Abstract
Objective
We compared failure patterns and survival after elective nodal irradiation (eni) or involved-field irradiation (ifi) in patients with thoracic esophageal squamous cell carcinoma (escc), clinical stage T2–4N0M0, to determine whether ifi is feasible for such patients.
Methods
Between 2005 and 2015, 126 patients with clinical stage T2–4N0M0 thoracic escc who received definitive concurrent chemoradiotherapy in Shandong Cancer Hospital and Institute and who had complete data, were analyzed retrospectively. Of those patients, 49 received ifi, and 77 received eni. In the ifi group, the radiation field included the primary tumour, with a 3-cm to 4-cm margin in the craniocaudal direction, and the elective irradiation was delivered to the adjacent regional lymphatics according to the location of the primary tumour. Patterns of failure were classified using the first site of failure, which included primary tumour failure, regional lymph node failure, and distant metastasis.
Results
Median progression-free survival was 20 months [95% confidence interval (ci): 7.87 months to 39.2 months] in the ifi group and 30 months (95% ci: 17.4 months to 44.6 months) in the eni group (p = 0.580). Median overall survival (os) was 36 months (95% ci: 21.9 months to 50.1 months) in the ifi group and 38 months (95% ci: 26.1 months to 49.9 months) in the eni group (p = 0.761). The estimated 1-year, 3-year, and 5-year os rates were, respectively, 87.8%, 49.4%, and 32.3% for the ifi patients and 92.2%, 52.0%, and 28.9% for the eni patients. Disease persistence and primary lesion recurrence after complete remission (cr) were the most frequent causes of treatment failure in the patients overall (83 of 124, 66.9%). Of the 66 patients achieving a clinical cr, 25 experienced recurrence of the primary lesion, 12 experienced distant relapse, 10 experienced regional nodal failure, and 2 experienced an isolated recurrence. No significant differences in the pattern of failure or in the incidences of grade 3 or greater treatment-related myelosuppression or esophagitis were found between the ifi and eni groups.
Conclusions
In patients with thoracic escc clinical stage T2–4N0M0 receiving definitive chemoradiotherapy, failure patterns and os were similar with either eni or ifi. Large prospective randomized studies are needed to further investigate and verify those results in this subgroup of patients.
Keywords: Esophageal squamous cell carcinoma, radiotherapy, elective nodal irradiation, involved-field irradiation
BACKGROUND
Radiation has an important role in the treatment of esophageal carcinoma in both the inoperable and pre-operative settings. The results of the Radiation Therapy Oncology Group 8501 trial and a few later trials1 showed that chemoradiotherapy (crt) achieves better local control and overall survival (os) than does radiotherapy alone. Although esophagectomy with neoadjuvant therapy has been the first choice of treatment for patients with stages ii–iii esophageal carcinoma, a few studies have reported that, for patients with resectable thoracic esophageal squamous cell carcinoma (escc), treatment outcomes after definitive crt or after surgery are comparable2.
Lymph node metastasis—and particularly regional lymph node involvement, which is an early process—is common in esophageal carcinoma. Delivery of a radiation dose to the uninvolved regional lymph node area at risk for microscopic disease is known as elective nodal irradiation (eni). Despite the high risk of nodal spread in esophageal carcinoma, the benefit of additional eni is controversial, especially with respect to os. On the other hand, the large radiation volume can increase acute toxicity and the late adverse events that involve the heart, lungs, and hematologic system3. Earlier studies by our group and others have shown that using 3-dimensional conformal radiotherapy without intentional eni is associated with a rate of isolated out-of-field failure of only 2%–13% in patients with escc4–7.
For patients with clinical stage N0 escc, the radiation volume should be dramatically decreased if involved-field irradiation (ifi) is being used. But surgical data suggest that locoregional nodal positivity is close to 31%–56% for T1b tumours, 58%–78% for T2 tumours, 74%–81% for T3 tumours, and 83%–100% for T4 tumours in patients with escc 8,9.The feasibility of ifi could therefore be challenged in that subgroup patients. In the present study, we retrospectively analyzed the treatment results and patterns of failure associated with ifi and with eni in patients with clinical stage T2–4N0M0 thoracic escc.
METHODS
The medical records of patients with esophageal cancer attending the Shandong Cancer Hospital and Institute between January 2005 and June 2015 were retrospectively reviewed. Only patients who had clinical N0 escc and who received definitive crt were included in the present analysis. All disease had been confirmed by biopsy and had not been treated. Staging examinations in every patient included esophagography, endoscopic ultrasonography (eus), and computed tomography (ct) imaging; some patients also underwent positron-emission tomography (pet) as part of pet – ct fusion imaging. Patients were excluded if they had clinically positive lymph nodes or distant metastasis, a history of any other malignant tumour, or fistulas before treatment. The institutional review board of the Shandong Cancer Hospital and Institute approved the study.
Staging
The T stage was determined using ct imaging and eus10,11. Stage T1–3 tumours are confined to the esophagus; T4 tumours extend beyond the esophageal wall into adjacent structures. For the present study, lesions staged as T1 by eus were excluded because of the small number of patients. A limitation of eus is esophageal stenosis, and in our hospital, determination of T stage used mainly the ct criteria: T2, mass enhancement, with an irregular margin and muscle layer preservation; T3, mass enhancement with disruption of the muscle layer; and T4, bulging of the esophageal tumour and obliteration of the fat layer between the esophageal tumour and the adjacent mediastinal organs. Aortic invasion was suggested if 90 degrees or more of the aorta was in contact with the tumour or if obliteration of the triangular fat space between the esophagus, aorta, and spine adjacent to the primary tumour was evident. Bronchoscopy was performed in some cases when tracheobronchial involvement was suspected.
The primary criterion for nodal involvement was size on ct imaging, with “normal size” being defined as less than 5 mm for supraclavicular nodes, less than 10 mm in the short axis for mediastinal nodes, 5 mm for retrocrural nodes, and 5 mm for left gastric nodes. Nodes with high uptake of fluorodeoxyglucose on pet images were also considered to be metastatic. Other criteria used to determine the presence of nodal metastasis included an enhancement pattern and the presence of extranodal tumour extensions.
Radiotherapy
All radiation treatments were delivered as 3-dimensional conformal radiotherapy or intensity-modulated radiation therapy with standard fractionation (1.8 Gy or 2.0 Gy fractions administered once daily, 5 days each week). Treatment plans were generated using a 3-dimensional planning system (adac Pinnacle 3, version 5.0: Philips Healthcare, Amsterdam, Netherlands).
For each patient, the primary tumour constituted the gross target volume (gtv), which was visualized using ct imaging, esophagography, and endoscopic extension. For ifi, the gtv used in the present study included a field containing the primary tumour, plus a 3.0 cm margin superior and inferior and a 0.8–1.0 cm radial margin [the esophageal clinical target volume (ctv)]. The planning target volume (ptv) was defined as the esophageal ctv plus a 0.5–1.0 cm margin. In patients receiving ifi, a definitive radiation dose totalling 50–64 Gy was delivered to the ptv. In patients receiving eni, the nodal ctv included the adjacent regional lymphatics depending on the location of the primary tumour. The ptv was defined as the esophageal ctv plus nodal ctv plus a margin of 0.5–1.0 cm. After a prophylactic radiation dose (40–50 Gy; median: 41.4 Gy), a booster dose of 10–20 Gy was delivered to the primary tumour only.
Chemotherapy
Only patients who received concurrent crt were included. The chemotherapy could be either monotherapy or a two-drug regimen, and adjuvant chemotherapy was permitted. The chemotherapeutic agents included 5-fluorouracil–cisplatin, docetaxel–cisplatin, paclitaxel–carboplatin, and single-agent paclitaxel, cisplatin, or S-1.
Treatment-Related Toxicity
Radiation treatment–related acute and late toxicities were reviewed and scored according to Radiation Therapy Oncology Group criteria. Major treatment toxicities included myelosuppression and esophagitis.
Result Assessment and Follow-Up
After completion of treatment, patients were reviewed in physical examination within 4–6 weeks, and then every 3 months for the first 2 years, every 6 months during years 3–5, and once annually thereafter. Measurement of serum tumour markers included carcinoembryonic antigen, squamous cell carcinoma–related antigen, and cytokeratin 19 fragment. Other studies routinely performed were ct imaging of neck, thorax, and upper abdomen; esophagography; esophagoscopy; and pet – ct fusion imaging when a recurrence was suspected after the other examinations.
Clinical response was evaluated by esophagography, esophagoscopy, and ct imaging11. For esophageal lesions, a cr was defined as no dysphagia, a normal esophagogram, and disappearance of all visible tumours, including ulceration, for at least 4 weeks, confirmed by normal endoscopic biopsy samples. Partial remission was defined as an improvement in dysphagia, a greater than 50% reduction in intra-esophageal tumour extension as assessed by esophagography, and a greater than 50% reduction in the area of the primary tumour as observed on esophagography. Progressive disease was defined as a greater than 25% increase in the area of the tumour.
Patterns of failure, defined using the first site of failure, were “local recurrence” (primary tumour), “regional failure” (regional lymph nodes), and “distant metastasis.” Patients who had an initial radiographic response to treatment and a stable mass at each follow-up visit were considered to have achieved local control; patients were considered to be experiencing local failure only if histologic or cytologic evidence was observed in the primary tumour. Lymph node metastases were diagnosed based on the appearance of new nodes in regions where no enlarged nodes had been identified before irradiation. Suspected supraclavicular node recurrences were confirmed by fine-needle aspiration biopsy. Elective nodal failure was defined as lymph node recurrence at the regional level. Nodal metastases outside the regional level were considered distant failures.
Statistical Analyses
The os duration was calculated from the first day of irradiation. If death had not already occurred, patient survival was censored at the last follow-up visit. Patterns of failure and treatment-related toxicities were also evaluated. Continuous variables are summarized using descriptive statistics such as means with standard deviation and medians with range. Categorical variables are expressed using frequencies and percentages. The Kaplan–Meier method was applied to estimate survival probabilities. The SPSS Statistics software application (version 17.0: SPSS, Chicago, IL, U.S.A.) was used for data analysis. The level of significance was set at p < 0.05.
RESULTS
Patient Characteristics
Table i lists clinical and treatment characteristics for the study groups. Patients in both groups had similar characteristics with respect to sex, clinical T stage, tumour location, and tumour size. Median age was 67 years (range: 43–76 years) in the ifi group and 64 years (range: 39–75 years) in the eni group. The mean radiation ptv was greater in patients receiving eni than in those receiving ifi (417 ± 126 mL vs. 171.6 ± 53 mL, p = 0.000).
TABLE I.
Characteristics of the study patients
| Characteristic | Irradiation type | p Value | |
|---|---|---|---|
|
| |||
| Involved-field | Elective nodal | ||
| Patients (n) | 49 | 77 | |
|
| |||
| Sex (n) | 0.904 | ||
| Men | 38 | 59 | |
| Women | 11 | 18 | |
|
| |||
| Age (years) | |||
| Median | 67 | 64 | |
| Range | 43–76 | 39–75 | |
|
| |||
| Clinical T stage (n) | 0.733 | ||
| T2–3 | 31 | 51 | |
| T4 | 18 | 26 | |
|
| |||
| Primary tumour location (n) | 0.846 | ||
| Upper thoracic | 27 | 39 | |
| Mid-thoracic | 14 | 28 | |
| Lower thoracic | 8 | 10 | |
|
| |||
| Tumour length (cm) | |||
| Median | 6.0 | 6.5 | |
| Range | 2.0–12 | 1.5–11 | |
|
| |||
| Radiation | |||
| PTV (mL) | 171.6±53 | 417±126 | 0.000 |
| Dose (Gy) | |||
| Median | 60 | 59.4 | |
| Range | 50.4–66 | 50.4–64 | |
|
| |||
| Salvage surgery (n) | 4 | 5 | |
PTV = planning target volume.
Acute Toxicities of CRT
Massive esophageal bleeding led to 2 treatment-related deaths (1.6%): 1 at 1.0 months after the start of definitive crt in the ifi group and 1 at 1.5 months in the eni group. The most commonly reported acute toxicities during concurrent crt in the patients overall were myelosuppression and esophagitis (Table ii).
TABLE II.
Acute toxicities of chemoradiotherapy (grade 3 or greater)
| Toxicity | Irradiation type | p Value | |||
|---|---|---|---|---|---|
|
| |||||
| Elective nodal | Involved-field | ||||
|
|
|
||||
| (n) | (%) | (n) | (%) | ||
| Leucocytopenia | 45 | 58.4 | 22 | 44.9 | 0.137 |
|
| |||||
| Anemia | 19 | 24.7 | 13 | 26.5 | 0.816 |
|
| |||||
| Thrombocytopenia | 21 | 27.3 | 11 | 22.4 | 0.544 |
|
| |||||
| Esophagitis | 12 | 15.6 | 6 | 12.2 | 0.602 |
|
| |||||
| Treatment-related death | 1 | 1.3 | 1 | 2.0 | 0.628 |
In the eni group, the worst hematologic toxicities during the treatment period were leucocytopenia of grade 3 or greater (n = 45, 58.4%), anemia (n = 19, 24.7%), and thrombocytopenia (n = 21, 27.3%). The worst nonhematologic toxicity was acute radiation esophagitis of grade 3 or greater (n = 12, 15.6%). In the ifi group, grade 3 toxicities of leucocytopenia, anemia, and thrombocytopenia occurred in 44.9%, 26.5%, and 22.4% of patients respectively. Acute radiation esophagitis of grade 3 or greater was seen in 6 patients (12.2%). No significant differences were found between the ifi and eni groups with respect to grade 3 or greater acute toxicities. No severe late effects (grade 3 or greater) involving the esophagus, lungs, skin, or spinal cord were seen.
Treatment Response
After excluding the 2 patients who died of treatment-related causes, 66 of the remaining 124 patients achieved a clinical cr (53.2%), and 58 (46.8%) experienced persistent disease with partial remission or stable disease. A cr was seen in 50% of patients in the ifi group, and in 55.2% of the eni group (p = 0.567). The clinical cr rate was higher in the patients staged T2–3 than in those staged T4 (63% vs. 34.9%, p = 0.003, Table iii).
TABLE III.
Response rates
| Response type | Overall | Irradiation type | p Value | T Stage | p Value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Involved-field | Elective nodal | T2–3 | T4 | ||||
| All (n) | 124 | 48 | 76 | 81 | 43 | ||
|
| |||||||
| Complete [n (%)] | 66 (53.2) | 24 (50) | 42 (55.2) | 0.567 | 51 (63.0) | 15 (34.9) | 0.003 |
|
| |||||||
| Non-complete [n (%)] | 58 (46.8) | 24 (50) | 34 (44.7) | 30 (37.0) | 28 (65.1) | ||
Patterns of Failure
Patients were followed until death or 96 months posttreatment (median: 48 months) for those still alive at the last follow-up contact in December 2015. Failure occurred in most of the patients who survived treatment (101 of 124), including 7 who were found to have second primary tumours (4 at a different esophageal site; 1 in the stomach; 1 in the hypopharynx; 1 in the lung). The most frequent causes of treatment failure in the overall cohort were disease persistence (58 patients with non-cr) and primary lesion recurrence after cr (25 of 66 patients), for an overall failure rate of 66.9% (83 of 124 patients).
Among the 66 patients achieving a clinical cr, distant relapse occurred in 12 (18.2%), including 9 experiencing distant recurrence only (13.6%). Regional nodal failure occurred in 10 patients (15.2%), only 2 of whom (3.0%) experienced isolated failures; the other 8 patients also experienced primary tumour or distant recurrence. Regional failure most commonly occurred in the upper mediastinum and supraclavicular areas (7 patients). The sites of distant metastasis were lungs (n= 4), retroperitoneal lymph nodes (n= 2), bone ( n= 1), liver ( n= 2), pleura ( n= 1), and multiple organs (n = 2). No significant difference was found between the ifi group and the eni group with respect to any pattern of failure (Table iv).
TABLE IV.
Failure patterns after complete response
| Variable | Irradiation type | p Value | |
|---|---|---|---|
|
| |||
| Involved-field | Elective nodal | ||
| Complete response | 24 | 42 | |
|
| |||
| Living | 10 | 18 | 0.925 |
|
| |||
| No evidence of disease | |||
| Living | 7 | 12 | 0.959 |
| Died | 2 | 2 | 0.462 |
|
| |||
| Site | |||
| Any | 15 | 28 | 0.733 |
| Primary alone | 6 | 11 | 0.915 |
| Regional alone | 1 | 1 | 0.599 |
| Distant alone | 3 | 6 | 0.839 |
| Primary + regional + distant | 1 | 2 | 0.702 |
| Primary + regional | 2 | 3 | 0.604 |
| Second primary tumour | 2 | 5 | 0.498 |
Survival
At the time of analysis, 28 patients remained alive, including 19 with no evidence of disease. The estimated median os duration was 38.0 months (95% ci: 30.1 months to 45.9 months). The estimated 1-year, 3-year, and 5-year os rates were, respectively, 90.5%, 51.0%, and 30.2%. The estimated progression-free survival (pfs) duration was 30.0 months (95% ci: 16.9 months to 30.1 months). The estimated 1-year, 3-year, and 5-year pfs rates were, respectively, 68.1%, 44.1%, and 26.1%.
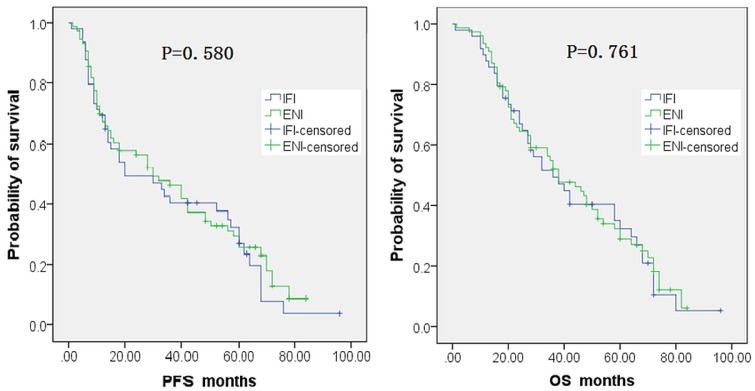
Median pfs was 20 months (95% ci: 7.87 months to 39.2 months) in the ifi group and 30 months (95% ci: 17.4 months to 44.6 months) in the eni group, a difference that was not statistically significant (p = 0.580, Kaplan–Meier method, Figure 1). Median os was 36 months (95% ci: 21.9 months to 50.1 months) in the ifi group and 38 months (95% ci: 26.1 months to months 49.9) in the eni group, a difference that was also not statistically significant (p = 0.761, Figure 1). The estimated 1-year, 3-year, and 5-year os rates were, respectively, 87.8%, 49.4%, and 32.3% in the ifi group and 92.2%, 52.0%, and 28.9% in the eni group.
FIGURE 1.
Progression-free (PFS) and overall survival (OS) curves for patients receiving involved-field (IFI) or elective nodal irradiation (ENI).
DISCUSSION
Definitive crt is a standard of care for resectable thoracic escc, but whether to use eni or ifi is still controversial. Finding the optimal radiotherapeutic target volume is very important to improve the therapeutic effect and reduce treatment toxicity.
In the present study, survival in the two groups was comparable to that achieved in other reports. Trials for local or advanced esophageal carcinoma treated with crt using eni reported os rates of 41%–88% at 1 year, 28%–63% at 2 years, 19%–48% at 3 years, and up to 26% at 5 years; median survival duration was 9.0–33 months depending on the disease stage12. In a study of ifi, Yamashita et al.13 reported 3-year pfs and os rates of 47.7% and 51.1% respectively, with median pfs and os durations of 34.6 months and 38.4 months respectively.
Although esophageal carcinoma is characterized by a high rate of pathologic nodal involvement in its early stage, our results showed that regional lymph node failure is not common after definitive crt in patients with clinical N0 escc receiving either eni or ifi. And we observed no significant difference in failure patterns and survival rates between our ifi and eni groups. In our cohort, 53.2% of the patients overall achieved a clinical cr, and 46.8% showed persistent disease (partial remission or stable disease). Of ifi and eni patients, 48.9% and 54.5% respectively achieved a cr. The cr rate was higher in the patients staged T2–3 than in those staged T4 (63% vs. 34.1%). Those results are consistent findings from other reports12. Our patients had no clinical lymph node metastasis, but a few studies have reported that the gtv for the primary tumour is the best predictor of survival and response in patients with esophageal cancer receiving radiotherapy14–16. Also, clinical cr has been correlated with survival in patients with locally advanced esophageal carcinoma treated with definitive crt 17–19.
In our cohort, primary tumour recurrence or disease persistence constituted the dominant pattern of failure in the patients overall. Even in the 66 patients achieving a cr, 25 experienced recurrence of the primary lesion, and 12 experienced distant metastasis. Similar outcomes have also been found in patients receiving definitive crt for locally advanced esophageal carcinoma with or without lymph node involvement. Welsh et al.20 reported failure patterns in patients with esophageal carcinoma treated with definitive crt using eni. At a median follow-up of 52.6 months, 50% had experienced local failure, and 48%, distant failure; only 31% had no evidence of failure. Of all local failures, 90% were within the gtv. In an ifi study by Di Fiore et al.17, the radiotherapy target volume was the macroscopic tumour and enlarged lymph nodes. For the 86 patients with a clinical cr, 34 (39.5%) experienced local disease recurrence, 37 (43.0%) experienced metastatic disease, and 19 experienced both. Of the patients with disease staged T1, most achieved a cr after definitive radiation therapy or crt. Nonetheless, half of all failures occurred in the local primary tumour; the secondary failure pattern was at distant lymph nodes or organs7,21.
In our cohort, a second primary cancer was observed in 7 patients (4 in the esophagus at a different site; 1 in the stomach; 1 in the hypopharynx; 1 in the lung). A long survival duration and similar risk factors could lead to those results. In the jcog9708 study, 18 of 72 patients with early-stage escc experienced a second primary cancer21. The authors, Yamamoto et al., also found that, for the stage i population, most local recurrences in the crt group were intramucosal metachronous carcinomas and that cure could be achieved after salvage treatment, with no effect on os 22.
The first failure site in 10 of our patients (8%) was the regional nodal area, with only 2 of those patients (1.6%) experiencing isolated recurrences. Although lymph node involvement on pathology assessment is common in early-stage escc, regional nodal failure is not common after definitive concurrent crt with or without eni. For disease staged T1, the incidence of regional nodal failure is low (1%–6%), with or without eni 7,21. In another ifi study based on fluorodeoxyglucose pet staging for inoperable escc with lymph node metastases, Yamashita et al.13 reported failure in lymph node regions not included in the target volume in only 2 of 63 patients.
Published reports indicate that hematologic toxicities are the most severe acute toxicities and that esophagus-related toxicity is the most common radiation-induced acute toxicity in concurrent crt with eni for esophageal cancer23. Some authors observed significantly reduced incidences of esophageal and lung toxicities with ifi 24. However, in our cohort, no significant differences in high-grade myelosuppression were observed between the ifi and eni groups. The volume of thoracic irradiation, age, and the chemotherapy drugs used might have affected the hematopoietic toxicity results. We believe that hematopoietic toxicity should be attributed mainly to the chemotherapy regimen. In our study, the eni ptv was larger than the ifi ptv, but the median patient age was 67 years in ifi group and 64 years in the eni group. Older age—notably, age greater than 65—has been reported to be the most important risk factor for developing myelosuppression25. The incidence of acute esophagitis was similar in our ifi and eni groups, and severe late toxicities involving heart and lungs was not found. To some extent, the latter results reflect the fact that lesions in our patients were located mostly in the upper and mid-thoracic esophageal area, which suggests that heart and lungs might be receiving less irradiation. Prospective studies should evaluate the incidences of severe acute and late toxicities in a different mix of patients.
A limitation of our study is its small sample size, which did not allow for the full stratification of T stage from T2 to T4. The retrospective character of the study is also a limitation.
Correct staging provides accurate information about the extent of the disease and guides the treatment plan. If ifi is used, detection of the involved lymph nodes before treatment is important when the intent is curative. In the present study, eus was used for staging starting in 2014, and only some patients underwent pet imaging. Each imaging modality has its advantages and disadvantages. For example, the normal esophageal wall is less than 3 mm on ct imaging, and individual layers cannot be identified, making it impossible to differentiate T1 and T2 tumours. Invasion of the peri-esophageal fat might be seen as ill-defined soft-tissue stranding, but T3 tumours cannot be accurately assessed. Those limitations might affect the outcomes data, and therefore ct, eus, and pet should be considered to be complementary diagnostic methods. Even so, undetected occult disease recurrence and micrometastases to regional lymph nodes can be present because of the intrinsic limitations in the accuracy of all those diagnostic methods.
The findings in our study are likely biased because of the distribution in the primary tumour location. Quite a few of the patients receiving definitive crt at our hospital had lesions located in the upper esophagus. Similarly, the median age of our patients, especially those in the ifi group, was greater than the age reported in most studies (typically about 60 years in escc patients). At our hospital, most younger patients received more aggressive treatment in the early clinical stages.
CONCLUSIONS
We retrospectively analyzed the feasibility of ifi in patients with clinical T2–4N0 escc. Our results show that failure patterns and survival rates do not significantly differ between the ifi and eni groups. Large prospective randomized studies are needed for further investigation and verification of those results in this subgroup of patients.
ACKNOWLEDGMENTS
This study was funded by the Natural Science Foundation of China (no. 81672995) and by the Science and Technology Program of Shandong Province (no.2016GSF201133).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Wong R, Malthaner R. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2006:CD002092. doi: 10.1002/14651858.CD002092.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Pöttgen C, Stuschke M. Radiotherapy versus surgery within multimodality protocols for esophageal cancer—a meta-analysis of the randomized trials. Cancer Treat Rev. 2012;38:599–604. doi: 10.1016/j.ctrv.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Itoh Y, Huwa N. Simultaneous combination of chemotherapy using protracted infusion of low-dose cisplatin and 5-fluorouracil with radiotherapy. Relationship between the size of the irradiation field and hemotoxicity. Anticancer Res. 2003;23:1709–11. [PubMed] [Google Scholar]
- 4.Zhao KL, Ma JB, Liu G, Wu KL, Shi XH, Jiang GL. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary? Int J Radiat Oncol Biol Phys. 2010;76:446–51. doi: 10.1016/j.ijrobp.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Li M, Meng X, et al. Involved-field irradiation in definitive chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol. 2014;9:64. doi: 10.1186/1748-717X-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: ffcd 9102. J Clin Oncol. 2007;25:1160–8. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi Y, Nishiyama K, Miyagi K, Suzuki O, Ito Y, Nakamura S. Patterns of failure associated with involved field radiotherapy in patients with clinical stage i thoracic esophageal cancer. Jpn J Clin Oncol. 2011;41:1007–12. doi: 10.1093/jjco/hyr069. [DOI] [PubMed] [Google Scholar]
- 8.Nishihira T, Sayama J, Ueda H, et al. Lymph flow and lymph node metastasis in esophageal cancer. Surg Today. 1995;25:307–17. doi: 10.1007/BF00311252. [DOI] [PubMed] [Google Scholar]
- 9.Motoyama S, Maruyama K, Sato Y, et al. Status of involved lymph nodes and direction of metastatic lymphatic flow between submucosal and T2–4 thoracic squamous cell esophageal cancers. World J Surg. 2009;33:512–17. doi: 10.1007/s00268-008-9781-8. [DOI] [PubMed] [Google Scholar]
- 10.Quint LE, Bogot NR. Staging esophageal cancer. Cancer Imaging. 2008;8(spec no.A):S33–42. doi: 10.1102/1470-7330.2008.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479–90. doi: 10.3748/wjg.14.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Zhang X, Zhao F, Luo Y, Kong L, Yu J. Involved-field radiotherapy for esophageal squamous cell carcinoma: theory and practice. Radiat Oncol. 2016;11:18. doi: 10.1186/s13014-016-0589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita H, Omori M, Takenaka R, et al. Involved-field irradiation concurrently combined with nedaplatin/5-fluorouracil for inoperable esophageal cancer on basis of 18fdg-pet scans: a phase ii study. Radiother Oncol. 2014;113:182–7. doi: 10.1016/j.radonc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen CZ, Chen JZ, Li DR, et al. Long-term outcomes and prognostic factors for patients with esophageal cancer following radiotherapy. World J Gastroenterol. 2013;19:1639–44. doi: 10.3748/wjg.v19.i10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li SH, Rau KM, Lu HI, et al. Pre-treatment maximal oesophageal wall thickness is independently associated with response to chemoradiotherapy in patients with T3–4 oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2012;42:958–64. doi: 10.1093/ejcts/ezs136. [DOI] [PubMed] [Google Scholar]
- 16.Lim JT, Truong PT, Berthelet E, et al. Endoscopic response predicts for survival and organ preservation after primary chemoradiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2003;57:1328–35. doi: 10.1016/S0360-3016(03)00751-X. [DOI] [PubMed] [Google Scholar]
- 17.Di Fiore F, Lecleire S, Rigal O, et al. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12:4185–90. doi: 10.3748/wjg.v12.i26.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tahara M, Ohtsu A, Hironaka S, et al. Clinical impact of criteria for complete response (cr) of primary site to treatment of esophageal cancer. Jpn J Clin Oncol. 2005;35:316–23. doi: 10.1093/jjco/hyi095. [DOI] [PubMed] [Google Scholar]
- 19.Adenis A, Tresch E, Dewas S, et al. Clinical complete responders to definite chemoradiation or radiation therapy for oesophageal cancer: predictors of outcome. BMC Cancer. 2013;13:413. doi: 10.1186/1471-2407-13-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh J, Settle SH, Amini A, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118:2632–40. doi: 10.1002/cncr.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato H, Sato A, Fukuda H, et al. A phase ii trial of chemoradiotherapy for stage i esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (jcog9708) Jpn J Clin Oncol. 2009;39:638–43. doi: 10.1093/jjco/hyp069. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto S, Ishihara R, Motoori M, et al. Comparison between definitive chemoradiotherapy and esophagectomy in patients with clinical stage i esophageal squamous cell carcinoma. Am J Gastroenterol. 2011;106:1048–54. doi: 10.1038/ajg.2011.42. [DOI] [PubMed] [Google Scholar]
- 23.Du D, Song T, Liang X, Fang M, Wu S. Concurrent chemoradiotherapy with elective lymph node irradiation for esophageal cancer: a systemic review and pooled analysis of the literature. Dis Esophagus. 2017;30:1–9. doi: 10.1111/dote.12471. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Miao C, Chen Z, et al. Can involved-field irradiation replace elective nodal irradiation in chemoradiotherapy for esophageal cancer? A systematic review and meta-analysis. Onco Targets Ther. 2017;10:2087–95. doi: 10.2147/OTT.S130285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]