Abstract
Introduction
Patients with cancer of unknown primary (cup) have pathologically confirmed metastatic tumours with unidentifiable primary tumours. Currently, very little is known about the relationship between the treatment of patients with cup and their survival outcomes. Thus, we compared oncologic treatment and survival outcomes for patients in Ontario with cup against those for a cohort of patients with metastatic cancer of known primary site.
Methods
Using the Ontario Cancer Registry and the Same-Day Surgery and Discharge Abstract databases maintained by the Canadian Institute for Health Information, we identified all Ontario patients diagnosed with metastatic cancer between 1 January 2000 and 31 December 2005. Ontario Health Insurance Plan treatment records were linked to identify codes for surgery, chemotherapy, or therapeutic radiation related to oncology. Multivariable Cox regression models were constructed, adjusting for histology, age, sex, and comorbidities.
Results
In 45,347 patients (96.3%), the primary tumour site was identifiable, and in 1743 patients (3.7%), cup was diagnosed. Among the main tumour sites, cup ranked as the 6th largest. The mean Charlson score was significantly higher (p < 0.0001) in patients with cup (1.88) than in those with a known primary (1.42). Overall median survival was 1.9 months for patients with cup compared with 11.9 months for all patients with a known-primary cancer. Receipt of treatment was more likely for patients with a known primary site (n= 35,012, 77.2%) than for those with cup (n = 891, 51.1%). Among patients with a known primary site, median survival was significantly higher for treated than for untreated patients (19.0 months vs. 2.2 months, p < 0.0001). Among patients with cup, median survival was also higher for treated than for untreated patients (3.6 months vs. 1.1 months, p < 0.0001).
Conclusions
In Ontario, patients with cup experience significantly lower survival than do patients with metastatic cancer of a known primary site. Treatment is associated with significantly increased survival both for patients with cup and for those with metastatic cancer of a known primary site.
Keywords: Cancer of unknown primary, cup, Ontario Cancer Registry, Ontario Health Insurance Plan, administrative data, survival analyses
INTRODUCTION
In most patients with metastatic cancer, the primary tumour is identified during a standard or extensive diagnostic work-up. But identification is not the case for an estimated 3%–5% of all incident cancers, which are termed “cancer of unknown primary” (cup)1. Currently, the widely accepted definition of cup requires the tumour at the metastatic site to be histologically confirmed2. Patients with cup have a poor prognosis, with survival estimates often being less than 1 year3–5.
Although population studies of cup are common, data about the relationship between treatment and patient outcomes are limited4–7. A Canadian study of a cup population found that 55% of patients did not receive any treatment, with younger age being significantly associated with treatment receipt8. In a U.S. cup population, most patients with cup (79.7%) did not receive any treatment; however, use of radiation therapy in those patients was associated with longer survival [hazard ratio (hr): 0.68; p < 0.001 compared with patients who did not receive radiation therapy]. A recent study undertaken by Australia’s Department of Veterans’ Affairs found that 30% of patients with cup compared with 70% of those with a known primary received treatment, experiencing a median survival of 37 days compared with 310 days respectively9. The literature comparing treatment and survival outcomes for patients with cup with those for patients with a known primary tumour is scarce.
Current clinical guidelines recommend wide-spectrum empiric chemotherapy for almost all cup patients3,10–12. However, a synthesis of clinical trials involving patients with cup questioned the therapeutic benefit of that approach, finding no evidence to support chemotherapy over best supportive care, no evidence for platinum-based chemotherapy over non-platinum-based chemotherapy, and no evidence to support a multi-agent chemotherapy regimen over single-agent treatment3.
Given the limited information about the association between treatment for cup and survival, we investigated the relationship. Using a cup population previously identified in Ontario, we compared survival outcomes for that population with outcomes for a cohort of Ontario patients with metastatic cancer of known primary site13. Using administrative data related to treatment, we stratified both populations based on the type of treatment received, and we investigated potential differences in survival outcomes.
METHODS
Data Sources
The Ontario Cancer Data Linkage project “cd-link” is a collaborative data-release program between the Institute for Clinical Evaluative Sciences (ices) and Cancer Care Ontario that provides research access to Ontario health administrative databases. Through a data use agreement, we gained access to data from ohip (the Ontario Health Insurance Plan), the Same-Day Surgery (sds) and Discharge Abstract (dad) databases maintained by the Canadian Institute for Health Information, and the Ontario Cancer Registry (ocr) for all Ontario patients diagnosed with cancer between 1 January 2000 and 31 December 2005. All data were linked and de-identified by ices. Table i shows the datasets and corresponding data used for cohort selection and analysis. Ethics approval was obtained from the University of Western Ontario Research Ethics Board before the databases were accessed.
TABLE I.
Ontario administrative databases and data used for cohort selection and analysis
| Data source | Data used |
|---|---|
| Ontario Cancer Registry | Origin site of malignancy,a age, sex, tumour histology,b diagnosis date, diagnostic confirmation, date of last contact with patient, death date |
| Same Day Surgery and Discharge Abstract Database | Date of hospital admission, primary tumour site,a metastatic cancer |
| OHIP Claims Database | Physician services,c date provided |
International Classification of Diseases, 10th revision.
International Classification of Diseases for Oncology.
OHIP Schedule of Benefits.
OHIP = Ontario Health Insurance Plan.
Study Cohorts
Two cohorts were studied: a previously identified population of patients with cup13 and a population of patients with metastatic cancer of known primary site. The methods used to identify the cup population have already been described13. A similar algorithm was used to identify the Ontario population of patients with metastatic cancer of known primary site. A unique patient identifier was used to link hospitalization records from the sds database and the dad with patient records from the ocr. Diagnosis codes (International Classification of Diseases, revision 10) were cross-validated between the databases. We used a flag for metastatic disease from the sds database and the dad to further validate the diagnosis code. Patients were removed if histology was missing or if the primary tumour diagnosis changed within 60 days of the initial diagnosis. Histology codes in the ocr were grouped using the International Classification of Diseases for Oncology (3rd edition).
Treatment Data
To obtain patient-specific treatment data, both study populations were linked with the ohip database, which includes all claims made by physicians and other health care providers for insured services to residents of the province. The resulting dataset was sorted to identify codes for surgery, chemotherapy, and therapeutic radiation related to oncology. Those codes were obtained from the ohip Schedule of Benefits for Physician Services (Table ii).
TABLE II.
Fee codes associated with oncologic chemotherapy, radiation, and surgery from the Ontario Health Insurance Plan Schedule of Benefits for Physician Services
| Treatment | Fee code |
|---|---|
| Chemotherapy | G381, G281, G345, G359 |
| Radiation | X310, X311, X312, X313, X323, X302 |
| Surgery | E165, E166, E167, E168, E172, E300, E382, E383, E386, E525, E529, E540, E542, E635, E688, E695, E720, E751, E781, E792, E823, E901, E902, E903, E906, E925, E931, E977, E981 M058, M105, M106, M135 N102, N103, N113, N151, N152, N153, N203, N211, N286, N295, N548, N549, N550, N553, N554, N560, N561 R010, R018, R019, R020, R031, R032, R033, R037, R040, R041, R048, R049, R050, R051, R081, R094, R107, R108, R109, R111, R114, R117, R118, R119, R120, R142, R143, R144, R146, R147, R148, R149, R156, R214, R216, R226, R246, R253, R266, R272, R293, R294, R295, R330, R505, R513, R514, R546, R591, R592, R641, R714, R796, R920 S093, S116, S291, S301, S312, S316, S400, S431, S432, S482, S483, S490, S590, S715, S795 Z117, Z353, Z354, Z522, Z570, Z571, Z753, Z754, Z755, Z761, Z784, Z785, Z632, Z633, Z634, Z857, Z858 |
Comorbidities
Hospitalization records from the sds database and the dad were used to identify comorbidities diagnosed from 1 year before the cancer diagnosis to 6 months after the diagnosis date. Comorbidity scores were calculated using the Charlson comorbidity index14. The comorbidities included were myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, rheumatic disease, peptic ulcer disease, mild liver disease, diabetes without complications, diabetes with complications, paraplegia and hemiplegia, renal disease, moderate or severe liver disease, and aids or hiv infection.
Statistical Analysis
All statistical analyses were performed using the SAS software application (version 9.3: SAS Institute, Cary, NC, U.S.A). The Kaplan–Meier method was used to generate survival curves. We obtained 5-year survival data for all patients. The date of cancer diagnosis was the start date for the survival analysis, and the primary endpoint of that analysis was overall survival. The log-rank test was used to assess differences between the survival curves, stratified by site and histology. Multivariable Cox regression analyses adjusted for age, sex, histology, and comorbidities were used to calculate hrs and 95% confidence intervals. A forward-selection approach was used to construct the models. Time-dependent variables were used to test the proportional hazards assumption. All statistical tests were two-tailed and were conducted at the 5% significance level. Cell sizes of fewer than 5 patients are not reported, as required by the cd-link data use agreement.
RESULTS
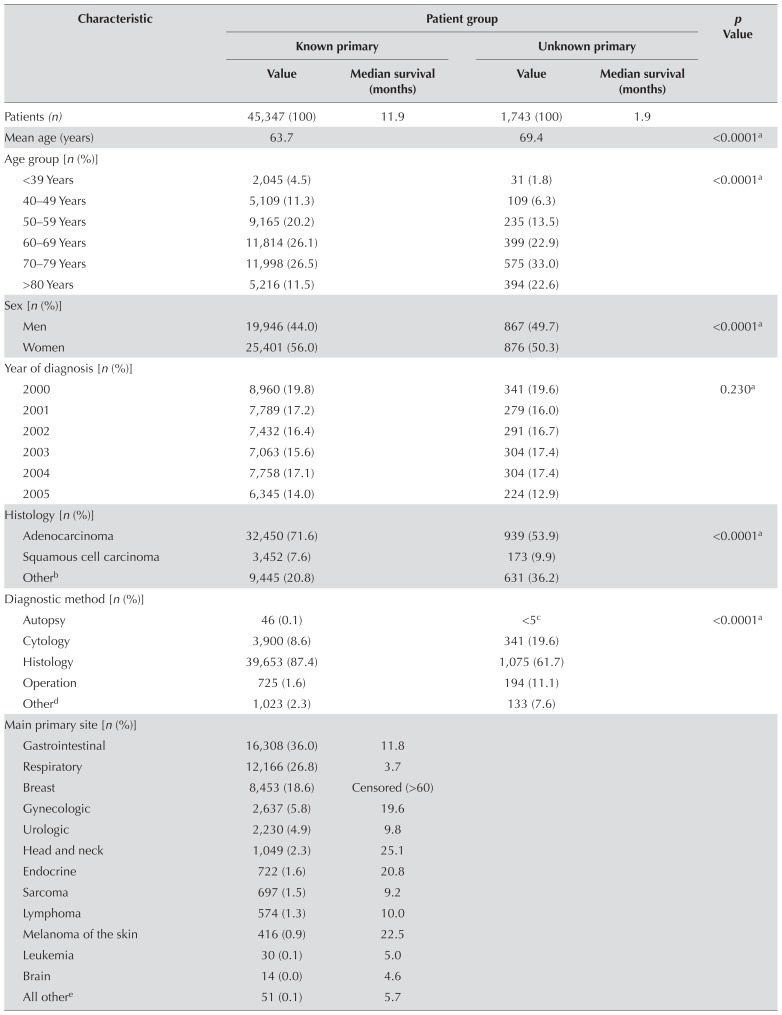
Table iii summarizes patient and tumour characteristics. From January 2000 to December 2005, 47,090 patients were diagnosed with histologically confirmed metastatic cancer. Of those patients, 45,347 (96.3%) had an identifiable primary tumour site, and 1743 (3.7%) were diagnosed with cup. Patients with cup were, on average, older (69.4 years) than the patients with a known primary (63.7 years) and more likely to be male (49.7% and 44.0% respectively, p < 0.0001). Among the main primary tumour types, cup ranked 6th behind gastrointestinal (n = 16,308), respiratory (n = 12,166), breast (n = 8453), gynecologic (n = 2637), and urologic (n = 2230) cancers. The mean Charlson score was significantly higher (p < 0.0001) for patients with cup (1.88) than for patients with a known primary (1.42), and the distribution of Charlson scores differed significantly between the two groups (p < 0.0001).
TABLE III.
Characteristics of patients with metastatic tumours of a known or unknown primary
| Characteristic | Patient group | p Value | |||
|---|---|---|---|---|---|
|
| |||||
| Known primary | Unknown primary | ||||
|
|
|
||||
| Value | Median survival (months) | Value | Median survival (months) | ||
| Patients (n) | 45,347 (100) | 11.9 | 1,743 (100) | 1.9 | |
|
| |||||
| Mean age (years) | 63.7 | 69.4 | <0.0001a | ||
|
| |||||
| Age group [n (%)] | |||||
| <39 Years | 2,045 (4.5) | 31 (1.8) | <0.0001a | ||
| 40–49 Years | 5,109 (11.3) | 109 (6.3) | |||
| 50–59 Years | 9,165 (20.2) | 235 (13.5) | |||
| 60–69 Years | 11,814 (26.1) | 399 (22.9) | |||
| 70–79 Years | 11,998 (26.5) | 575 (33.0) | |||
| >80 Years | 5,216 (11.5) | 394 (22.6) | |||
|
| |||||
| Sex [n (%)] | |||||
| Men | 19,946 (44.0) | 867 (49.7) | <0.0001a | ||
| Women | 25,401 (56.0) | 876 (50.3) | |||
|
| |||||
| Year of diagnosis [n (%)] | |||||
| 2000 | 8,960 (19.8) | 341 (19.6) | 0.230a | ||
| 2001 | 7,789 (17.2) | 279 (16.0) | |||
| 2002 | 7,432 (16.4) | 291 (16.7) | |||
| 2003 | 7,063 (15.6) | 304 (17.4) | |||
| 2004 | 7,758 (17.1) | 304 (17.4) | |||
| 2005 | 6,345 (14.0) | 224 (12.9) | |||
|
| |||||
| Histology [n (%)] | |||||
| Adenocarcinoma | 32,450 (71.6) | 939 (53.9) | <0.0001a | ||
| Squamous cell carcinoma | 3,452 (7.6) | 173 (9.9) | |||
| Otherb | 9,445 (20.8) | 631 (36.2) | |||
|
| |||||
| Diagnostic method [n (%)] | |||||
| Autopsy | 46 (0.1) | <5c | <0.0001a | ||
| Cytology | 3,900 (8.6) | 341 (19.6) | |||
| Histology | 39,653 (87.4) | 1,075 (61.7) | |||
| Operation | 725 (1.6) | 194 (11.1) | |||
| Otherd | 1,023 (2.3) | 133 (7.6) | |||
|
| |||||
| Main primary site [n (%)] | |||||
| Gastrointestinal | 16,308 (36.0) | 11.8 | |||
| Respiratory | 12,166 (26.8) | 3.7 | |||
| Breast | 8,453 (18.6) | Censored (>60) | |||
| Gynecologic | 2,637 (5.8) | 19.6 | |||
| Urologic | 2,230 (4.9) | 9.8 | |||
| Head and neck | 1,049 (2.3) | 25.1 | |||
| Endocrine | 722 (1.6) | 20.8 | |||
| Sarcoma | 697 (1.5) | 9.2 | |||
| Lymphoma | 574 (1.3) | 10.0 | |||
| Melanoma of the skin | 416 (0.9) | 22.5 | |||
| Leukemia | 30 (0.1) | 5.0 | |||
| Brain | 14 (0.0) | 4.6 | |||
| All othere | 51 (0.1) | 5.7 | |||
|
| |||||
| Mean score on the CCI | 1.42 | 1.88 | <0.0001f | ||
|
| |||||
| CCI score group [n (%)] | |||||
| 0 | 15,342 (33.8) | 475 (27.3) | <0.0001f | ||
| 1 | 13,179 (29.1) | 429 (24.6) | |||
| 2 | 7,797 (17.2) | 309 (17.7) | |||
| 3 | 4,475 (9.9) | 223 (12.8) | |||
| 4 | 2,330 (5.1) | 141 (8.1) | |||
| 5–8 | 2,138 (4.7) | 151 (8.7) | |||
| >9 | 86 (0.2) | 15 (0.9) | |||
By Fisher exact test.
Unspecified carcinoma, undifferentiated, sarcoma, lymphoma, other hematologic, melanoma, and other specified carcinoma.
Cells representing fewer than 5 patients are not reported in accordance with the cd-link data user agreement. “Diagnostic Method–Autopsy” has been included as part of “Other” for patients with unknown primary.
Unknown and pathology report outside of the country. Includes autopsy for patients with unknown primary.
Bone, joints, soft tissue, heart, eye adnexa, and ill-defined sites.
By t-test.
CCI = Charlson comorbidity index.
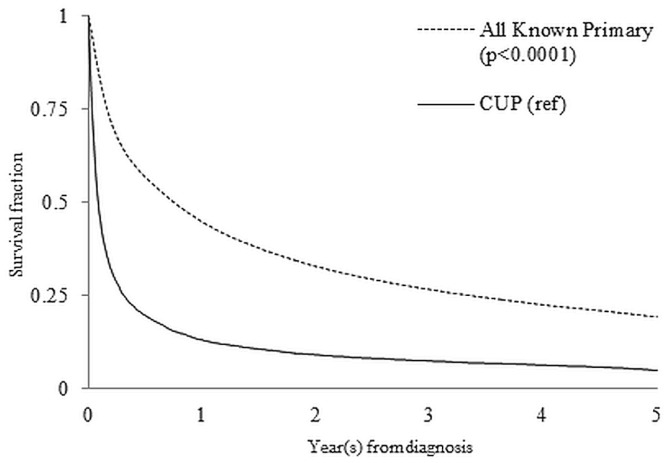
Figure 1 shows survival for all patients over a 5-year period. The median overall survival was 1.9 months for patients with cup compared with 11.9 months for all patients a known primary cancer (Table iii). Among the known primary sites, median survival was lowest for metastatic liver cancer (2.9 months) and highest for breast cancer (censored, >60 months).
FIGURE 1.
Kaplan–Meier 5-year survival curves for patients with a cancer of unknown primary (CUP) and with known primary tumours.
Table iv shows the characteristics of the patients with cup and with metastatic cancer of known primary. Receipt of treatment of any type was more likely for patients with a known primary site (n = 35,012, 77.2%) than for patients with cup (n= 891, 51.1%). Patients with cup were most likely to receive a single type of treatment (73.1%); patients with a known primary were more likely to receive two or three different types of treatment (55.3%). Of the patients with cup, those who received treatment were younger on average (67.7 years) than those who did not receive treatment (71.2 years, p< 0.0001). Squamous-cell histology was more common in patients with cup who received treatment (16.2%) than in those who did not receive treatment (3.4%).
TABLE IV.
Characteristics of patients with metastatic tumours of known or unknown primary, by whether they received or did not receive treatment with surgery, chemotherapy, or radiation
| Characteristic | Received treatment | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Yes | No | |||||
|
|
|
|||||
| Known primary [n (%)] | Unknown primary [n (%)] | p Value | Known primary [n (%)] | Unknown primary [n (%)] | p Value | |
| Patients | 35,012 | 891 | 10,335 | 852 | ||
|
| ||||||
| Mean age (years) | 62.0 | 67.7 | <0.0001a | 69.5 | 71.2 | <0.0001a |
|
| ||||||
| Age group | ||||||
| <39 Years | 1,787 (5.1) | 17 (1.9) | 258 (2.5) | 14 (1.6) | ||
| 40–49 Years | 4,626 (13.2) | 76 (8.5) | 483 (4.7) | 33 (3.9) | ||
| 50–59 Years | 7,866 (22.5) | 143 (16.1) | 1,299 (12.6) | 92 (10.8) | ||
| 60–69 Years | 9,348 (26.7) | 213 (23.9) | 2,466 (23.9) | 186 (21.8) | ||
| 70–79 Years | 8,446 (24.1) | 267 (30.0) | 3,552 (34.4) | 308 (36.2) | ||
| >80 Years | 2,939 (8.4) | 175 (19.6) | 2,277 (22.0 | 219 (25.7 | ||
|
| ||||||
| Sex | <0.0001a | 0.0270a | ||||
| Women | 20,374 (58.2) | 428 (48.0) | 5,027 (48.6) | 448 (52.6) | ||
| Men | 14,638 (41.8) | 463 (52.0) | 5,308 (51.4) | 404 (47.4) | ||
|
| ||||||
| Histology | <0.0001b | <0.0001b | ||||
| Adenocarcinoma | 25,846 (73.8) | 472 (53.0) | 6,604 (63.9) | 467 (54.8) | ||
| Squamous cell carcinoma | 2,487 (7.1) | 144 (16.2) | 965 (9.3) | 29 (3.4) | ||
| Other | 6,679 (19.1) | 275 (30.9) | 2,766 (26.8) | 356 (41.8) | ||
|
| ||||||
| Mean score on the CCI | 1.3 | 1.8 | <0.0001a | 1.7 | 2.0 | <0.0001a |
|
| ||||||
| CCI score group | ||||||
| 0 | 12,608 (36.0) | 269 (30.2) | <0.0001b | 2,734 (26.5) | 206 (24.2) | <0.0001b |
| 1 | 10,242 (29.3) | 224 (25.1) | 2,937 (28.4) | 205 (24.1) | ||
| 2 | 5,816 (16.6) | 140 (15.7) | 1,981 (19.2) | 169 (19.8) | ||
| 3 | 3,217 (9.2) | 111 (12.5) | 1,258 (12.2) | 112 (13.2) | ||
| 4 | 1,614 (4.6) | 68 (7.6) | 716 (6.9) | 73 (8.6) | ||
| 5–8 | 1,455 (4.2) | 73 (8.2) | 683 (6.6) | 78 (9.2) | ||
| >9 | 60 (0.2) | 6 (0.7) | 26 (0.3) | 9 (1.1) | ||
|
| ||||||
| Median survival (months) | 19.0 | 3.6 | 2.2 | 1.1 | ||
|
| ||||||
| Treatment type | ||||||
| None | Not applicable | <0.0001b | 10,335 (100) | 852 (100) | ||
| Surgery only | 6,237 (17.8) | 372 (41.8) | ||||
| CTx only | 6,503 (18.6) | 141 (15.8) | ||||
| RT only | 2,889 (8.3) | 138 (15.5) | ||||
| CTx and RT | 3,533 (10.1) | 44 (4.9) | Not applicable | |||
| Surgery and RT | 2,356 (6.7) | 107 (12.0) | ||||
| Surgery and CTx | 7,009 (20.0) | 65 (7.3) | ||||
| Surgery, RT, and CTx | 6,485 (18.5 | 24 (2.7) | ||||
By t-test.
By Fisher exact test.
CCI = Charlson comorbidity index; CTx = chemotherapy; RT = radiation therapy.
Receiving any treatment was associated with longer median survival in both patient populations. Patients with a known primary tumour who received any treatment type had a median survival of 19.0 months; those who received no treatment had a median survival of 2.2 months. A similar trend was observed in patients with cup: treated patients had a median survival of 3.6 months, and untreated patients had a median survival of 1.1 months.
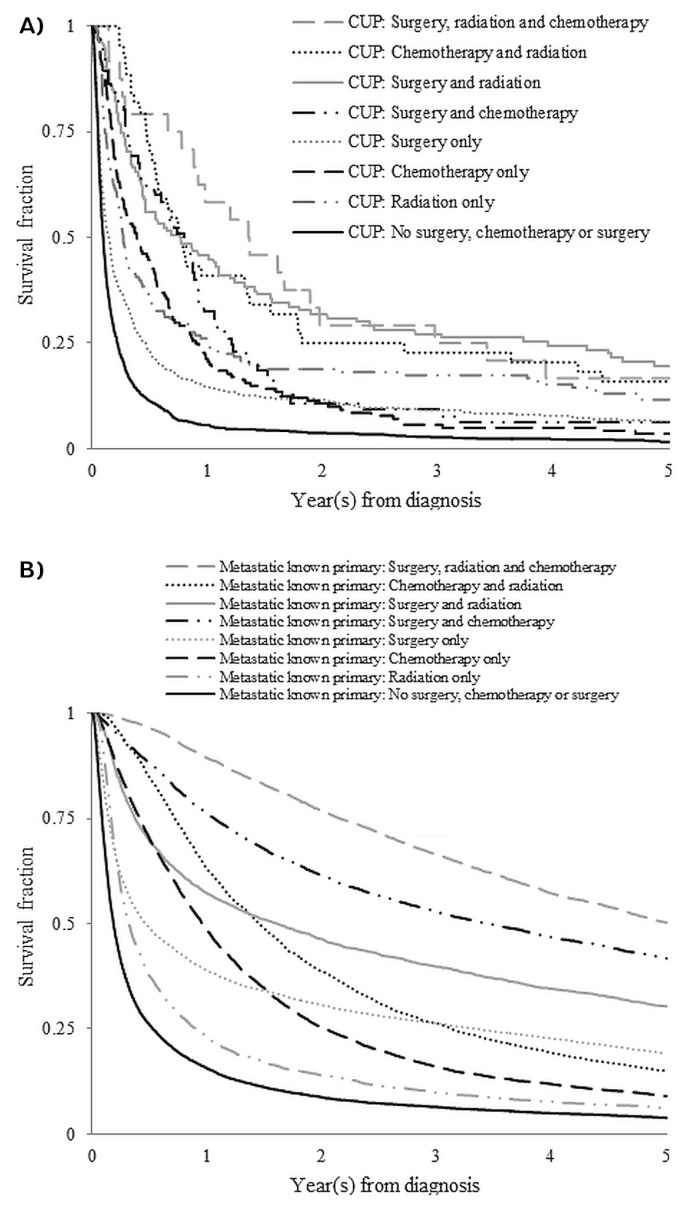
For each treatment type, 2-year hrs and survival curves were constructed (Table v, Figure 2). Patients with cup who received no treatment were considered the reference for each set of comparisons, and these trends were observed:
TABLE V.
Hazard ratios (HRs) at 2 years for patients with metastatic tumours of known or unknown primary, by type of treatment receiveda
| Treatment (frequency or combination) | Known primary | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Yes (n = 35,012) | p Valueb | No (n = 891) | p Valueb | |||
|
|
|
|||||
| [n (%)] | HR | [n (%)] | HR | |||
| Radiation (RT) | ||||||
| 0 | 30,084 (66.3) | 0.42 | <0.0001 | 1,430 (82.0) | Reference | |
| 1–2 | 13,138 (29.0) | 0.31 | <0.0001 | 274 (15.7) | 0.42 | <0.0001 |
| 3–4 | 1,638 (3.6) | 0.29 | <0.0001 | 33 (1.9) | 0.40 | <0.0001 |
| >5 | 487 (1.1) | 0.26 | <0.0001 | 6 (0.3) | 0.34 | 0.0084 |
|
| ||||||
| Chemotherapy (CTx) | ||||||
| None | 21,817 (48.1) | 0.59 | <0.0001 | 1,469 (84.3) | Reference | |
| Single agent | 2,877 (6.3) | 0.36 | <0.0001 | 55 (3.2 | 0.47) | <0.0001 |
| Multi-agent | 20,653 (45.5) | 0.30 | <0.0001 | 219 (12.6) | 0.60 | <0.0001 |
|
| ||||||
| Surgery | ||||||
| 0 | 23,260 (51.3) | 0.51 | <0.0001 | 1,175 (67.4) | Reference | |
| 1 | 6,469 (14.3) | 0.34 | <0.0001 | 207 (11.9) | 0.82 | 0.0077 |
| >2 | 15,618 (34.4) | 0.25 | <0.0001 | 361 (20.7) | 0.48 | <0.0001 |
|
| ||||||
| Combination | ||||||
| None | 10,335 (22.8) | 0.55 | <0.0001 | 852 (48.9) | Reference | |
| Surgery only | 6,237 (13.8) | 0.26 | <0.0001 | 372 (21.3) | 0.54 | <0.0001 |
| CTx only | 6,503 (14.3) | 0.25 | <0.0001 | 141 (8.1) | 0.42 | <0.0001 |
| RT only | 2,889 (6.4) | 0.39 | <0.0001 | 138 (7.9) | 0.36 | <0.0001 |
| CTx and RT | 3,533 (7.8) | 0.20 | <0.0001 | 44 (2.5) | 0.25 | <0.0001 |
| Surgery and RT | 2,356 (5.2) | 0.19 | <0.0001 | 107 (6.1) | 0.22 | <0.0001 |
| Surgery and CTx | 7,009 (15.5) | 0.16 | <0.0001 | 65 (3.7) | 0.35 | <0.0001 |
| Surgery, RT, and CTx | 6,485 (14.3) | 0.14 | <0.0001 | 24 (1.4) | 0.22 | <0.0001 |
Models adjusted for age, sex, histology, and comorbidities.
Values reflect comparisons between the reference for each treatment type and the treatment group.
FIGURE 2.
Kaplan–Meier 5-year survival curves for patients (A) with a cancer of unknown primary (CUP) by type of treatments received and (B) with metastatic tumours of known primary by type of treatments received.
■ First, receiving any treatment was associated with a better survival outcome. For patients with cup, the reference group in each treatment block had the highest hr, and untreated patients with a known primary had the highest hr observed in each treatment block for the relevant patient population.
■ Increasing intensity of treatment was associated with a lower hr in all patients. In the patients with cup who received radiation, the hr declined from 0.42 (p< 0.0001) for 1–2 treatments, to 0.40 (p < 0.0001) for 3–4 treatments, and to 0.34 (p= 0.0084) for 5 or more treatments.
■ Even with comparable treatment, survival outcomes were worse for patients with cup than for patients with a known primary site. For example, patients who received no surgery, a single surgery, or 2 or more surgeries were compared. The resulting 2-year hrs were 1.00 (reference), 0.82 (p = 0.0077), and 0.48 (p < 0.0001) for patients with cup and 0.51 (p < 0.0001), 0.34 (p < 0.0001), and 0.25 (p < 0.0001) for patients with a known primary.
■ Survival outcomes associated with the use of more than one type of therapy were also investigated. Compared with the use of any single therapy, the addition of a second therapy was associated with further survival benefit. All 3 therapies used in tandem were no worse than any 2 therapies. For patients with a known primary site, the 2-year hr trend was similar to that observed for patients with cup.
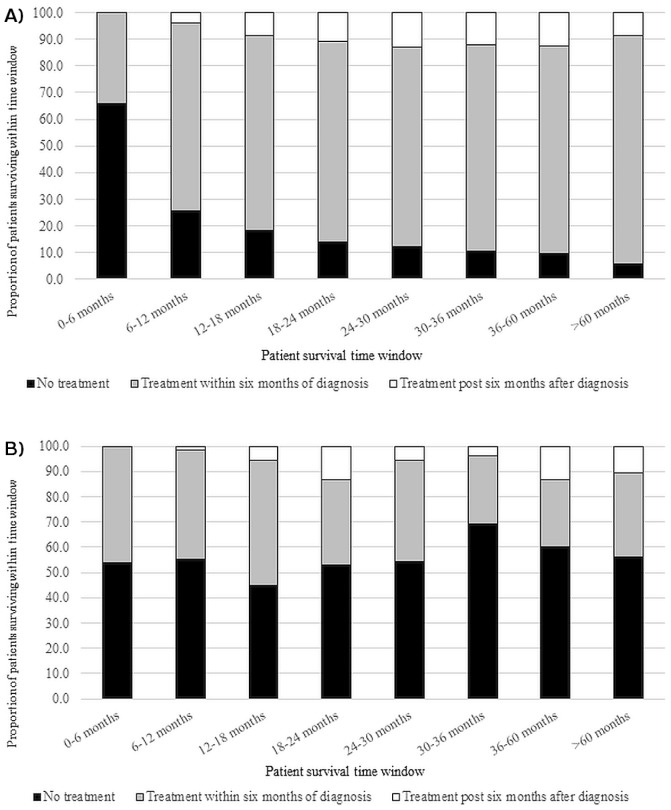
The time of initial treatment relative to initial diagnosis was examined in both patient populations. Figure 3 shows the proportions of patients allocated to these 3 groups: treatment received within 6 months of diagnosis, treatment received 6 months or more after diagnosis, or no treatment received. Patient survival was then plotted for those subpopulations in 6-month increments. The surviving proportion of patients with a known primary who received no treatment declined from more than 60% up to 6 months to just 5% at more than 60 months. The increase in the proportion of patients who received treatment within 6 months of diagnosis largely accounted for that change, starting at 34.2% for 6-month survival and rising to 85.6% for more than 60-month survival. For patients with cup, survival did not appear to have any relation with first treatment date. The proportion of patients with cup who received no treatment remained above 50% for most survival durations.
FIGURE 3.
Observed 5-year survival for patients (A) with a known primary and (A) with a cancer of unknown primary, both stratified by commencement of treatment within 6 months of diagnosis, commencement of treatment at least 6 months after diagnosis, or no treatment received.
DISCUSSION
Using health administrative databases from Ontario, we identified a population of patients diagnosed with metastatic cancer of known primary origin between January 2000 and December 2005. We described differences in the use of treatment for that population and for a previously identified population of patients with cup, summarizing chemotherapy, radiation therapy, and surgeries received by both cohorts. We found that, in Ontario, patients with cup experience significantly decreased survival and are less likely than patients with metastatic cancer of a known primary to receive treatment of any type. Compared with their untreated counterparts, patients with cup who received treatment of any type experienced superior survival. Early treatment was associated with extended survival in patients with a known primary, but a parallel gain in survival was largely absent in patients with cup.
The increased survival observed in patients having metastatic cancer of known primary site compared with patients having cup could be attributable to several factors. Knowledge of the primary site might significantly alter clinical decision-making3, and treatment might be more common in patients with a known primary site if oncologists and patients are more willing to accept available treatment when the primary tumour is known15. Second, compared with patients having a known primary, patients having cup tended to be older and to have more comorbidities. Those factors might have led to those patients being less thoroughly investigated, resulting in a diagnosis of cup and a corresponding reluctance to seek treatment. Alternatively, the comorbidities in patients with cup might have lowered the likelihood of therapeutic intervention. Finally, a large percentage of the known primary tumours were breast cancers, and compared with other cancer types, were associated with the best survival. However, the favourable survival of breast cancer patients might be attributable to the inclusion of patients whose metastases were limited to lymph nodes (a potential result of ascertaining metastasis information from hospital abstracts, given that information about cancer staging was unavailable in the ocr for the study accrual period).
Our estimate of the proportion of patients with metastatic cancer of known primary not receiving treatment is similar to estimates described elsewhere. A study of 8 common solid tumour types recorded in the U.S. National Cancer Database found that 20.6% of all patients (n = 159,284) with those types of tumours received no treatment16. The authors attributed most nontreatment to poor functional status, comorbidities, and patient preference. We also observed that a larger proportion of patients with cup than of patients with a known primary site had higher scores on the Charlson comorbidity index, suggesting that comorbidities might be a key factor for nontreatment in both patient cohorts.
Methodologically, the present work is similar to an Australian comparison between patients with cup and those with known primary tumours9. The main difference between the two studies is that our cohort was drawn from the general population and the Australian study surveyed Australia’s Department of Veterans’ Affairs. Despite that difference, key findings were similar: Both studies found that patients with cup are less likely than patients with a known primary to be treated and that survival outcomes are poorer for patients with cup than for patients with a known primary tumour.
Some discussion has arisen about the role of chemotherapy in the treatment of patients with cup. Clinical guidelines recommend the use of empiric platinum-based combination therapies for most patients with cup10–12. When such patients are treated with combination chemotherapy, they experience an estimated median survival of 9–13 months17. Our analyses show a significant reduction in the 2-year survival hrs in patients treated with single-agent chemotherapy. We observed no additional survival benefit associated with multi-agent chemotherapy regimens in patients with cup. That observation is consistent with conclusions from another study that patients with cup receiving carboplatin–paclitaxel, cisplatin–gemcitabine, or gemcitabine monotherapy showed no significant differences in survival by treatment type18. However, the limited number of administrative codes for chemotherapy make it difficult to determine whether a single combination therapy or several different combination therapies were used in sequence for patients with cup.
The benefit of treatment has been described in the cup literature, but those results have been limited to single therapy types2,18,19. We found that patients receiving chemotherapy in addition to radiation therapy or surgery (or both) experienced a further survival benefit. However, we did not measure the intensity of radiation therapy, only whether a patient received radiation therapy. We are not aware of any other study that has looked at the survival benefit associated with multiple treatment types for patients with cup. The clinical significance of our finding could be minimal, because the addition of surgery or radiation might not be tenable in some patients.
Although we found strong associations for knowledge of the primary tumour with treatment and survival outcomes, we are unable to prove causation. The choice to treat a patient—especially a patient without a primary tumour site—could be related to patient health. Alternatively, patients with cup who receive treatment might be fundamentally different from those who do not. There are some indications that such fundamental differences might be present in our population. First, of the patients with cup who received treatment, 16.2% had squamous cell carcinoma; of cup patients who did not receive treatment, just 3.4% had squamous histology tumours. Squamous histology represents a favourable subgroup in cup, and prior studies have found median survival to be as high as 20.4 months in that subgroup13. Second, the proportion of patients with cup who received no treatment did not change dramatically with patient survival, but the proportion of patients with a known primary site who were not treated decreased sharply.
Although the present work describes the use of the most common types of therapy associated with cup, other modalities, including immunotherapy and targeted therapy, are available. However, we believe that our analysis describes the treatment received by a significant proportion of patients with cup largely because current clinical guidelines rarely recommend treatment outside of chemotherapy, surgery, or radiation therapy10–12. The most recent cup guideline from the European Society for Medical Oncology indicates the use of chemotherapy, radiation, or surgery in 7 of the 8 classifications of favourable cup cases and for all classifications of unfavourable cup cases18. Furthermore, for patients for whom targeted treatment is recommended, the use of surgery, chemotherapy, or radiation is also prescribed.
CONCLUSIONS
The present work shows that, in cup, a survival benefit is associated with all treatment types analyzed. The observed increase in survival was not uniform across patient groups. Compared with their cup counterparts, patients with a metastatic tumour of known primary were far more likely to receive treatment and experienced a greater survival benefit from that treatment. The direct relationship between treatment and survival remains less clear for cup patients. Advancing clinical guidelines for cup patients toward specific and specialized care, while also expanding treatment to those who would normally go untreated, is vital for this patient population.
ACKNOWLEDGMENTS
Portions of this work were described in Chong S. Kim’s Master’s thesis “Survival outcomes and treatment utilization among patients with known and unknown primary tumours in Ontario,” available in the Electronic Thesis and Dissertation Repository of the University of Western Ontario.
This study was funded by the Canadian Institutes of Health Research and was supported through provision of data by ices and Cancer Care Ontario and through funding to ices in the form of an annual grant from the Ministry of Health and Long-Term Care (mohltc) and the Ontario Institute for Cancer Research (oicr). The opinions, results, and conclusions reported in this paper are those of the authors. No endorsement by ices, Cancer Care Ontario, oicr, or the mohltc is intended or should be inferred.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: PKR is a co-founder of Cytognomix Inc.; SMM has received unrestricted research grants from Merck, GlaxoSmithKline, Sanofi Pasteur, Pfizer, and Roche–Assurex for unrelated studies, and has also received fees as an advisory board member for Sanofi Pasteur; EW has received fees as an advisory board member from Amgen, Bayer, Eisai, Merck, and Roche, and EW’s institution receives funding from AstraZeneca, Bristol–Myers Squibb, Eisai, Merck, and Roche for trials in which he is a co-investigator; GSZ has received fees as an advisory board member for Amgen and GlaxoSmithKline, and executive education fees from Bayer; the remaining authors have no conflicts to disclose.
REFERENCES
- 1.Greco FA, Oien K, Erlander M, et al. Cancer of unknown primary: progress in the search for improved and rapid diagnosis leading toward superior patient outcomes. Ann Oncol. 2012;23:298–304. doi: 10.1093/annonc/mdr306. [DOI] [PubMed] [Google Scholar]
- 2.Urban D, Rao A, Bressel M, Lawrence YR, Mileshkin L. Cancer of unknown primary: a population-based analysis of temporal change and socioeconomic disparities. Br J Cancer. 2013;109:1318–24. doi: 10.1038/bjc.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amela EY, Lauridant-Philippin G, Cousin S, Ryckewaert T, Adenis A, Penel N. Management of “unfavourable” carcinoma of unknown primary site: synthesis of recent literature. Crit Rev Oncol Hematol. 2012;84:213–23. doi: 10.1016/j.critrevonc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Brewster DH, Lang J, Bhatti LA, Thomson CS, Oien KA. Descriptive epidemiology of cancer of unknown primary site in Scotland, 1961–2010. Cancer Epidemiol. 2014;38:227–34. doi: 10.1016/j.canep.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Hemminki K, Bevier M, Hemminki A, Sundquist J. Survival in cancer of unknown primary site: population-based analysis by site and histology. Ann Oncol. 2012;23:1854–63. doi: 10.1093/annonc/mdr536. [DOI] [PubMed] [Google Scholar]
- 6.Bevier M, Sundquist J, Hemminki K. Incidence of cancer of unknown primary in Sweden. Eur J Cancer Prev. 2012;21:596–601. doi: 10.1097/CEJ.0b013e3283523468. [DOI] [PubMed] [Google Scholar]
- 7.Kaaks R, Sookthai D, Hemminki K, et al. Risk factors for cancers of unknown primary site: results from the prospective epic cohort. Int J Cancer. 2014;135:2475–81. doi: 10.1002/ijc.28874. [DOI] [PubMed] [Google Scholar]
- 8.Seve P, Sawyer M, Hanson J, Broussolle C, Dumontet C, Mackey JR. The influence of comorbidities, age, and performance status on the prognosis and treatment of patients with metastatic carcinomas of unknown primary site. Cancer. 2006;106:2058–66. doi: 10.1002/cncr.21833. [DOI] [PubMed] [Google Scholar]
- 9.Schaffer AL, Pearson SA, Dobbins TA, Er CC, Ward RL, Vajdic CM. Patterns of care and survival after a cancer of unknown primary (cup) diagnosis: a population-based nested cohort study in Australian Government Department of Veterans’ Affairs clients. Cancer Epidemiol. 2015;39:578–84. doi: 10.1016/j.canep.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Fizazi K, Greco FA, Pavlidis N, Pentheroudakis G. Cancers of unknown primary site: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(suppl 6):vi64–8. doi: 10.1093/annonc/mdr389. [DOI] [PubMed] [Google Scholar]
- 11.Pavlidis N, Briasoulis E, Pentheroudakis G on behalf of the esmo Guidelines Working Group. Cancers of unknown primary site: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v228–31. doi: 10.1093/annonc/mdq193. [DOI] [PubMed] [Google Scholar]
- 12.Collado Martín R, García Palomo A, de la Cruz Merino L, Borrega García P, Barón Duarte FJ on behalf of the Spanish Society for Medical Oncology. Clinical guideline seom: cancer of unknown primary site. Clin Transl Oncol. 2014;16:1091–7. doi: 10.1007/s12094-014-1244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CS, Hannouf MB, Sarma S, et al. Identification and survival outcomes of a cohort of patients with cancer of unknown primary in Ontario, Canada. Acta Oncol. 2015;54:1781–7. doi: 10.3109/0284186X.2015.1020965. [DOI] [PubMed] [Google Scholar]
- 14.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New icd-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–94. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Pavlidis N, Pentheroudakis G. Cancer of unknown primary site: 20 questions to be answered. Ann Oncol. 2010;21(suppl 7):vii303–7. doi: 10.1093/annonc/mdq278. [DOI] [PubMed] [Google Scholar]
- 16.Small AC, Tsao CK, Moshier EL, et al. Prevalence and characteristics of patients with metastatic cancer who receive no anticancer therapy. Cancer. 2012;118:5947–54. doi: 10.1002/cncr.27658. [DOI] [PubMed] [Google Scholar]
- 17.Daud AI. Removing the unknown from the carcinoma of unknown primary. J Clin Oncol. 2013;31:174–5. doi: 10.1200/JCO.2012.45.7630. [DOI] [PubMed] [Google Scholar]
- 18.Löffler H, Puthenparambil J, Hielscher T, Neben K, Krämer A. Patients with cancer of unknown primary: a retrospective analysis of 223 patients with adenocarcinoma or undifferentiated carcinoma. Dtsch Arztebl Int. 2014;111:481–7. doi: 10.3238/arztebl.2014.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demirci U, Coskun U, Karaca H, et al. Docetaxel and cisplatin in first line treatment of patients with unknown primary cancer: a multicenter study of the Anatolian Society of Medical Oncology. Asian Pac J Cancer Prev. 2014;15:1581–4. doi: 10.7314/APJCP.2014.15.4.1581. [DOI] [PubMed] [Google Scholar]