Abstract
Background
Radiation therapy (rt) is a longstanding treatment modality for cancer. In addition, immune checkpoint blockade has been a significant development in the field of immunotherapy, modifying key immunosuppressive pathways of cancer cells.
Methods
The aim of the present work was to review current concepts of rt and immunotherapy synergism, the abscopal effect, and the molecular effects of rt in the tumour microenvironment, its influence on immune stimulation, and potential clinical outcomes that might evolve from ongoing studies. We also discuss potential predictors of clinical response.
Results
Up-to-date literature concerning the mechanisms, interactions, and latest knowledge about rt and immunotherapy was reviewed and summarized, and is presented here.
Conclusions
The possibility of using hyperfractionated rt to combine an abscopal effect with the enhanced effect of immune treatment using checkpoint blockade is a very promising method for future tumour treatments.
Keywords: Radiation therapy, abscopal effect, immunotherapy, checkpoint blockade, combination therapy, PD-L1
INTRODUCTION
Radiation therapy (rt) is a longstanding modality in cancer treatment. The biologic effects of radiation apply to neoplastic and normal tissues alike. The effects of radiation depend on selected biologic parameters, such as oxygen supply and cell cycle. The radiation effect can be modified by chemical agents such as radiosensitizers, radioprotectors, and chemotherapeutic agents. Repair of sublethal injury, regenerative processes, redistribution within the cell-division cycle and the re-oxygenation of tumour tissue have to be taken into account when treating a patient1. The main goal of optimized rt is to maximize the therapeutic ratio, with tumour tissues receiving high doses of radiation and normal healthy tissues being spared. However, certain radiation-induced bystander effects in non-irradiated cells have biologic results that can be beneficial for treatment outcome1–4.
In the abscopal effect (ab, position away from, and scopos, a target for shooting at), described by R.H. Mole in 1953, tumour cells outside the field of primary irradiation regress or even disappear5. Several clinical case studies have reported the regression of nonirradiated metastases after conventional rt with or without combined immunotherapy6,7. The effect was observed in several tumours, including melanoma, lung cancer, renal cancer, hepatocellular cancer, and chronic lymphocytic leukemia6–11. To date, the complete picture of the biologic mechanisms underlying those radiation-induced bystander effects remains elusive, but the most likely explanation is that regression of nonirradiated tumour is a consequence of systemic immune activation induced by immunogenic cell death in irradiated tumour tissue12.
Remarkable progress has been made in the field of immunotherapy with immune checkpoint blockade, which regulates key immunosuppressive pathways of cancer cells. Current targets of checkpoint blockades are ctla-4 and PD-1—molecules crucial for the peripheral CD8+ T-cell tolerance induced by antigen-presenting cells. The ctla-4 protein affects the priming phase of the immune response. It is transported to the surface when the T-cell receptor recognizes an antigenic peptide in association with the major histocompatibility complex of the antigen-presenting cells. For complete T-cell stimulation, the CD28 receptor of the T cell and the B7 ligand of the antigen-presenting cell have to be bound for a co-stimulatory pathway13. The higher affinity of ctla-4 inhibits the proliferation of T cells by outcompeting CD28 receptors for ligand binding. T-Cell immune tolerance mediated by ctla-4 can also be achieved with the production of cytokines such as transforming growth factor β in regulatory T cells14.
Another key inhibitory receptor, PD-1, is found on the surface of T cells and B cells and binds to PD-1 ligands 1 and 2 (PD-L1 and PD-L2). PD-L1 is widely expressed on hematopoietic and nonhematopoietic cells. The main role of the PD-1/PD-L1 system is to limit the response of effector T cells and thus immune-mediated tissue damage. PD-L1 is also expressed in solid tumours of various types and in hematologic malignancies. Tumour cells with PD-L1 expression can escape the T-cell–related immune reaction regulated by cytokines such as tumour necrosis factor α and interferon γ15.
In clinical trials using checkpoint blockade, anti–ctla-4 and anti–PD-1 monoclonal antibodies were associated with improved survival outcomes in patients with advanced solid tumours, particularly melanoma and non-small-cell lung cancer (nsclc).
In this review, we summarize the current concepts of synergism between rt and immunotherapy, the molecular effects of rt in the tumour microenvironment, the impact of those effects on immune activation, and potential clinical applications in trials exploring this important therapeutic opportunity. Finally, potential predictors of clinical response are discussed. Checkpoint blockade and the abscopal effect are emphasized.
DISCUSSION
RT Combined with Immunotherapy
Research into cancer therapeutics has focused largely on two distinct lines of inquiry. In one approach, efforts to understand the underlying cells—autonomous genetic drivers of tumorigenesis—have led to the development of clinically important targeted agents that induce profound, but often not durable, tumour responses in genetically defined populations. In the second parallel approach, exploration of the mechanisms of tumour-protective immunity has provided several therapeutic strategies—most notably the immune checkpoint antibodies that reverse the negative regulators of T-cell function and that induce durable clinical responses in subsets of patients with various tumour types. The integration of those potentially complementary research fields provides new opportunities to improve cancer treatments. As demonstrated in preclinical models and proved in concept trials in humans, combining radiation with immune therapy is a highly rational approach that can clearly increase the antitumour immune response when given together with other immune interventions16,17.
Clinical concerns about that approach relate to two main situations. The first is whether the combination will be less effective because of reduced cell kill by either the rt or the immunotherapies. The second is whether the toxicity will be too high, either that directly induced by rt or that resulting from the immunogenic agent. Historically, based on older treatment techniques with large fields that included substantial bone marrow volume or circulating blood volume, rt has been considered to be immunosuppressive, resulting in reduced blood cell counts1. In addition, because of the relative radiosensitivity of hematopoietic cells, whole-body rt regimens are used to induce lympho- and myeloablation before stem-cell transplantation18,19.
PD-1/PD-L1
PD-1 is an inhibitory cell surface receptor that acts as an immune checkpoint. Its ligand, PD-L1, is expressed on diverse cell types, including antigen-presenting, epithelial, and endothelial cells. Studies have shown that PD-1/PD-L1–targeted therapies have clinical activity against metastatic bladder cancer, head-and-neck cancers, Hodgkin lymphoma, nsclc, and renal cell cancer20. The anti–PD-1 therapies pembrolizumab and nivolumab are currently approved as treatment for unresectable or metastatic melanoma, and as first- and second-line treatment for nsclc after chemotherapy. Nivolumab has recently been approved for Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem-cell transplantation and for metastatic renal cell carcinoma. Atezolizumab and durvalumab are monoclonal anti–PD-L1 antibodies that are currently under active investigation in clinical trials. The U.S. Food and Drug Administration recently approved atezolizumab for locally advanced or metastatic progressing urothelial carcinoma during or after platinum chemotherapy. REGN2810 is a monoclonal antibody that binds to PD-1 and inhibits PD-L1–mediated activation of downstream pathways. In preclinical studies, the combination of rt and targeted PD-1/PD-L1 therapy has been demonstrated to activate cytotoxic T cells, reduce levels of myeloid-derived suppressor cells, and induce an abscopal response21,22.
Based on those promising results, numerous ongoing clinical trials are exploring the combination of PD-1/PD-L1 inhibition with rt. The core studies are in phase i or ii. Two open phase iii trials (NCT02768558 and NCT02617589 at http://ClinicalTrials.gov/) are examining the combination of nivolumab with rt in locally advanced nsclc and glioblastoma; results are pending.
In general, checkpoint blockade and rt effects are based on ligands of tumour cell PD-L1 and PD-L2 receptors on T cells; ctla-4, on ligands on antigen-presenting B7 cells (CD80, CD86); and using checkpoint blockade, on anti–PD-1/PD-L1 (agents such as atezolizumab, nivolumab, pembrolizumab) and anti–ctla-4 (ipilimumab). From the other side, the effect of rt is to increase antigen presentation and CD8+ T-cell infiltration, stimulating tumour-specific cytotoxic T lymphocytes in distant sites that lead to the abscopal effect23.
Based on preclinical and human trials, combining rt with immune therapy is highly rational because the combination can increase antitumour immunity when given together with other immune interventions. Azad and colleagues24 showed in vitro that rt can stimulate the immune system. Pan02 murine PDAC (pancreatic cancer) cells treated with rt and gemcitabine resulted in upregulated PD-L1. In vitro, PD-L1 inhibition did not alter radio- and chemosensitivity. In vivo, the addition of an anti–PD-L1 agent to high-dose rt (12.5×3, 20 Gy) significantly improved tumour response, which provided a rationale for testing the use of rt with immune checkpoint inhibitors in pancreatic carcinoma.
At this time, a limited number of clinical trials have reported results from combined rt and checkpoint blockade, most of which used an anti–ctla-4 monoclonal antibody. A preclinical study demonstrated that rt is synergistic with anti–ctla-4 antibody and induces systemic antitumour responses in a poorly immunogenic carcinoma refractory to anti–ctla-4 monotherapy25.
Koller et al.26 evaluated patients treated with ipilimumab with or without irradiation for advanced melanoma. Median overall survival (os), overall response, complete response, and median progression-free survival were significantly improved in the concurrent ipilimumab–rt arm. Moreover, no increase in toxicities was observed in the ipilimumab–rt group compared with the group receiving ipilimumab alone26.
The first trial to focus on small-cell lung cancer (sclc) is the phase ii stimuli trial, which is incorporating induction with concurrent ipilimumab and nivolumab at about 6–8 weeks after the 4th cycle of standard-of-care concurrent chemoradiation for sclc (see NCT02046733 at http://ClinicalTrials.gov/). The strategy is based on the rationale of combining peripheral T-cell priming by ipilimumab to increase intratumoural T cells with maintenance PD-1 blockade in an attempt to sustain the population of activated cells. One major question might be whether 4 cycles of chemotherapy with concurrent radiation will lead to suboptimal neoantigen presentation and priming by the time ipilimumab is delivered, 2 months after the last dose of therapy, which might not yield the benefit that administering ipilimumab earlier in the regimen would. This study is open across multiple centres in Europe and is estimated to be completed in 2019.
Similarly, a single-institution phase i dose-escalation study underway at the MD Anderson Cancer Center is giving pembrolizumab concurrently with rt in two populations (see NCT02402920 at http://ClinicalTrials.gov/). The first population consists of patients with untreated sclc, and notably, pembrolizumab will be administered upfront with concurrent chemoradiotherapy with platinum–etoposide for a standard 4 cycles, with twice-daily rt to the chest in 150 cGy fractions for a total dose of 45 Gy. The second population will consist of patients with untreated sclc for whom the treatment paradigm will include pembrolizumab starting with cycle 3 of chemotherapy. The same dose of rt will be given, but the timing of the irradiation is left open at this point. It is likely that the extensive-stage sclc population will be very heterogeneous and that the timing of rt will depend on the response to chemotherapy and the functional status of each patient. Given that this is a phase i trial, dose-limiting toxicities will be the main focus in judging whether concurrent rt in this potentially ill population will be feasible, providing helpful data about pulmonary function and toxicities exacerbated by radiation. The trial should provide a foundation for the design of future studies to move checkpoint blockade agents into the first-line setting and for determining whether twice-daily rt in combination with checkpoint inhibition is a manageable strategy.
Shaverdian et al.27 analyzed the effects of pembrolizumab and rt. Their data suggest that, in patients with advanced nsclc, pembrolizumab treatment results in longer progression-free survival and os when combined with prior rt than when rt has not been given, with an acceptable safety profile.
Another phase i/ii trial enrolled men with metastatic castration-resistant prostate cancer and escalated the dose of ipilimumab, with or without concurrent single-fraction (8 Gy) rt targeting an osseous metastasis28. That approach was further evaluated in a subsequent multi-institutional phase iii trial (NCT00861614 at http://ClinicalTrials.gov/), in which 799 patients diagnosed with metastatic castration-resistant prostate cancer were treated with single-fraction (8 Gy) rt to an osseous metastasis and randomized to ipilimumab or placebo29. The primary endpoint of improved os was not achieved, although the p value was close to the cut-off for significance (p= 0.0530). On subset analysis, patients with non-visceral metastatic disease treated with ipilimumab combined with rt experienced an incremental improvement in os, suggesting that non-visceral metastases are a more appropriate target for rt in combination with ipilimumab. Furthermore, the study demonstrated that the combination of rt and ipilimumab was well-tolerated overall, with minimal added toxicity. Some ongoing studies are focusing on combined rt and immunotherapy (Table i).
TABLE I.
Partial list of ongoing studies30
| ClinicalTrials.gov identifier | Study name | Phase |
|---|---|---|
| NCT02710253 | Salvage Radiation Therapy in Treating Patients with Metastatic Cancer That Has Progressed After Systemic Immunotherapy | II |
| NCT02400814 | Atezolizumab and Stereotactic Ablative Radiotherapy in Treating Patients with Stage IV Non-Small-Cell Lung Cancer | I |
| NCT02843165 | Checkpoint Blockade Immunotherapy with or without Stereotactic Body Radiation Therapy in Treating Patients with Primary or Metastatic Tumor | II |
| NCT01970527 | Phase II Trial of Stereotactic Body Radiotherapy Followed by Ipilimumab in Treating Patients with Stage IV Melanoma | II |
| NCT02696993 | Nivolumab and Radiation Therapy with or without Ipilimumab in Treating Patients with Brain Metastases from Non-Small-Cell Lung Cancer | I/II |
| NCT01436968 | Phase 3 Study of ProstAtak Immunotherapy with Standard Radiation Therapy for Localized Prostate Cancer | III |
| NCT02239900 | Ipilimumab and Hypofractionated Stereotactic Body Radiation Therapy in Treating Patients with Advanced Solid Malignancies | I/II |
| NCT02444741 | Pembrolizumab and Stereotactic Body Radiation Therapy or Non-stereotactic Wide-Field Radiation Therapy in Treating Patients with Non-Small-Cell Lung Cancer | I/II |
| NCT03162731 | Nivolumab, Ipilimumab, and Radiation Therapy in Treating Patients with Stage IVA-B Head and Neck Cancer | |
| NCT02437071 | Pembrolizumab with Radiation Therapy or Ablation Therapy in Treating Patients with Metastatic Colorectal Cancer | II |
| NCT03122496 | Durvalumab, Tremelimumab, and Stereotactic Body Radiation Therapy in Treating Patients with Metastatic Anaplastic Thyroid Cancer | I |
| NCT02830594 | Pembrolizumab and Palliative Radiation Therapy in Treating Patients with Metastatic Esophagus, Stomach, or Gastroesophageal Junction Cancer | II |
| NCT02829931 | Hypofractionated Stereotactic Radiation Therapy and Nivolumab in Treating Patients with Recurrent High Grade Glioma | I |
| NCT02781506 | Nivolumab with or without Stereotactic Ablative Radiation Therapy in Treating Patients with Metastatic Kidney Cancer | II |
| NCT01996202 | Ipilimumab and Radiation Therapy in Treating Patients with High-Risk of Recurrence or Locally Advanced Melanoma | |
| NCT02959463 | Pembrolizumab after Radiation Therapy in Treating Patients with Pleural Malignant Mesothelioma | I |
| NCT03051672 | Pembrolizumab and Palliative Radiation Therapy in Treating Patients with Metastatic Invasive Breast Cancer | II |
| NCT02303990 | Pembrolizumab and Hypofractionated Radiation Therapy in Treating Patients with Advanced or Metastatic Cancer | I |
Abscopal Effect
The abscopal effect is a rare phenomenon in rt, leading to impressive tumour regression outside the rt field. The phenomenon is described in the treatment of metastatic cancer, where localized treatment of a tumour causes not only shrinking of the treated tumour, but also shrinking of tumours outside the scope of the localized treatment. Mole’s proposed term, “abscopal,” refers to radiation effects “at a distance from the irradiated volume but within the same organism”5. Initially associated with single-tumour localized rt, the term has also come to encompass other types of localized treatments, such as electroporation and intratumour injection of therapeutics. Although the phenomenon is extremely rare, its effect on the tumour can be stunning, leading to the disappearance of malignant growths throughout the entire body.
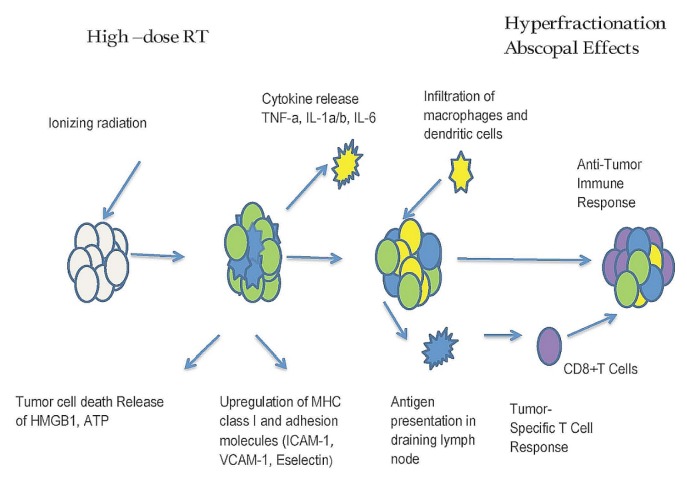
Scientists are not certain how the abscopal effect works to eliminate cancer in patients. Studies in mice suggest that the effect might depend on activation of the immune system. Postow et al.31 reported changes measured in the immune system of a patient with metastatic melanoma over the course of treatment. Their findings support the idea that localized treatment might broadly stimulate the immune system to fight cancer. Currently, various cells of the immune system, including T cells and dendritic cells, are believed to play a primary role. Figure 1 presents the proposed mechanism.
FIGURE 1.
Proposed mechanism of the abscopal effect. RT = radiation therapy; TNF-a = tumour necrosis factor α; IL-1a/b = interleukins 1α and 1β; IL-6 = interleukin 6; HMGB1 = high mobility group box 1 protein; ATP = adenosine triphosphate; MHC = major histocompatibility complex; ICAM = intercellular adhesion molecule; VCAM = vascular cell adhesion protein.
A review by Hu et al.32 of 23 clinical cases of the abscopal effect after rt alone noted that reported cases occurred mostly in immunogenic tumours, such as renal cell carcinoma, melanoma, and hepatocellular carcinoma. However, with the continued development and use of immunotherapy strategies incorporating combinations of targeted immunomodulators and immune checkpoint blockade with rt, the abscopal effect is becoming increasingly relevant in less immunogenic tumours such as breast cancer.
The abscopal effect is believed to arise from the capability of local rt to elicit systemic immune effects that control the nonirradiated tumour burden. In the tumour microenvironment, rt acts as an immune modulator through several mechanisms. Localized rt induces cell death and release of immunogenic factors via a process called “immunogenic cell death,” which subsequently triggers the release of a number of endogenous damage-associated molecular patterns. Those molecular patterns—which include calreticulin, hmgb1 (high-mobility group box 1 protein), and atp (adenosine triphosphate)—contribute to the priming of the immune system by triggering dendritic cells (dcs), resulting in improved antigen presentation to T cells30,33. Specifically, during immunogenic cell death, dying cells translocate calreticulin to the cell surface and are processed by calregulin, facilitating tumour antigen presentation and cytotoxic T-lymphocyte stimulation34. The release of hmgb1 acts as a pro-inflammatory mediator, stimulating monocyte production of the cytokines tumour necrosis factor α and interleukins 1, 6, and 835. In addition, hmgb1 improves tumour antigen presentation by binding to the toll-like receptor 4 on calregulin and preventing the accelerated degradation of antigens within calregulin36,37. Released atp binds to the purine receptors on calregulin, leading to inflammatory-like activation and interleukin 1β release38. The dna released from dying cells can also activate the sting (stimulator of interferon gene) pathway in calregulin, initiating type i interferon production and enhancing dc cross-priming39.
Moreover, rt has been shown to stimulate tumour-cell release of chemokines cxcl16 and cxcl10, to increase the expression of adhesion molecules E-selectin and intercellular adhesion molecule 1 in endothelial cells, and to upregulate major histocompatibility complex 1, Fas, intercellular adhesion molecule 1, and nkg2d ligands40–42. Lastly, when combined with adoptive therapy, rt might render tumours accessible to infiltration and help to normalize the vasculature in the tumour microenvironment43. Low-dose radiation has also been reported to recruit nos2-expressing macrophages to the tumours, subsequently enhancing T-cell infiltration and normalizing the tumour vasculature44.
Kotter et al.45 demonstrated that combining rt with further immune stimulation, such as hyperthermia, induces immune-mediated antitumour responses. The release of Hsp70 (heat shock protein 70) was increased by hyperthermia. Apoptosis induction and release of danger signals were both dependent on caspase-3; it could be that hyperthermia enhances the abscopal effect.
Using an oligonucleotide aptamer platform, radiation-induced vascular endothelial growth factor–targeted 4-1BB co-stimulation was shown to potentiate both local tumour control and abscopal responses with equal or greater efficiency than could 4-1BB, ctla-4, or PD-1 antibodies alone. Although 4-1BB and ctla-4 antibodies elicited organ-wide inflammatory responses and tissue damage, vascular endothelial growth factor–targeted 4-1BB co-stimulation produced no observable toxicity. Those findings suggest that radiation-induced tumour-targeted immunotherapy can improve the therapeutic index and extend the reach of immunomodulatory agents46.
The study of Habets et al.47 of tumour-bearing Balb/C mice treated with the dc-stimulating growth factor Flt3L failed to demonstrate either modulation or composition of the humoral immune response. Furthermore, the study used flow cytometry to evaluate the immune infiltrate and immunoglobulin isotype content of tumour tissue, finding no differences between the treatment groups that were indicative of local antibody production. Interestingly, the 67NR mammary carcinoma in Balb/C mice was demonstrated to be associated with a pre-existing antibody response, and in tumour-bearing Balb/C mice with abscopal tumour regression, such pre-existing antibody responses were observed not to be altered upon fractionated rt or dc stimulation with Flt3L (or both). This research indicates that evaluating the humoral immune response in the setting of abscopal tumour regression is not invariably associated with therapeutic effects47.
Saba et al.48 published the first report of an abscopal effect against multiple myeloma. In a female patient who originally presented with advanced multiple myeloma in 1996 at the age of 50, multiple chemotherapeutic regimens, including high-dose melphalan with autologous stem-cell transplantation, had failed. After receiving palliative rt to a symptomatic gastric plasmacytoma, this patient achieved sustained complete remission. She has remained in remission for more than 15 years.
CURRENT AND FUTURE PERSPECTIVES
The current scientific evidence indicates that conventional rt affects the immunologic profile of tumours in a particular manner which, in turn, might induce beneficial effects at both the local and systemic levels (that is, an abscopal effect). The interaction between rt and the immune system is being explored with the aim of combining immune and radiation (including particle) treatments that might, in many cases, have a greater clinical effect than any of the therapies alone.
Radiotherapy is an integral part of cancer treatment. The immune-activating properties of (especially) hypofractionated irradiation are, in addition to its well-known effects on the cell cycle and the clonogenic potential of tumour cells, in the spotlight of clinicians. Combining rt with further immune stimulation induces immune-mediated antitumour responses.
Understanding the immune mechanisms associated with tumour establishment and the ways in which rt affects inflammation and immunity has led to the rise of novel treatment strategies. Several preclinical and clinical studies support the use of rt in combination with immunotherapy, obtaining better local and systemic tumour control. Studies that are currently ongoing will provide information about the optimal rt approach, but the development of reliable predictors of response from preclinical and early phases of clinical studies is necessary to avoid discarding treatment strategies with significant clinical benefit49.
Newly available immune checkpoint blockers50, capable of reverting tumour immune tolerance, are revolutionizing the anticancer armamentarium. Recent evidence also established that ionizing radiation could produce antitumour immune responses and might also synergize with immune checkpoint blockers. Multiple radioimmunotherapy combinations are currently being assessed in early clinical trials. Past examples have highlighted the need for treatment personalization, and there are unmet needs for immunologic biomarkers to be deciphered, potentially allowing for the selection of patients who could benefit from these promising, but expensive, combinations. Recent studies have identified immune assays that are potentially predictive and prognostic at the cellular (tumour microenvironment composition), genomic (mutation or neoantigen load), and peripheral blood levels. Here, we have collected the available evidence about personalized immune biomarker–directed rt strategies that could potentially be used for patient selection in the era of radioimmunotherapy50.
Preclinical in vivo studies using small animals are considered crucial in translational cancer research and clinical implementation of novel treatments51. Such studies are of paramount relevance in radiobiology, especially for any technological developments connected to delivering high doses in single- or oligo-fractionated regimens, such as stereotactic ablative radiotherapy. In that context, clinical success in cancer treatment has to be guaranteed, sparing normal tissue and preventing the spread of disease or local recurrence. Marconi et al.51 introduced a new dose–response relationship based on publications relevant to preclinical models of delivered dose, fractionation schedule, biologic effects on non-irradiated tissues, and the abscopal effect. In particular, using a log-likelihood method, the team evaluated whether the occurrence of abscopal effects might be related to the biologically effective dose. To that end, the present review considered studies involving various tumour histotypes, including fibrosarcoma, melanoma, and cancers of the breast, colon, lung, pancreas, and head and neck. For all tumours, the α/β ratio was assumed to be 10 Gy, as generally adopted for neoplastic cells51.
The influence of dose fractionation and timing, particularly with respect to immune activation, has not yet been satisfactorily investigated. In the present review, we summarized current concepts of modern rt and evaluated the potential of rt for immune activation. Focus was placed on radiation-induced forms of tumour cell death and the immunogenicity of tumour cells. The non-targeted abscopal effect can contribute to the antitumour response in a specific and systemic manner and has the ability to target relapsing tumour cells as well as metastases.
Although a beneficial outcome seems to be associated with stereotactic ablative body rt compared with classical rt fractionation in preclinical animal models, in vitro model systems suggest an advantage for classical fractionated rt in immune activation. Furthermore, the optimal approach might vary depending on the tumour site or genetic signature, or both. Those observations highlight the urgent need for clinical trials that will identify whether high-dose rt is superior to classical fractionated rt in terms of antitumour immune response—and, particularly, outcomes—when rt is combined with immunotherapy in selected tumour entities52.
The combination of rt and various checkpoint blockade agents has been demonstrated in vitro and in vivo in various clinical studies18–23,49. The possibility of combining an abscopal effect from hyperfractionated rt with the enhanced effect of immune treatment with checkpoint blockade is a very promising approach to treating tumours in the future.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.McBride WH, Withers HR. Biologic basis of radiation therapy. In: Perez CA, Brady LW, editors. Principles and Practice of Radiation Oncology. Philadelphia, PA: Lippincott Williams and Wilkins; 2004. pp. 96–136. [Google Scholar]
- 2.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–26. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med. 2013;5:173sr2. doi: 10.1126/scitranslmed.3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–41. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 6.Siva S, Macmanus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Bramhall RJ, Mahady K, Peach AHS. Spontaneous regression of metastatic melanoma—clinical evidence of the abscopal effect. Eur J Surg Oncol. 2014;40:34–41. doi: 10.1016/j.ejso.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–72. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuma K, Yamashita H, Niibe Y, Hayakawa K, Nakagawa K. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J Med Case Rep. 2011;5:111. doi: 10.1186/1752-1947-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45:493–7. doi: 10.1080/02841860600604611. [DOI] [PubMed] [Google Scholar]
- 11.Lakshmanagowda PB, Viswanath L, Thimmaiah N, Dasappa L, Supe SS, Kallur P. Abscopal effect in a patient with chronic lymphocytic leukemia during radiation therapy: a case report. Cases J. 2009;2:204. doi: 10.1186/1757-1626-2-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden EB, Apetoh L. Radiotherapy and immunogenic cell death. Semin Radiat Oncol. 2015;25:11–17. doi: 10.1016/j.semradonc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 14.Sansom DM. CD28, ctla-4 and their ligands: who does what and to whom? Immunology. 2000;101:169–77. doi: 10.1046/j.1365-2567.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert C, Schachter J, Long GV, et al. on behalf of the keynote-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 17.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett A. Total body irradiation (tbi) before bone marrow transplantation in leukaemia: a co-operative study from the European Group for Bone Marrow Transplantation. Br J Radiol. 1982;55:562–7. doi: 10.1259/0007-1285-55-656-562. [DOI] [PubMed] [Google Scholar]
- 19.Deeg HJ, Sullivan KM, Buckner CD, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission: toxicity and long-term follow-up of patients conditioned with single dose or fractionated total body irradiation. Bone Marrow Transplant. 1986;1:151–7. [PubMed] [Google Scholar]
- 20.Gangadhar TC, Salama AK. Clinical applications of PD-1–based therapy: a focus on pembrolizumab (MK-3475) in the management of melanoma and other tumor types. Onco Targets Ther. 2015;8:929–37. doi: 10.2147/OTT.S53164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng L, Liang H, Burnette B, et al. Irradiation and anti–PD-L1 treatment synergistically promote antitumour immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen specific PD-1–mediated antitumour immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3:345–55. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo T, Kim IA. Radiotherapy and immune checkpoint blockades: a snapshot in 2016. Radiat Oncol J. 2016;34:250–9. doi: 10.3857/roj.2016.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azad A, Yin Lim S, D’Costa Z, et al. PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol Med. 2017;9:167–80. doi: 10.15252/emmm.201606674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and ctla-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 26.Koller KM, Mackley HB, Liu J, et al. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol Ther. 2017;18:36–42. doi: 10.1080/15384047.2016.1264543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the keynote-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase i/ii study. Ann Oncol. 2013;24:1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon ED, Drake CG, Scher HI, et al. on behalf of the CA184-043 investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–25. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu ZI, McArthur HL, Ho AY. The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr Breast Cancer Rep. 2017;9:45–51. doi: 10.1007/s12609-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–16. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 35.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (hmg-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between hmgb1 and tlr4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 37.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 38.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the nlrp3 inflammasome in dendritic cells induces il-1β–dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 39.Deng L, Liang H, Xu M, et al. sting-dependent cytosolic dna sensing promotes radiation-induced type i interferon-dependent antitumour immunity in immunogenic tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances ctl lytic activity and ctl adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 41.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 42.Ruocco MG, Pilones KA, Kawashima N, et al. Suppressing T cell motility induced by anti-ctla-4 monotherapy improves antitumour effects. J Clin Invest. 2012;122:3718–30. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–70. [PubMed] [Google Scholar]
- 44.Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an inos+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Kotter B, Frey B, Winderl M, et al. The in vitro immunogenic potential of caspase-3 proficient breast cancer cells with basal low immunogenicity is increased by hypofractionated irradiation. Radiat Oncol. 2015;10:197. doi: 10.1186/s13014-015-0506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrand B, Verma B, Levay A, et al. Radiation-induced enhancement of antitumour T-cell immunity by vegf-targeted 4–1BB costimulation. Cancer Res. 2017;77:1310–21. doi: 10.1158/0008-5472.CAN-16-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habets TH, Oth T, Houben AW, et al. Fractionated radiotherapy with 3×8 Gy induces systemic anti-tumour responses and abscopal tumour inhibition without modulating the humoral anti-tumour response. PLoS One. 2016;11:e0159515. doi: 10.1371/journal.pone.0159515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saba R, Saleem N, Peace D. Long-term survival consequent on the abscopal effect in a patient with multiple myeloma. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-215237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.United States, Department of Health and Human Services, National Institutes of Health (nih) Clinical Trials [Web page] Bethesda, MD: NIH; 2015. [Available at: https://www.nih.gov/research-training/clinical-trials; cited 2 January 2018] [Google Scholar]
- 50.Levy A, Nigro G, Sansonetti PJ, Deutsch E. Candidate immune biomarkers for radioimmunotherapy. Biochim Biophys Acta. 2017;1868:58–68. doi: 10.1016/j.bbcan.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Marconi R, Strolin S, Bossi G, Stigari L. A meta-analysis of the abscopal effect in preclinical models: Is the biologically effective dose a relevant physical trigger? PLoS One. 2017;12:e0171559. doi: 10.1371/journal.pone.0171559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deloch L, Derer A, Hartmann J, Frey B, Fietkau R, Gaipl US. Modern radiotherapy concepts and the impact of radiation on immune activation. Front Oncol. 2016;6:141. doi: 10.3389/fonc.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]