Abstract
Objective:
To estimate the prevalence of HIV drug resistance over time and identify risk factors for multi-class resistance.
Design: Prospective clinical cohort of HIV-infected patients at the University of North Carolina.
Methods:
Among ART-experienced patients in care 2000–2016, we estimated annual prevalences of cumulative resistance, defined as ≥1 major mutation, by drug class. Clinical data and multiple imputation were used when genotypic data was missing, and mutations were carried forward in time. We estimated resistance odds ratios comparing characteristics of patients in care in 2016.
Results:
3,682 patients contributed 23,169 person-years. Prevalence of ≥1 major resistance mutation, irrespective of viral suppression, peaked in 2005 with 49% (95% CI 46, 52) and decreased to 38% (35, 40) in 2016. Resistance to NRTIs, PIs, and NNRTIs also peaked in 2005–2007 and decreased to 28% (26, 31), 14% (12, 16), and 27% (24, 29) in 2016, respectively. In 2016, prevalence of INSTI resistance was 2% (1, 3) and triple-class resistance 10% (9, 12). Over the study period, cumulative resistance was frequent among patients with detectable viremia, but uncommon among patients initiating ART post-2007. Among 1,553 patients in care in 2016, ART initiation at an older age, with an INSTI, and with higher CD4 were associated with resistance to fewer or no classes.
Conclusion:
Prevalence of resistance to older ART classes has decreased in the last ten years in this clinical cohort, while INSTI resistance has increased but remained very low. Patients with viremia continue to have a high burden of resistance even if they initiated ART recently.
Keywords: HIV; antiretroviral therapy, highly active; drug resistance; United States; HIV integrase inhibitors
INTRODUCTION
Antiretroviral therapy (ART) reduces HIV morbidity and mortality and prevents forward HIV transmission.1,2 Poor ART adherence may lead to sub-therapeutic drug levels, incomplete suppression of viral replication, and selection of resistance,3,4 which can limit the success of subsequent therapy and result in further selection of resistance.5 Moreover, HIV strains with resistance-conferring mutations can be transmitted to others.6 Genotypic resistance testing, performed before initiating treatment or after virologic failure, helps guide clinicians in choosing an efficacious drug regimen.7 In addition, genotypic resistance testing of samples collected via population-based surveys provides drug resistance estimates in regions where genotypes may not be available for patient care.8–10
Drug resistance prevalence is affected by changes in treatment guidelines, including use of newer agents and genotypic resistance testing, as well as patient turnover due to care entry, loss to follow-up, or death. Capturing complete resistance history, even in currently suppressed patients, is essential to assess the burden of all resistance with a potential clinical impact on patients, for example after regimen modification or simplification. However, contemporary resistance prevalence is not well known, because few observational studies have been conducted since the introduction of newer boosted protease inhibitors (PIs)11,12 and integrase strand transfer inhibitors (INSTIs),13–15 which have high barriers to resistance, limiting the selection of resistance mutations at virologic failure.11–13,16–19
Characterizing resistance prevalence is also challenging because resistance testing for individual patient care is not consistently done in all viremic patients. Prevalence estimates relying solely on available genotypes are susceptible to changes in the use of resistance testing and may exclude important groups of patients,20 and they fail to capture archived variants harboring resistance mutations.21–24 In this study, we used clinical information, available resistance tests, and multiple imputation to estimate changes in resistance prevalence between 2000 and 2016, and identify risk factors for multi-class resistance in an HIV clinical cohort.
METHODS
Study population
We included all ART-experienced patients in the University of North Carolina Center for AIDS Research HIV Clinical Cohort (UCHCC) with at least one HIV RNA viral load (VL) measurement after ART initiation between January 1, 2000 and December 31, 2016, including patients who first entered HIV care prior to 2000. The UCHCC is a prospective clinical cohort of over 5,000 HIV-infected adults followed at UNC Hospitals after 1996.25 Laboratory testing is collected electronically in real time. Diagnoses and medication information are abstracted twice yearly from medical records, and resistance mutations are abstracted from genotype reports as they are performed. Prospective data collection for the UCHCC and this secondary data analysis have both been approved by the UNC Institutional Review Board.
Measures
ART-experienced patients contributed data to a given calendar year of analysis if they had at least one VL measurement in that year (defined as being in care), even if they subsequently discontinued ART. Patients who were out of care and had no VL in a given calendar year were excluded for that year but could contribute to subsequent years. For each calendar year, patients were considered viremic if they met any of the following: two consecutive VL>500 copies/mL at least 90 days post-ART initiation; one VL>500 and no evidence of care the following calendar year; or a genotype test performed post-ART initiation. We defined antiretroviral drug resistance by agent class as having one or more major (bolded) mutation in the 2017 IAS-USA list,26 including genotypes performed both before and after ART initiation. Genotypes performed prior to ART initiation were available for 31% of patients in this study. The drug classes examined were nucleoside reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitor (NNRTI), protease inhibitor (PI), and integrase strand transfer inhibitor (INSTI). Resistance to entry and fusion inhibitors was not considered in this study.
Analysis
For every calendar year from 2000 to 2016, we estimated the prevalence of resistance among all patients receiving care that year, as well as patients who were viremic that year. Prevalence for each year was calculated by dividing the total number of patients with resistance (both new and prior resistance) by the number of eligible patients that year. We also estimated resistance prevalence in the subset of patients who initiated ART in 2007 or later. For each group, we estimated resistance to at least one, two, three, or four classes, and resistance to NRTIs, PIs, NNRTIs, and INSTIs. INSTI and four-class resistance were only estimated starting in 2007. Among patients with triple-class resistance, we estimated the annual proportion of patients with viremia.
Our algorithm for estimating resistance prevalence using clinical information to impute missing genotypic information was adapted from published work (Fig. 1).27 Resistance mutations from genotype testing were carried forward in time. Suppressed patients, including those with no prior genotype test, were assumed to have no new mutations. For patients with viremia in a given year, resistance status for a drug class was considered missing if: no new genotype was obtained, resistance to that class was not already known, and the patient was not known to be off ART. Patients with missing resistance status were assumed not to have any transmitted resistance mutations to classes to which they had never been exposed, with the exception of NNRTI. This decision was based on research conducted in the UCHCC, where we estimated that, during most of the study period, approximately 5–10% of newly infected patients each year had transmitted resistance mutations to NNRTIs, whereas estimates for PIs and NRTIs were much lower.28 Patients with missing resistance who became suppressed without a change in ART regimen were assumed to have no new mutations.
Figure 1.
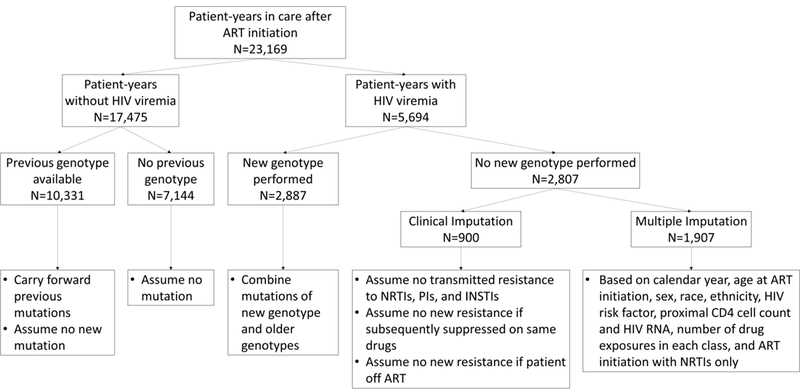
Algorithm to impute missing data on resistance mutations.
After applying these assumptions based on available clinical information, 10% of patient-years still had an unknown resistance status for some of the drug classes, and <1% of patient-years had an unknown resistance status for all four classes. For the remaining missing resistance, we conducted fifty multiple imputations with the SAS procedure MI and the Markov Chain Monte Carlo method with a single chain, assuming a multivariate normal distribution.29 Model estimates and statistical tests from multiply imputed data sets were combined using Rubin’s rules.30 The variables used for multiple imputation were calendar year, age at ART initiation, sex, race, ethnicity, HIV risk factor, proximal CD4 cell count and VL, number of drug exposures in each class, and a history of ART initiation with NRTIs only. Only viremic patient-years were included in the multiple imputation model, as associations between these characteristics and resistance can vary by HIV RNA suppression.27 Once resistance to a class was imputed, it was considered present in all future years.
After imputing missing resistance status for all patient-years, we estimated the prevalence of drug resistance for each calendar year from 2000 and 2016, including both new and prior resistance. We fit separate logistic regression models with GEE to compare patient-years in different calendar periods, and to estimate time trends in each prevalence estimate, using year as a linear predictor and including linear splines with a knot when a peak was observed. For patients in care in 2016, we used cumulative logistic regression (i.e. ordinal logistic regression) with unequal slopes to model the odds of having resistance to 0, ≥1, ≥2, or ≥3 classes. We estimated unadjusted odds ratios and 95% confidence intervals comparing patient demographic and clinical characteristics. In a secondary analysis, we estimated adjusted odds ratios with a multivariable model including important characteristics selected a priori. P values were two-sided and <0.05 was considered statistically significant. All analyses were conducted using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
The study population comprised 3,682 patients who contributed 23,168 person-years in care after initiating ART (Table 1). Overall, 30% of patient-years were contributed by women, 42% by men who have sex with men (MSM), and 58% by African American patients. Half of patient-years were contributed by patients who initiated ART prior to 1999 (interquartile range [IQR] 1996, 2005), and 34% by patients whose first ART regimen contained only NRTI agents. Compared to person-years in the 2000–2005 calendar period, person-time in the 2012–2016 period was more likely contributed by patients who were older (median age 48 [IQR 39, 55] vs. 42 [IQR 36, 48]), less likely to be viremic (11% vs. 44%), having initiated ART more recently (median year 2004 [IQR 1998, 2010] vs. 1997 [IQR 1995, 2000]), with higher CD4 cell counts (median 286 [IQR 106, 443] vs. 237 [IQR 67, 404]) and less likely to have used NRTI-only regimens (22% vs. 48%) (All P <0.05).
Table 1.
Demographic and clinical characteristics of ART-experienced patient-years in care, 2000–2016.
| Characteristic | All Patient-Years N=23,169 |
2000–2005 N=7,020 |
2006–2011 N=8,172 |
2012–2016 N=7,977 |
|---|---|---|---|---|
| Sexual risk group, N (%) | ||||
| Heterosexual male | 6,497 (28%) | 2,278 (32%) | 2,298 (28%) | 1,921 (24%) |
| Female | 6,837 (30%) | 2,171 (31%) | 2,400 (29%) | 2,266 (28%) |
| MSM | 9,835 (42%) | 2,571 (37%) | 3,474 (43%) | 3,790 (48%) |
| IDUa, N (%) | 2,849 (12%) | 1,118 (16%) | 1,008 (12%) | 723 (9%) |
| Race/ethnicity, N (%) | ||||
| White | 7,601 (33%) | 2,336 (33%) | 2,667 (33%) | 2,598 (33%) |
| African American | 13,504 (58%) | 4,143 (59%) | 4,707 (58%) | 4,654 (58%) |
| Hispanic or other | 2,064 (9%) | 541 (7%) | 798 (10%) | 725 (9%) |
| Initial ART regimen | ||||
| NRTI-only | 7,761 (34%) | 3,396 (48%) | 2,572 (31%) | 1,793 (22%) |
| Ritonavir-boosted PI | 3,100 (13%) | 411 (6%) | 1,355 (17%) | 1,334 (17%) |
| Unboosted PI | 3,488 (15%) | 1,498 (21%) | 1,190 (15%) | 802 (10%) |
| NNRTI | 6,173 (27%) | 1,237 (18%) | 2,314 (28%) | 2,622 (33%) |
| INSTI | 902 (4%) | 0 (0%) | 111 (1%) | 791 (10%) |
| Otherb | 1,745 (8%) | 478 (7%) | 630 (8%) | 637 (8%) |
| Year of ART start, median (IQR) | 1999 (1996, 2005) | 1997 (1995, 2000) | 2000 (1996, 2005) | 2004 (1998, 2010) |
| Age at ART start, median years (IQR) | 36 (29, 44) | 36 (30, 43) | 36 (30, 44) | 36 (29, 44) |
| CD4 at ART startc, median cells/mm3 (IQR) | 253 (78, 416) | 237 (67, 404) | 240 (68, 403) | 286 (106, 443) |
| Current HIV viremiad, N (%) | 5,694 (25%) | 3,055 (44%) | 1,797 (22%) | 842 (11%) |
| Current CD4 counte, median cells/mm3 (IQR) | 504 (301, 729) | 401 (211, 628) | 496 (304, 714) | 596 (395, 807) |
| Current age, median years (IQR) | 45 (37, 52) | 42 (36, 48) | 46 (38, 52) | 48 (39, 55) |
Abbreviations. ART: antiretroviral therapy. IDU: injection drug use. INSTI: integrase strand transfer inhibitor. IQR: interquartile range. MSM: men who have sex with men. NNRTI: non-nucleoside reverse transcriptase inhibitor. NRTI: nucleoside reverse transcriptase inhibitor. PI: protease inhibitor.
IDU identified as a risk factor for HIV acquisition.
Includes NRTI-sparing regimens (244 person-years), regimens containing more than two classes of agents (724 person-years), regimens containing only an anchor agent (165 person-years), and unknown regimens (612).
Missing for 5,422 (23%) person-years.
Defined as two HIV RNA >500 copies/mL post-ART initiation, or one RNA >500 copies/mL post-ART initiation with no care in the subsequent calendar year, or having a genotype test performed post-ART initiation.
Missing for 581 (3%) person-years.
Prevalence of Resistance over Time
Over the study period, the estimated prevalence of resistance to NRTI and PI drugs increased between 2000 and 2005 to a peak of 42% and 23% and decreased to 28% and 14% in 2016, respectively (Fig. 2A, all P <0.05). Resistance to NNRTIs increased between 2000 and 2007 to a peak of 30% and decreased to 27% in 2016 (both P <0.05). Prevalence of resistance to at least one, two, and three classes of drugs all increased from 2000 to 2005 and decreased from 2005 to 2016 (Fig. 2B, all P <0.05 for both increase and decrease). Their peaks in 2005 were 49%, 32%, and 14%, and they declined to 38%, 22%, and 10% in 2016, respectively. INSTI and four-class resistance increased from 2007 to 2016 but remained low with prevalences of 2% and 1% in 2016, respectively (both P <0.05). Eleven patients had observed mutations for all four classes of drugs, cumulatively on all available genotypes (Table, Supplemental Digital Content 1). Their most common mutations were M184V (10 patients) for NRTI, M46I (6 patients) for PI, K103N (8 patients) for NNRTI, and Q148H (5 patients) for INSTI. Almost two-thirds of these patients initiated ART either with only NRTIs (45%) or an unboosted PI (18%), and the median CD4 count of these eleven patients was 53 (IQR 9, 330) at ART initiation.
Figure 2.
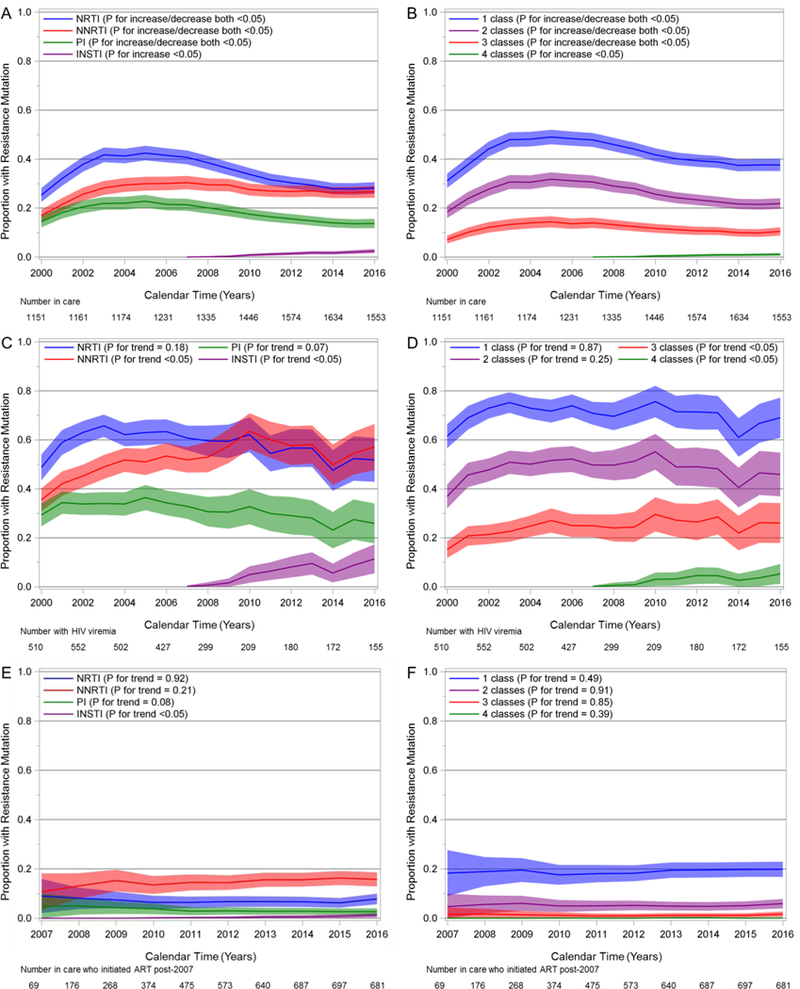
Estimated prevalence of resistance mutations and 95% confidence bands by drug class and multiclass combinations. Among all ART-experienced patients in care, 2000–2016 (Panels A and B); among ART-experienced patients in care with HIV viremia, 2000–2016 (Panels C and D); among patients in care who initiated ART in 2007 or later, 2007–2016 (Panels E and F).
The proportion of patients in care who had detectable viremia in a given year decreased over the study period from 44% in 2000 to 10% in 2016 (not shown, P <0.05). Among patients who had viremia during a given calendar year, resistance to NNRTIs and INSTIs increased over the study period (Fig. 2C, both P <0.05), while resistance to NRTIs and PIs did not change significantly (P = 0.18 and 0.07, respectively). In 2016, resistance prevalence was 52% for NRTI, 57% for NNRTI, 26% for PI, and 11% for INSTI agents. Resistance to at least one drug class remained above 50% throughout the period with no evidence of change (P = 0.87), reaching 69% in 2016 (Fig. 2D). Two-class resistance did not change over time either (P = 0.25), while three-class and four-class resistance increased (both P <0.05). These were estimated to be 46%, 26%, and 5% in 2016, respectively.
In patients who initiated ART in 2007 or later, regardless of viremia, the prevalence of resistance to each class remained low between 2007 and 2016 and was estimated to be 8% for NRTI, 3% for PI, 16% for NNRTI, 1% for INSTI drugs in 2016 (Fig. 2E). Only INSTI resistance increased between 2007 and 2016 (P <0.05). One-class resistance prevalence was 20% in 2016, while multi-class resistance was uncommon, with 6% for two, 2% for three, and <1% for four classes, and these did not change significantly over time (Fig. 2F). However, among patients initiating ART since 2007 with viremia in a given year, one-class resistance prevalence was 53% (95% Confidence Interval [CI] 40, 66) in 2016 with no detected change over time (Fig., Supplemental Digital Content 2, P = 0.83). INSTI resistance prevalence increased to 7% (95% CI 0, 14) in 2016 (P <0.05), but resistance to other classes or several classes did not appear to change. However, the number of patients in this subgroup was small, and our prevalence estimates were imprecise.
Among patients estimated to have triple-class resistance, the proportion with HIV viremia each year decreased over the study period (Fig. 3, P <0.05). In 2000, 94% of 84 patients with triple-class resistance had viremia, compared to 67% of 172 patients in 2005, 37% of 170 patients in 2010, and 25% of 163 patients in 2016.
Figure 3.
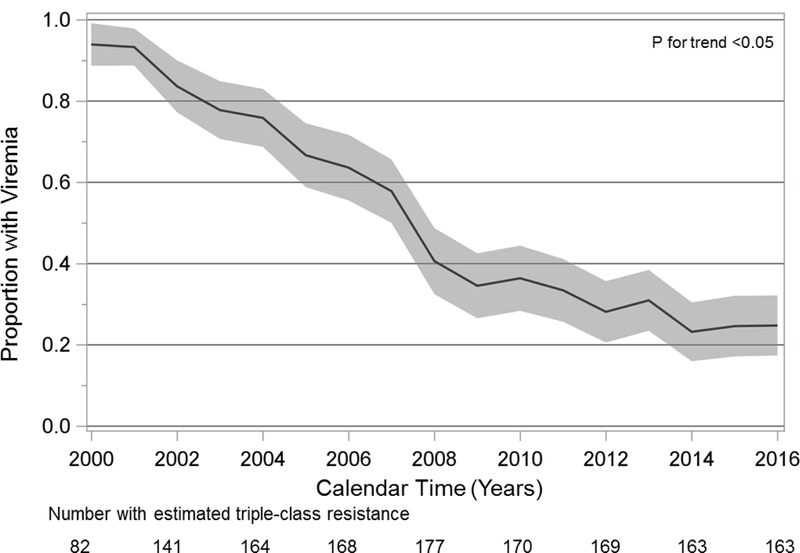
Prevalence of HIV viremia among patients with estimated triple-class drug resistance, 2000–2016.
Risk Factors for Resistance in 2016
In 2016, 1,553 ART-experienced patients were in care and 155 (10%) of them were viremic (Table 2). One-third of these patients initiated ART prior to 2000, and 14%, 15%, and 30% with an INSTI-, bPI-, or NNRTI-based regimen, respectively. Thirty percent had been exposed to over eight ART agents. In separate models for each characteristic, examining odds of no resistance, single, dual, and triple class resistance, race/ethnicity, female sex, and IDU were not associated with having any major resistance, while being MSM, older age at ART initiation, and higher nadir CD4 counts were associated with no or lower levels of resistance (Table 2). Patients who had detectable viremia in 2016 had 3.58 times (95% CI 2.43, 5.27) the odds of two-class resistance, compared to suppressed patients. Compared to patients who had initiated ART with an NNRTI-based regimen, patients who had initiated with an INSTI had lower odds of resistance (odds ratio [OR] 0.25 for two classes; 95% CI 0.10, 0.59), while those who had initiated with unboosted PIs, NRTI-only and other regimens had greater odds of resistance. Exposure to more than eight drugs substantially increased the likelihood of resistance with an OR of two-class resistance of 16.15 (95% CI 11.74, 22.22). In a secondary analysis, in a multivariable model including race/ethnicity, sexual risk group, IDU, detectable HIV viral load, nadir CD4 count, age at ART initiation, and first ART regimen, most associations were unchanged with the exception of MSM status and INSTI and bPI regimens which were similar to unadjusted estimates but less precise and no longer statistically significant (Table, Supplemental Digital Content 3).
Table 2.
Demographic and clinical characteristics of ART-experienced patients in care in 2016, and odds ratios of HIV drug resistance with 95% confidence intervals.
| Number of Resistant Drug Classes |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristic | N (%) or median (IQR) |
1 or more (N = 584) | 2 or more (N = 339) | 3 or more (N = 163) | |||
| OR (95% CI)a | P | OR (95% CI)a | P | OR (95% CI)a | P | ||
| Total | 1,553 (100%) | ||||||
| Sexual risk factor | |||||||
| Heterosexual male | 368 (24%) | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Female | 448 (29%) | 0.98 (0.73, 1.31) | 0.89 | 0.88 (0.63, 1.24) | 0.47 | 1.04 (0.65, 1.68) | 0.86 |
| MSM | 737 (47%) | 0.77 (0.59, 1.00) | <0.05 | 0.71 (0.51, 0.97) | <0.05 | 0.78 (0.50, 1.24) | 0.29 |
| IDUb | 128 (8%) | 1.29 (0.88, 1.88) | 0.19 | 1.39 (0.89, 2.16) | 0.15 | 1.49 (0.80, 2.78) | 0.21 |
| Race/ethnicity | |||||||
| White | 498 (32%) | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| African American | 916 (59%) | 1.11 (0.88, 1.41) | 0.37 | 1.13 (0.85, 1.50) | 0.39 | 1.25 (0.82, 1.89) | 0.30 |
| Hispanic or other | 139 (9%) | 1.15 (0.77, 1.72) | 0.49 | 0.97 (0.59, 1.60) | 0.91 | 0.56 (0.22, 1.46) | 0.24 |
| Initial ART regimen | |||||||
| NRTI-only | 331 (21%) | 4.26 (3.12, 5.81) | <0.01 | 5.87 (4.03, 8.54) | <0.01 | 5.95 (3.46, 10.21) | <0.01 |
| Ritonavir-boosted PI | 238 (15%) | 1.46 (1.02, 2.08) | <0.05 | 1.28 (0.78, 2.09) | 0.33 | 1.15 (0.53, 2.50) | 0.73 |
| Unboosted PI | 150 (10%) | 4.32 (2.89, 6.47) | <0.01 | 5.37 (3.38, 8.53) | <0.01 | 5.49 (2.87, 10.51) | <0.01 |
| NNRTI | 473 (30%) | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| INSTI | 227 (15%) | 0.62 (0.41, 0.93) | <0.05 | 0.25 (0.10, 0.59) | <0.01 | 0.12 (0.02, 0.88) | <0.05 |
| Other | 134 (9%) | 1.98 (1.31, 2.99) | <0.01 | 2.52 (1.53, 4.16) | <0.01 | 3.05 (1.49, 6.24) | <0.01 |
| Exposure to >8 ARVs | 470 (30%) | 8.44 (6.52, 10.92) | <0.01 | 16.15 (11.74, 22.22) | <0.01 | 26.58 (14.32, 49.34) | <0.01 |
| Detectable HIV viral loadc | 155 (10%) | 4.31 (2.88, 6.45) | <0.01 | 3.58 (2.43, 5.27) | <0.01 | 3.66 (2.30, 5.83) | <0.01 |
| Year of ART start, per 1-year increase | 2005 (1998, 2011) | 0.90 (0.88, 0.91) | <0.01 | 0.86 (0.84, 0.88) | <0.01 | 0.85 (0.83, 0.88) | <0.01 |
| Age at ART start, per 10-year increase | 35 (28, 44) | 0.76 (0.68, 0.84) | <0.01 | 0.70 (0.62, 0.80) | <0.01 | 0.71 (0.59, 0.86) | <0.01 |
| Nadir CD4 count, per 100-cell increase | 188 (50, 351) | 0.74 (0.69, 0.79) | <0.01 | 0.64 (0.59, 0.70) | <0.01 | 0.55 (0.47, 0.64) | <0.01 |
| Agec, per 10-year increase | 50 (40, 57) | 1.15 (1.06, 1.26) | <0.01 | 1.28 (1.15, 1.42) | <0.01 | 1.34 (1.14, 1.58) | <0.01 |
| CD4 countc, per 100-cell increase | 614 (406, 847) | 0.90 (0.86, 0.93) | <0.01 | 0.86 (0.82, 0.90) | <0.01 | 0.83 (0.78, 0.89) | <0.01 |
Abbreviations. ART: antiretroviral therapy. ARV: antiretrovirals. CI: confidence interval. IDU: injection drug use. INSTI: integrase strand transfer inhibitor. IQR: interquartile range. MSM: men who have sex with men. NNRTI: non-nucleoside reverse transcriptase inhibitor. NRTI: nucleoside reverse transcriptase inhibitor. OR: odds ratio. PI: protease inhibitor.
Estimates, 95% confidence intervals, and P values from logistic regression models with cumulative logits and unequal slopes (i.e. ordinal logistic regression). Each model includes one characteristic.
IDU identified as a risk factor for HIV acquisition.
Measured in 2016.
DISCUSSION
In this study, the prevalence of resistance to NRTI, NNRTI, and PI agents increased from 2000 to 2005–2007 and subsequently decreased through 2016. The proportion of patients with HIV viremia decreased over time, though those with viremia had a consistently high prevalence of resistance including multi-class resistance. Resistance to INSTI drugs and four-class resistance were consistently low although slight increases were observed since 2007. Most patients initiating ART in more recent years remained virologically suppressed, however the patients with detectable virus experienced a high burden of resistance to at least one class, especially NNRTIs and NRTIs.
Decreases in resistance beginning in the mid-2000s can be attributed to several changes in ART regimens used in that period. Atazanavir and darunavir, approved in 2003 and 2006, respectively, have high barriers to resistance, are effective against virus resistant to earlier PIs, and can be given once daily, facilitating adherence.12,17,31–33 Additionally, efavirenz became available in a single-tablet regimen in 2006, and etravirine, active against virus resistant to earlier NNRTIs, was approved in 2008.34,35 Boosted PIs and efavirenz were frequently prescribed as first-line therapy in our cohort through the early 2010s,36 likely resulting in the high rates of virologic suppression and lower resistance acquisition we observed. Additionally, the introduction of these agents, as well as INSTIs,34,37 allowed patients harboring resistant HIV variants to achieve and maintain virologic suppression, limiting acquisition of additional resistance mutations.
NNRTI resistance persisted through the end of the study period, as did resistance among patients with viremia. This persistence is likely linked to patients experiencing virologic failure on NNRTI-based regimens, which have low barriers to resistance with emerging variants frequently having both NNRTI and NRTI resistance.38,39 Transmitted resistance to NNRTIs was observed in 5–10% of patients in our cohort annually in the 2000s and may have contributed as well.28 In addition, there was a large uptake of raltegravir and elvitegravir in our cohort.36 While these potent drugs lead to high rates of viral suppression,14,15,36 20–50% of virologic failures on these agents in clinical trials have emergence of resistance, frequently including both INSTI and NRTI mutations.40,41 If similar resistance emergence occurred in our patients, this may have contributed to the unchanging resistance prevalence in viremic patients, as well as to the small increase in INSTI resistance between 2007 and 2016 in our entire cohort.
Dolutegravir has a high barrier to resistance than first-generation INSTIs, with no emergence of resistance to INSTI or NRTI in first-line studies and rare case reports in clinical practice.13,17,18,42 The recently approved INSTI bictegravir may have a similar barrier to resistance.43–45 Uptake of these agents in clinical practice, especially in new single-tablet formulations, will likely limit emergence of variants with INSTI and NRTI resistance and help maintain INSTI prevalence at the low levels observed in this study. Notably, transmitted resistance to INSTIs remains very rare in North Carolina46 and is unlikely to contribute substantially to increases in overall INSTI resistance.
Studies limited to clinical specimens of patients with virologic failure have reported high prevalence of resistance to NRTI, PI, and NNRTI drugs and multi-class combinations, as we found in our cohort among patients with detectable viremia.47–50 In addition, a North Carolina study of available genotypes reported a 7% INSTI resistance prevalence for viremic individuals assumed to be treatment-experienced, similar to the estimate in viremic patients in 2016 in our study.46 A large study of North American cohorts (including UCHCC data), which first developed the imputation algorithm adapted in our study, also found an increasing trend in resistance prevalence for the period 2000–2005 among all patients, regardless of viremia.27 However, their estimates were lower than the ones presented here, likely because they defined resistance as intermediate level or above per the Stanford algorithm rather than a single major mutation.51 In a large Swiss cohort, using different imputation methods to estimate resistance, investigators reported a similar decrease in overall and triple-class resistance, and lower resistance prevalences for patients who have initiated ART more recently.52,53
Our cohort previously reported that exposure to more ART drugs and ART initiation with unboosted PIs were strong predictors of triple-class resistance.54 In this study, we found that patients who had been exposed to fewer drugs and initiated ART in recent years with potent INSTI-based regimens had lower odds of prevalent resistance. MSM status and older age at ART initiation were also associated with lower odds of resistance, consistent with studies showing lower risk of acquiring mutations for these patients.55–58 These associations likely reflect patient characteristics linked to better treatment adherence,33,59,60 and therefore virologic suppression. Additionally, we found an association between high nadir CD4 counts and lower resistance, which may be explained by patients in more recent years entering care earlier with higher CD4 counts and lower viral loads, as well as by a possible lower incidence of drug toxicity when initiating ART with high CD4 counts that may lead to better adherence and fewer treatment interruptions.61 Higher nadir CD4 may also reflect health-seeking behavior leading to earlier HIV testing and care and to better treatment adherence.33,59,60,62–64
To our knowledge, this is the first US study to examine the prevalence of drug resistance in an entire clinical cohort since the uptake of INSTI agents. Our approach builds on a previously developed algorithm used to address missing genotypes and extends prior studies by including more recent observation time and new agents. This study benefits from granular longitudinal data used to impute missing resistance and represent what clinicians might see in their patient populations over time, although this imputation relied on strong clinical and statistical assumptions. In contrast, studies with only one genotype available per patient are likely to miss archived mutations and underestimate the burden of potentially clinical relevant resistance, and studies restricted to viremic patients can overestimate true prevalence.20 Capturing previously acquired resistance is especially important in populations where patients have had many ART exposures, as past mutations can resurface and impact treatment decisions such as regimen changes or simplifications to fewer than three drugs.
One limitation of this study is that our estimates could be underestimating resistance prevalence if mutations occurred prior to the introduction of genotype testing or prior to entering care at UNC. Secondly, this study used data from a single clinical site in the southeastern US and may not be representative of other geographical settings. Future studies will need to confirm the trends we observed in other patient populations as well as determine whether the increasing uptake of second-generation INSTIs has the expected impact on drug resistance prevalence.
In the UCHCC, HIV drug resistance has become less prevalent, and INSTI resistance remains rare. Patients who initiated ART in the last decade have consistently low resistance prevalence estimates, and patients who have accumulated triple-class resistance are increasingly able to achieve virologic suppression. Among patients currently in care, those who have initiated ART in recent years with potent regimens, have been exposed to fewer agents, and were not as immunosuppressed at initiation are less likely to harbor highly resistant virus. These trends are likely to persist with continued uptake of potent drugs with high resistance barriers. Meanwhile, non-adherent patients failing therapy may continue to develop resistance in spite of novel regimens. Continuing to monitor HIV drug resistance in the clinical setting is important to identify trends and factors that can limit effective treatment options and can reduce the impact of ART on patient and community health.
Supplementary Material
Table. Characteristics of patients with cumulative resistance mutations in four classes on available genotype tests. docx
Figure. Estimated prevalence of resistance mutations and 95% confidence bands by drug class and multiclass combinations among patients in care with HIV viremia who initiated ART between 2007 and 2016. pptx
Table. Multivariable odds ratios of HIV drug resistance with 95% confidence intervals for patients in care in 2016. docx
ACKNOWLEDGEMENTS
Funding/Support: This study was funded by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program (Grant Award Number P30 AI50410). Traineeship for TD provided by the National Institute of Allergy and Infectious Diseases (Grant Award Number T32 AI007001).
REFERENCES
- 1.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–366. [DOI] [PubMed] [Google Scholar]
- 4.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20(2):223–231. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzi P, Opravil M, Hirschel B, et al. Impact of drug resistance mutations on virologic response to salvage therapy. Swiss HIV Cohort Study. AIDS. 1999;13(2):F17–21. [DOI] [PubMed] [Google Scholar]
- 6.Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347(6):385–394. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Top HIV Med. 2008;16(3):266–285. [PubMed] [Google Scholar]
- 8.Global Action Plan on HIV Drug Resistance 2017–2012. Geneva: World Health Organization;2017. [Google Scholar]
- 9.Hunt GM, Ledwaba J, Basson AE, et al. Surveillance of transmitted HIV-1 drug resistance in Gauteng and KwaZulu-Natal Provinces, South Africa, 2005–2009. Clin Infect Dis. 2012;54 Suppl 4:S334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manasa J, Danaviah S, Lessells R, et al. Increasing HIV-1 Drug Resistance Between 2010 and 2012 in Adults Participating in Population-Based HIV Surveillance in Rural KwaZulu-Natal, South Africa. AIDS Res Hum Retroviruses. 2016;32(8):763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22(12):1389–1397. [DOI] [PubMed] [Google Scholar]
- 12.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53(3):323–332. [DOI] [PubMed] [Google Scholar]
- 13.Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818. [DOI] [PubMed] [Google Scholar]
- 14.Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615. [DOI] [PubMed] [Google Scholar]
- 15.Lennox JL, Dejesus E, Berger DS, et al. Raltegravir versus Efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr. 2010;55(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daar ES, Tierney C, Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154(7):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molina JM, Clotet B, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;2(4):e127–136. [DOI] [PubMed] [Google Scholar]
- 18.Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–935. [DOI] [PubMed] [Google Scholar]
- 19.Eron JJ, Clotet B, Durant J, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill VC, Lynch T, Ramazani S, Krentz HB. Reporting on the prevalence of antiretroviral drug resistance in a regional HIV population over 20 years: a word of caution. Antivir Ther. 2017;22(4):277–286. [DOI] [PubMed] [Google Scholar]
- 21.Gallien S, Charreau I, Nere ML, et al. Archived HIV-1 DNA resistance mutations to rilpivirine and etravirine in successfully treated HIV-1-infected individuals pre-exposed to efavirenz or nevirapine. J Antimicrob Chemother. 2015;70(2):562–565. [DOI] [PubMed] [Google Scholar]
- 22.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–1295. [DOI] [PubMed] [Google Scholar]
- 23.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–1300. [DOI] [PubMed] [Google Scholar]
- 24.Turriziani O, Bucci M, Stano A, et al. Genotypic resistance of archived and circulating viral strains in the blood of treated HIV-infected individuals. J Acquir Immune Defic Syndr. 2007;44(5):518–524. [DOI] [PubMed] [Google Scholar]
- 25.Napravnik S, Eron JJ Jr., McKaig RG, Heine AD, Menezes P, Quinlivan E Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care. 2006;18 Suppl 1:S45–50. [DOI] [PubMed] [Google Scholar]
- 26.Wensing AM, Calvez V, Gunthard HF, et al. 2017. Update of the Drug Resistance Mutations in HIV-1. Top Antivir Med. 2017;24(4):132–133. [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham AG, Lau B, Deeks S, et al. Missing data on the estimation of the prevalence of accumulated human immunodeficiency virus drug resistance in patients treated with antiretroviral drugs in north america. Am J Epidemiol. 2011;174(6):727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanik EL, Napravnik S, Hurt CB, et al. Prevalence of transmitted antiretroviral drug resistance differs between acutely and chronically HIV-infected patients. J Acquir Immune Defic Syndr. 2012;61(2):258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer J Analysis of Incomplete Multivariate Data. New York: Chapman & Hall; 1997. [Google Scholar]
- 30.Rubin D Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 2004. [Google Scholar]
- 31.Arasteh K, Yeni P, Pozniak A, et al. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antivir Ther. 2009;14(6):859–864. [DOI] [PubMed] [Google Scholar]
- 32.Ammassari A, Trotta MP, Murri R, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr. 2002;31 Suppl 3:S123–127. [DOI] [PubMed] [Google Scholar]
- 33.Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49(9):1441–1449. [DOI] [PubMed] [Google Scholar]
- 35.Katlama C, Clotet B, Mills A, et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir Ther. 2010;15(7):1045–1052. [DOI] [PubMed] [Google Scholar]
- 36.Davy-Mendez T, Eron JJ, Zakharova O, Wohl DA, Napravnik S. Increased Persistence of Initial Treatment for HIV Infection With Modern Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2017;76(2):111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eron JJ, Cooper DA, Steigbigel RT, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis. 2013;13(7):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378(9787):238–246. [DOI] [PubMed] [Google Scholar]
- 39.Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011;378(9787):229–237. [DOI] [PubMed] [Google Scholar]
- 40.Eron JJ Jr., Rockstroh JK, Reynes J, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis. 2011;11(12):907–915. [DOI] [PubMed] [Google Scholar]
- 41.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–2448. [DOI] [PubMed] [Google Scholar]
- 42.Fulcher JA, Du Y, Sun R, Landovitz RJ. Emergence of integrase resistance mutations during initial therapy with TDF/FTC/DTG. Paper presented at: Conference on Retroviruses and Opportunistic Infections; February 13–16, 2017; Seattle, Washington. [Google Scholar]
- 43.Sax PE, DeJesus E, Crofoot G, et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;4(4):e154–e160. [DOI] [PubMed] [Google Scholar]
- 44.Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380–1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073–2082. [DOI] [PubMed] [Google Scholar]
- 45.Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380–1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063–2072. [DOI] [PubMed] [Google Scholar]
- 46.Menza TW, Billock R, Samoff E, Eron JJ, Dennis AM. Pre-treatment integrase strand transfer inhibitor resistance in north carolina from 2010–2016. AIDS. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richman DD, Morton SC, Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18(10):1393–1401. [DOI] [PubMed] [Google Scholar]
- 48.Buchacz K, Baker R, Ward DJ, et al. Trends in Decline of Antiretroviral Resistance among ARV-Experienced Patients in the HIV Outpatient Study: 1999–2008. AIDS Res Treat. 2012;2012:230290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paquet AC, Solberg OD, Napolitano LA, et al. A decade of HIV-1 drug resistance in the United States: trends and characteristics in a large protease/reverse transcriptase and co-receptor tropism database from 2003 to 2012. Antivir Ther. 2014;19(4):435–441. [DOI] [PubMed] [Google Scholar]
- 50.Snedecor SJ, Sudharshan L, Nedrow K, et al. Burden of nonnucleoside reverse transcriptase inhibitor resistance in HIV-1-infected patients: a systematic review and meta-analysis. AIDS Res Hum Retroviruses. 2014;30(8):753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhee SY, Gonzales MJ, Kantor R, Betts BJ, Ravela J, Shafer RW. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003;31(1):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherrer AU, von Wyl V, Yang WL, et al. Emergence of Acquired HIV-1 Drug Resistance Almost Stopped in Switzerland: A 15-Year Prospective Cohort Analysis. Clin Infect Dis. 2016;62(10):1310–1317. [DOI] [PubMed] [Google Scholar]
- 53.von Wyl V, Yerly S, Burgisser P, et al. Long-term trends of HIV type 1 drug resistance prevalence among antiretroviral treatment-experienced patients in Switzerland. Clin Infect Dis. 2009;48(7):979–987. [DOI] [PubMed] [Google Scholar]
- 54.Napravnik S, Keys JR, Quinlivan EB, Wohl DA, Mikeal OV, Eron JJ, Jr. Triple-class antiretroviral drug resistance: risk and predictors among HIV-1-infected patients. AIDS. 2007;21(7):825–834. [DOI] [PubMed] [Google Scholar]
- 55.Phillips AN, Dunn D, Sabin C, et al. Long term probability of detection of HIV-1 drug resistance after starting antiretroviral therapy in routine clinical practice. AIDS. 2005;19(5):487–494. [DOI] [PubMed] [Google Scholar]
- 56.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191(3):339–347. [DOI] [PubMed] [Google Scholar]
- 57.Collaborative UK Group on HIV Drug Resistance. Long-term probability of detecting drug-resistant HIV in treatment-naive patients initiating combination antiretroviral therapy. Clin Infect Dis. 2010;50(9):1275–1285. [DOI] [PubMed] [Google Scholar]
- 58.Lodwick R, Costagliola D, Reiss P, et al. Triple-class virologic failure in HIV-infected patients undergoing antiretroviral therapy for up to 10 years. Arch Intern Med. 2010;170(5):410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beer L, Heffelfinger J, Frazier E, et al. Use of and Adherence to Antiretroviral Therapy in a Large U.S. Sample of HIV-infected Adults in Care, 2007–2008. Open AIDS J. 2012;6:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2001;26(1):82–92. [DOI] [PubMed] [Google Scholar]
- 61.Lichtenstein KA, Armon C, Buchacz K, et al. Initiation of antiretroviral therapy at CD4 cell counts >/=350 cells/mm3 does not increase incidence or risk of peripheral neuropathy, anemia, or renal insufficiency. J Acquir Immune Defic Syndr. 2008;47(1):27–35. [DOI] [PubMed] [Google Scholar]
- 62.Gay CL, Napravnik S, Eron JJ, Jr. Advanced immunosuppression at entry to HIV care in the southeastern United States and associated risk factors. Aids. 2006;20(5):775–778. [DOI] [PubMed] [Google Scholar]
- 63.Dennis AM, Napravnik S, Sena AC, Eron JJ. Late entry to HIV care among Latinos compared with non-Latinos in a southeastern US cohort. Clin Infect Dis. 2011;53(5):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robertson M, Wei SC, Beer L, et al. Delayed entry into HIV medical care in a nationally representative sample of HIV-infected adults receiving medical care in the USA. AIDS Care. 2016;28(3):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table. Characteristics of patients with cumulative resistance mutations in four classes on available genotype tests. docx
Figure. Estimated prevalence of resistance mutations and 95% confidence bands by drug class and multiclass combinations among patients in care with HIV viremia who initiated ART between 2007 and 2016. pptx
Table. Multivariable odds ratios of HIV drug resistance with 95% confidence intervals for patients in care in 2016. docx


