Abstract
Background
Inhibition of the anaplastic lymphoma kinase (alk) oncogenic driver in advanced non-small-cell lung carcinoma (nsclc) improves survival. In 2015, Canadian thoracic oncology specialists published a consensus guideline about the identification and treatment of ALK-positive patients, recommending use of the alk inhibitor crizotinib in the first line. New scientific literature warrants a consensus update.
Methods
Clinical trials of alk inhibitor were reviewed to assess benefits, risks, and implications relative to current Canadian guidance in patients with ALK-positive nsclc.
Results
Randomized phase iii trials have demonstrated clinical benefit for single-agent alectinib and ceritinib used in treatment-naïve patients and as second-line therapy after crizotinib. Phase ii trials have demonstrated activity for single-agent brigatinib and lorlatinib in further lines of therapy. Improved responses in brain metastases were observed for all second- and next/third-generation alk tyrosine kinase inhibitors in patients progressing on crizotinib. Canadian recommendations are therefore revised as follows:
■ Patients with advanced nonsquamous nsclc have to be tested for the presence of an ALK rearrangement.
■ Treatment-naïve patients with ALK-positive disease should initially be offered single-agent alectinib or ceritinib, or both sequentially.
■ Crizotinib-refractory patients should be treated with single-agent alectinib or ceritinib, or both sequentially.
■ Further treatments could include single-agent brigatinib or lorlatinib, or both sequentially.
■ Patients progressing on alk tyrosine kinase inhibitors should be considered for pemetrexed-based chemotherapy.
■ Other systemic therapies should be exhausted before immunotherapy is considered.
Summary
Multiple lines of alk inhibition are now recommended for patients with advanced nsclc with an ALK rearrangement.
Keywords: Non-small-cell lung cancer, nsclc, anaplastic lymphoma kinase, ALK, tyrosine kinase inhibitors, tkis, cns, metastases
INTRODUCTION
Lung cancer is the most common cause of cancer-related death in Canada (26%), with an estimated 28,600 new cases diagnosed in 20171. Approximately 85% of those cases are non-small-cell cancer (nsclc), with 70% of those being of nonsquamous histology; most cases are found to be locally advanced or metastatic at diagnosis2–4. Distinctive chromosomal rearrangements in the ALK gene (ALK-positive) were first described in 20075 and occur in approximately 2%–5% of patients with nsclc6.
The most common ALK rearrangement is a fusion between the N-terminal half of eml4 and the intracellular kinase domain of alk (eml4–alk)7,8, leading to an active oncogenic driver. Other variations of ALK rearrangements exist. Additional ALK-related oncogenic drivers include point mutations in the kinase domain and alk overexpression9,10. Patients with ALK-positive nsclc are typically younger and tend to be light or never-smokers9; brain metastases are present at diagnosis in approximately 25% of patients11.
Many small-molecule tyrosine kinase inhibitors of alk (alk tkis) have been developed. The first-generation tki crizotinib inhibits cell-surface receptor tyrosine kinases including alk, met, and ros111. Crizotinib treated ALK-positive patients eventually develop resistance through mechanisms including acquired mutations in the alk tyrosine kinase domain, ALK gene amplification, and activation of other signalling pathways12–14; the brain is the most common site of progression, occurring in approximately 60%–70% of crizotinib-treated patientsa. The second-generation tkis alectinib and ceritinib and the next/third-generation atp (adenosine triphosphate)–competitive alk tkis brigatinib and lorlatinib, designed to overcome resistance, are currently under development for use in ALK-positive disease.
CRIZOTINIB IN THE FIRST-LINE SETTING
PROFILE 1014
Based on promising results from a phase i study15, the phase iii profile 1007 trial compared second-line crizotinib (n = 173) with standard-of-care chemotherapy (n = 174, pemetrexed or docetaxel) in advanced ALK-positive nsclc after progression on 1 prior platinum-based chemotherapy regimen16. The primary endpoint of median progression-free survival (pfs) was met, favouring crizotinib over chemotherapy [7.7 months vs. 3.0 months; hazard ratio (hr): 0.49; 95% confidence interval (ci): 0.37 to 0.64; p< 0.0001]. Results led, in May 2013, to Health Canada approval of second-line crizotinib for patients with ALK-positive disease after progression on platinum doublet therapy.
To assess crizotinib in the first line, the pivotal phase iii profile 1014 trial randomized 343 treatment-naïve patients with advanced ALK-positive nonsquamous nsclc to receive either crizotinib or platinum–pemetrexed chemotherapy without pemetrexed maintenance17. The primary endpoint, pfs by independent radiologic review, was significantly longer with crizotinib than with chemotherapy (median: 10.9 months vs. 7.0 months; hr: 0.45; 95% ci: 0.35 to 0.60; p < 0.001). The overall response rate (orr) was higher for crizotinib than for chemotherapy (74% vs. 45%, p < 0.001). Crizotinib was also associated with reduced lung cancer symptoms and improved quality of life. Based on those results, Health Canada in July 2015 approved crizotinib for treatment-naïve patients with ALK-positive nsclc. At a median follow-up of approximately 46 months in both arms, median overall survival (os) was numerically improved for crizotinib compared with chemotherapy (not yet reached vs. 47.5 months; hr: 0.76; 95% ci: 0.55 to 1.05; p = 0.098), although the difference did not reach significance18. After adjustment for crossover in the crizotinib (19.2%) and chemotherapy (84.2%) groups, a more pronounced os benefit was observed (hr: 0.35; 95% ci: 0.081 to 0.72). The longest os was associated with crizotinib followed by a second-line alktki; the shortest was associated with chemotherapy followed by treatments not involving an alk tki. Discontinuation attributable to treatment-related adverse events (aes) occurred in 5% of patients receiving crizotinib and in 8% of patients receiving chemotherapy17.
Treatment Beyond Progression
Oligometastatic progression on crizotinib can be treated with local therapy, surgery, or radiation. If clinical benefit is apparent, alk tkis can also be continued beyond progression in advanced ALK-positive nsclc. That option is based on retrospective data showing significantly longer median os from the start of crizotinib in 120 such patients who continued crizotinib compared with those who discontinued it (n = 74; 16.4 months vs. 3.9 months; hr: 0.27; 95% ci: 0.17 to 0.42; p< 0.0001)—an observation that remained significant after adjustment for confounding factors19. Although that retrospective analysis might be subject to selection bias and differences in disease biology, it remains a clinically important concept. For patients with minimal disease burden and no cranial involvement, an individualized treatment strategy developed by a multidisciplinary group might include treatment beyond progression.
SECOND-GENERATION ALK TKIS AFTER PROGRESSION ON CRIZOTINIB
Ceritinib: ASCEND-5 and -8
The pivotal phase iii ascend-5 trial confirmed the efficacy of ceritinib shown in earlier phase i/ii trials12,20,21 in patients progressing on crizotinib (Table i)22. Patients who had received 1 (88%) or 2 (12%) lines of chemotherapy and who had progressed on crizotinib (n = 231) were randomized to ceritinib (n= 115) or single-agent chemotherapy (n = 116). Compared with chemotherapy, ceritinib was associated with significantly improved median pfs (5.4 months vs. 1.6 months; hr: 0.49; 95% ci: 0.36 to 0.67; p < 0.0001) and with improved orr (39.1% vs. 6.9%). The most commonly reported aes in the ceritinib group were gastrointestinal (diarrhea, nausea, vomiting). Discontinuation because of aes occurred in 5% of patients receiving ceritinib and in 7% of patients receiving chemotherapy. Thus, ascend-5 was the first randomized phase iii study to establish the option of further targeted therapy after crizotinib for advanced ALK-positive disease.
TABLE I.
Efficacy of second and next/third-generation ALK inhibitors after progression on crizotinib
| Variable | Reference (study name) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Shaw et al., 201722 (ASCEND-5) | Novello et al., 201823 (ALUR) | Ahn et al., 201724 (ALTA) | Shaw et al., 201825 (B7461001) | ||||||
| Investigational agent | Ceritinib (second generation) | Alectinib (second generation) | Brigatinib (next/third-generation) | Lorlatinib (next/third-generation) | |||||
|
| |||||||||
| Study type | Phase III | Phase III | Randomized phase II | Phase II (cohorts 2–5)a | |||||
|
| |||||||||
| Review type | IRC | Investigator | Investigator | IRC | |||||
|
| |||||||||
| Line of treatment | Third (88%) Fourth (12%) |
Third | ≥Second | ≥Second | |||||
|
| |||||||||
| Prior therapies | ≥1 Lines of CTx and crizotinib | Platinum CTx and crizotinib | Crizotinib-refractory with (74%) or without (26%) prior CTx | Crizotinib ±CTx | Non-crizotinib TKI±CTx | 2–3 prior TKIs±CTx | |||
|
| |||||||||
| Dosage | Ceritinib 750 mg daily | Pemetrexed 500 mg/m2 or docetaxel 75 mg/m2 every 3 weeks | Alectinib 600 mg twice daily | Pemetrexed 500 mg/m2 or docetaxel 75 mg/m2 every 3 weeks | Brigatinib 180 mg dailyb (Brig-90/180) | Brigatinib 90 mg daily (Brig-90) | Lorlatinib 100 mg daily | ||
|
| |||||||||
| Patients (n) | 115 | 116 | 72 | 35 | 110 | 112 | 59 | 27 | 111 |
|
| |||||||||
| Median follow-up (months) | 16.6 | 16.4 | Not reportedc | 18.6 | 16.8 | Not reported | |||
|
| |||||||||
| Intention-to-treat ORR (%) | 39.1 | 6.9 | 37.5 | 2.9 | 55 | 46 | 69 | 33 | 39 |
| 95% Confidence interval | 30.2 to 48.7 | 3.0 to 13.1 | 26 to 50 | 0 to 15 | 44 to 66d | 35 to 57d | 56 to 81 | 16 to 54 | 30 to 49 |
|
| |||||||||
| Median PFS (months) | 5.4 | 1.6 | 9.6 | 1.4 | 15.6 | 9.2 | Not yet reached | 5.5 | 6.9 |
| Hazard ratio | 0.49 | 0.15 | 0.64e | ||||||
| 95% Confidence interval | 0.36 to 0.67 | 0.08 to 0.29 | 0.45 to 0.91 | Not reported | |||||
| p Value | <0.0001 | <0.001 | Not reported | ||||||
|
| |||||||||
| Median OS (months) | 18.1f,g | 20.1f,g | 12.6f | Not yet reachedf | 27.6 | Not yet reached | Not reported | ||
| Hazard ratio | 1.0 | 0.89 | 0.67 | Not reported | |||||
| 95% Confidence interval | 0.67 to 1.49 | 0.35 to 2.24 | 0.42 to 1.06 | ||||||
| p Value | 0.50 | Not reported | Not reported | Not reported | |||||
Treatment cohorts included cohorts 2 and 3A [prior crizotinib only or prior crizotinib plus 1–2 lines of prior CTx (n = 59)]; cohort 3B [prior non-crizotinib ALK TKI with or without CTx (n = 27)]; and cohorts 4 and 5 [2–3 prior ALK TKIs with or without CTx (n = 111)].
After a 7-day lead-in with brigatinib 90 mg daily.
Median safety follow-up was 6.5 months for the alectinib arm and 5.8 months for the CTx arm.
97.5% Confidence interval for the primary endpoint.
At a median follow-up of 8.0 months, median PFS was 12.9 months for Brig-90/180 and 9.2 months for Brig-90 (hazard ratio: 0.55; 95% confidence interval: 0.35 to 0.86)26.
OS data were immature at the time of analysis.
Investigator-assessed.
IRC = independent review committee; CTx = chemotherapy; TKI = tyrosine kinase inhibitor; PFS = progression-free survival; OS = overall survival; ORR = overall response rate.
In earlier studies and in the ascend-5 trial, ceritinib was administered at 750 mg daily without food (750 mg fasting). The goal of the phase i ascend-8 trial was to determine whether ceritinib at 450 mg or 600 mg taken with a low-fat meal (450 mg or 600 mg fed) could improve the gastrointestinal aes without compromising efficacy27. Compared with the 600 mg fed or 750 mg fasting doses, ceritinib 450 mg fed resulted in similar pharmacokinetic levels and treatment exposure at steady state, with fewer dose reductions or interruptions28. Although both doses were tolerable (discontinuation because of aes was 7.9% and 5.6% for the 450 mg fed and 750 mg fasting doses respectively), patients taking the 450 mg fed dose, compared with those taking the 750 mg fasting dose, also experienced fewer grade 3 or 4 gastrointestinal aes, including diarrhea (1.1% vs. 7.8%), nausea (0% vs. 5.6%), and vomiting (0% vs. 4.4%). In treatment-naïve patients, the 450 mg fed compared with the 750 mg fasting dose resulted in median pfs durations of 17.6 months and 10.9 months as assessed by a blinded independent review committee (irc). The orr and time to response were similar in the two arms. The 450 mg fed dose was approved by many regulatory bodies, including the U.S. Food and Drug Administration29.
The ascend-5 trial demonstrated that ceritinib is an effective alk inhibitor after crizotinib22. Similar efficacy and better tolerability of the lower dose of ceritinib was also confirmed in ascend-827,28. The lower dose improved the cost–benefit ratio of ceritinib therapy, and in 2017, it was approved by Health Canada and the pan-Canadian Oncology Drug Review as a second-line option for patients progressing on crizotinib.
Alectinib: ALUR
The pivotal phase iii alur trial (n = 107) confirmed the efficacy of alectinib shown by earlier trials30,31 in patients with ALK-positive nsclc who had progressed on both platinum-based chemotherapy and crizotinib (Table i)23. Patients were randomized 2:1 to either alectinib or standard second-line chemotherapy (pemetrexed or docetaxel). Investigator-assessed median pfs was significantly better in the alectinib group than in the chemotherapy group (9.6 months vs. 1.4 months; hr: 0.15; 95% ci: 0.08 to 0.29; p < 0.001), with a substantially improved orr (37.5% vs. 2.9%). Discontinuation because of aes occurred in 5.7% of patients at receiving alectinib and in 8.8% of patients receiving chemotherapy. Alectinib showed a significant pfs benefit in patients with crizotinib-refractory disease and received Health Canada approval for that indication on 31 October 201632.
NEXT/THIRD-GENERATION ALK TKIS AFTER PROGRESSION ON CRIZOTINIB
Brigatinib: ALTA
Brigatinib is a next/third-generation alk tki designed for potent activity against a broad range of alk–inhibitor resistant mutations33. In preclinical models, brigatinib was associated with inhibition of all ALK resistance mutations tested, including the solvent-front mutation G1202R, which confers resistance to crizotinib, ceritinib, and alectinib34,35.
Following on from an earlier phase i/ii trial36,37, the randomized phase ii alta trial prospectively assessed the efficacy and safety of brigatinib in 222 crizotinib-refractory patients (74% had received prior chemotherapy) with advanced ALK-positive nsclc, comparing a dose of 180 mg daily preceded by a 7-day 90 mg lead-in regimen (Brig-90/180, n = 110) with a dose of 90 mg once daily (Brig-90, n= 112) 26. The primary endpoint was investigator-assessed orr, with pfs being a key secondary endpoint. The orr was 54% for the Brig-90/180 group and 45% for the Brig-90 group, with median pfs durations of 12.9 months and 9.2 months respectively (hr: 0.55; 95% ci: 0.35 to 0.86). Early-onset pulmonary aes (median: within 2 days) occurred in 14 of 219 patients (6.4%). No events occurred after escalation to 180 mg in the Brig-90/180 arm, and in 7 of 14 patients, brigatinib re-treatment or continued treatment at a lower dose was instituted without pulmonary issues. Updated findings showed orrs of 55% for Brig-90/180 and 46% for Brig-90 (Table i)24. Median pfs increased to 15.6 months in the Brig-90/180 group (95% ci: 11.1 months to 19.4 months), which was even higher when assessed by the irc (16.7 months; 95% ci: 11.6 months to not reached), while the median pfs remained at 9.2 months in the Brig-90 group [95% ci: 7.4 months to 11.1 months (investigator) or 12.8 months (irc)]. Discontinuation because of aes occurred in 8.2% of patients receiving Brig-90/180 and in 2.7% of those receiving Brig-9026. The Brig-90/180 regimen is the recommended dosing scheme.
Lorlatinib
Lorlatinib is a next/third-generation alk tki that is highly active in preclinical models of lung cancer harbouring chromosomal rearrangements of ALK, including cell lines with mutations that result in resistance to other alk inhibitors, and it was specifically designed to penetrate the blood–brain barrier38,39.
After determining a 100 mg optimal daily dose for lorlatinib, the ongoing phase i/ii trial included multiple patient cohorts with advanced nsclc and ALK rearrangements, many of whom were heavily pretreated (including 1–3 prior alk tkis with or without prior chemotherapy). An expanded analysis of the irc-assessed orr in 197 patients receiving 1 or more prior tkis was recently presented (cohorts 2–5, Table i)25. In 59 patients previously treated with crizotinib with or without chemotherapy (cohorts2–3A), the systemic orr was 69% (95% ci: 56% to 81%), and the median pfs was not yet reached (95% ci: 12.5 months to not yet reached). In 27 patients previously treated with one second-generation tki plus chemotherapy (cohort 3B), the systemic orr was 33% (95% ci: 16% to 54%), and the median pfs was 5.5 months (95% ci: 2.9 months to 9.0 months). In 111 patients previously treated with 2 or more alk tkis with or without chemotherapy (cohorts 4 and 5), the systemic orr was 39% (95% ci: 30% to 49%), and the median pfs was 6.9 months (95% ci: 5.4 months to 9.5 months). Among all patients in the phase ii study (n = 275), treatment-related aes leading to discontinuation occurred in 3% of patients. Lorlatinib showed substantial activity in patients with heavily pretreated ALK-positive nsclc.
FIRST-LINE TREATMENT WITH SECOND- AND NEXT/THIRD-GENERATION ALK TKIS
Ceritinib (Second Generation): ASCEND-4
The phase iii ascend-4 trial randomized 376 treatment-naïve patients with ALK-positive advanced nsclc to receive ceritinib 750 mg daily (n = 189) or platinum–pemetrexed with or without pemetrexed maintenance (n = 187)40. The primary endpoint assessed by the blinded irc was met, showing that, compared with chemotherapy, ceritinib was associated with a significant improvement in median pfs (16.6 months vs. 8.1 months; hr: 0.55; 95% ci: 0.42 to 0.73; p < 0.00001), with orrs of 72.5% (ceritinib) and 26.7% (chemotherapy) and similar improvements in duration of response and time to response. Patients treated with ceritinib experienced improved overall quality of life, with significantly prolonged time to definitive deterioration for lung cancer–specific symptoms, and fewer patients discontinued therapy because of treatment-related aes in the ceritinib group (5%) than in the chemotherapy group (11%). A significant and clinically meaningful improvement in pfs was shown for first-line ceritinib compared with chemotherapy in patients with advanced ALK-rearranged nsclc.
Alectinib (Second Generation): ALEX
The phase iii alex trial randomized 303 treatment-naïve patients with ALK-positive advanced nsclc to receive either alectinib 600 mg twice daily or crizotinib 250 mg twice daily41. At a median follow-up of 18.6 months for alectinib and 17.6 months for crizotinib, the irc showed a significantly longer median pfs for alectinib compared with crizotinib (25.7 months vs.10.4 months; hr: 0.50; 95% ci: 0.36 to 0.70; p < 0.001). The investigator-assessed orr in the alectinib group was 82.9%; it was 75.5% in patients treated with crizotinib (p= 0.09). An updated analysis with nearly 8 months’ additional follow-up confirmed those findings, showing improvements in the primary endpoint of investigator-assessed pfs (median: 34.8 months vs. 10.9 months; hr: 0.43; 95% ci: 0.32 to 0.58; p value not reported) and orr (82.9% vs. 75.5%, p value not reported) for alectinib compared with crizotinib (Table ii)42. Moreover, the Japanese phase iii j-alex trial showed an impressive pfs improvement for patients receiving alectinib at a dose of 300 mg twice daily (hr: 0.34; 99.7% ci: 0.17 to 0.71; p < 0.0001)43. Discontinuation for any-cause aes occurred in 11% of patients receiving alectinib and in 13% of patients receiving full-dose crizotinib41. First-line alectinib was associated with both a significantly longer pfs and a favourable safety profile.
TABLE II.
Efficacy of first-line first- or second-generation ALK inhibitors in treatment-naïve patients with ALK-positive disease
| Variable | Reference (study name) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Solomon et al., 201417 (PROFILE 1014) | Soria et al., 201740 (ASCEND-4) | Camidge et al., 201842 (ALEX, poster) | ||||
| Investigational agent | Crizotinib (1st generation) | Ceritinib (2nd generation) | Alectinib (2nd generation) | |||
|
| ||||||
| Phase | III | III | III | |||
|
| ||||||
| Review | IRC | IRC | Investigator | |||
|
| ||||||
| Treatment | Crizotinib 250 mg twice daily | Pemetrexed 500 mg/m2 plus platinum CTx every 3 weeks | Ceritinib 750 mg daily | Pemetrexed 500 mg/m2 plus platinum CTx every 3 weeks | Alectinib 600 mg twice daily | Crizotinib 250 mg twice daily |
|
| ||||||
| Patients (n) | 172 | 171 | 189 | 187 | 152 | 151 |
|
| ||||||
| Median follow-up (months) | ~46 | Not reported | 27.8 | 22.8 | ||
|
| ||||||
| Intention-to-treat ORR (%) | 74 | 45 | 72.5 | 26.7 | 82.9 | 75.5 |
| 95% Confidence interval | 67 to 81 | 37 to 53 | 65.5 to 78.7 | 20.5 to 33.7 | 76.0 to 88.5 | 67.8 to 82.1 |
| p Value | <0.001 | Not reported | Not reported | |||
|
| ||||||
| Median PFS (months) | 10.9 | 7.0 | 16.6 | 8.1 | 34.8a | 10.9a |
| Hazard ratio | 0.45 | 0.55 | 0.43 | |||
| 95% confidence interval | 0.35 to 0.60 | 0.42 to 0.73 | 0.32 to 0.58 | |||
| p Value | <0.001 | <0.00001b | Not reported | |||
|
| ||||||
| Median OS (months) | Not yet reachedc | 47.5c | Not estimablec | 26.2c | Not estimablec | Not estimablec |
| Hazard ratio | 0.76 | 0.73 | 0.76 | |||
| 95% confidence interval | 0.55 to 1.05 | 0.50 to 1.08 | 0.50 to 1.15 | |||
| p Value | 0.0978 | Not reported | Not reported | |||
The event-free survival rate at 12 months was 68.4% with alectinib (95% confidence interval: 61.0% to 75.9%) compared with 48.7% with crizotinib (95% confidence interval: 40.4% to 56.9%); the IRC-assessed median PFS was 25.7 months compared with 10.4 months (hazard ratio: 0.50; 95% confidence interval: 0.36 to 0.70; p < 0.001).
The investigator-assessed median PFS was 16.8 months compared with 7.2 months (hazard ratio: 0.49; 95% confidence interval: 0.37 to 0.64; p < 0.00001).
The OS data were immature at the time of analysis.
IRC = independent review committee; CTx = chemotherapy; PFS = progression-free survival; OS = overall survival; ORR = overall response rate.
Brigatinib and Lorlatinib (Next/Third Generation)
Ongoing trials evaluating the efficacy of next/third-generation alk tkis in the first line are underway. Brigatinib is being compared with crizotinib in the international randomized multicentre phase iii alta-1L trial with alk tki–naïve patients with ALK-positive advanced nsclc (see NCT02737501 at http://ClinicalTrials.gov/), in which 270 patients have been randomized to the Brig-90/180 regimen or to crizotinib 250 mg twice daily. The primary endpoint, pfs as assessed by a blinded irc, was met on 28 July 201844.
Lorlatinib is also being compared with crizotinib with respect to efficacy and safety in the phase iii crown trial in ALK-positive metastatic nsclc (see NCT03052608 at http://ClinicalTrials.gov/). The 280 enrolled patients are being randomized to lorlatinib 100 mg daily or to crizotinib 250 mg twice daily.
BRAIN METASTASES AND ALK TKIS
ALK-positive central nervous system (cns) metastases are initially present in approximately 25% of patients with ALK-positive nsclc11, and the cns is the most common site of progression for patients taking crizotinib, with approximately 60%–70% of patients eventually developing this complicationb. Because the presence and treatment of cns metastases can have debilitating consequences for patients, treatment of this patient subset deserves special attention.
Crizotinib
In the profile 1014 trial (first-line crizotinib vs. platinum–pemetrexed), brain metastases were present at baseline in 26% of the group receiving crizotinib and in 27% of the group receiving chemotherapy17. Brain responses were not reported, but intracranial lesions progressed or new ones developed in 15% of the patients in each arm. Analysis of the 79 patients (23%) with stable treated brain metastases showed that time to progression in the brain nonsignificantly favoured crizotinib (hr: 0.45; 95% ci: 0.19 to 1.07; p = 0.063), that the intracranial disease control rate was significantly higher with crizotinib than with chemotherapy at both 12 weeks (85% vs. 45%, p < 0.001) and 24 weeks (56% vs. 25%, p = 0.006), and that median pfs was significantly improved in patients with treated brain metastases (9.0 months vs. 4.0 months; hr: 0.40; 95% ci: 0.23 to 0.69; p < 0.001)11.
The alex trial mandated imaging of the brain at baseline and every 8 weeks throughout the trial; in the 22 patients who had measurable cns metastases at baseline and who were treated with crizotinib, a 50% cns response was seen (Table iii), although the duration of response was only 5.5 months41.
TABLE III.
Central nervous system (CNS) response with first- and second-line ALK inhibitors
| Setting and agent | Reference (study name, phase) | Pts with measureable brain metastases at baseline (n) | CNS ORR [n/N (%)] with | p Value | |
|---|---|---|---|---|---|
|
| |||||
| ALK inhibitor | Chemotherapy | ||||
| First line | |||||
|
| |||||
| Crizotinib | Solomon et al., 201611 (PROFILE 1014, III) | 79a | Not reportedb | Not reportedb | Not reportedb |
| Peters et al., 201741 (ALEX, III) | 22c | 11/22 (50) | Not applicabled | Not reported | |
|
| |||||
| Alectinib | Peters et al., 201741 (ALEX, III) | 21c | 17/21 (81) | Not applicabled | Not reported |
|
| |||||
| Ceritinib | Soria et al., 201740 (ASCEND-4, III) | 44e | 16/22 (72.7) | 6/22 (27.3) | Not reported |
|
| |||||
| Second line | |||||
|
| |||||
| Brigatinib | Ahn et al., 201724 (ALTA, II, randomized) | Brig-90/180f: 18 | 12/18 (67) | Not applicable | Not reported |
| Brig-90g: 26 | 13/26 (50) | ||||
|
| |||||
| Ceritinib | Shaw et al., 201722 (ASCEND-5, III) | 37e | 6/17 (35) | 1/20 (5) | Not reported |
|
| |||||
| Alectinib | Novello et al., 201823 (ALUR, III) | 40c | 13/24 (54.2) | 0/16 (0) | <0.001 |
|
| |||||
| Lorlatinib | Shaw et al., 201825 (II, expansion, pooled cohorts 2–5) | 132h | 70/132 (53)i | Not available | Not reported |
Patients with stable treated brain metastases.
Compared with chemotherapy, crizotinib was associated with significantly improved intracranial disease control, including stable disease, at 12 weeks (85% vs. 45%, p < 0.001) and at 24 weeks (56% vs. 25%, p = 0.006).
Patients with measurable CNS disease at baseline.
Trial compared crizotinib with alectinib (CNS ORR: 50% vs. 81% respectively).
Eligible patients with active brain metastases and at least 1 post-baseline assessment.
Brigatinib 180 mg daily after a 7-day lead-in with brigatinib 90 mg daily.
Brigatinib 90 mg daily.
Brain metastases present at baseline.
Intracranial ORR.
Pts = patients; ORR = objective response rate.
Ceritinib
Of patients in the phase iii ascend-5 post-crizotinib trial who had active-target brain lesions and at least 1 post-baseline tumour assessment, 17 (15%) received ceritinib and 20 (17%) received single-agent chemotherapy. Of those patients, 6 (35%) in the ceritinib arm and just 1 (5%) in the chemotherapy arm experienced an overall intracranial response22.
The phase iii ascend-4 trial (first-line ceritinib vs. platinum–pemetrexed) included 22 patients in each arm with baseline measurable brain metastases and at least 1 post-baseline confirmed assessment40. Of those patients, 16 (72.7%) receiving ceritinib and 6 (27.3%) receiving chemotherapy experienced an overall intracranial response. In ascend-4, only patients with confirmed cns metastases were mandated to receive cns imaging with computed tomography (ct) or magnetic resonance imaging (mri) at baseline. Thus, a comparison of the incidence of new brain metastases between the treatment arms was not feasible.
Alectinib
Unlike crizotinib and ceritinib, alectinib is not a substrate of P-glycoprotein, a key efflux transporter located at the blood–brain barrier. Alectinib is therefore hypothesized to better penetrate cns sites. In both preclinical and early clinical investigations, alectinib showed promising cns activity30,31,45,46.
In the phase iii alur trial comparing alectinib with single-agent chemotherapy after chemotherapy–crizotinib, cns response was the key secondary endpoint23. All patients were required to undergo imaging by ct or mri every 6 weeks for the duration of the trial (to coincide with scheduled chemotherapy visits). Measurable lesions in the cns were seen in 24 patients (33%) in the alectinib group and in 16 patients (46%) in the chemotherapy group, with a cns orr of 54.2% being observed in those treated with alectinib (95% ci: 33% to 74%) compared with 0% in the group receiving chemotherapy (95% ci: 0% to 21%; p < 0.001).
The phase iii alex trial was appropriately designed to observe both the incidence of cns metastases and the cns response for first-line alectinib compared with crizotinib41; brain imaging every 8 weeks was mandatory throughout the study. Brain metastases were seen at baseline in 64 patients (42%) randomized to alectinib and in 58 patients (38%) randomized to crizotinib. Measurable baseline cns lesions were observed in 21 patients (13.8%) receiving alectinib and in 22 (14.6%) receiving crizotinib, with a cns response being observed in 17 patients receiving alectinib (81%; 95% ci: 58% to 95%) and in 11 receiving crizotinib (50%; 95% ci: 28% to 72%). The median duration of cns response was considerably longer in the patients receiving alectinib (17.3 months; 95% ci: 14.8 months to not yet reached) than in those receiving crizotinib (5.5 months; 95% ci: 2.1 months to 17.3 months), as was the time to cns progression in the intention-to treat population (n= 303; hr: 0.16; 95% ci: 0.10 to 0.28; p < 0.001). Progression events in the cns were seen in 18 patients receiving alectinib (12%) and in 68 patients receiving crizotinib (45%). The more recently reported 12-month cumulative incidence rates of cns progression in patients without baseline cns metastases were 4.6% for the alectinib group (95% ci: 1.5% to 10.6%) compared with 31.5% for the crizotinib group (95% ci: 22.1% to 41.3%)47. This detailed prospective analysis confirms the greater ability of alectinib to prevent cns progression.
Brigatinib
Brigatinib has impressive cns activity despite being a substrate for P-glycoprotein. In the phase ii alta trial evaluating two doses of brigatinib in patients previously treated with crizotinib, 69% (n= 154) had brain metastases at baseline; measurable brain lesions were observed in 18 patients receiving Brig-90/180 (16.4%) and in 26 patients receiving Brig-90 (23.2%)26. A recent update showed that intracranial orrs were seen in 12 patients receiving Brig-90/180 (67%) and in 13 patients receiving Brig-90 (50%)24. At a median follow-up of 18.6 months in the Brig-90/180 arm, the median duration of cns response was 16.6 months; in the 73 patients in that arm with any brain metastases at baseline, an irc-assessed intracranial pfs of 18.4 months (95% ci: 12.6 months to not yet reached) was observed.
Results from the phase iii alta-1L trial (NCT02737501 at http://ClinicalTrials.gov/) comparing brigatinib with crizotinib in patients with no prior tki therapy and 1 or no prior systemic anticancer regimens in the advanced setting are eagerly awaited to confirm the foregoing results. The study protocol mandates disease assessment of the brain by ct or mri (or both) at screening, at baseline, every 8 weeks through cycle 14, and every 3 cycles thereafter until disease progression. Like the alex study of alectinib, alta-1L will provide a greater understanding of cns response with brigatinib and the ability of brigatinib to reduce the occurrence of brain metastases.
Lorlatinib
Lorlatinib was specifically designed to penetrate the blood brain–barrier38,39, and in the ongoing phase i/ii trial of lorlatinib in patients previously treated with at least 1 prior alk tki (n = 197, cohorts 2–5), 67% of patients (n = 132) had brain metastases at baseline, with an overall intracranial orr of 53% (Table iii)25. Among the patients previously treated with crizotinib with or without chemotherapy (n = 37, cohorts 2–3A), the intracranial orr was 68% (95% ci: 50% to 82%). Among those previously treated with 1 second-generation tki plus chemotherapy (n = 12, cohort 3B), the intracranial orr was 42% (95% ci: 15% to 72%), and in the patients previously treated with 2 or more alk tkis with or without chemotherapy (n = 83, cohorts 4 and 5), the intracranial orr was 48% (95% ci: 37% to 59%).
The ongoing randomized open-label two-arm phase iii crown study (NCT03052608 at http://ClinicalTrials.gov/) is comparing lorlatinib with crizotinib in the first-line treatment of patients with metastatic ALK-positive nsclc. The primary objective is blinded irc-assessed pfs; important blinded irc-assessed secondary objectives include cns orr from the time of study initiation up to 33 months and intracranial time to progression. Baseline mri screening and follow-up brain imaging every 8 weeks by either mri or ct is required.
Improved cns responses have been observed for all second- and next/third-generation alk tkis in patients progressing on crizotinib. Cross-trial comparisons and the direct comparison of crizotinib with alectinib in the alex trial suggest that crizotinib has the least cns activity among all the alktkis.
ALK TESTING
Detection of ALK rearrangements is necessary to select patients for optimal treatment of nsclc with alk inhibitors; testing should be performed in all patients eligible for targeted therapy at the time of diagnosis of advanced nsclc when a component of adenocarcinoma is noted or suspected48. Eligible pathologic diagnoses include adenocarcinoma, large-cell carcinoma, sarcomatoid carcinoma, adenosquamous carcinoma, and nsclc not otherwise specified. Patients not eligible for ALK testing are those with “pure” squamous-cell, small-cell, and large-cell neuroendocrine carcinoma49–53. However, ALK testing can be considered for atypical patients, such as a lifetime never-smoking individual with squamous-cell carcinoma. Testing has to be performed before systemic therapy is initiated. Tissue samples from the primary tumour or metastases are equally suitable for analysis. Biopsies, resection specimens, and cytology specimens with an available cellblock are all suitable for ALK testing using immunohistochemistry (ihc) and fluorescence in situ hybridization (fish).
Initially, the standard method for detection of ALK gene rearrangements in the United States was fish using the U.S. Food and Drug Administration–approved ALK Break Apart FISH Probe Kit (Abbott Molecular, Abbott Park, IL, U.S.A.). In Canada, fish, ihc, and other assays are available for detecting ALK rearrangements. However, fish is both expensive and labour-intensive, making it challenging to implement as the primary diagnostic test for the identification of ALK rearrangements in all molecular pathology laboratories nationwide. A network of pulmonary and molecular pathologists and cytogeneticists working in academic centres across Canada conducted the Canadian ALK study to address the challenge of standardization and optimization of detection tests for ALK-positive nsclc54. Cases deemed weakly positive or equivocal for alk by ihc were then tested by ALK fish for confirmation. The results supported the use of appropriately validated ihc laboratory– developed ihc assays using the 5A4 alk antibody clone to screen for ALK-positive nsclc. A second Canadian Immunohistochemistry Quality Control study showed good results and high concordance for alk ihc testing at 21 participating Canadian laboratories. Moreover, recent data from the alex trial indicate that, compared with ALK fish, alk ihc might identify more patients who benefit from alk tkis and that alk ihc–positive patients might benefit even if ALK fish results are negative55.
In June 2017, the U.S. Food and Drug Administration approved the Ventana ALK (D5F3) CDx IHC Assay (Roche Diagnostics, Risch-Rotkreuz, Switzerland) for the qualitative detection of alk protein in formalin-fixed paraffin-embedded nsclc tissue stained with a Ventana BenchMark XT or BenchMark Ultra automated staining instrument56. Currently, in Canada, positive alk ihc is sufficient for obtaining access to alk tkis. Nevertheless, the use of alk ihc alone requires high levels of reliability, and ongoing quality assurance and adoption should be linked to strict validation standards and ongoing quality assurance48,57.
OPTIMAL SEQUENCING OF THERAPY
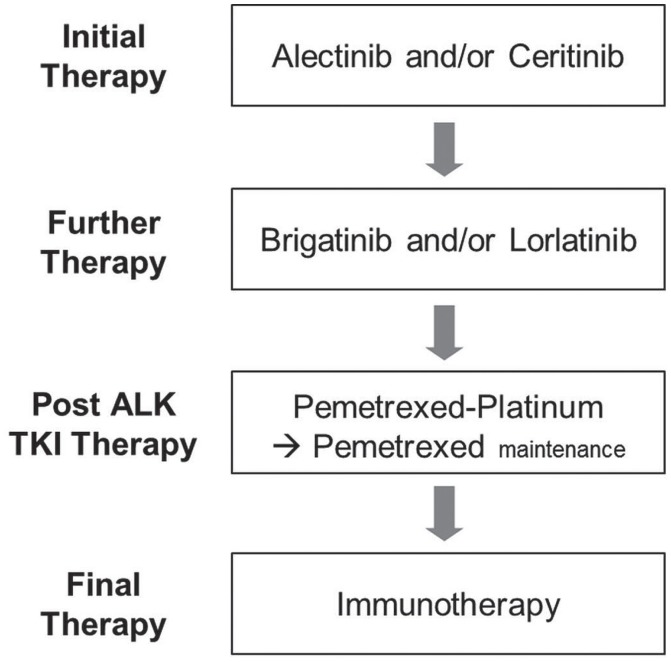
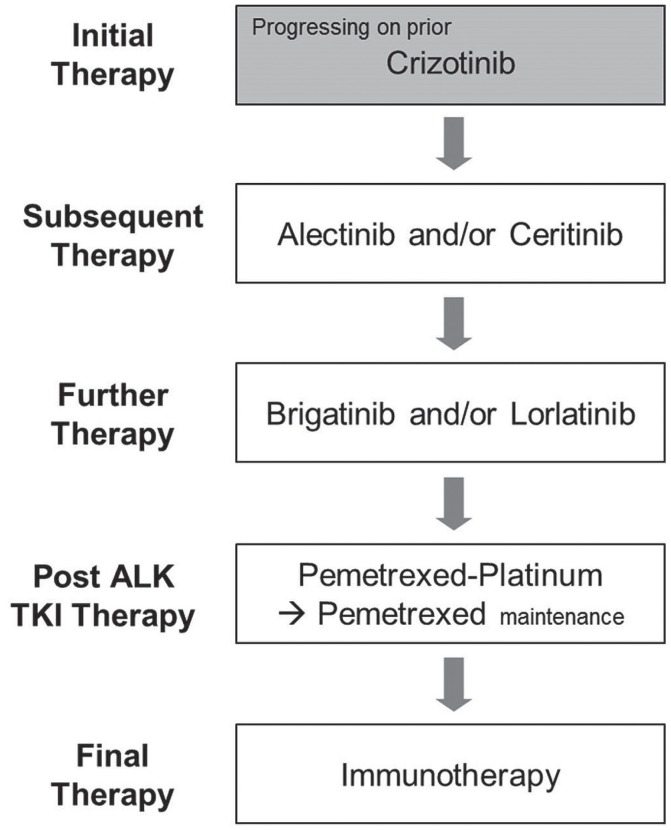
The optimal sequencing of alk tkis in patients with ALK-positive advanced nsclc continues to evolve, with a suggested sequencing outlined in Figures 1 and 2 for treatment-naïve and crizotinib-refractory patients respectively.
FIGURE 1.
Therapy options for treatment-naïve patients with advanced nonsquamous ALK-positive non-small-cell lung cancer. TKI = tyrosine kinase inhibitor.
FIGURE 2.
Therapy options by line for later lines in patients with advanced ALK-positive non-small-cell lung cancer currently receiving and progressing on crizotinib. TKI = tyrosine kinase inhibitor.
Initial Therapy
In Canada, recommendations for new therapies must meet regulatory requirements and must, in phase iii trials, demonstrate improved clinical outcomes compared with the current standard of care. Crizotinib was approved by Health Canada in April 2012 and has since been the standard of care for treatment-naïve patients with ALK-positive nsclc. However, initial treatment is currently changing given that alectinib and ceritinib both have phase iii data to support first-line use (Figure 1).
The alex trial is the only study to have compared a second-generation agent, alectinib, with the current first-line standard, crizotinib. The well-designed protocol— which showed an improvement by a factor of almost 2.5 in investigator-assessed median pfs (34.8 months vs. 10.9 months)42, a significantly reduced incidence of cns progression47, and a favourable safety profile41 for alectinib compared with crizotinib—provides compelling evidence for the use of alectinib as first-line therapy.
Outcomes from the ascend-4 trial also show good support for the use of ceritinib in this setting, although no randomized comparison with crizotinib was performed. The trial demonstrated strong activity for ceritinib compared with platinum–pemetrexed chemotherapy, with a more-than-doubled median pfs (16.6 months vs. 8.1 months)40. Gastrointestinal-related aes were considerably higher in the ceritinib 750 mg arm, although most were grade 1 or 2 and manageable; treatment discontinuation was required in only 3 patients (2%). Results from ascend-8 in previously treated patients also show that lower-dose ceritinib (450 mg) is equally effective, with an improved toxicity profile and cost–benefit ratio27,28. Again, cross-trial comparisons should be interpreted with caution, although it is notable that in these relatively comparable populations, the median pfs for ceritinib in ascend-4 was higher by a factor of 1.5 than the median for high-dose crizotinib in the profile 1014 study17,40.
Level i evidence supports the use of alectinib as a new standard of care for treatment-naïve patients with ALK-positive nsclc, and indirect data support ceritinib as an active and cost-effective option (Figure 1). Because case reports suggest that sequential use of these agents in either order might also impart clinical benefit, both are reasonable choices for initial therapy58–62.
Further Treatments
Recently, data from the ascend-5 and alur trials have respectively demonstrated activity for ceritinib and alectinib in patients progressing on crizotinib-based therapy22,23, establishing both as viable later-line treatment options (Figure 2). Although cross-trial comparisons should be interpreted with caution, and although those studies included slightly different patient populations [the alur trial was conducted strictly in third-line patients (100% having received both a platinum doublet and crizotinib), and ascend-5 included a mix of third-line (88% same population as alur) and fourth-line patients (12% having received 2 lines of chemotherapy and crizotinib)], the median pfs for alectinib appears to be slightly higher than the median for ceritinib, and a network meta-analysis suggested less toxicity with alectinib63. It must be noted that alur was conducted in a slightly more favourable population23, and outcomes from ascend-8 have shown that lower-dose ceritinib (450 mg) administered with food is as efficacious as the 750 mg dose used in the ascend-5 trial, with an improved safety profile22,27,28. Ceritinib and alectinib were both recently approved by the pan-Canadian Oncology Drug Review and are currently in price negotiations at the pan-Canadian Pharmaceutical Alliance. Both should be considered after progression on crizotinib.
Brigatinib has demonstrated activity in second-line or later disease after progression on crizotinib26, and lorlatinib has shown benefit after multiple lines of prior alk tki therapy25. Results from phase i/ii studies suggest that either can be used in patients progressing on prior alk inhibitors26,25. Data for the use of brigatinib after progression on alectinib are currently lacking, and therefore the optimal sequencing is currently unknown (Figures 1 and 2). Lorlatinib has demonstrated activity after multiple lines of alk tkis25, which could potentially indicate a preference for the use of brigatinib. However, preclinical and clinical evidence shows activity for lorlatinib against G1202R25,64, and preclinical data to date show activity for brigatinib against this most frequent and challenging ALK resistance mutation34. Further treatments could therefore include single-agent brigatinib or lorlatinib, or both sequentially. Rebiopsy to identify resistance mutations to guide therapy is not currently recommended, although that practice might play a role in the future.
Given that patients with advanced nsclc and an ALK rearrangement might have tumours that are quite sensitive to pemetrexed platinum-doublet chemotherapy65, that approach should be considered as an option to be used sequentially after brigatinib and lorlatinib (Figures 1 and 2). Although the exact sequence of these regimens in a new era of first-line alectinib or ceritinib has yet to be determined, the new options provide many treatment sequence alternatives. If single-agent pembrolizumab is being considered in patients with high PD-L1 expression, it should be noted that ALK-positive patients were excluded from the phase iii first-line pembrolizumab trial, and other data suggest a low likelihood of response in such patients because their mutational burden is low66–69. Although clinical trials are ongoing, findings to date underscore the importance of exhausting other systemic therapies before considering immunotherapy.
SUMMARY
Emerging data have expanded the role for alk inhibition in patients with ALK-positive nsclc, and Canadian recommendations have been updated accordingly:
■ Patients with advanced nonsquamous nsclc have to be tested for the presence of an ALK rearrangement.
■ Treatment-naïve patients with ALK-positive disease should initially be offered single-agent alectinib or ceritinib, or both sequentially.
■ Crizotinib-refractory patients should be treated with single-agent alectinib or ceritinib, or both sequentially.
■ Further treatments could include single-agent brigatinib or lorlatinib, or both sequentially.
■ Patients progressing on alk tkis should be considered for pemetrexed-based chemotherapy.
■ Other systemic therapies should be exhausted before immunotherapy is considered.
ACKNOWLEDGMENTS
We thank Kaleidoscope Strategic, Inc., for their editorial and administrative assistance in the preparation of this article. Pfizer, Roche, Novartis, and Takeda are also thanked for the unrestricted educational grants that made this initiative possible.
Footnotes
Novartis. Data on file [CLDK378X2101 full clinical study report as of 2 August 2013, and CLDK378A2201 full clinical study report as of 26 February 2014].
Novartis. Data on file [CLDK378X2101 full clinical study report as of 2 August 2013, and CLDK378A2201 full clinical study report as of 26 February 2014].
CONFLICT OF INTEREST DISCLOSURES
BM serves on advisory boards for Novartis, Pfizer, and Roche; JA serves on advisory boards for Novartis, Pfizer, Takeda, and Roche, and has given talks for, or served on advisory boards for, Astra-Zeneca, Boehringer Ingelheim, Bristol–Meyers Squibb, Merck, Novartis, and Pfizer; RA serves on advisory boards for Novartis, Pfizer, and Roche; DGB serves on advisory boards for Novartis, Pfizer, and Roche; NB serves on advisory boards for Takeda, Novartis, Pfizer, and Roche; RB serves on advisory boards for Roche, Takeda, Merck, and AstraZeneca; CB serves on advisory boards for AstraZeneca, Boehringer Ingelheim, Bristol–Meyers Squibb, Merck, and Pfizer; VH serves on advisory boards for Amgen, Astra-Zeneca, Boehringer Ingelheim, Bristol–Meyers Squibb, Eli Lilly, Merck, Novartis, Roche, and Pfizer; RJ serves on advisory boards for Novartis, Pfizer, and Roche; DNI has received honoraria from, or has been part of an advisory board for, AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Eli Lilly, Merck, Novartis, and Pfizer; WM serves on advisory boards for Novartis, Pfizer, and Roche; ZP has served on advisory boards for AstraZeneca, Merck, and Roche; RS serves on advisory boards for AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Merck, Novartis, AbbVie, Roche, and Takeda; MT has served on advisory boards for Takeda; MST has received research funding from Roche; MV serves on advisory boards for Amgen, AstraZeneca, Bristol–Myers Squibb, Boehringer Ingelheim, Celgene, Novartis, Eli Lilly, Takeda, Taiho, Hoffman–La Roche, Pfizer, and Merck, and is a member of the speaker’s bureau for AstraZeneca, Boehringer Ingelheim, Amgen, Merck and Eli Lilly; ZX serves on the advisory board for and has received grants from Pfizer; GL serves on advisory boards for and has received honoraria from AstraZeneca, Novartis, Pfizer, Roche, Merck, AbbVie, Bristol–Myers Squibb, and Takeda, and has received grants from AstraZeneca and Roche; the remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2017. Toronto, ON: Canadian Cancer Society; 2017. [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non–small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75(suppl):191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::AID-CNCR2820751307>3.0.CO;2-Y. [Erratum in: Cancer 1995;75:2979] [DOI] [PubMed] [Google Scholar]
- 5.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 6.Williams AS, Greer W, Bethune D, Craddock KJ, Flowerdew G, Xu Z. ALK+ lung adenocarcinoma in never smokers and long-term ex-smokers: prevalence and detection by immunohistochemistry and fluorescence in situ hybridization. Virchows Arch. 2016;469:533–40. doi: 10.1007/s00428-016-2005-y. [DOI] [PubMed] [Google Scholar]
- 7.Richards MW, O’Regan L, Roth D, et al. Microtubule association of eml proteins and the eml4–alk variant 3 oncoprotein require an N-terminal trimerization domain. Biochem J. 2015;467:529–36. doi: 10.1042/BJ20150039. [DOI] [PubMed] [Google Scholar]
- 8.Vendrell JA, Taviaux S, Beganton B, et al. Detection of known and novel ALK fusion transcripts in lung cancer patients using next-generation sequencing approaches. Sci Rep. 2017;7:12510. doi: 10.1038/s41598-017-12679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallberg B, Palmer RH. Mechanistic insight into alk receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13:685–700. doi: 10.1038/nrc3580. [Erratum in: Nat Rev Cancer 2013;13:820] [DOI] [PubMed] [Google Scholar]
- 10.Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17:2081–6. doi: 10.1158/1078-0432.CCR-10-1591. [DOI] [PubMed] [Google Scholar]
- 11.Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from profile 1014. J Clin Oncol. 2016;34:2858–65. doi: 10.1200/JCO.2015.63.5888. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non–small cell lung cancer. Clin Cancer Res. 2012;18:1472–82. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–19. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [Erratum in: N Engl J Med 2015;373:1582] [DOI] [PubMed] [Google Scholar]
- 17.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [Erratum in: N Engl J Med 2015;373:1582] [DOI] [PubMed] [Google Scholar]
- 18.Mok TSK, Kim DW, Wu YL, et al. Overall survival (os) for first-line crizotinib versus chemotherapy in ALK+ lung cancer: updated results from profile 1014 [abstract LBA50] Ann Oncol. 2017;28(suppl 5):v605–49. [Google Scholar]
- 19.Ou SH, Janne PA, Bartlett CH, et al. Clinical benefit of continuing alk inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive nsclc. Ann Oncol. 2014;25:415–22. doi: 10.1093/annonc/mdt572. [DOI] [PubMed] [Google Scholar]
- 20.Kim DW, Mehra R, Tan DSW, et al. Ceritinib in advanced anaplastic lymphoma kinase (ALK)- rearranged (ALK+) non-small cell lung cancer (nsclc): results of the ascend-1 trial [abstract 8003] J Clin Oncol. 2014;32 [Available online at: http://ascopubs.org/doi/abs/10.1200/jco.2014.32.15_suppl.8003; cited 28 August 2018] [Google Scholar]
- 21.Felip E, Kim D, Mehra R, et al. Efficacy and safety of ceritinib in patients (pts) with advanced anaplastic lymphoma kinase (ALK)- rearranged (ALK+) non–small cell lung cancer (nsclc) Ann Oncol. 2014;25(suppl 4):iv426–70. doi: 10.1093/annonc/mdu349.74. [DOI] [Google Scholar]
- 22.Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ascend-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18:874–86. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 23.Novello S, Mazieres J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)–positive non-small-cell lung cancer: results from the phase iii alur study. Ann Oncol. 2018;29:1409–16. doi: 10.1093/annonc/mdy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn M, Camidge DR, Tiseo M, et al. Brigatinib in crizotinib-refractory ALK+ nsclc: updated efficacy and safety results from alta, a randomized phase 2 trial [abstract OA 05.05] J Thorac Oncol. 2017;12(suppl 2):S1755–S6. doi: 10.1016/j.jtho.2017.09.350. [DOI] [Google Scholar]
- 25.Shaw AT, Martini JF, Besse B, et al. Efficacy of lorlatinib in patients (pts) with advanced ALK- positive non–small cell lung cancer (nsclc) and ALK kinase domain mutations [abstract CT044] Cancer Res. 2018;78(suppl) doi: 10.1158/1538-7445.AM2018-4858. [Available online at: http://cancerres.aacrjournals.org/content/78/13_Supplement/CT044; cited 1 September 2018] [DOI] [Google Scholar]
- 26.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase ii trial. J Clin Oncol. 2017;35:2490–8. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 27.Cho BC, Kim DW, Bearz A, et al. ascend-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)–rearranged meta-static non–small cell lung cancer (nsclc) J Thorac Oncol. 2017;12:1357–67. doi: 10.1016/j.jtho.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Cho BC, Obermannová R, Bearz A, et al. Efficacy and updated safety of ceritinib (450 mg or 600 mg) with low-fat meal vs. 750 mg fasted in ALK+ metastatic nsclc [abstract OA 05.07] J Thorac Oncol. 12:S1757. [Google Scholar]
- 29.Novartis Zykadia (ceritinib) capsules, for oral use [prescribing information] Basel Switzerland: Novartis; 2017. [Available online at: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/zykadia.pdf; cited 7 May 2018] [Google Scholar]
- 30.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–28. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 31.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–42. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cision Canada. Health Canada approves Alecensaro—a targeted oral treatment—for patients with aggressive and rare form of lung cancer [news release] Ottawa, ON: CisionCanada; 2016. [Available online at: https://www.newswire.ca/news-releases/health-canada-approves-alecensaro---a-targeted-oral-treatment---for-patients-with-aggressive-and-rare-form-of-lung-cancer-599296371.html; cited 11 July 2018] [Google Scholar]
- 33.Huang WS, Liu S, Zou D, et al. Discovery of brigatinib (AP26113), a phosphine oxide–containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem. 2016;59:4948–64. doi: 10.1021/acs.jmedchem.6b00306. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Anjum R, Squillace R, et al. The potent alk inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation alk inhibitors in preclinical models. Clin Cancer Res. 2016;22:5527–38. doi: 10.1158/1078-0432.CCR-16-0569. [DOI] [PubMed] [Google Scholar]
- 35.Cui JJ, Zhai D, Deng W, et al. alk/ros1/trk inhibitor TPX-0005 effectively overcomes clinical resistance solvent front mutations [abstract MA 07.09] J Thorac Oncol. 2017;12(suppl 2):S1829. doi: 10.1016/j.jtho.2017.09.510. [DOI] [Google Scholar]
- 36.Bazhenova LA, Gettinger SN, Langer CJ, et al. Brigatinib (brg) in patients (Pts) with anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (nsclc) in a phase 1/2 trial. Ann Oncol. 2016;27(suppl 6):416–54. [Google Scholar]
- 37.Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:1683–96. doi: 10.1016/S1470-2045(16)30392-8. [DOI] [PubMed] [Google Scholar]
- 38.Solomon B, Shaw A, Ou S, et al. Phase 2 study of lorlatinib in patients with advanced ALK+/ROS1+ non-small-cell lung cancer [abstract OA 05.06] J Thorac Oncol. 2017;12(suppl 2):S1756. doi: 10.1016/j.jtho.2017.09.351. [DOI] [Google Scholar]
- 39.Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadia zacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (alk) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57:4720–44. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
- 40.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ascend-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:P917–29. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 41.Peters S, Camidge DR, Shaw AT, et al. on behalf of the alex trial investigators. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 42.Camidge DR, Peters S, Mok T, et al. Updated efficacy and safety data from the global phase iii alex study of alectinib (alc) vs. crizotinib (cz) in untreated advanced ALK+ nsclc [abstract 9043] J Clin Oncol. 2018;36 [Available online at: https://meetinglibrary.asco.org/record/160811/abstract; cited 28 August 2018] [Google Scholar]
- 43.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (j-alex): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 44.The ASCO Post. alta-1L trial of brigatinib vs. crizotinib inALK- positive advanced nsclc meets primary endpoint [Web news article] Huntington, NY: The ASCO Post; 2018. [Available at: http://www.ascopost.com/News/59115; cited 8 August 2018] [Google Scholar]
- 45.Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase ii global study. J Clin Oncol. 2016;34:661–8. doi: 10.1200/JCO.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 46.Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumour activity of the selective alk inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74:1023–8. doi: 10.1007/s00280-014-2578-6. [DOI] [PubMed] [Google Scholar]
- 47.Gadgeel S, Peters S, Mok TSK, et al. Alectinib vs. crizotinib in treatment-naïve ALK+ nsclc: cns efficacy results from the alex study [abstract 1298O_PR] Ann Oncol. 2017;28(suppl 5) doi: 10.1093/annonc/mdy405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018;13:323–58. doi: 10.1016/j.jtho.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Aisner DL, Marshall CB. Molecular pathology of non–small cell lung cancer: a practical guide. Am J Clin Pathol. 2012;138:332–46. doi: 10.1309/AJCPFR12WJKCEEZZ. [DOI] [PubMed] [Google Scholar]
- 50.Naidoo J, Santos-Zabala ML, Iyriboz T, et al. 1142PD—large cell neuroendocrine carcinomas (lcnec) of the lung: pathologic features, treatment and outcomes. Ann Oncol. 2014;25(suppl 4):iv398–405. doi: 10.1093/annonc/mdu345.11. [DOI] [Google Scholar]
- 51.Nakamura H, Tsuta K, Yoshida A, et al. Aberrant anaplastic lymphoma kinase expression in high-grade pulmonary neuroendocrine carcinoma. J Clin Pathol. 2013;66:705–7. doi: 10.1136/jclinpath-2012-201329. [DOI] [PubMed] [Google Scholar]
- 52.Ionescu DN. Impact of the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology clinical practice guidelines for EGFR and ALK testing in lung cancer in Canada. Curr Oncol. 2013;20:220–6. doi: 10.3747/co.20.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montagut C, Arriola E, Galvan AB, et al. ALK chromosomal alterations in neuroendocrine tumors [abstract 10585] J Clin Oncol. 2011;29 doi: 10.1200/jco.2011.29.15_suppl.10585. [Available online at: http://meetinglibrary.asco.org/content/81898-102; cited 1 September 2018] [DOI] [Google Scholar]
- 54.Cutz JC, Craddock KJ, Torlakovic E, et al. Canadian Anaplastic Lymphoma Kinase study: a model for multicenter standardization and optimization of ALK testing in lung cancer. J Thorac Oncol. 2014;9:1255–63. doi: 10.1097/JTO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 55.Mok T, Peters S, Camidge DR, et al. Patients with ALK ihc-positive/fish-negative nsclc benefit from alk tki treatment: response data from the global alex trial. J Thorac Oncol. 2017;12(suppl 2):S1826–40. doi: 10.1016/j.jtho.2017.09.503. [DOI] [Google Scholar]
- 56.Cision Canada. Roche announces FDA approval of companion diagnostic to identify ALK- positive non–small cell lung cancer patients [Web new article] Ottawa, ON: Cision Canada; 2017. [Available at: https://www.prnewswire.com/news-releases/roche-announces-fda-approval-of-companion-diagnostic-to-identify-alk-positive-non-small-cell-lung-cancer-patients-300466815.html; cited 13 May 2018] [Google Scholar]
- 57.Melosky B, Blais N, Cheema P, et al. Standardizing biomarker testing for Canadian patients with advanced lung cancer. Curr Oncol. 2018;25:73–82. doi: 10.3747/co.25.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Makuuchi Y, Hayashi H, Haratani K, et al. A case of ALK-rearranged non–small cell lung cancer that responded to ceritinib after development of resistance to alectinib. Oncotarget. 2018;9:23315–19. doi: 10.18632/oncotarget.25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toyokawa G, Murakami H, Tokushige K, Hatano B, Nishio M. The efficacy of ceritinib for ALK-rearranged nonsmall cell lung cancer previously treated with alectinib—an analysis of Japanese and global phase i studies. Haigan. 2017;57:175–83. doi: 10.2482/haigan.57.175. [DOI] [Google Scholar]
- 60.Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages cns relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10:232–6. doi: 10.1097/JTO.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oya Y, Yoshida T, Kuroda H, et al. Clinical efficacy of alectinib in patients with ALK-rearranged non–small cell lung cancer after ceritinib failure. Anticancer Res. 2017;37:6477–80. doi: 10.21873/anticanres.12103. [DOI] [PubMed] [Google Scholar]
- 62.Tchekmedyian N, Ali SM, Miller VA, Haura EB. Acquired ALK L1152R mutation confers resistance to ceritinib and predicts response to alectinib. J Thorac Oncol. 2016;11:e87–8. doi: 10.1016/j.jtho.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 63.Steenrod A, Orme M, MacGilchrist KS, et al. Alectinib in treatment-naïve anaplastic lymphoma kinase–positive (ALK+) metastatic non-small-cell lung cancer (mnsclc): systematic literature review (slr) and network meta-analysis (nma) [abstract 1642] Cancer Res. 2018;78(suppl) doi: 10.1158/1538-7445.AM2018-1642. [Available online at: http://cancerres.aacrjournals.org/content/78/13_Supplement/1642; cited 4 September 2018] [DOI] [Google Scholar]
- 64.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation alk inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–33. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non–small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6:774–80. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spigel DR, Schrock AB, Fabrizio D, et al. Total mutation burden (tmb) in lung cancer (lc) and relationship with response to PD-1/PD-L1 targeted therapies [abstract 9017] J Clin Oncol. 2016;34 doi: 10.1200/JCO.2016.34.15_suppl.9017. [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.9017; cited 28 August 2018] [DOI] [Google Scholar]
- 67.Reck M, Rodriguez-Abreu D, Robinson AG, et al. on behalf of the keynote-024 investigators. Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 68.Moya-Horno I, Viteri S, Karachaliou N, Rosell R. Combination of immunotherapy with targeted therapies in advanced non–small cell lung cancer (nsclc) Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758834017745012. 1758834017745012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non–small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22:4585–93. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]