Abstract
Background
Malnutrition is a common and critical problem that influences outcome in cancer patients. Body composition reflects a patient’s metabolic profile and physiologic reserves, which might be the true determinant of prognosis. In the present study, which aimed to identify valuable new prognostic indicators, we investigated the association between computed tomography–quantified body composition and short-term outcomes after gastrectomy for gastric cancer.
Methods
Skeletal muscle index, mean muscle attenuation, and ratio of visceral-to-subcutaneous adipose tissue area (vsr) were calculated from preoperative computed tomography images. Low skeletal muscle index, low mean muscle attenuation, and high vsr were respectively termed “sarcopenia,” “myosteatosis,” and “visceral obesity.” The association of body composition with postoperative complications and serum markers of nutrition and inflammation after radical gastrectomy were analyzed.
Results
The overall complication rate was significantly higher in the sarcopenia (62.5% vs. 27.3%, p = 0.001) and myosteatosis groups (38.2% vs. 4%, p = 0.002). Patients with visceral obesity had a higher incidence of inflammatory complications (20.3% vs. 6.5%, p = 0.01). Multivariate logistic regression analysis demonstrated that sarcopenia (p = 0.013), myosteatosis (p = 0.017), and low serum retinol-binding protein (p = 0.019) were independent risk factors for overall complications. Compared with control subjects, patients with sarcopenia had lower postoperative levels of serum retinol-binding protein (p = 0.007), and patients with visceral obesity had higher levels of C-reactive protein (p = 0.026).
Conclusions
Sarcopenia, myosteatosis, and visceral obesity were significantly associated with increased rates of postoperative complications and affected the postoperative nutrition and inflammation status of patients with gastric cancer.
Keywords: Key Words Body composition, sarcopenia, myosteatosis, visceral obesity, gastrectomy, complications
INTRODUCTION
Globally, gastric cancer is one of the most commonly diagnosed cancers and a leading cause of cancer-related death1. In 2013 in China, it was estimated that 427,000 new cases of gastric cancer and 301,000 deaths from the disease occurred nationwide, accounting for half the global incidence and deaths2. The most effective therapy for potentially curable gastric cancer is surgical resection3. However, radical surgery is associated with high rates of complications and operative mortality, severely negatively affecting prognosis in these patients4,5. Objective and precise prognostic assessments before radical gastrectomy are therefore critical so that physicians can predict postoperative clinical outcomes and guide the therapeutic protocol.
Malnutrition and weight loss are common problems in cancer patients6,7, the pathophysiology of which consists of a mixture of reduced food intake and disturbance of the metabolic and inflammatory responses8. Those factors have been recognized to increase the risk for surgical complications9 and to be associated with longer hospital stays, increased health care costs, lower quality of life, and shorter survival. Assessing the nutrition status of these patients before surgery and rendering the appropriate nutrition support is therefore important to optimize status, decrease complications, and improve clinical outcomes.
Identification of patients who are at nutritional risk and who have malnutrition is the first step in the nutrition care pathway. Commonly used tools for nutrition assessment such as body mass index (bmi) or Nutritional Risk Screening (nrs) 2002 are limited because of their inability to assess individual components of body weight such as regional fat distribution and muscle volume and composition. On the other hand, nutrition assessments based on body composition measurements (bcms) can reflect body shape and composition, metabolic profile, and physiologic reserve, which might affect the perioperative inflammatory response and nutrition metabolism and be a true determinant of prognosis10.
It has been reported that visceral obesity, rather than bmi, is an independent risk factor for recurrence of hepatocellular carcinoma in patients with non-viral disease11. Loss of muscle mass, called sarcopenia, has been found in 19%–74% of patients with solid tumours12, and it is an independent risk factor for complications and survival after surgical resection13. The mean muscle attenuation (ma), measured in mean Hounsfield units during routine computed tomography (ct) imaging, indicates muscle composition. Low ma, known as myosteatosis, indicates increased intramuscular lipid content that contributes to muscle weakness14,15. Myosteatosis has previously been reported to be associated with postoperative mortality after hepatocellular carcinoma resection16.
Thus, in the present study, we explored the association of body composition assessed by preoperative ct with postoperative complications and markers of nutrition and inflammation in patients undergoing radical surgery for gastric cancer. We aimed to identify prognostic bcms that can predict short-term outcomes after gastrectomy and guide the therapeutic protocol.
METHODS
Patients and Data Collection
The study protocol was approved by the Ethics Committee of Jinling Hospital. All procedures involving human participants conformed to the ethics standards of the institutional or national research committee (or both) and the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all patients participating in the study.
The study included all consecutive patients with gastric cancer undergoing open radical gastrectomy at the Department of General Surgery at Jinling Hospital from September 2015 to March 2017. Inclusion criteria were age 18–80 years, histologically proven gastric adenocarcinoma before surgery, availability of digitally-stored ct imaging taken within 15 days before surgery, and no history of previous abdominal surgery. Patients with metastatic cancer and those undergoing laparoscopic-assisted surgery or combined organ resection were excluded. The operations were performed by a single group of specialized surgeons with extensive experience in radical resections for gastric cancer. All patients were managed according to the Japanese gastric cancer treatment guidelines (version 3, 2010)17.
The following data were collected by trained surgeons and maintained in a digital database: clinicopathologic features (age, sex, bmi, nrs 2002 score, presence of diabetes and other comorbidities, neoadjuvant chemotherapy, type of resection and reconstruction, histologic type, and TNM tumour stage); body composition variables and laboratory parameters associated with nutrition and inflammation status [albumin, prealbumin, transferrin, retinol-binding protein (rbp), C-reactive protein (crp), procalcitonin, and interleukin 6]; and postoperative outcomes (complications, time of intestinal exhaust, gastric drainage, abdominal drainage, albumin use, and postoperative hospital stay). Postoperative complications were graded using the Clavien–Dindo system18. Overall complications were defined as those of Clavien–Dindo grade 2 or higher. Inflammatory complications such as infection at the surgical site, pneumonia, infection of the gastrointestinal system, and bloodstream infection were defined using the National Healthcare Safety Network criteria established by the U.S. Centers for Disease Control and Prevention19. Cancer staging was based on the 7th edition of the TNM classification system published by the Union for International Cancer Control20.
Imaging Analysis
The OsiriX open-source software (version 8.5.2: Pixmeo sarl, Geneva, Switzerland) was used to analyze the ct imaging according to a previously described protocol16,21. A single slice at L3, with both transverse processes visible, was extracted to determine the skeletal muscle and abdominal adipose tissue area. These tissue-specific thresholds, as previously described, were used: −29 HU to 150 HU for skeletal muscle; −190 HU to −30 HU for subcutaneous adipose tissue; and −150 HU to −50 HU for visceral adipose tissue. Each specific tissue area was normalized to the square of the patient’s height (m2), resulting in a skeletal muscle index (smi), a subcutaneous adipose tissue index, and a visceral adipose tissue index. We calculated the ma by averaging the Hounsfield units of the L3 skeletal muscle to assess skeletal muscle composition and the visceral-to-subcutaneous ratio of adipose tissue area (vsr) to explore abdominal adipose tissue distributions. Sarcopenia was accepted when the smi was 34.9 cm2/m2 or less for women and 40.8 cm2/m2 or less for men (cut-off values determined in a very large cohort of Chinese patients22). Myosteatosis was accepted when the ma was 44.4 HU or less in men and 39.3 HU or less in women, and visceral obesity was accepted when the vsr was 1.33 or greater in men and 0.93 or greater in women (based on a prior report from Japan16).
Statistics
Quantitative variables are expressed as means and standard deviations (normally distributed data) or medians with interquartile ranges (non-normally distributed data). Categorical variables are expressed as numbers and percentages. Groups were compared using the Student t-test for normally distributed data, the Pearson chi-square test or Fisher exact test for categorical variables, and the Mann–Whitney U-test for non-normally distributed continuous data and ranked data. Univariate and multivariate analyses of postoperative complications were performed using logistic regression, and the results are presented as odds ratios (ors) with 95% confidence intervals (cis). Variables significant in the univariate model were entered into the multivariate models. Repeated-measures linear regression models were used to account for the dependency of the observations over time and to analyze the effect of body composition over time on changes in markers of nutrition and inflammation. Values of p < 0.05 were considered statistically significant. All data were analyzed using the IBM SPSS Statistics software application (version 23.0: IBM, Armonk, NY, U.S.A.).
RESULTS
Patient Characteristics
Of the 187 patients who met the inclusion criteria, 31 (16.6%) were excluded (7 with metastatic cancer incurable by radical surgery, 10 who had undergone combined organ resection, and 14 who had undergone laparoscopic-assisted surgery), leaving 156 patients [115 men (73.7%), 41 women (26.3%)] available for analysis.
Table i summarizes the demographic and clinical characteristics of the patients. Mean age in the cohort was 59.1 years. The TNM stage distribution showed 48 patients with stage i disease (30.8%), 27 with stage ii disease (17.3%), and 81 with stage iii disease (51.9%). Neoadjuvant chemotherapy was administered to 35 patients (22.4%) with unresectable locally advanced gastric cancer. Although 31.7% of the patients were found to be at nutritional risk (nrs 2002 score ≥3), only 6.4% (10 patients) had a bmi less than 18.5. The mean preoperative values for serum markers of nutrition, inflammatory cytokines, and other laboratory parameters were within normal range.
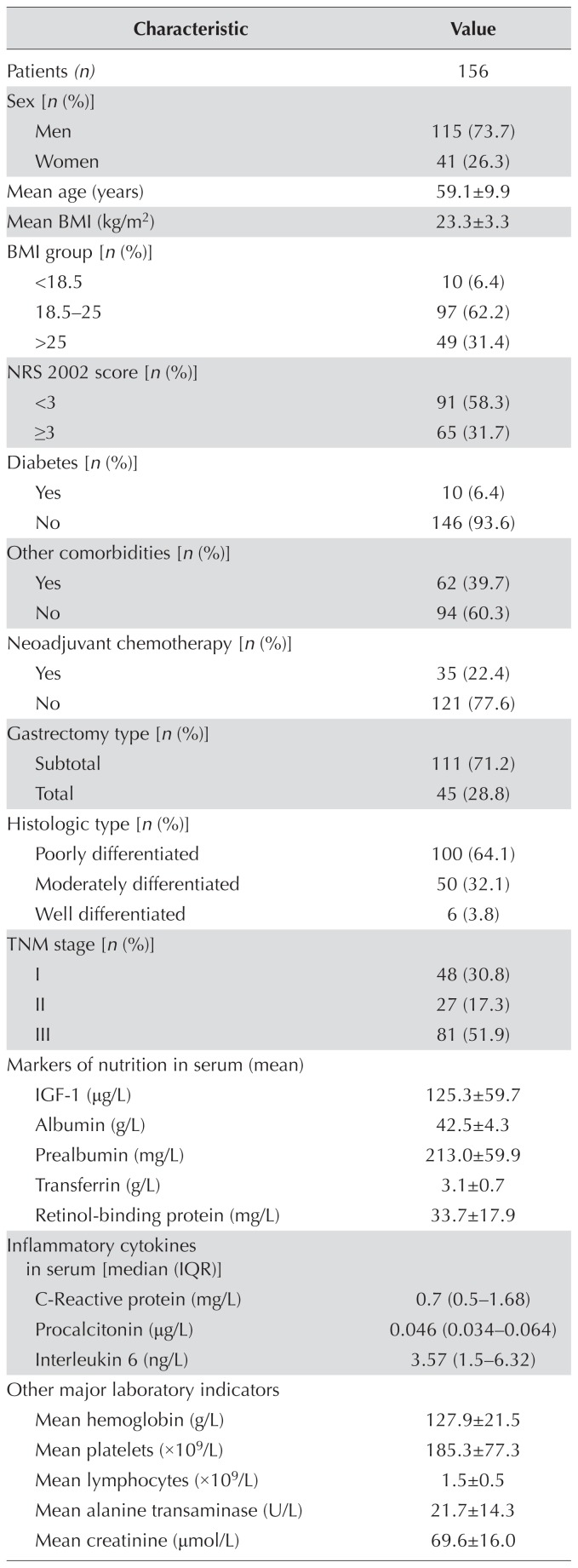
TABLE I.
Baseline characteristics of the study patients
| Characteristic | Value |
|---|---|
| Patients (n) | 156 |
|
| |
| Sex [n (%)] | |
| Men | 115 (73.7) |
| Women | 41 (26.3) |
|
| |
| Mean age (years) | 59.1±9.9 |
|
| |
| Mean BMI (kg/m2) | 23.3±3.3 |
|
| |
| BMI group [n (%)] | |
| <18.5 | 10 (6.4) |
| 18.5–25 | 97 (62.2) |
| >25 | 49 (31.4) |
|
| |
| NRS 2002 score [n (%)] | |
| <3 | 91 (58.3) |
| ≥3 | 65 (31.7) |
|
| |
| Diabetes [n (%)] | |
| Yes | 10 (6.4) |
| No | 146 (93.6) |
|
| |
| Other comorbidities [n (%)] | |
| Yes | 62 (39.7) |
| No | 94 (60.3) |
|
| |
| Neoadjuvant chemotherapy [n (%)] | |
| Yes | 35 (22.4) |
| No | 121 (77.6) |
|
| |
| Gastrectomy type [n (%)] | |
| Subtotal | 111 (71.2) |
| Total | 45 (28.8) |
|
| |
| Histologic type [n (%)] | |
| Poorly differentiated | 100 (64.1) |
| Moderately differentiated | 50 (32.1) |
| Well differentiated | 6 (3.8) |
|
| |
| TNM stage [n (%)] | |
| I | 48 (30.8) |
| II | 27 (17.3) |
| III | 81 (51.9) |
|
| |
| Markers of nutrition in serum (mean) | |
| IGF-1 (μg/L) | 125.3±59.7 |
| Albumin (g/L) | 42.5±4.3 |
| Prealbumin (mg/L) | 213.0±59.9 |
| Transferrin (g/L) | 3.1±0.7 |
| Retinol-binding protein (mg/L) | 33.7±17.9 |
|
| |
| Inflammatory cytokines in serum [median (IQR)] | |
| C-Reactive protein (mg/L) | 0.7 (0.5–1.68) |
| Procalcitonin (μg/L) | 0.046 (0.034–0.064) |
| Interleukin 6 (ng/L) | 3.57 (1.5–6.32) |
|
| |
| Other major laboratory indicators | |
| Mean hemoglobin (g/L) | 127.9±21.5 |
| Mean platelets (×109/L) | 185.3±77.3 |
| Mean lymphocytes (×109/L) | 1.5±0.5 |
| Mean alanine transaminase (U/L) | 21.7±14.3 |
| Mean creatinine (μmol/L) | 69.6±16.0 |
| Mean skeletal muscle index (cm2/m2) | |
| Overall | 48.0±8.8 |
| In men | 50.7±7.9 |
| In women | 40.6±6.6 |
|
| |
| Mean muscle attenuation (HU) | |
| Overall | 34.9±6.4 |
| In men | 36.4±5.8 |
| In women | 30.8±6.4 |
|
| |
| Mean SATI (cm2/m2) | |
| Overall | 38.5±23.0 |
| In men | 32.6±17.0 |
| In women | 55.5±28.0 |
|
| |
| Mean VATI (cm2/m2) | |
| Overall | 42.1±27.8 |
| In men | 43.9±28.9 |
| In women | 37.4±23.5 |
|
| |
| Mean VSR | |
| Overall | 1.16±0.7 |
| In men | 1.3±0.6 |
| In women | 0.7±0.6 |
BMI = body mass index; NRS = Nutritional Risk Screening; IGF-1 = insulin-like growth factor 1; SATI = subcutaneous adipose tissue index; VATI = visceral adipose tissue index; VSR = visceral-to-subcutaneous ratio of adipose area.
Using the OsiriX software, body composition variables were calculated based on ct imaging (supplementary Figure 1). The mean smi, ma, subcutaneous adipose tissue index, visceral adipose tissue index, and vsr were, respectively, 50.7 ± 7.9 cm2/m2, 36.4 ± 5.8 HU, 32.6 ± 17.0 cm2/m2, 43.9 ± 28.9 cm2/m2, and 1.3 ± 0.6 in men, and 40.6 ± 6.6 cm2/m2, 30.8 ± 6.4 HU, 55.5 ± 28.0 cm2/m2, 37.4 ± 23.5 cm2/m2, and 0.7 ± 0.6 in women.
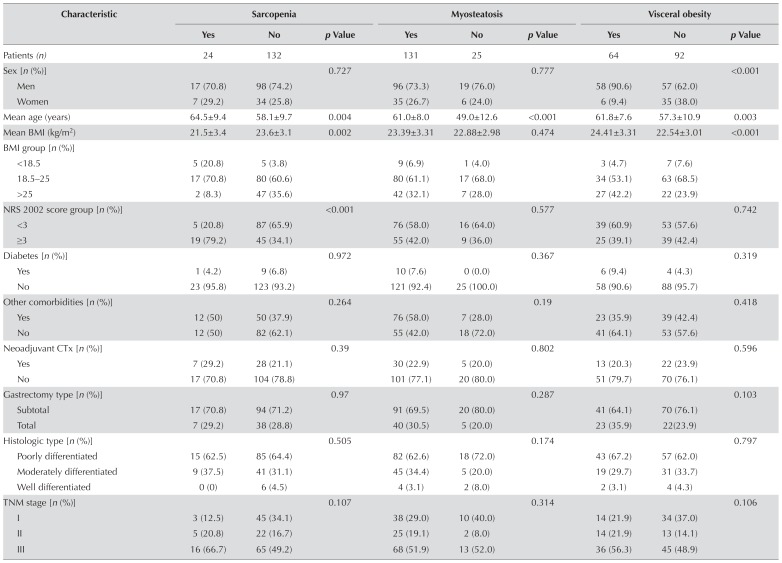
We subsequently investigated the associations between body composition and clinicopathologic characteristics in the patients (Table ii). Patients were divided into groups with and without sarcopenia, myosteatosis, and visceral obesity based on the criteria previously discussed. According to those criteria, 24 patients (15.4%) had sarcopenia, 131 patients (84.0%) had myosteatosis, and 64 patients (41.0%) had visceral obesity. Visceral obesity was more frequently seen in men (p < 0.001). Patients with sarcopenia (p = 0.004), myosteatosis (p < 0.001), and visceral obesity (p = 0.003) were significantly older than patients without those conditions. Patients with sarcopenia had a significantly lower bmi (p = 0.002) and nrs 2002 score (p < 0.001). Serum markers of nutrition, including insulin-like growth factor 1 (p= 0.022), albumin ( p= 0.003), prealbumin (p < 0.001), and hemoglobin (p = 0.013) were also significantly lower in the sarcopenia group. However, patients with visceral obesity had a significantly higher bmi (p< 0.001), serum crp (p= 0.049), and serum creatinine ( p= 0.005), and lower serum rbp (p = 0.048). Serum crp and interleukin 6 were higher in patients with myosteatosis, but not significantly so (p= 0.058 and p= 0.062 respectively). Of sarcopenia, myosteatosis, and visceral obesity, none was significantly associated with tumour histologic type, TNM stage, or preoperative comorbidities.
TABLE II.
Association between body composition and clinicopathologic characteristics in the study patients
| Characteristic | Sarcopenia | Myosteatosis | Visceral obesity | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Yes | No | p Value | Yes | No | p Value | Yes | No | p Value | |
| Patients (n) | 24 | 132 | 131 | 25 | 64 | 92 | |||
|
| |||||||||
| Sex [n (%)] | 0.727 | 0.777 | <0.001 | ||||||
| Men | 17 (70.8) | 98 (74.2) | 96 (73.3) | 19 (76.0) | 58 (90.6) | 57 (62.0) | |||
| Women | 7 (29.2) | 34 (25.8) | 35 (26.7) | 6 (24.0) | 6 (9.4) | 35 (38.0) | |||
|
| |||||||||
| Mean age (years) | 64.5±9.4 | 58.1±9.7 | 0.004 | 61.0±8.0 | 49.0±12.6 | <0.001 | 61.8±7.6 | 57.3±10.9 | 0.003 |
|
| |||||||||
| Mean BMI (kg/m2) | 21.5±3.4 | 23.6±3.1 | 0.002 | 23.39±3.31 | 22.88±2.98 | 0.474 | 24.41±3.31 | 22.54±3.01 | <0.001 |
|
| |||||||||
| BMI group [n (%)] | |||||||||
| <18.5 | 5 (20.8) | 5 (3.8) | 9 (6.9) | 1 (4.0) | 3 (4.7) | 7 (7.6) | |||
| 18.5–25 | 17 (70.8) | 80 (60.6) | 80 (61.1) | 17 (68.0) | 34 (53.1) | 63 (68.5) | |||
| >25 | 2 (8.3) | 47 (35.6) | 42 (32.1) | 7 (28.0) | 27 (42.2) | 22 (23.9) | |||
|
| |||||||||
| NRS 2002 score group [n (%)] | <0.001 | 0.577 | 0.742 | ||||||
| <3 | 5 (20.8) | 87 (65.9) | 76 (58.0) | 16 (64.0) | 39 (60.9) | 53 (57.6) | |||
| ≥3 | 19 (79.2) | 45 (34.1) | 55 (42.0) | 9 (36.0) | 25 (39.1) | 39 (42.4) | |||
|
| |||||||||
| Diabetes [n (%)] | 0.972 | 0.367 | 0.319 | ||||||
| Yes | 1 (4.2) | 9 (6.8) | 10 (7.6) | 0 (0.0) | 6 (9.4) | 4 (4.3) | |||
| No | 23 (95.8) | 123 (93.2) | 121 (92.4) | 25 (100.0) | 58 (90.6) | 88 (95.7) | |||
|
| |||||||||
| Other comorbidities [n (%)] | 0.264 | 0.19 | 0.418 | ||||||
| Yes | 12 (50) | 50 (37.9) | 76 (58.0) | 7 (28.0) | 23 (35.9) | 39 (42.4) | |||
| No | 12 (50) | 82 (62.1) | 55 (42.0) | 18 (72.0) | 41 (64.1) | 53 (57.6) | |||
|
| |||||||||
| Neoadjuvant CTx [n (%)] | 0.39 | 0.802 | 0.596 | ||||||
| Yes | 7 (29.2) | 28 (21.1) | 30 (22.9) | 5 (20.0) | 13 (20.3) | 22 (23.9) | |||
| No | 17 (70.8) | 104 (78.8) | 101 (77.1) | 20 (80.0) | 51 (79.7) | 70 (76.1) | |||
|
| |||||||||
| Gastrectomy type [n (%)] | 0.97 | 0.287 | 0.103 | ||||||
| Subtotal | 17 (70.8) | 94 (71.2) | 91 (69.5) | 20 (80.0) | 41 (64.1) | 70 (76.1) | |||
| Total | 7 (29.2) | 38 (28.8) | 40 (30.5) | 5 (20.0) | 23 (35.9) | 22(23.9) | |||
|
| |||||||||
| Histologic type [n (%)] | 0.505 | 0.174 | 0.797 | ||||||
| Poorly differentiated | 15 (62.5) | 85 (64.4) | 82 (62.6) | 18 (72.0) | 43 (67.2) | 57 (62.0) | |||
| Moderately differentiated | 9 (37.5) | 41 (31.1) | 45 (34.4) | 5 (20.0) | 19 (29.7) | 31 (33.7) | |||
| Well differentiated | 0 (0) | 6 (4.5) | 4 (3.1) | 2 (8.0) | 2 (3.1) | 4 (4.3) | |||
|
| |||||||||
| TNM stage [n (%)] | 0.107 | 0.314 | 0.106 | ||||||
| I | 3 (12.5) | 45 (34.1) | 38 (29.0) | 10 (40.0) | 14 (21.9) | 34 (37.0) | |||
| II | 5 (20.8) | 22 (16.7) | 25 (19.1) | 2 (8.0) | 14 (21.9) | 13 (14.1) | |||
| III | 16 (66.7) | 65 (49.2) | 68 (51.9) | 13 (52.0) | 36 (56.3) | 45 (48.9) | |||
|
| |||||||||
| Nutrition indicators in serum (mean) | |||||||||
| IGF-1 (μg/L) | 104.7±66.2 | 134.8±57.2 | 0.022 | 129.0±57.4 | 136.1±70.1 | 0.585 | 139.0±63.2 | 123.9±56.3 | 0.12 |
| Albumin (g/L) | 40.1±4.9 | 42.9±4.1 | 0.003 | 42.4±4.4 | 42.9±4.2 | 0.599 | 42.6±4.7 | 42.4±4.1 | 0.802 |
| Prealbumin (mg/L) | 171.1±56.1 | 220.6±57.5 | <0.001 | 210.6±60.0 | 225.4±58.9 | 0.258 | 213.6±60.8 | 212.6±59.6 | 0.923 |
| Transferrin (g/L) | 2.9±0.5 | 3.1±0.8 | 0.109 | 3.1±0.8 | 2.9±0.5 | 0.246 | 3.2±1.0 | 3.1±0.4 | 0.384 |
| RBP (mg/L) | 28.3±16.9 | 34.6±18 | 0.112 | 33.5±19.0 | 34.6±11.6 | 0.785 | 30.3±12.8 | 36.0±20.5 | 0.048 |
|
| |||||||||
| Serum inflammatory cytokines | |||||||||
| C-Reactive protein (mg/L) | 0.057 | 0.058 | 0.049 | ||||||
| Median | 1.15 | 0.6 | 0.8 | 0.5 | 0.85 | 0.65 | |||
| IQR | (0.5–8.5) | (0.5–1.5) | (0.5–1.95) | (0.5–0.9) | (0.5–2.75) | (0.5–1.28) | |||
| Procalcitonin (μg/L) | 0.487 | 0.752 | 0.481 | ||||||
| Median | 0.046 | 0.046 | 0.045 | 0.049 | 0.045 | 0.047 | |||
| IQR | (0.033–0.058) | (0.034–0.065) | (0.034–0.066) | (0.043–0.058) | (0.034–0.065) | (0.034–0.064) | |||
| Interleukin 6 (ng/L) | 0.235 | 0.062 | 0.445 | ||||||
| Median | 4.66 | 3.49 | 3.7 | 1.95 | 3.97 | 3.25 | |||
| IQR | (2.28–6.81) | (1.5–6.28) | (1.5–6.52) | (1.5–4.0) | (1.5–6.36) | (1.5–6.29) | |||
|
| |||||||||
| Other major laboratory indicators (mean) | |||||||||
| Hemoglobin (g/L) | 118±22.4 | 130±20.9 | 0.013 | 126.9±20.6 | 133.5±25.0 | 0.155 | 129.7±23.2 | 126±20.3 | 0.392 |
| Platelets (×109/L) | 199±87 | 182.8±75.5 | 0.344 | 180.2±77.0 | 212.0±75.1 | 0.059 | 179.2±73.4 | 189.5±80.1 | 0.419 |
| Lymphocytes (×109/L) | 1.5±0.7 | 1.5±0.5 | 0.974 | 1.5±0.6 | 1.7±0.4 | 0.17 | 1.5±0.5 | 1.5±0.5 | 0.537 |
| ALT (U/L) | 18.5±9.4 | 22.2±15 | 0.236 | 21.7±14.6 | 21.6±12.9 | 0.984 | 21.7±13.6 | 21.6±14.9 | 0.963 |
| Creatinine (μmol/L) | 66.7±15.1 | 70.2±16.2 | 0.328 | 69.2±15.2 | 71.6±19.8 | 0.494 | 73.9±15.7 | 66.7±15.6 | 0.005 |
BMI = body mass index; NRS = Nutritional Risk Screening; CTx = chemotherapy; IGF-1 = insulin-like growth factor 1; RBP = retinol-binding protein; IQR = interquartile range; ALT = alanine transaminase.
Short-Term Surgical Outcomes
In terms of short-term surgical outcomes, 51 patients (32.7%) experienced postoperative complications, and 19 (12.2%) experienced inflammatory complications. The inflammatory complications included anastomotic leakage (n = 7), wound infection (n = 1), intra-abdominal infection (n = 3), pneumonia (n = 6), and bloodstream infection (n = 2). The median postoperative hospital stay was 8 days (Table iii).
TABLE III.
Postoperative outcomes
| Characteristic | All patients (n=156) | Sarcopenia | Myosteatosis | Visceral obesity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Yes (n=24) | No (n=132) | p Value | Yes (n=131) | No (n=25) | p Value | Yes (n=64) | No (n=92) | p Value | ||
| Complicationsa [n (%)] | ||||||||||
| All | 51 (32.7) | 15 (62.5) | 36 (27.3) | 0.001 | 50 (38.2) | 1 (4.0) | 0.002 | 23 (35.9) | 28 (30.4) | 0.471 |
| Stage 2 | 26 (16.7) | 9 (37.5) | 18 (13.6) | 26 (19.8) | 0 (0.0) | 13 (20.3) | 13 (14.1) | |||
| Stage 3a | 17 (10.9) | 5 (20.8) | 12 (9.1) | 16 (12.2) | 1 (4.0) | 6 (9.4) | 11 (12.0) | |||
| Stage 3b | 7 (4.5) | 1 (4.2) | 6 (4.5) | 7 (5.3) | 0 (0.0) | 3 (4.7) | 4 (4.3) | |||
| Stage 4 | 1 (0.6) | 0 (0.0) | 1 (0.8) | 1 (0.8) | 0 (0.0) | 1 (1.6) | 0 (0.0) | |||
| Inflammatory | 19 (12.2) | 4 (16.7) | 15 (11.4) | 0.695 | 19 (14.5) | 0 (0.0) | 0.089 | 13 (20.3) | 6 (6.5) | 0.01 |
|
| ||||||||||
| Exsufflation time (days) | 0.136 | 0.26 | 0.574 | |||||||
| Median | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| IQR | (3–4) | (3–4) | (3–4) | (3–4) | (2–4) | (3–4) | (3–4) | |||
|
| ||||||||||
| Gastric drainage (mL) | 0.216 | 0.209 | 0.837 | |||||||
| Median | 80 | 140 | 80 | 70 | 130 | 80 | 80 | |||
| IQR | (25–247) | (36–408) | (21–215) | (22–210) | (30–300) | (30–290) | (22–232.5) | |||
|
| ||||||||||
| Abdominal drainage (mL) | 0.467 | 0.003 | 0.609 | |||||||
| Median | 525 | 560 | 520 | 580 | 275 | 545 | 515 | |||
| IQR | (275–1180) | (324–1545) | (267–1080) | (300–1250) | (151–419) | (270–1260) | (277–1045) | |||
|
| ||||||||||
| Albumin use (g) | 0.002 | 0.037 | 0.85 | |||||||
| Median | 40 | 70 | 35 | 40 | 0 | 40 | 30 | |||
| IQR | (0–60) | (23–70) | (0–60) | (0–60) | (0–50) | (0–75) | (0–60) | |||
|
| ||||||||||
| Postoperative hospitalization (days) | 0.065 | 0.006 | 0.135 | |||||||
| Median | 8 | 11 | 8 | 8.5 | 7 | 9 | 8 | |||
| IQR | (7–11.8) | (7–17) | (7–11) | (7–12) | (6–8) | (7–12) | (6–10.5) | |||
Assessed by Clavien–Dindo grade.
IQR = interquartile range.
The associations between body composition parameters and clinical outcomes were also investigated (Table iii). The results showed that the overall complication rate was significantly higher in the sarcopenia group (62.5% vs. 27.3%, p = 0.001) and in the myosteatosis group (38.2% vs. 4%, p = 0.002). Patients with visceral obesity had a higher incidence of inflammatory complications (20.3% vs. 6.5%, p= 0.01). Increased albumin infusions were needed postoperatively in both the sarcopenia and myosteatosis groups. The myosteatosis group had more abdominal drainage and a longer postoperative hospital stay. Other short-term postoperative outcomes were not significantly different in the body composition groups.
Factors Associated with Postoperative Complications
In univariate analysis (Table iv), the overall rate of postoperative complications was associated with a higher nrs 2002 score (p = 0.021), more advanced tumour stage (stage ii, p= 0.005; stage iii, p = 0.017), lower serum prealbumin (p= 0.044) and serum rbp (p= 0.003), sarcopenia (p = 0.001), and myosteatosis (p = 0.009). No significant associations between postoperative complications and the other variables were found.
TABLE IV.
Univariate and multivariate logistic regression analysis of factors associated with total postoperative complications
| Variable | Patients [n (%)] | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| All | With complicationsa | OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| Sex | Women | 41 | 14 (34.1) | Reference | |||||
| Men | 115 | 37 (32.2) | 0.9 | 0.4 to 1.9 | 0.817 | ||||
|
| |||||||||
| Age group | <65 Years | 105 | 29 (27.6) | Reference | |||||
| ≥65 Years | 51 | 22 (43.1) | 2.0 | 0.9 to 4.0 | 0.054 | ||||
|
| |||||||||
| BMI group | 18.5–25 | 97 | 32 (33.0) | Reference | |||||
| <18.5 | 10 | 6 (60.0) | 3.0 | 0.8 to 11.6 | 0.102 | ||||
| >25 | 49 | 13 (26.5) | 0.7 | 0.3 to 1.6 | 0.426 | ||||
|
| |||||||||
| NRS 2002 score group | <3 | 92 | 24 (26.1) | Reference | |||||
| ≥3 | 64 | 27 (42.2) | 1.6 | 1.1 to 2.5 | 0.021 | ||||
|
| |||||||||
| Diabetes | No | 146 | 49 (33.6) | Reference | |||||
| Yes | 10 | 2 (20.0) | 0.5 | 0.1 to 2.4 | 0.385 | ||||
|
| |||||||||
| Other comorbidities | No | 94 | 29 (30.9) | Reference | |||||
| Yes | 62 | 22 (35.5) | 1.2 | 0.6 to 2.4 | 0.546 | ||||
|
| |||||||||
| Neoadjuvant CTx | No | 121 | 36 (29.8) | Reference | |||||
| Yes | 35 | 15 (42.9) | 1.7 | 0.8 to 3.8 | 0.148 | ||||
|
| |||||||||
| Resection type | Subtotal | 111 | 32 (28.8) | Reference | |||||
| Total | 45 | 19 (42.2) | 1.8 | 0.9 to 3.7 | 0.108 | ||||
|
| |||||||||
| Histologic type | Well differentiated | 6 | 1 (16.7) | Reference | |||||
| Moderately differentiated | 50 | 18 (36.0) | 2.4 | 0.3 to 21.0 | 0.443 | ||||
| Poorly differentiated | 100 | 32 (32.0) | 2.8 | 0.3 to 26.0 | 0.362 | ||||
|
| |||||||||
| TNM stage | I | 48 | 8 (16.7) | Reference | |||||
| II | 27 | 13 (48.1) | 4.6 | 1.6 to 13.5 | 0.005 | ||||
| III | 81 | 30 (37.0) | 2.9 | 1.2 to 7.1 | 0.017 | ||||
|
| |||||||||
| IGF-1 | ≥75 μg/L | 128 | 40 (31.3) | Reference | |||||
| <75 μg/L | 28 | 11 (39.3) | 1.4 | 0.6 to 3.3 | 0.413 | ||||
|
| |||||||||
| Albumin | ≥35 g/L | 146 | 46 (31.5) | Reference | |||||
| <35 g/L | 10 | 5 (50.0) | 2.2 | 0.6 to 7.9 | 0.237 | ||||
|
| |||||||||
| Prealbumin | ≥150 mg/L | 135 | 40 (29.6) | Reference | |||||
| <150 mg/L | 21 | 11 (52.4) | 2.6 | 1.0 to 6.6 | 0.044 | ||||
|
| |||||||||
| Transferrin | ≥2.5 g/L | 146 | 47 (32.2) | Reference | |||||
| <2.5 g/L | 10 | 4 (40.0) | 1.4 | 0.4 to 5.2 | 0.612 | ||||
|
| |||||||||
| Retinol-binding protein | ≥25 mg/L | 110 | 28 (25.5) | Reference | |||||
| <25 mg/L | 46 | 23 (50.0) | 2.9 | 1.4 to 6.0 | 0.003 | 2.5 | 1.2 to 5.5 | 0.019 | |
|
| |||||||||
| Hemoglobin | ≥110 g/L | 127 | 41 (32.3) | Reference | |||||
| <110 g/L | 29 | 10 (34.5) | 1.1 | 0.5 to 2.6 | 0.82 | ||||
|
| |||||||||
| Platelets | ≥100×109/L | 135 | 41 (30.4) | Reference | |||||
| <100×109/L | 21 | 10 (47.6) | 2.1 | 0.8 to 5.3 | 0.122 | ||||
|
| |||||||||
| Lymphocytes | ≥1.0×109/L | 132 | 41 (31.1) | Reference | |||||
| <1.0×109/L | 24 | 10 (41.7) | 1.6 | 0.7 to 3.9 | 0.311 | ||||
|
| |||||||||
| Sarcopenia | No | 132 | 36 (27.3) | Reference | |||||
| Yes | 24 | 15 (62.5) | 4.0 | 1.9 to 8.6 | 0.001 | 3.4 | 1.3 to 8.8 | 0.013 | |
|
| |||||||||
| Myosteatosis | No | 25 | 1 (4.0) | Reference | |||||
| Yes | 131 | 50 (32.7) | 14.8 | 1.9 to 112.9 | 0.009 | 12.7 | 1.6 to 93.0 | 0.017 | |
|
| |||||||||
| Visceral obesity | No | 92 | 28 (30.4) | Reference | |||||
| Yes | 64 | 23 (35.9) | 1.3 | 0.7 to 2.5 | 0.472 | ||||
Clavien-Dindo grade 2 or greater.
OR = odds ratio; CI = confidence interval; BMI = body mass index; NRS = Nutritional Risk Screening; CTx = chemotherapy; IGF-1 = insulin-like growth factor 1.
The multivariate logistic regression analysis demonstrated that lower serum rbp (or: 2.5; 95% ci: 1.2 to 5.5; p= 0.019), sarcopenia (or: 3.4; 95% ci: 1.3 to 8.8; p= 0.013), and myosteatosis (or: 12.7; 95% ci: 1.6 to 93.0; p = 0.017) were independently associated with overall complications after surgery for gastric cancer. Among the various variables listed in Table v, only visceral obesity (or: 3.7; 95% ci: 1.3 to 10.2; p= 0.013) was associated with inflammatory complications.
TABLE V.
Univariate logistic regression analysis of factors associated with postoperative inflammatory complications
| Variable | Patients [n (%)] | OR | 95% CI | p Value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| All | With complicationsa | |||||
| Sex | Women | 41 | 2 (4.9) | Reference | ||
| Men | 115 | 17 (14.8) | 3.4 | 0.7 to 15.3 | 0.114 | |
|
| ||||||
| Age | <65 Years | 105 | 12 (11.4) | Reference | ||
| ≥65 Years | 51 | 7 (13.7) | 1.2 | 0.5 to 3.3 | 0.681 | |
|
| ||||||
| BMI group | 18.5–25 | 97 | 10 (10.3) | Reference | ||
| <18.5 | 10 | 0 (0.0) | — | 0.999 | ||
| ≥25 | 49 | 9 (18.4) | 2.0 | 0.7 to 5.2 | 0.177 | |
|
| ||||||
| NRS 2002 score group | <3 | 92 | 9 (9.8) | Reference | ||
| ≥3 | 64 | 10 (15.6) | 1.7 | 0.7 to 4.5 | 0.276 | |
|
| ||||||
| Diabetes | No | 146 | 18 (12.3) | Reference | ||
| Yes | 10 | 1 (10.0) | 0.8 | 0.1 to 6.6 | 0.828 | |
|
| ||||||
| Other comorbidities | No | 94 | 10 (10.6) | Reference | ||
| Yes | 62 | 9 (14.5) | 1.4 | 0.6 to 3.7 | 0.47 | |
|
| ||||||
| Neoadjuvant CTx | No | 121 | 14 (11.6) | Reference | ||
| Yes | 35 | 5 (14.3) | 1.3 | 0.4 to 3.8 | 0.666 | |
|
| ||||||
| Resection type | Subtotal | 111 | 15 (13.5) | Reference | ||
| Total | 45 | 4 (8.9) | 0.6 | 0.2 to 2.0 | 0.427 | |
|
| ||||||
| Histologic type | Well differentiated | 6 | 1 (16.7) | Reference | ||
| Moderately differentiated | 50 | 7 (14.0) | 0.8 | 0.1 to 8.0 | 0.86 | |
| Poorly differentiated | 100 | 11 (11.0) | 0.6 | 0.1 to 5.8 | 0.618 | |
|
| ||||||
| TNM stage | I | 48 | 5 (10.4) | Reference | ||
| II | 27 | 3 (11.1) | 1.1 | 0.2 to 4.9 | 0.926 | |
| III | 81 | 11 (13.6) | 1.4 | 0.4 to 4.2 | 0.599 | |
|
| ||||||
| IGF-1 | ≥75 μg/L | 128 | 16 (12.5) | Reference | ||
| <75 μg/L | 28 | 3 (10.7) | 0.8 | 0.2 to 3.1 | 0.794 | |
|
| ||||||
| Albumin | ≥35 g/L | 146 | 18 (12.3) | Reference | ||
| <35 g/L | 10 | 1 (10.0) | 0.8 | 0.1 to 6.6 | 0.828 | |
|
| ||||||
| Prealbumin | ≥150 mg/L | 135 | 15 (11.1) | Reference | ||
| <150 mg/L | 21 | 4 (19.0) | 1.9 | 0.6 to 6.3 | 0.307 | |
|
| ||||||
| Transferrin | ≥2.5 g/L | 146 | 18 (12.3) | Reference | ||
| <2.5 g/L | 10 | 1 (10.0) | 0.8 | 0.1 to 6.6 | 0.828 | |
|
| ||||||
| Retinol-binding protein | ≥25 mg/L | 110 | 12 (10.9) | Reference | ||
| <25 mg/L | 46 | 7 (15.2) | 1.5 | 0.5 to 4.0 | 0.455 | |
|
| ||||||
| Hemoglobin | ≥110 g/L | 127 | 15 (11.8) | Reference | ||
| <110 g/L | 29 | 4 (13.8) | 1.2 | 0.4 to 3.9 | 0.769 | |
|
| ||||||
| Platelets | ≥100×109/L | 135 | 15 (11.1) | Reference | ||
| <100×109/L | 21 | 4 (19.0) | 1.9 | 0.6 to 6.3 | 0.307 | |
|
| ||||||
| Lymphocytes | ≥1.0×109/L | 132 | 14 (10.6) | Reference | ||
| <1.0×109/L | 24 | 5 (20.8) | 2.2 | 0.7 to 6.9 | 0.167 | |
|
| ||||||
| Sarcopenia | No | 132 | 15 (11.4) | Reference | ||
| Yes | 24 | 4(16.7) | 1.6 | 0.5 to 5.2 | 0.468 | |
|
| ||||||
| Myosteatosis | No | 25 | 0 (0.0) | Reference | ||
| Yes | 131 | 19 (14.5) | — | 0.998 | ||
|
| ||||||
| Visceral obesity | No | 92 | 6 (6.5) | Reference | ||
| Yes | 64 | 13 (20.3) | 3.6 | 1.3 to 10.2 | 0.013 | |
Inflammatory complications.
OR = odds ratio; CI = confidence interval; BMI = body mass index; NRS = Nutritional Risk Screening; CTx = chemotherapy; IGF-1 = insulin-like growth factor 1.
Relationship Between Body Composition and Perioperative Changes in Markers of Nutrition and Inflammation
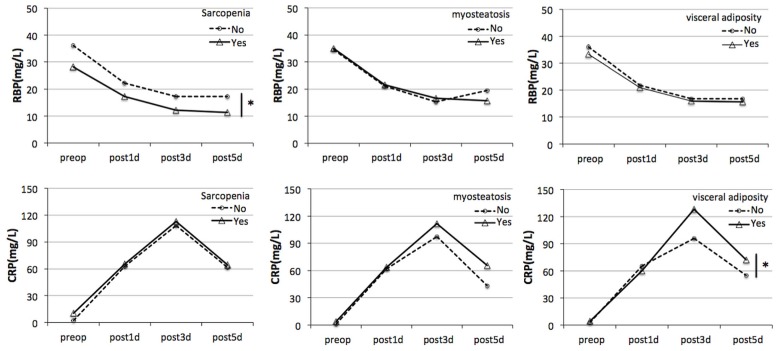
Serum rbp and crp were measured preoperatively and on days 1, 3, and 5 postoperatively to estimate perioperative change in markers of nutrition and inflammation. In patients without sarcopenia, serum rbp declined sharply on postoperative day 1 and was lowest on day 3, after which it began to slowly recover. In patients with sarcopenia, serum rbp reached a lower level and recovered later (p = 0.007). In patients with and without myosteatosis and visceral obesity, no differences in the change of serum rbp were observed. Postoperatively, serum crp rose significantly on day 1, as expected, peaking on day 3; it declined thereafter. In the group with visceral obesity, serum crp rose higher on day 3 and declined less than it did in the group without visceral obesity (p = 0.026). A difference in the pattern of crp change was not observed in other two groups (Figure 1).
FIGURE 1.
Relationship between body composition and perioperative changes in markers of nutrition and inflammation. Serum retinol-binding protein (RBP) and C-reactive protein (CRP) were measured preoperatively (preop) and on postoperative days 1, 3, and 5 (post1d, post3d, post5d). The differences in serum RBP and CRP over time were compared for the patients with and without sarcopenia, myosteatosis, and visceral obesity. *p < 0.05.
DISCUSSION
In the present study, we prospectively analyzed the associations of three main bcms with postoperative outcomes in patients with operable gastric cancer. The results showed that sarcopenia, myosteatosis, and visceral obesity were poor prognostic factors for short-term outcomes. In particular, our study is, to the best of our knowledge, the first to find a significant association between visceral obesity and inflammatory complications after radical gastrostomy for gastric cancer. We also showed that body composition might affect markers of nutrition and inflammation, which means that it might influence the body’s response to operative stress.
Increasing modern evidence shows that body composition, rather than bmi, is the stronger prognostic indicator of patient outcomes. Several prior studies have found that loss of muscle (sarcopenia), defined as a low smi, is independently associated with poor clinical outcomes in cancer patients, including excess chemotherapy toxicity23,24, increased risk of surgical complications25,26, and even poor long-term survival27,28. Several studies have investigated the effect of sarcopenia on outcomes in gastric cancer patients21,29,30. However, the results of those studies were inconsistent, possibly because of the different cut-off values used to define sarcopenia and the heterogeneity of the patient cohorts and study designs. Our prospective study focused specifically on patients with operable disease, and all surgeries were performed by a single group of surgeons. To define sarcopenia, we adopted smi cut-off values of 40.8 cm2/m2 or less for men and 34.9 cm2/m2 or less for women (obtained from a very large study about gastric cancer in patients from China22). In our study, 24 patients (15.4%) were diagnosed with sarcopenia. It is well known that poor nutrition status is associated with an increased postoperative complication rate. Our results confirmed that sarcopenia serves as a reflection of poor nutrition status and is strongly associated with a lower bmi, a higher nrs 2002 score, and lower levels of other serum markers of nutrition, including insulin-like growth factor 1, albumin, prealbumin, and hemoglobin. Sarcopenia was also an independent risk factor for overall postoperative complications.
Based on work by the European Working Group on Sarcopenia in Older People31 and the Asian Working Group for Sarcopenia32, sarcopenia has been defined as low muscle mass plus low muscle strength or low physical performance (or both). Not only decreased muscle size, but also an increased proportion of intramuscular fat can contribute to reduction in muscle strength. Most earlier studies tended to focus exclusively on skeletal muscle size, but in the present study, we introduced ma. Determined by ct imaging, ma is a noninvasive measure of muscle density in which lower values reflect increased muscle lipid content. In prior studies, ma has been found to account for differences in muscle strength independent of muscle mass, making it an indicator of muscle strength15. A significant association between low ma and reduced overall or progression-free survival has been reported in patients with gastrointestinal or respiratory tract cancer14, renal cell carcinoma33, melanoma14, and epithelial ovarian cancer34. However, any associations of ma with the rate of postoperative complications in patients with gastric cancer had not been fully investigated. Given the lack of a large study of ma in gastric cancer patients, the low ma cut-off value adopted in our study was based on a large cohort of Japanese patients with hepatocellular carcinoma16. We found that both low smi (sarcopenia) and low ma (myosteatosis) were independent predictors for more complications of Clavien–Dindo grade 2 or higher. Furthermore, we observed that patients with myosteatosis did not present with an obviously worse nutrition status as measured by bmi, nrs 2002 score, or the usual serum markers of nutrition. However, based on their elevated serum crp (p = 0.058) and interleukin 6 (p = 0.062), they seemed to present in a state of systemic inflammation that was associated with a greater occurrence of postoperative inflammatory complications (p = 0.089). However, that association did not reach statistical significance, possibly because of the limited sample size or lack of an optimal cut-off value for ma.
In addition to sarcopenia and myosteatosis, our study focused on visceral obesity as another important body composition factor. Visceral adipose tissue is an important metabolic tissue that secretes factors that systemically alter the immunologic, metabolic, and endocrine milieu35. Excess visceral adipose tissue gives rise to a state of chronic systemic inflammation with associated insulin resistance and dysmetabolism35. Earlier studies have demonstrated associations between visceral obesity and an increased risk of breast cancer36, colorectal cancer37, and esophageal adenocarcinoma38. A higher vsr was found to be associated with increased tumour progression and reduced survival in cancer patients11,16. Our study uncovered a significant relationship between visceral adipose tissue and inflammatory complications and a greater postoperative level of serum crp. Those observations indicate that visceral adipose tissue might exacerbate the postoperative acute-phase inflammatory response, affect the immune response, and ultimately result in poorer outcomes.
Cancer cachexia results not only from reduced nutrient intake or availability, but also from metabolic abnormalities triggered by the cancer and the patient’s antineoplastic therapies. Those factors stimulate systematic inflammation and cytokine networks39 that in turn result in significant loss of body weight, alterations in body composition, and declining physical function. Our findings showed that patients with sarcopenia had a lower postoperative level of serum rbp and that rbp recovery was slower in them than in patients without sarcopenia. Compared with patients not having visceral obesity, those with visceral obesity were observed to have a higher postoperative maximal level of serum crp and a prolonged systemic inflammatory response. Similar findings were reported in another study40. Those findings suggested that bcms could reflect variation in physiologic reserves, metabolic profile, and inflammatory and immune responses, and might consequently have a close association with clinical outcomes.
Limitations of our study include the small number of patients, the single-centre setting, and the lack of long-term survival data. Using long-term follow-up, we will continue to investigate this issue, combining various individual bcms so as to obtain more accurate variables potentially reflecting body metabolism and clinical prognosis.
CONCLUSIONS
In the present study, we observed that sarcopenia, myosteatosis, and visceral obesity were not only significantly associated with increased postoperative complication rates, but that they were also associated with the pattern of change in perioperative serum markers of nutrition and inflammation in patients with primary operable gastric cancer.
Supplemental Materials
ACKNOWLEDGMENTS
This work was supported by the Research Special Fund for Public Welfare Industry of Health (201202022) from the National Health and Family Planning Commission of the People’s Republic of China. Sheng Yang, Department of Public Health, Nanjing Medical University, contributed to the optimization of the statistical methods of this study.
Footnotes
Supplemental material available at http://www.current-oncology.com
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SW, Yang ZX, Zheng RS, Zeng HM, Chen WQ, He J. Incidence and mortality of stomach cancer in China, 2013 [Chinese] Zhonghua Zhong Liu Za Zhi. 2017;39:547–52. doi: 10.3760/cma.j.issn.0253-3766.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Thrumurthy SG, Chaudry MA, Hochhauser D, Mughal M. The diagnosis and management of gastric cancer. BMJ. 2013;347:f6367. doi: 10.1136/bmj.f6367. [DOI] [PubMed] [Google Scholar]
- 4.Papenfuss WA, Kukar M, Oxenberg J, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol. 2014;21:3008–14. doi: 10.1245/s10434-014-3664-z. [DOI] [PubMed] [Google Scholar]
- 5.Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99:1681–7. doi: 10.1002/bjs.8924. [DOI] [PubMed] [Google Scholar]
- 6.Zheng H, Huang Y, Shi Y, Chen W, Yu J, Wang X. Nutrition status, nutrition support therapy, and food intake are related to prolonged hospital stays in China: results from the Nutrition Day 2015 Survey. Ann Nutr Metab. 2016;69:215–25. doi: 10.1159/000451063. [DOI] [PubMed] [Google Scholar]
- 7.Gyan E, Raynard B, Durand JP, et al. Malnutrition in patients with cancer. JPEN J Parenteral Enteral Nutr. 2017 doi: 10.1177/0148607116688881. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer (Oxford) 2008;44:1124–32. doi: 10.1016/j.ejca.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda Y, Yamamoto K, Hirao M, et al. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann Surg Oncol. 2015;22(suppl 3):S778–85. doi: 10.1245/s10434-015-4820-9. [DOI] [PubMed] [Google Scholar]
- 10.Ahima RS, Lazar MA. Physiology. The health risk of obesity— better metrics imperative. Science. 2013;341:856–8. doi: 10.1126/science.1241244. [DOI] [PubMed] [Google Scholar]
- 11.Ohki T, Tateishi R, Shiina S, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected nash. Gut. 2009;58:839–44. doi: 10.1136/gut.2008.164053. [DOI] [PubMed] [Google Scholar]
- 12.Levolger S, van Vugt JL, de Bruin RW, IJzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102:1448–58. doi: 10.1002/bjs.9893. [DOI] [PubMed] [Google Scholar]
- 13.Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: a review of the current literature. J Surg Oncol. 2015;112:503–9. doi: 10.1002/jso.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985) 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral obesity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–40. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–23. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horan TC, Andrus M, Dudeck MA. cdc/nhsn surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Biondi A, Hyung WJ. Seventh edition of TNM classification for gastric cancer. J Clin Oncol. 2011;29:4338–9. doi: 10.1200/JCO.2011.36.9900. [DOI] [PubMed] [Google Scholar]
- 21.Tegels JJ, van Vugt JL, Reisinger KW, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112:403–7. doi: 10.1002/jso.24015. [DOI] [PubMed] [Google Scholar]
- 22.Zhuang CL, Huang DD, Pang WY, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore) 2016;95:e3164. doi: 10.1097/MD.0000000000003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shachar SS, Deal AM, Weinberg M, et al. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res. 2017;23:3537–43. doi: 10.1158/1078-0432.CCR-16-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 25.Sabel MS, Terjimanian M, Conlon AS, et al. Analytic morphometric assessment of patients undergoing colectomy for colon cancer. J Surg Oncol. 2013;108:169–75. doi: 10.1002/jso.23366. [DOI] [PubMed] [Google Scholar]
- 26.Zhou CJ, Zhang FM, Zhang FY, et al. Sarcopenia: a new predictor of postoperative complications for elderly gastric cancer patients who underwent radical gastrectomy. J Surg Res. 2017;211:137–46. doi: 10.1016/j.jss.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Voron T, Tselikas L, Pietrasz D, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 2015;261:1173–83. doi: 10.1097/SLA.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 28.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–86. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuda Y, Yamamoto K, Hirao M, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. 2016;19:986–93. doi: 10.1007/s10120-015-0546-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang SL, Zhuang CL, Huang DD, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: a prospective study. Ann Surg Oncol. 2016;23:556–64. doi: 10.1245/s10434-015-4887-3. [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. on behalf of the European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Antoun S, Lanoy E, Iacovelli R, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer. 2013;119:3377–84. doi: 10.1002/cncr.28218. [DOI] [PubMed] [Google Scholar]
- 34.Aust S, Knogler T, Pils D, et al. Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PloS One. 2015;10:e0140403. doi: 10.1371/journal.pone.0140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc. 2012;71:181–9. doi: 10.1017/S002966511100320X. [DOI] [PubMed] [Google Scholar]
- 36.Schapira DV, Clark RA, Wolff PA, Jarrett AR, Kumar NB, Aziz NM. Visceral obesity and breast cancer risk. Cancer. 1994;74:632–9. doi: 10.1002/1097-0142(19940715)74:2<632::AID-CNCR2820740215>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 37.Yamaji T, Iwasaki M, Sasazuki S, et al. Visceral fat volume and the prevalence of colorectal adenoma. Am J Epidemiol. 2009;170:1502–11. doi: 10.1093/aje/kwp311. [DOI] [PubMed] [Google Scholar]
- 38.Beddy P, Howard J, McMahon C, et al. Association of visceral obesity with oesophageal and junctional adenocarcinomas. Br J Surg. 2010;97:1028–34. doi: 10.1002/bjs.7100. [DOI] [PubMed] [Google Scholar]
- 39.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–9. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 40.Doyle SL, Mongan AM, Donohoe CL, et al. Impact of visceral obesity and metabolic syndrome on the postoperative immune, inflammatory, and endocrine response following surgery for esophageal adenocarcinoma. Dis Esophagus. 2017;30:1–11. doi: 10.1093/dote/dox008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.