treatment response as those with classical Parkinson’s disease, though with early-onset (i.e., <50 years) and variable presence of typical Lewy bodies on postmortem [Booij et al., 2010; Butcher et al., 2013; Mok et al., 2016; Zaleski et al., 2009]. Diagnostic delays were reported in individuals with comorbid schizophrenia, likely related to difficulties differentiating Parkinson’s disease symptoms from antipsychotic related parkinsonism [Butcher et al., 2013]. Profound cognitive deterioration of unknown etiology has also been reported in some 22q11.2DS patients with intellectual disability and psychosis [Evers et al., 2014b].
Other movement disorders and motor abnormalities
Psychiatric disorders and/or their associated treatments may cause or contribute to the manifestation of motor symptoms in 22q11.2DS (Table 1). For example, parkinsonism, dystonias or dyskinesias can represent innate features of schizophrenia in the absence of antipsychotics [Fervaha et al., 2015; Peralta et al., 2010; Walther and Strik 2012]. The antipsychotic medications that are the mainstay of treatment for schizophrenia have the potential to induce, exacerbate, or, interestingly, diminish pre-existing movement disorders [Peluso et al., 2012]. Other psychotropic medications commonly used in 22q11.2DS such as antidepressants and anticonvulsants can also cause adverse motor effects [Damsa et al., 2004; Kennedy and Lhatoo 2008]. Case reports suggest an increased vulnerability to antipsychotic-related movement disorders in 22q11.2DS that may include drug-induced parkinsonism, non-epileptic myoclonus, and dystonia [Boot et al., 2015; Butcher et al., 2015b; Kontoangelos et al., 2015].
Parkinsonian motor symptoms may also be manifestations of 22q11.2DS itself. For example, a recent study of adults with 22q11.2DS revealed elevated rates of bradykinesia, rigidity, postural instability, and tremor relative to healthy age-matched controls [Butcher et al., 2017]. Bradykinesia was the most common feature, affecting those with and without psychotic illness. Longitudinal studies will be needed to evaluate to what extent these symptoms may represent prodromal signs of Parkinson’s disease.
Psychogenic/functional disorders
Psychogenic/functional neurological disorders may, with caution, be considered in the differential diagnoses for patients with 22q11.2DS (Table 1). Anecdotally, these cases have infrequently presented in clinical practice (e.g., psychogenic non-epileptic seizures or parkinsonism). A psychogenic/functional neurological disorder should only be considered in consultation with a clinician having significant expertise in the relevant fields (e.g., epilepsy, movement disorders) and preferably also with expertise in 22q11.2DS, given the complexities of management [Espay and Lang 2015; Milan-Tomas et al., 2018].
General principles of management for neuropsychiatric disorders in 22q11.2DS
The management of neuropsychiatric disorders in adults with 22q11.2DS follows the same biopsychosocial principles as those applied in the general population; this has been described in detail elsewhere [Fung et al., 2015].
Assessment
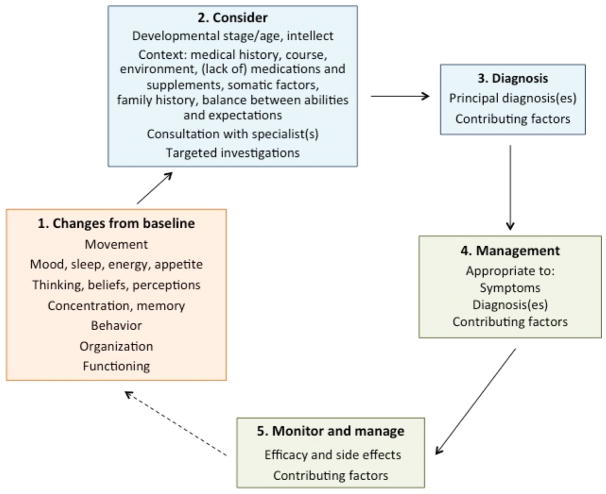
Assessment and monitoring for neuropsychiatric expression in 22q11.2DS requires awareness of issues such as the patient’s baseline state and functioning, intellectual level, and the disorders most likely to present at their neurodevelopmental stage (Figure 1) [Fung et al., 2015]. Assessment begins with obtaining a history from and examining the individual, although some patients tend to minimize problems. For patients with moderate or severe intellectual disability [Evers et al., 2014b; Ferrell et al., 2004] limitations in communication can present additional challenges. Collateral information, including from family members, treating clinicians, and diaries logging symptoms and behaviors, is usually essential for diagnosis and helping to track the development or worsening of neuropsychiatric features. Standardized clinical rating scales (e.g., Unified Parkinson Disease Rating Scale (UPDRS), Clinical Global Impression (CGI) Scale) [Boot et al., 2015; Butcher et al., 2017] and video recordings (e.g., of movement abnormalities) and/or electro-encephalogram (EEG) may be a useful supplement to the history for making a diagnosis, and/or monitoring progress and response to treatment, particularly for patients seen infrequently and by various clinicians.
Figure 1.
Management principles for neuropsychiatric features, including catatonia, in individuals with 22q11.2 deletion syndrome.
Pharmacological management
For psychiatric illnesses associated with 22q11.2DS, management according to general population clinical practice guidelines is indicated [Bassett et al., 2011; Fung et al., 2015]. There are however some additional considerations, particularly for antipsychotic medications. These include the potential for a lowered seizure threshold [Butcher et al., 2015a; Wither et al., 2017], vulnerability to movement disorders [Boot et al., 2015; Butcher et al., 2013; Butcher et al., 2017], and a propensity to obesity [Voll et al., 2017]. Notably, none of these considerations precludes the use of any particular group of psychotropic medications in 22q11.2DS. For example, a “start low and go slow” approach to dosing and concomitant anticonvulsant use may help ameliorate the increased seizure risk associated with what is arguably the most effective antipsychotic, clozapine, that has demonstrated efficacy in 22q11.2DS [Butcher et al., 2015a]. Clozapine, often under-utilized in clinical practice [Farooq and Taylor 2011], is of especially low risk to cause or aggravate motor disturbances [Caroff et al., 2011] and has been used to manage tremor and psychosis in Parkinson’s disease [Thomas and Friedman 2010].
Similarly, standard pharmacological treatments for neurological disorders are recommended, e.g., dopaminergic therapy for Parkinson’s disease (Table 1). Published case reports suggest typical response to standard anticonvulsant medications for managing seizure disorders [Gonzalez and Bautista 2009; Kao et al., 2004]. Some anticonvulsants may also help ameliorate psychiatric symptoms [Kaufman 2011]. However it is important to keep in mind that certain antiepileptic drugs such as valproate are associated with parkinsonism [Brugger et al., 2016]. Notably, oculogyric crisis, a dystonic complication of antipsychotics, may be misinterpreted as a seizure, leading to unnecessary use or increase in doses of anticonvulsant medications.
Non-pharmacological interventions
To date, no study has directly investigated non-pharmacological therapies for a psychiatric disorder in 22q11.2DS [Buijs et al., 2018]. This includes evidence-based effective treatments such as electroconvulsive therapy (ECT) and cognitive-behavioral therapy. However, there are a few preliminary studies of 22q11.2DS involving computer-based and group interventions aimed at improving the neuropsychological deficits that may be associated with psychiatric disorders [Buijs et al., 2018]. Areas of relative strength in adult functioning in 22q11.2DS that could help inform expectations and planning are outlined elsewhere [Butcher et al., 2012].
Concomitant medical features
Appropriate management of other concomitant medical comorbidities are recommended for all patients with 22q11.2DS (Table 1) [Fung et al., 2015]. For example, hypocalcemia can arise at any age and affects the majority (>60%) of adult patients [Cheung et al., 2014b; McDonald-McGinn et al., 2015]. While often asymptomatic, hypocalcemia can be associated with neuropsychiatric symptoms, including irritability, fatigue, tremor, muscle cramps, stiffness, and seizures [Bassett et al., 2011; Kao et al., 2004; Weinzimer 2001; Yu 2014]. Notably, some anticonvulsants, e.g., carbamazepine, phenytoin, may diminish vitamin D levels [Teagarden et al., 2014], potentially increasing the risk of hypocalcemia [Cheung et al., 2014b] and its associated complications. Thyroid disease affects up to one in four patients with 22q11.2DS [Bassett et al., 2005; Cheung et al., 2014a], and similarly may have symptoms or side effects of treatment that can be confused with psychiatric symptoms or overlap with motor features. Disruptions in sleep pattern and the increased risk for obstructive sleep apnea, an understudied feature in 22q11.2DS [Kennedy et al., 2014], and congenital musculoskeletal abnormalities, also warrant consideration in the context of neuropsychiatric symptoms including motor functioning (Table 1).
Catatonia as a complex neuropsychiatric manifestation in 22q11.2DS
In the context of prominent neuropsychiatric expression in 22q11.2DS and a recent study reporting on copy number variations in adults with catatonia [Breckpot et al., 2016], we examined catatonic features as part of neuropsychiatric expression (Table 2). Catatonia is a complex psychomotor phenotype, defined in the DSM-5 [Tandon et al., 2013] as the presence of three or more of the following: 1) catalepsy (i.e., passive induction of a posture held against gravity); (2) waxy flexibility (i.e., slight and even resistance to positioning by examiner); (3) stupor (no psychomotor activity; not actively relating to environment); (4) agitation, not influenced by external stimuli; (5) mutism (i.e., no, or very little, verbal response); (6) negativism (i.e., opposing or not responding to instructions or external stimuli); (7) posturing (i.e., spontaneous and active maintenance of a posture against gravity); (8) mannerisms (i.e., odd caricature of normal actions); (9) stereotypies (i.e., repetitive, abnormally frequent, non-goal directed movements); (10) grimacing; (11) echolalia (i.e., mimicking another’s speech), and (12) echopraxia (i.e., mimicking another’s movements). The DSM-5 allows diagnosis of catatonia as a specifier to multiple neuropsychiatric disorders (including schizophrenia and other psychotic disorders, major mood disorders) or general medical conditions, or as “catatonia not otherwise specified” [Tandon et al., 2013; Solmi et al., 2017].
Table 2.
Catatonic features in individuals with 22q11.2 deletion syndrome.
| Case | Age, y; Sex |
Intellect | Lifetime history of seizure disorder |
Lifetime history of other neurological/ motor issues |
Psychiatric disorders (AAO, y) |
Catatonia | Medications at last assessment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AAO, y |
Features of catatoniaa,b |
Course and response to treatment |
Antipsychotic | Other neuropsychiatric |
||||||
| Current case series | ||||||||||
| 1 | 19, M | Mild ID | - | - | SZ (18), SAD and OCD (14), GAD (12) with panic attacks | ~14 | b Stupor, mutism, not eating | Good response to antipsychotic | risperidone | escitalopram |
| 2 | 17, M | Mild ID | - | - | SZ (16), MDD (16) | 16 | a Stupor, agitation, mutism, negativism, verbal stereotypy | Decreased catatonic features with increased dosage of antipsychotic | risperidone | valproic acid |
| 3 | 18, F | DD | - | Tremors, dyskinesia, insomnia, cognitive decline | PNOS (16) | 16 | Mutism, not eating | Good response to antipsychotic; Limited response at age 16 y to steroid and IVIG therapy, for suspected autoimmune encephalopathy | risperidone | lorazepam, experimentald |
| 4 | 21, F | BL-mild ID | Generalized seizures | - | MDD (12), GAD (12), panic disorder | 16 | Stupor | Gradual reduction in symptoms without any specific treatment | - | sertraline, zoplicone |
| 5 | 33, F | Mild ID | - | Tardive choreiform movements, akathisia | SZ (14) | 19 | b Posturing, stereotypies | Good response to antipsychotic | risperidone, paliperidone | lorazepam |
| 6 | 22, F | NVLD | - | Antipsychotic-induced tremors, akathisia, and acute dystonia | SZA (BP type, 21), GAD (16) | 22 | a Stupor, negativism, motor stereotypies, not eating | Good response to antipsychotic | risperidone | valproic acid |
| 7 | 22, M | Mild ID | - | Restless legs syndrome, tremors, stiffness | SZ (21) | 22 | b Stupor, mannerisms, altered arousal with eyes closed | Catatonic and floridly psychotic at last assessment | lurasidone | clonazepam |
| 8 | 38, F | Mild ID | - | Bilateral tremor (hands) | SZA (22), SAD | 22 | b Stupor, negativism, self-injury, not eating | On antipsychotic gradual improvement over time | olanzapine | valproic acid |
| 9 | 28, F | Mild ID | - | Tremor (hand), myoclonic jerks (arms), tardive dyskinesia | SZ (21), OCD (22) | >22 | a Waxy flexibility, mutism, negativism, posturing, mannerisms, motor and verbal stereotypies | Catatonic and psychotic symptoms at last assessment | paliperidone | fluvoxamine, clonazepam |
| 10 | 40, F | BL | Generalized seizures | Recurrent oculogyric crises | SZ (21), MDD (19) | ~25 | Psychomotor agitation “bordering on catatonic excitement” | Dramatic improvement with ECT and antipsychotic | quetiapine | citalopram, bupropion, lorazepam |
| 11 | 34, F | BL | Generalized seizures | Tremors, cogwheel rigidity, marked speech degradation (slurring, mumbling), query PD/DIP | SZ (15) | <34 | a Stupor, mutism, grimacing, echophenomena, self-injury, not eating | Catatonic and floridly psychotic symptoms at last assessment | chlorpromazine,zuclopenthixol | valproic acid, benztropine |
| 12c | 50, M | BL-mild ID | Generalized seizures | PDb (diagnosed age 48 years), dystonia | SZ (18) | 45 | b Stupor, stereotypies (possible), lethargy, NMS (suspected) | Good response to levodopa/carbidopa; Limited response previously to ECT | - | levodopa/carbidopa, entacapone, pramipexole, fluoxetine, amitriptyline, clonidine, zoplicone, lorazepam |
| 13 | 60, F | Mild ID | - | Mild facial hemiparesis, rigidity, dysphagia, antipsychotic-induced dystonia, orofacial dyskinesias, query Lewy body disease | SZA (57) | ~60 | b Posturing, stereotypies, NMS (suspected) | Ongoing, as are the psychotic symptoms | olanzapine | venlafaxine, trazodone |
| Published case reports | ||||||||||
| Usiskin et al., 1999 | 15, F | BL | NR | Dystonic reaction to risperidone | SZ (12) | 12 | a Waxy flexibility, stupor, mutism | Good response to antipsychotic | olanzapine | NR |
| Faedda et al., 2015 | 15, F | Mild ID | - | Tics, bradykinesia (AAO 12) | PNOS (14), OCD (12), Anxiety | 14 | a Stupor, agitation, mutism, negativism, posturing, grimacing, echolalia,,self-injury, incontinence, not eating | Good response of catatonic features to lorazepam; Partial response of psychotic illness to olanzapine, ziprasidone; No response at age 14 y to IVIG therapy for suspected autoimmune encephalopathy, or age 12 to 14 y to several psychotropic/antiparkinsonian medications (none antipsychotic) | ziprasidone | lorazepam |
| Graf et al., 2001 | 18, F | NR | NR | NR | PNOS (<18) | NR | “Catatonia”, agitation | Good response to antipsychotic and experimental drugc | quetiapine | Experimental drugc |
| Sachdev et al., 2002 | 22, M | Mild-mod ID | Generalized seizures | Myoclonic jerks | PNOS (19) | 19 | a Posturing, stereotypies, grimacing | Fair to good response to antipsychotic | olanzapine | carbamazepine, clonazepam |
| Sieberer et al., 2005 | 22, F | Mild ID | NR | NR | PNOS (22) | 22 | b Stupor, mutism | Good response to lorazepam and antipsychotic | risperidone | NR |
Abbreviations: AAO, age at onset; BL, borderline; BP, bipolar; DD, developmental delay; DIP, drug-induced parkinsonism; ECT, electroconvulsive therapy; F, female; GAD, generalized anxiety disorder; ID, intellectual disability; IVIG, intravenous immunoglobulin; M, male; MDD, major depressive disorder; mod, moderate; NMS, neuroleptic malignant syndrome; NR, not reported; NVLD, non-verbal learning disability; OCD, obsessive compulsive disorder; PD, Parkinson’s disease; PNOS, psychotic disorder not otherwise specified; SAD, social anxiety disorder; SZ, schizophrenia; SZA, schizoaffective disorder; y, years
Met criteria for a diagnosis of catatonia based on retrospectively-applied DSM-5 criteria. See text for details.
Met two of 12 criteria for DSM-5 catatonia. See text for details.
Case 12: Previously published in Parkinson’s disease case series [Butcher et al., 2015a; Butcher et al., 2013]
Experimental drugs: Case 3, minocycline [Dean et al., 2012]; Case in Graf et al., 2001, metyrosine (alpha-methyl-para-tyrosine), a competitive inhibitor of tyrosine hydroxylase, the rate-limiting enzyme of catecholamine synthesis [Bloemen et al., 2008].
NB. All but two of the 13 cases in the new case series had a history of at least one endocrine disorder (hypocalcemia, n=9; hypoparathyroidism, n=6; hypothyroidism, n=4; type 2 diabetes, n=4; hypomagnesium, n=3; data not shown), consistent with the multisystem nature of 22q11.2DS [Fung et al., 2015].
Catatonia is important to recognize both because of its severity and immediate treatment implications. Catatonia can be life-threatening in the form of “malignant” catatonia associated with autonomic dysfunction, hyperthermia, and altered consciousness [Rasmussen et al., 2016] that may be indistinguishable from neuroleptic malignant or toxic serotonin syndromes [Fink and Taylor 2009]. Specific management with proven efficacy includes lorazepam, often provided intravenously (4 to 20 mg/day), or ECT [Fink and Taylor 2009; Luchini et al., 2015; Peralta et al., 2010]. These may be less effective however when catatonia is associated with schizophrenia compared with mood or other disorders [Ungvari et al., 2010].
Case series of patients with 22q11.2DS presenting with catatonic features
In addition to summarizing the five previous reports of 22q11.2DS patients with catatonic features [Faedda et al., 2015; Graf et al., 2001; Sachdev 2002; Sieberer et al., 2005; Usiskin et al., 1999], we present findings from a new case series. We retrospectively reviewed documentation of direct assessments and lifetime medical records at two major North American 22q11.2DS centers (Dalglish Family 22q Clinic for Adults and the Children’s Hospital of Philadelphia 22q and You Center). We included all individuals with molecularly confirmed 22q11.2 deletions where the term “catatonia” or symptoms attributed to catatonia were described. We extracted information on the features of catatonia, response to treatment, and relevant demographic and clinical characteristics including lifetime neuropsychiatric phenotypes. There were no standardized scales for catatonia identified in the charts reviewed. We used the data available and applied DSM-5 criteria to determine whether a retrospective diagnosis of catatonia was warranted. We present results for the 13 subjects (9 female) in our new case series and the 5 previously published cases (4 female) together in Table 2, given the small numbers involved. Also, to place the results for 22q11.2DS in the broader context of the genetics of catatonia, we used OMIM, supplemented by a recent study of catatonia and copy number variation [Breckpot et al., 2016] and reference list searching to identify and synthesize reports of other genetic variants identified in patients with catatonia (Table 3).
Table 3.
Catatonia and genetic anomalies.
| Genetic anomaly | Genes implicated | Details | Reference |
|---|---|---|---|
| Recurrent rare copy number variations and chromosomal anomalies | |||
| 22q11.2 deletion | Multiple | 18 reported cases with catatonic features and/or clinically diagnosed catatoniaa | Current study (including 5 previous reports; Table 2) |
| 15q11q13 maternal duplication or maternal UPD Prader-Willi syndrome | Multiple | 8 cases: 6 (of 29) cases with 15q11q13 duplication and schizophrenia or other psychotic disorders had catatonia; one adolescent female with psychotic illness, mood disorder, and mild ID with recurrent catatonia responsive to lorazepam and haloperidol (initially) and later to ECT; one adolescent male with psychotic illness responsive to lorazepam and risperidone |
[Isles et al., 2016]; [Poser and Trutia 2015]; [Dhossche and Bouman 1997] |
| Trisomy 21 (Down syndrome) | Multiple | 8 cases: 4 adolescents with mood disorders, borderline to severe ID, responsive to benzodiazepines with ECT; 2 adolescent females, one with mood disorder NOS and borderline ID responsive to ECT, one with mood disorder NOS, PDD, severe ID, responsive to lorazepam and fluoxetine; 1 adult female (mosaic type) with depressed mood responsive to ECT; 1 adult male with unspecified psychosis, mood disorder, severe ID responsive to ECT | [Ghaziuddin et al., 2015]; [Jap and Ghaziuddin 2011]; [Jacobs et al., 2016]; [Torr and D’Abrera 2014]; [Breckpot et al., 2016] |
| 22q13.3 deletion | SHANK3 | 4 cases: 2 adult females with PNOS, severe/profound ID, ASD/PDD, responsive to lorazepam/ECT; 2 brothers with moderate to severe ID clinically diagnosed with Clark-Baraitser syndrome | [Breckpot et al., 2016]; [Tabolacci et al., 2005] |
| 16p11.2 duplication | Multiple | 1 case: adult female (typical 600 kb duplication) with schizophrenia, moderate ID, epilepsy, parkinsonism responsive to lorazepam | [Breckpot et al., 2016] |
| 22q11.2 duplication | Multiple | 1 case: adult male with schizophrenia, mild ID, epilepsy, and parkinsonism responsive to ECT (typical 3 Mb duplication) | [Breckpot et al., 2016] |
| Other rare copy number variations | |||
| 9q34.3 deletions | EHMT1 | 5 of 5 cases surviving past age 19 years had dramatic behavioral changes in adolescence with psychiatric diagnoses (all with severe ID): “psychosis, bipolar mood disorder and/or autistic catatonia” | [Kleefstra et al., 2009] |
| 14q11.2 duplication | SUPT16H, CHD8 | 1 case with schizophrenia, moderate ID, and epilepsy, responsive to clozapine | [Breckpot et al., 2016] |
| Rare single gene variants | |||
| Trinucleotide repeat expansion (Huntington’s disease) | HTT | 4 cases: Adult female with Westphal variant (juvenile) Huntingon’s disease, psychotic symptoms, responsive to ECT, lorazepam, augmented with amantadine and levodopa; Adult female with psychotic symptoms, mild depression, anxiety, who had become increasingly psychotic and agitated, not eating or bathing, responsive to ECT; Adult male with psychotic symptoms, suicidal ideation, responsive to ECT, lorazepam, antipsychotic, antidepressant treatment; Adolescent male (refused molecular testing; parent with Huntington’s) with schizophrenia | [Merida-Puga et al., 2011] [Magid et al., 2014] [Cusin et al., 2013] [Consoli et al., 2012] |
| Hexanucleotide repeat expansion | C9orf72 | 2 cases: Adult male with traumatic brain injury, depression with suicide attempt, responsive to antidepressants, aripiprazole augmentation, lorazepam, and ECT; Adult male with major depression/possible dementia and drug-induced parkinsonism. Notably, this mutation is associated with frontotemporal dementia and ALS. | [Holm 2014]; [Bieniek et al., 2014] |
| Trinucleotide repeat expansion (Fragile X premutation) | FMR1 | 1 case: Adult male with bipolar disorder, psychotic symptoms, ADHD, ASD, PDD NOS, OCD, and Tourette syndrome, responsive to benzodiazepines with ECT | [Winarni et al., 2015] |
| Heterozygous mutations (microdeletion and nonsense) | SHANK3 | 22q13.3 locus; 2 cases with atypical bipolar disorder, ASD, and severe ID, responsive to lithium carbonate | [Serret et al., 2015] |
| Missense mutation (homozygosity not assessed) | PRODH | 22q11.2 locus; 1 case with schizophrenia and hyperprolinemia, partially responsive to ECT | [Consoli et al., 2012] |
| Heterozygous missense mutations | KCNT1 | 9q34.3 locus; Nocturnal frontal lobe epilepsy (ENFL5; OMIM #615005), childhood onset, with reported psychosis and catatonia in some cases | [Heron et al., 2012] |
| Homozygous missense mutations | HARS | 5q31.3 locus; Usher syndrome, type III B (USH3B; OMIM #614504) in Old Order Amish families; several cases with psychosis responsive to antipsychotics, 1 with catatonia | [Puffenberger et al., 2012] |
| Homozygous nonsense mutation | MMACHC | 1p34.1 locus; Methylmalonic aciduria and homocystinuria, cblC type (OMIM #277400; 609831.0003), in 2 unrelated girls from 2 consanguineous South Asian families, one with “catatonic psychotic behavior”, seizures, and mild ID, responsive to cobalamin | [Ben-Omran et al., 2007] |
| Heterozygous missense mutationa | PRNP | 20p13 locus; Fatal familial insomnia (OMIM #600072) prion protein disease in adult (18 y) male with psychotic mood disorder; medications and ECT worsened course | [Dimitri et al., 2006] |
| Genome-wide linkage to familial periodic catatonia | |||
| (No mutations identified) | Uncertain; ?VSP19 | 15q15 locus; Deemed a schizophrenia susceptibility locus (SCZD10) for chromosome 15q15-related periodic catatonia (OMIM #605419) using linkage evidence from multiplex periodic catatonia families with a further potential locus at 22q13 in these families; VSP19 was proposed as a 15q15 locus candidate gene for schizophrenia and catatonia from de novo variants identified in a trio study | [Stober et al., 2002] [Xu et al., 2012] |
Abbreviations: ADHD, attention deficit hyperactivity disorder; ALS, amyotrophic lateral sclerosis; ASD, autism spectrum disorder; ECT, electroconvulsive therapy; ID, intellectual disability; k, kilobase; Mb, megabase; NOS, not otherwise specified; OCD, obsessive compulsive disorder; OMIM, Online Mendelian Inheritance in Man; PDD, pervasive developmental disorder; PNOS, psychotic disorder not otherwise specified; UPD, uniparental disomy
PRNP D178N/129 (causative haplotype for fatal familial insomnia involves presence of normal variant M129 in addition to Asp178Asn mutation)
Table 2 summarizes the results recorded for the total 18 cases (n=13, 72% female) identified. Catatonic symptoms had a median onset of 22 (range 12–60) years. Seven patients, including four from the new case series, met criteria for DSM-5 catatonia (i.e., three or more catatonia features); seven others (six in the new case series) had two of these features documented. There were diverse intellectual levels and lifetime histories of neurological and psychiatric disorders for these patients. While all but one patient had a lifetime history of schizophrenia or other psychotic disorder, the later age at onset of catatonic features and/or response to treatment suggested a possible relationship to Parkinson’s disease or other neurological findings in some patients (e.g., case 12, Table 2).
Notably, relatively few of the patients were reported to have received specific treatment for catatonic features (Table 2). Response to ECT was reported to be dramatic for one patient with just one catatonic feature documented, but limited for the patient who responded to standard levodopa/carbidopa treatment of Parkinson’s disease. Although there were reports of positive response of catatonic features to second generation antipsychotic medications for 11 of the 14 with catatonic features having onset age 25 years or younger, in the context of a major psychotic illness, 7 of these patients were also receiving a benzodiazepine at last assessment. Other signs that diagnosis and management appeared challenging included unsuccessful trials of IVIG therapy for suspected autoimmune encephalopathy in 2 patients and poor outcomes for 4 others, including one where neuroleptic malignant syndrome was suspected (Table 2).
Genetics and proposed mechanisms of catatonia
This report adds to the growing literature on genetic anomalies that may be associated with catatonia, including other recurrent copy number variations and chromosomal abnormalities (Table 3). Although perhaps related to the prevalence of 22q11.2 deletions, no other genetic anomaly has more reported cases of catatonia (Table 3). As may be expected for a complex neuropsychiatric condition such as catatonia, there may be numerous genetic and other causative/risk factors that lead to the same clinical endpoint. Identification of genetic risk variants may provide novel insights into its underlying pathophysiology, which remains poorly understood. There is evidence of dysfunction in multiple neurotransmitter systems reported for 22q11.2DS [Boot et al., 2008; Butcher et al., 2017; da Silva Alves et al., 2011; Evers et al., 2014a]. Similarly, abnormalities in multiple central neurotransmitter systems, including dopamine, glutamate, and gamma-aminobutryic acid (GABA), have been implicated in catatonia, in part by the response to benzodiazepines and ECT [Fornaro 2011]. Interestingly, there is a report of catatonia in a patient with a PRODH mutation (Table 3) [Consoli et al., 2012]. The PRODH gene is located within the 22q11.2 deletion region and encodes a mitochondrial enzyme implicated in motor abnormalities and with functions including modulation of glutamatergic and GABA-ergic transmission [Guna et al., 2015].
Advantages and limitations
This report provides an up-to-date synthesis of neuropsychiatric conditions associated with 22q11.2DS and management considerations in the context of the associated comorbidities. Also, it provides a comprehensive summary of catatonic features, and neuropsychiatric comorbidities and management, in 13 new and 5 previously reported patients. Numbers are small however and only a minority met full DSM-5 criteria for catatonia. The retrospective case series was unavoidably limited to what was reported as “catatonia” and/or catatonic features in the available charts. Catatonia is often missed clinically [Ghaziuddin et al., 2012], thus it is likely that other cases remain unidentified. On the other hand, particular caution may be required when diagnosing catatonia in patients with 22q11.2DS, given that the neuropsychiatric and medical comorbidities associated with 22q11.2DS have signs and symptoms, and treatments, that may overlap with and/or exacerbate symptoms of catatonia, as noted in general for catatonia [Bhati et al., 2007; Penland et al., 2006; Rasmussen et al., 2016]. The positive response to antipsychotic medications documented may suggest that the expression of catatonic features was related to the expression of psychotic illness in some of these 22q11.2DS cases. In general, experts focus on benzodiazepine and/or ECT to manage catatonia, recommending caution with primary antipsychotic treatment [Fink and Taylor 2009; Paparrigopoulos et al., 2009]. This study was not designed as a study of treatment however, and assessment of treatment response was mainly cross-sectional and limited by the data recorded.
Implications and conclusions
Despite the limitations, with respect to catatonic features, the results collectively suggest that catatonia may be of clinical relevance to patients with 22q11.2DS, perhaps particularly in those with psychotic illness. This appears consistent with findings for the 15q11q13 duplication (Table 3), another rare recurrent copy number variation where there is high penetrance for schizophrenia and expression may include catatonia [Isles et al., 2016]. The observed female excess suggests the possibility of a sex effect on risk for catatonic features in 22q11.2DS, consistent with some reports for catatonia in schizophrenia [Ungvari et al., 2010]. The findings illustrate the need for prospective cohort studies to optimally assess the prevalence of catatonia and response to treatment in 22q11.2DS. Raised awareness of the possible association of catatonia in 22q11.2DS should aid recognition and may help improve both management and more accurate and timely diagnosis of catatonia and the associated conditions(s) that could contribute to the expression of catatonic features in this genetic population. These would include consideration of developmental stage/age and comorbidities (Figure 1), and other motor disturbances including those sometimes reported in catatonia in the general population, e.g., parkinsonism and other extrapyramidal symptoms, dyskinesia, and dystonia [Bush et al., 1997; Northoff et al., 1999; Shill and Stacy 2000]. In all cases, standard assessments for catatonia, including careful history, physical examination, and catatonia rating scales (e.g., Bush-Francis Catatonia Rating Scale [Bush et al., 1996] or others [Solmi et al., 2017]) will be helpful to evaluate presence, severity and course of symptoms.
Large prospective longitudinal studies are needed to better delineate the full neuropsychiatric phenotype in 22q11.2DS, and to help inform patient and clinician expectations and genetic counseling strategies. The wide range of co-morbid features in 22q11.2DS may have an important impact on the overall neuropsychiatric presentation, including the expression of catatonia. Regular monitoring of psychiatric and neurological features and functioning in the adult 22q11.2DS population is recommended to facilitate early diagnoses of later onset features and the implementation of best practices and effective treatments.
Acknowledgments
Financial support and sponsorship
Supported by funding from: the Canadian Institutes of Health Research (MOP nos. 97800 and 111238; ASB); the NIMH (5U01MH101723-02; ASB, EB); the University of Toronto McLaughlin Centre (ASB); and the Dalglish Fellowship in 22q11.2 deletion syndrome (EB). ASB holds the Dalglish Chair in 22q11.2 Deletion Syndrome at the Toronto General Hospital.
References
- Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-III) Washington, D.C: American Psychiatric Press; 1980. [Google Scholar]
- Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiat. 2003;160:1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, Husted J, Hodgkinson KA, Oechslin E, Harris L, Silversides C. Premature death in adults with 22q11.2 deletion syndrome. Journal of Medical Genetics. 2009;46:324–330. doi: 10.1136/jmg.2008.063800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, Husted J, Weksberg R, Caluseriu O, Webb GD, Gatzoulis MA. Clinical features of 78 adults with 22q11 Deletion Syndrome. American Journal of Medical Genetics Part A. 2005;138:307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Costain G, Marshall CR. Neuropsychiatric aspects of 22q11.2 deletion syndrome: considerations in the prenatal setting. Prenatal Diagnosis. 2017;37:61–69. doi: 10.1002/pd.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, Marino B, Oskarsdottir S, Philip N, Sullivan K, Swillen A, Vorstman J International 22q11.2 Deletion Syndrome C. Practical guidelines for managing patients with 22q11.2 deletion syndrome. Journal of Pediatrics. 2011;159:332–339. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Omran TI, Wong H, Blaser S, Feigenbaum A. Late-onset cobalamin-C disorder: a challenging diagnosis. American Journal of Medical Genetics Part A. 2007;143A:979–984. doi: 10.1002/ajmg.a.31671. [DOI] [PubMed] [Google Scholar]
- Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy & Behavior. 2009;15:15–21. doi: 10.1016/j.yebeh.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Bhati MT, Datto CJ, O’Reardon JP. Clinical manifestations, diagnosis, and empirical treatments for catatonia. Psychiatry (Edgmont) 2007;4:46–52. [PMC free article] [PubMed] [Google Scholar]
- Bieniek KF, van Blitterswijk M, Baker MC, Petrucelli L, Rademakers R, Dickson DW. Expanded C9ORF72 hexanucleotide repeat in depressive pseudodementia. JAMA Neurol. 2014;71:775–781. doi: 10.1001/jamaneurol.2013.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen O, de Koning M, Boot E, Booij J, Van amelsvoort T. Challenge and Therapeutic Studies Using Alpha-Methyl-para-Tyrosine (AMPT) in Neuropsychiatric Disorders: A Review. Central Nervous System Agents in Medicinal Chemistry. 2008;8:249–256. [Google Scholar]
- Booij J, van Amelsvoort T, Boot E. Co-occurrence of early-onset Parkinson disease and 22q11.2 deletion syndrome: Potential role for dopamine transporter imaging. American Journal of Medical Genetics Part A. 2010;152A:2937–2938. doi: 10.1002/ajmg.a.33665. [DOI] [PubMed] [Google Scholar]
- Boot E, Booij J, Zinkstok J, Abeling N, de Haan L, Baas F, Linszen D, van Amelsvoort T. Disrupted dopaminergic neurotransmission in 22q11 deletion syndrome. Neuropsychopharmacology. 2008;33:1252–1258. doi: 10.1038/sj.npp.1301508. [DOI] [PubMed] [Google Scholar]
- Boot E, Butcher NJ, van Amelsvoort TA, Lang AE, Marras C, Pondal M, Andrade DM, Fung WL, Bassett AS. Movement disorders and other motor abnormalities in adults with 22q11.2 deletion syndrome. American Journal of Medical Genetics Part A. 2015;167A:639–645. doi: 10.1002/ajmg.a.36928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckpot J, Vercruyssen M, Weyts E, Vandevoort S, D’Haenens G, Van Buggenhout G, Leempoels L, Brischoux-Boucher E, Van Maldergem L, Renieri A, Mencarelli MA, D’Angelo C, Mericq V, Hoffer MJ, Tauber M, Molinas C, Castiglioni C, Brison N, Vermeesch JR, Danckaerts M, Sienaert P, Devriendt K, Vogels A. Copy number variation analysis in adults with catatonia confirms haploinsufficiency of SHANK3 as a predisposing factor. European Journal of Medical Genetics. 2016;59:436–443. doi: 10.1016/j.ejmg.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Brugger F, Bhatia KP, Besag FM. Valproate-Associated Parkinsonism: A Critical Review of the Literature. CNS Drugs. 2016;30:527–540. doi: 10.1007/s40263-016-0341-8. [DOI] [PubMed] [Google Scholar]
- Buijs PCM, Bassett AS, Boot E. Non-pharmacological treatment of psychiatric disorders in individuals with 22q11.2 deletion syndrome; a systematic review. American Journal of Medical Genetics Part A. 2018 doi: 10.1002/ajmg.a.38612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatrica Scandinavica. 1996;93:129–136. doi: 10.1111/j.1600-0447.1996.tb09814.x. [DOI] [PubMed] [Google Scholar]
- Bush G, Petrides G, Francis A. Catatonia and other motor syndromes in a chronically hospitalized psychiatric population. Schizophrenia Research. 1997;27:83–92. doi: 10.1016/S0920-9964(97)00084-4. [DOI] [PubMed] [Google Scholar]
- Butcher NJ, Chow EW, Costain G, Karas D, Ho A, Bassett AS. Functional outcomes of adults with 22q11.2 deletion syndrome. Genetics in Medicine. 2012;14:836–843. doi: 10.1038/gim.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher NJ, Fung WL, Fitzpatrick L, Guna A, Andrade DM, Lang AE, Chow EW, Bassett AS. Response to clozapine in a clinically identifiable subtype of schizophrenia. British Journal of Psychiatry. 2015a;206:484–491. doi: 10.1192/bjp.bp.114.151837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher NJ, Kiehl TR, Hazrati LN, Chow EWC, Rogaeva E, Lang AE, Bassett AS. Association between early-onset Parkinson disease and 22q11.2 deletion syndrome: Identification of a novel genetic form of Parkinson disease and its clinical implications. JAMA Neurology. 2013;70:1359–1366. doi: 10.1001/jamaneurol.2013.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher NJ, Marras C, Pondal M, Rusjan P, Boot E, Christopher L, Repetto GM, Fritsch R, Chow EWC, Masellis M, Strafella AP, Lang AE, Bassett AS. Neuroimaging and clinical features in adults with a 22q11.2 deletion at risk of Parkinson’s disease. Brain. 2017;140:1371–1383. doi: 10.1093/brain/awx053. [DOI] [PubMed] [Google Scholar]
- Butcher NJ, Marras C, Pondal M, Rusjan P, Christopher L, Strafella AP, Lang AE, Bassett AS. Investigating prodromal markers of Parkinson’s disease in adults with hemizygous 22q11.2 deletions. Movement Disorders. 2015b;30(S1):S1035. [Google Scholar]
- Caroff SN, Hurford I, Lybrand J, Campbell EC. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurologic Clinics. 2011;29:127–148. viii. doi: 10.1016/j.ncl.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EN, George SR, Andrade DM, Chow EW, Silversides CK, Bassett AS. Neonatal hypocalcemia, neonatal seizures, and intellectual disability in 22q11.2 deletion syndrome. Genetics in Medicine. 2014a;16:40–44. doi: 10.1038/gim.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EN, George SR, Costain GA, Andrade DM, Chow EW, Silversides CK, Bassett AS. Prevalence of hypocalcemia and its associated features in 22q11.2 deletion syndrome. Clin Endocrinol. 2014b;81:190–196. doi: 10.1111/cen.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EW, Watson M, Young DA, Bassett AS. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophrenia Research. 2006;87:270–278. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli A, Raffin M, Laurent C, Bodeau N, Campion D, Amoura Z, Sedel F, An-Gourfinkel I, Bonnot O, Cohen D. Medical and developmental risk factors of catatonia in children and adolescents: a prospective case-control study. Schizophrenia Research. 2012;137:151–158. doi: 10.1016/j.schres.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Cusin C, Franco FB, Fernandez-Robles C, DuBois CM, Welch CA. Rapid improvement of depression and psychotic symptoms in Huntington’s disease: a retrospective chart review of seven patients treated with electroconvulsive therapy. General Hospital Psychiatry. 2013;35:678 e673–675. doi: 10.1016/j.genhosppsych.2013.01.015. [DOI] [PubMed] [Google Scholar]
- da Silva Alves F, Boot E, Schmitz N, Nederveen A, Vorstman J, Lavini C, Pouwels P, de Haan L, Linszen D, van Amelsvoort T. Proton magnetic resonance spectroscopy in 22q11 deletion syndrome. PloS One. 2011;6(6) doi: 10.1371/journal.pone.0021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsa C, Bumb A, Bianchi-Demicheli F, Vidailhet P, Sterck R, Andreoli A, Beyenburg S. “Dopamine-dependent” side effects of selective serotonin reuptake inhibitors: a clinical review. Journal of Clinical Psychiatry. 2004;65:1064–1068. doi: 10.4088/jcp.v65n0806. [DOI] [PubMed] [Google Scholar]
- Dean OM, Data-Franco J, Giorlando F, Berk M. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26:391–401. doi: 10.2165/11632000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Dhossche D, Bouman N. Catatonia in an Adolescent with Prader-Willi Syndrome. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 1997;9:247–253. doi: 10.1023/a:1022308511313. [DOI] [PubMed] [Google Scholar]
- Dimitri D, Jehel L, Durr A, Levy-Soussan M, Andreux V, Laplanche JL, Fossati P, Cohen D. Fatal familial insomnia presenting as psychosis in an 18-year-old man. Neurology. 2006;67:363–364. doi: 10.1212/01.wnl.0000225181.98341.74. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Lang AE. Phenotype-specific diagnosis of functional (psychogenic) movement disorders. Current Neurology and Neuroscience Reports. 2015;15:32. doi: 10.1007/s11910-015-0556-y. [DOI] [PubMed] [Google Scholar]
- Evers LJ, Curfs LM, Bakker JA, Boot E, da Silva Alves F, Abeling N, Bierau J, Drukker M, van Amelsvoort TA. Serotonergic, noradrenergic and dopaminergic markers are related to cognitive function in adults with 22q11 deletion syndrome. International Journal of Neuropsychopharmacology. 2014a;17:1159–1165. doi: 10.1017/S1461145714000376. [DOI] [PubMed] [Google Scholar]
- Evers LJ, van Amelsvoort TA, Candel MJ, Boer H, Engelen JJ, Curfs LM. Psychopathology in adults with 22q11 deletion syndrome and moderate and severe intellectual disability. Journal of Intellectual Disability Research. 2014b;58:915–925. doi: 10.1111/jir.12117. [DOI] [PubMed] [Google Scholar]
- Faedda GL, Wachtel LE, Higgins AM, Shprintzen RJ. Catatonia in an adolescent with velo-cardio-facial syndrome. American Journal of Medical Genetics Part A. 2015;167A:2150–2153. doi: 10.1002/ajmg.a.37087. [DOI] [PubMed] [Google Scholar]
- Farooq S, Taylor M. Clozapine: dangerous orphan or neglected friend? British Journal of Psychiatry. 2011;198:247–249. doi: 10.1192/bjp.bp.110.088690. [DOI] [PubMed] [Google Scholar]
- Ferrell RB, Wolinsky EJ, Kauffman CI, Flashman LA, McAllister TW. Neuropsychiatric syndromes in adults with intellectual disability: issues in assessment and treatment. Curr Psychiatry Rep. 2004;6:380–390. doi: 10.1007/s11920-004-0025-9. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Agid O, Takeuchi H, Lee J, Foussias G, Zakzanis KK, Graff-Guerrero A, Remington G. Extrapyramidal symptoms and cognitive test performance in patients with schizophrenia. Schizophrenia Research. 2015;161:351–356. doi: 10.1016/j.schres.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Fiksinski AM, Breetvelt EJ, Duijff SN, Bassett AS, Kahn RS, Vorstman JAS. Autism Spectrum and psychosis risk in the 22q11.2 deletion syndrome. Findings from a prospective longitudinal study. Schizophrenia Research. 2017;188:59–62. doi: 10.1016/j.schres.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Archives of General Psychiatry. 2009;66:1173–1177. doi: 10.1001/archgenpsychiatry.2009.141. [DOI] [PubMed] [Google Scholar]
- Fornaro M. Catatonia: a narrative review. Central Nervous System Agents in Medicinal Chemistry. 2011;11:73–79. doi: 10.2174/187152411794961031. [DOI] [PubMed] [Google Scholar]
- Fung WL, Butcher NJ, Costain G, Andrade DM, Boot E, Chow EW, Chung B, Cytrynbaum C, Faghfoury H, Fishman L, Garcia-Minaur S, George S, Lang AE, Repetto G, Shugar A, Silversides C, Swillen A, van Amelsvoort T, McDonald-McGinn DM, Bassett AS. Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genetics in Medicine. 2015;17:599–609. doi: 10.1038/gim.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung WL, McEvilly R, Fong J, Silversides C, Chow E, Bassett A. Elevated prevalence of generalized anxiety disorder in adults with 22q11.2 deletion syndrome. American Journal of Psychiatry. 2010;167:998. doi: 10.1176/appi.ajp.2010.09101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatrica Scandinavica. 2012;125:33–38. doi: 10.1111/j.1600-0447.2011.01778.x. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin N, Nassiri A, Miles JH. Catatonia in Down syndrome; a treatable cause of regression. Neuropsychiatric Disease and Treatment. 2015;11:941–949. doi: 10.2147/NDT.S77307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez W, Bautista RE. Seizures and EEG findings in an adult patient with DiGeorge syndrome: a case report and review of the literature. Seizure. 2009;18(9):648–651. doi: 10.1016/j.seizure.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Graf WD, Unis AS, Yates CM, Sulzbacher S, Dinulos MB, Jack RM, Dugaw KA, Paddock MN, Parson WW. Catecholamines in patients with 22q11.2 deletion syndrome and the low-activity COMT polymorphism. Neurology. 2001;57:410–416. doi: 10.1212/wnl.57.3.410. [DOI] [PubMed] [Google Scholar]
- Guna A, Butcher NJ, Bassett AS. Comparative mapping of the 22q11.2 deletion region and the potential of simple model organisms. Journal of Neurodevelopmental Disorders. 2015;7:18. doi: 10.1186/s11689-015-9113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercher L, Bruenner G. Living with a child at risk for psychotic illness: the experience of parents coping with 22q11 deletion syndrome: an exploratory study. American Journal of Medical Genetics Part A. 2008;146A:2355–2360. doi: 10.1002/ajmg.a.32466. [DOI] [PubMed] [Google Scholar]
- Heron SE, Smith KR, Bahlo M, Nobili L, Kahana E, Licchetta L, Oliver KL, Mazarib A, Afawi Z, Korczyn A, Plazzi G, Petrou S, Berkovic SF, Scheffer IE, Dibbens LM. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nature Genetics. 2012;44:1188–1190. doi: 10.1038/ng.2440. [DOI] [PubMed] [Google Scholar]
- Holm AC. Neurodegenerative and psychiatric overlap in frontotemporal lobar degeneration: a case of familial frontotemporal dementia presenting with catatonia. International Psychogeriatrics. 2014;26:345–347. doi: 10.1017/S1041610213001403. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery and Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles AR, Ingason A, Lowther C, Walters J, Gawlick M, Stober G, Rees E, Martin J, Little RB, Potter H, Georgieva L, Pizzo L, Ozaki N, Aleksic B, Kushima I, Ikeda M, Iwata N, Levinson DF, Gejman PV, Shi J, Sanders AR, Duan J, Willis J, Sisodiya S, Costain G, Werge TM, Degenhardt F, Giegling I, Rujescu D, Hreidarsson SJ, Saemundsen E, Ahn JW, Ogilvie C, Girirajan SD, Stefansson H, Stefansson K, O’Donovan MC, Owen MJ, Bassett A, Kirov G. Parental origin of interstitial duplications at 15q11.2–q13.3 in schizophrenia and neurodevelopmental disorders. PLoS Genet. 2016;12:e1005993. doi: 10.1371/journal.pgen.1005993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Schwartz A, McDougle CJ, Skotko BG. Rapid clinical deterioration in an individual with Down syndrome. American Journal of Medical Genetics Part A. 2016;170:1899–1902. doi: 10.1002/ajmg.a.37674. [DOI] [PubMed] [Google Scholar]
- Jap SN, Ghaziuddin N. Catatonia among adolescents with Down syndrome: a review and 2 case reports. Journal of ECT. 2011;27:334–337. doi: 10.1097/YCT.0b013e31821d37c6. [DOI] [PubMed] [Google Scholar]
- Kao A, Mariani J, McDonald-McGinn DM, Maisenbacher MK, Brooks-Kayal AR, Zackai EH, Lynch DR. Increased prevalence of unprovoked seizures in patients with a 22q11.2 deletion. American Journal of Medical Genetics Part A. 2004;129:29–34. doi: 10.1002/ajmg.a.30133. [DOI] [PubMed] [Google Scholar]
- Karas D, Costain G, Chow E, Bassett A. Perceived burden and neuropsychiatric morbidities in adults with 22q11.2 deletion syndrome. Journal of Intellectual Disability Research. 2014;58:198–210. doi: 10.1111/j.1365-2788.2012.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nature Reviews: Neuroscience. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman KR. Antiepileptic drugs in the treatment of psychiatric disorders. Epilepsy & Behavior. 2011;21:1–11. doi: 10.1016/j.yebeh.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22:739–760. doi: 10.2165/00023210-200822090-00003. [DOI] [PubMed] [Google Scholar]
- Kennedy WP, Mudd PA, Maguire MA, Souders MC, McDonald-McGinn DM, Marcus CL, Zackai EH, Solot CB, Mason TB, Jackson OA, Elden LM. 22q11.2 Deletion syndrome and obstructive sleep apnea. International Journal of Pediatric Otorhinolaryngology. 2014;78:1360–1364. doi: 10.1016/j.ijporl.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Kleefstra T, van Zelst-Stams WA, Nillesen WM, Cormier-Daire V, Houge G, Foulds N, van Dooren M, Willemsen MH, Pfundt R, Turner A, Wilson M, McGaughran J, Rauch A, Zenker M, Adam MP, Innes M, Davies C, Lopez AG, Casalone R, Weber A, Brueton LA, Navarro AD, Bralo MP, Venselaar H, Stegmann SP, Yntema HG, van Bokhoven H, Brunner HG. Further clinical and molecular delineation of the 9q subtelomeric deletion syndrome supports a major contribution of EHMT1 haploinsufficiency to the core phenotype. Journal of Medical Genetics. 2009;46:598–606. doi: 10.1136/jmg.2008.062950. [DOI] [PubMed] [Google Scholar]
- Kontoangelos K, Maillis A, Maltezou M, Tsiori S, Papageorgiou CC. Acute Dystonia in a Patient with 22q11.2 Deletion Syndrome. Ment Illn. 2015;7:5902. doi: 10.4081/mi.2015.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchini F, Medda P, Mariani MG, Mauri M, Toni C, Perugi G. Electroconvulsive therapy in catatonic patients: Efficacy and predictors of response. World J Psychiatry. 2015;5:182–192. doi: 10.5498/wjp.v5.i2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magid M, Trevino K, Reid WH, Jalalat S, Husain MM, Kahn DA. Emergency ECT in an incapacitated, medically compromised patient with Huntington’s disease. Journal of Psychiatric Practice. 2014;20:470–475. doi: 10.1097/01.pra.0000456596.43492.48. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE, Scambler PJ, Bassett AS. 22q11.2 deletion syndrome. Nature Reviews Disease Primer. 2015:15071. doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merida-Puga J, Ramirez-Bermudez J, Aguilar-Venegas LC, Fricchione GL, Espinola-Nadurille M. Westphal variant Huntington disease and refractory catatonia: a case report. Cognitive and Behavioral Neurology. 2011;24:204–208. doi: 10.1097/WNN.0b013e318240080d. [DOI] [PubMed] [Google Scholar]
- Milan-Tomas A, Persyko M, Del Campo M, Shapiro CM, Farcnik K. An Overview of Psychogenic Non-Epileptic Seizures: Etiology, Diagnosis and Management. Canadian Journal of Neurological Sciences. 2018:1–7. doi: 10.1017/cjn.2017.283. [DOI] [PubMed] [Google Scholar]
- Mok KY, Sheerin U, Simon-Sanchez J, Salaka A, Chester L, Escott-Price V, Mantripragada K, Doherty KM, Noyce AJ, Mencacci NE, Lubbe SJ, Williams-Gray CH, Barker RA, van Dijk KD, Berendse HW, Heutink P, Corvol JC, Cormier F, Lesage S, Brice A, Brockmann K, Schulte C, Gasser T, Foltynie T, Limousin P, Morrison KE, Clarke CE, Sawcer S, Warner TT, Lees AJ, Morris HR, Nalls MA, Singleton AB, Hardy J, Abramov AY, Plagnol V, Williams NM, Wood NW International Parkinson’s Disease Genomics C. Deletions at 22q11.2 in idiopathic Parkinson’s disease: a combined analysis of genome-wide association data. Lancet Neurology. 2016;15:585–596. doi: 10.1016/S1474-4422(16)00071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Koch A, Wenke J, Eckert J, Boker H, Pflug B, Bogerts B. Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Movement Disorders. 1999;14:404–416. doi: 10.1002/1531-8257(199905)14:3<404::aid-mds1004>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Paparrigopoulos T, Tzavellas E, Ferentinos P, Mourikis I, Liappas J. Catatonia as a risk factor for the development of neuroleptic malignant syndrome: report of a case following treatment with clozapine. World Journal of Biological Psychiatry. 2009;10:70–73. doi: 10.1080/15622970701287369. [DOI] [PubMed] [Google Scholar]
- Peluso MJ, Lewis SW, Barnes TR, Jones PB. Extrapyramidal motor side-effects of first- and second-generation antipsychotic drugs. British Journal of Psychiatry. 2012;200:387–392. doi: 10.1192/bjp.bp.111.101485. [DOI] [PubMed] [Google Scholar]
- Penland HR, Weder N, Tampi RR. The catatonic dilemma expanded. Ann Gen Psychiatry. 2006;5:14. doi: 10.1186/1744-859X-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta V, Campos MS, De Jalon EG, Cuesta MJ. Motor behavior abnormalities in drug-naive patients with schizophrenia spectrum disorders. Movement Disorders. 2010;25:1068–1076. doi: 10.1002/mds.23050. [DOI] [PubMed] [Google Scholar]
- Philip N, Bassett AS. Cognitive, behavioural and psychiatric phenotype in 22q11.2 deletion syndrome. Behavior Genetics. 2011;41:403–412. doi: 10.1007/s10519-011-9468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser HM, Trutia AE. Treatment of a Prader-Willi Patient with Recurrent Catatonia. Case Reports in Psychiatry. 2015:1–4. doi: 10.1155/2015/697428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, Cassidy RP, Fiorentini CJ, Heiken KF, Lawrence JJ, Mahoney MH, Miller CJ, Nair DT, Politi KA, Worcester KN, Setton RA, Dipiazza R, Sherman EA, Eastman JT, Francklyn C, Robey-Bond S, Rider NL, Gabriel S, Morton DH, Strauss KA. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PloS One. 2012;7:e28936. doi: 10.1371/journal.pone.0028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: Our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6:391–398. doi: 10.5498/wjp.v6.i4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P. Schizophrenia-like illness in velo-cardio-facial syndrome: a genetic subsyndrome of schizophrenia? Journal of Psychosomatic Research. 2002;53:721–727. doi: 10.1016/s0022-3999(02)00431-2. [DOI] [PubMed] [Google Scholar]
- Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, Van den Bree MBM, Owen M, Murphy KC, Niarchou M, Kates WR, Antshel KM, Fremont W, McDonald-McGinn DM, Gur RE, Zackai EH, Vorstman J, Duijff SN, Klaassen PWJ, Swillen A, Gothelf D, Green T, Weizman A, Van Amelsvoort T, Evers R, Boot E, Shashi V, Hooper SR, Bearden CE, Jalbrzikowski M, Armando M, Vicari S, Murphy DG, Ousley O, Campbell LE, Simon TJ, Eliez S Consortium tIqDS. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. American Journal of Psychiatry. 2014;171:627–639. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serret S, Thummler S, Dor E, Vesperini S, Santos A, Askenazy F. Lithium as a rescue therapy for regression and catatonia features in two SHANK3 patients with autism spectrum disorder: case reports. BMC Psychiatry. 2015;15:107. doi: 10.1186/s12888-015-0490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shill HA, Stacy MA. Malignant catatonia secondary to sporadic encephalitis lethargica. Journal of Neurology, Neurosurgery and Psychiatry. 2000;69:402–403. doi: 10.1136/jnnp.69.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer M, Haltenhof H, Haubitz B, Pabst B, Miller K, Garlipp P. Basal ganglia calcification and psychosis in 22q11.2 deletion syndrome. European Psychiatry: the Journal of the Association of European Psychiatrists. 2005;20:567–569. doi: 10.1016/j.eurpsy.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Solmi M, Pigato GG, Roiter B, Guaglianone A, Martini L, Fornaro M, Monaco F, Carvalho AF, Stubbs B, Veronese N, Correll CU. Prevalence of Catatonia and Its Moderators in Clinical Samples: Results from a Meta-analysis and Meta-regression Analysis. Schizophrenia Bulletin. 2017 doi: 10.1093/schbul/sbx157. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stober G, Seelow D, Ruschendorf F, Ekici A, Beckmann H, Reis A. Periodic catatonia: confirmation of linkage to chromosome 15 and further evidence for genetic heterogeneity. Human Genetics. 2002;111:323–330. doi: 10.1007/s00439-002-0805-4. [DOI] [PubMed] [Google Scholar]
- Tabolacci E, Zollino M, Lecce R, Sangiorgi E, Gurrieri F, Leuzzi V, Opitz JM, Neri G. Two brothers with 22q13 deletion syndrome and features suggestive of the Clark-Baraitser syndrome. Clinical Dysmorphology. 2005;14:127–132. [PubMed] [Google Scholar]
- Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, Malaspina D, Owen MJ, Schultz S, Tsuang M, Van Os J, Carpenter W. Definition and description of schizophrenia in the DSM-5. Schizophrenia Research. 2013;150:3–10. doi: 10.1016/j.schres.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Teagarden DL, Meador KJ, Loring DW. Low vitamin D levels are common in patients with epilepsy. Epilepsy Research. 2014;108:1352–1356. doi: 10.1016/j.eplepsyres.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AA, Friedman JH. Current use of clozapine in Parkinson disease and related disorders. Clinical Neuropharmacology. 2010;33:14–16. doi: 10.1097/WNF.0b013e3181c47168. [DOI] [PubMed] [Google Scholar]
- Torr J, D’Abrera JC. Maintenance electroconvulsive therapy for depression with catatonia in a young woman with Down syndrome. Journal of ECT. 2014;30:332–336. doi: 10.1097/YCT.0000000000000116. [DOI] [PubMed] [Google Scholar]
- Ungvari GS, Caroff SN, Gerevich J. The catatonia conundrum: evidence of psychomotor phenomena as a symptom dimension in psychotic disorders. Schizophrenia Bulletin. 2010;36:231–238. doi: 10.1093/schbul/sbp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiskin SI, Nicolson R, Krasnewich DM, Yan W, Lenane M, Wudarsky M, Hamburger SD, Rapoport JL. Velocardiofacial syndrome in childhood-onset schizophrenia. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1536–1543. doi: 10.1097/00004583-199912000-00015. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort T, Henry J, Morris R, Owen M, Linszen D, Murphy K, Murphy D. Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophrenia Research. 2004;70:223–232. doi: 10.1016/j.schres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Voll SL, Boot E, Butcher NJ, Cooper S, Heung T, Chow EW, Silversides CK, Bassett AS. Obesity in adults with 22q11.2 deletion syndrome. Genetics in Medicine. 2017;19:204–208. doi: 10.1038/gim.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JS, Breetvelt EJ, Thode KI, Chow EW, Bassett AS. Expression of autism spectrum and schizophrenia in patients with a 22q11.2 deletion. Schizophrenia Research. 2013;143:55–59. doi: 10.1016/j.schres.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66:77–92. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- Weinzimer SA. Endocrine aspects of the 22q11.2 deletion syndrome. Genetics in Medicine. 2001;3:19–22. doi: 10.1097/00125817-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Winarni TI, Schneider A, Ghaziuddin N, Seritan A, Hagerman RJ. Psychosis and catatonia in fragile X: Case report and literature review. Intractable Rare Dis Res. 2015;4:139–146. doi: 10.5582/irdr.2015.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wither RG, Borlot F, MacDonald A, Butcher NJ, Chow EWC, Bassett AS, Andrade DM. 22q11.2 deletion syndrome lowers seizure threshold in adult patients without epilepsy. Epilepsia. 2017;58:1095–1101. doi: 10.1111/epi.13748. [DOI] [PubMed] [Google Scholar]
- Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y, Levy S, Gogos JA, Karayiorgou M. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nature Genetics. 2012;44:1365–1369. doi: 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. Endocrine disorders and the neurologic manifestations. Ann Pediatr Endocrinol Metab. 2014;19:184–190. doi: 10.6065/apem.2014.19.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleski C, Bassett AS, Tam K, Shugar AL, Chow EW, McPherson E. The co-occurrence of early onset Parkinson disease and 22q11.2 deletion syndrome. American Journal of Medical Genetics Part A. 2009;149A:525–528. doi: 10.1002/ajmg.a.32650. [DOI] [PMC free article] [PubMed] [Google Scholar]