Abstract
Extracellular vesicles (EVs) are membrane-derived vesicles which can be released by different cell types, including hepatocytes, hepatic stellate cells and immune cells in normal and pathological conditions. EVs carry lipids, proteins, coding and non-coding RNAs and mitochondrial DNA causing modifications on the recipient cells. These vesicles are considered potential biomarkers and therapeutic agents for human diagnostic and prognostic due to their function as intercellular mediators of cell-cell communication within the liver and between other organs. However, the development and optimization of methods for EVs isolation is required to characterize their biological functions as well as their potential as a treatment option in the clinic. Nevertheless, many questions remain unanswered related to the function of EVs under physiological and pathological conditions. In the current editorial, the results obtained in different studies that investigated the role of intrahepatic EVs during liver diseases, including drug-induced liver injury, non-alcoholic fatty liver, non-alcoholic steatohepatitis, alcoholic liver disease and hepatocellular carcinoma and extrahepatic EVs in remote organs during pathological events such as pulmonary disease, cardiovascular diseases, neurodegenerative disorders e.g., Alzheimer’s disease, Parkinson’s disease and multiple sclerosis as well as in immunopathological processes, are discussed. Although much light needs to be shed on the mechanisms of EVs, these membrane-derived vesicles represent both a novel promising diagnostic, and a therapeutic tool for clinical use that we emphasize in the current editorial.
Keywords: Extracellular vesicles, MicroRNA, Hepatocytes, Drug-induced liver injury, Alcoholic liver disease, Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Hepatocellular carcinoma
Core tip: It has become increasingly clear that extracellular vesicles (EVs) are particularly important intercellular messenger vesicles during pathophysiological processes. EVs can provide more information about the processes that occur in remote organs during the development of diseases contributing to improving our tools for diagnosis, prognosis and therapy.
INTRODUCTION
The emergence of extracellular vesicles (EVs) as critical mediators of cell-cell communication has gained great interest from the scientific community due to its implication for human diagnostic and therapeutic applications[1,2]. The role of EVs in intercellular transport was reported for the first time in 1980[3]. However, in the past decades, EVs have exponentially attracted the interest of researchers.
There are different mechanisms of formation of these vesicles, creating a complex repertoire of EVs which are secreted and differ in size and origin, such as exosomes, ectosomes, apoptotic bodies, oncosomes and large oncosomes[1]. Exosomes are the smallest EVs (30-100 nm). The process of formation the exosomes is originated during endosome maturation[2]. First, the early endosome is formed by invagination of the plasma membrane and the consequent fusion of endocytic vesicles. The endocytic vesicles can follow two pathways: (1) The endocytic material is recycled and returns to the plasmatic membrane; and (2) exosomes become multivesicular bodies (MVBs) which are a type of late endosomes containing membrane-bound vesicles (intraluminal vesicles)[4].
MVBs are formed by the invagination of the limiting membrane, a process during which a small portion of cytosol is trapped into the vesicle. Finally, there are MVBs which are degraded in the lysosome or release their membrane-bound vesicles known as exosomes to extracellular media by the fusion of MVBs to the plasma membrane (Figure 1).
Figure 1.
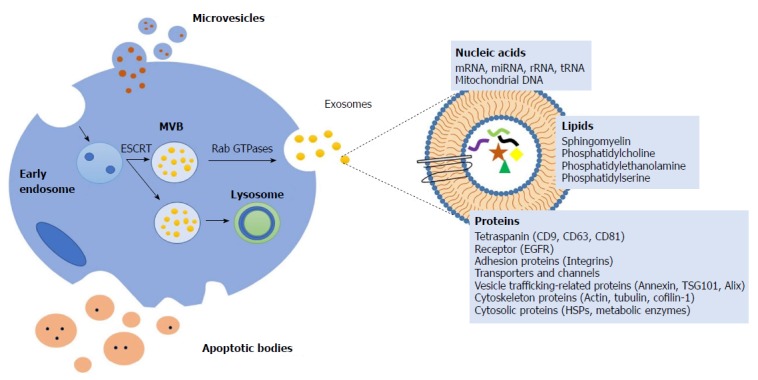
Mechanisms of formation extracellular vesicles and composition. The early endosome is generated by invagination of the plasma membrane. The consequent fusion of endocytic vesicles mediated by the endosomal sorting complex responsible for transport (ESCRTs), formed multivesicular bodies (MVBs). MVBs can be degraded in the lysosome or released the intraluminal vesicles known as exosomes by the fusion of MVBs to the plasma membrane mediated by Rab GTPases. Microvesicles are generated by outward budding from the plasmatic membrane. Apoptotic bodies are generated during programmed cell death or apoptosis. The composition of extracellular vesicles (EVs) includes proteins (tetraspanins, receptors including epidermal growth factor receptor (EGFR), adhesion proteins, transporters and channels, vesicle trafficking-related proteins, cytoskeleton proteins and cytosolic proteins), lipids (sphingomyelin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine) and nucleic acids (messenger RNA (mRNA), microRNA (miRNA), ribosomal RNA (rRNA), transfer RNA (tRNA), mitochondrial DNA (mtDNA)). ESCRTs: Endosomal sorting complex responsible for transport; MVBs: Multivesicular bodies; EVs: Extracellular vesicles; EGFR: Epidermal growth factor receptor; mRNA: Messenger RNA; miRNA: MicroRNA; rRNA: Ribosomal RNA; tRNA: Transfer RNA; mtDNA: Mitochondrial DNA.
The process of generation of vesicles is mediated by the endosomal sorting complex responsible for transport and other components, such as ceramide lipids and tetraspanins. Rab GTPases are involved in exosome secretion but the requirements for specific Rabs may differ depending on the cell type[5,6].
Ectosomes (also known as microvesicles) are a population of extracellular vesicles whose size is 50-1000 nm[7]. They are formed by outward budding of the cell plasma membrane[8]. These vesicles are shed by different cell types and express a subset of cell surface proteins that depend on the component of the cells plasma membranes of origin[9].
Apoptotic bodies are presented in a wide range of sizes (50-2000 nm). Programmed cell death or apoptosis triggers the formation and release of apoptotic bodies[10].
Oncosomes and large oncosomes are presented in a range of size between 100-500 nm and they are generated by budding of the plasma membrane. These types of vesicles are only released by cancer cells[11] carrying oncogenic cargo which modulate tumor environment promoting the proliferation, differentiation and metabolism of tumors[12].
Composition of EVs
Independently of their biogenesis, the composition of EVs includes proteins, lipids, and nucleic acids (coding and non-coding RNA and mitochondrial DNA)[13]. Lipidomic analysis shows that the membrane of EVs contains abundant cholesterol, sphingomyelin, ceramide, saturated fatty acids and phosphatidylserine. Furthermore, proteomic analysis shows that EVs share common marker proteins, such as heat shock proteins (Hsp70 and Hsp90), tetraspanins (CD9, CD63, CD81, CD82), endosomal sorting complex required for transport (Alix and Tsg101) and membrane trafficking and merging proteins (GTPases, Flotillin and Annexins) (Figure 2)[14].
Figure 2.
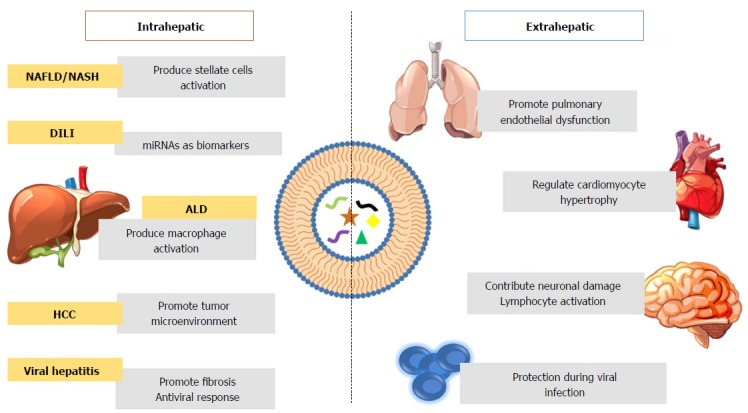
Role of extracellular vesicles during pathologic processes inside and outside the liver. Intrahepatic extracellular vesicles in non-alcoholic fatty liver (NAFLD), non-alcoholic steatohepatitis (NASH), drug-induced liver injury (DILI), alcoholic liver disease (ALD) and hepatocellular carcinoma (HCC). Extrahepatic EVs play a fundamental role in pulmonary disease, cardiovascular diseases (CVDs), neurodegenerative disorders and immunopathological disorders. EVs: Extracellular vesicles; NAFLD: Non-alcoholic fatty liver; NASH: Non-alcoholic steatohepatitis; DILI: Drug-induced liver injury; ALD: Alcoholic liver disease; HCC: Hepatocellular carcinoma; CVDs: Cardiovascular diseases.
Location of EVs
EVs are released to the extracellular media circulating in the adjacent extracellular space and appear in biological fluids, such as blood, saliva, breast milk, bronchial lavage fluid, cerebral spinal fluid, amniotic fluid and urine[15]. However, due to their heterogeneous size, there is a current lack of purification methods. Moreover, these molecules are included in a big group known as EVs since they are also very difficult to isolate and fully discriminate[16].
Circulating EVs can be captured by other cells via three ways: Direct membrane fusion, receptor mediated fusion or endocytosis. The recipient cells accept their cargo and, consequently, may suffer modifications in their normal cellular processes[17,18]. EVs-mediated pathological processes can be interrupted by inhibiting EVs release. Emerging studies have recently shown that the inhibition of neutral sphingomyelinase 2 (nSmase2) with GW4869 block exosome release or exosome mediated signalling in different cell types[19].
EVs in liver
The liver has great interest in the scientific research due to this implication in many processes, such as detoxification of blood, filtering all harmful elements and in production, processing and transport of lipids. Furthermore, the liver is a multicellular organ formed by parenchymal cells (hepatocytes) and non-parenchymal cells including Kupffer cells (KCs), sinusoidal endothelial cells (SECs), hepatic stellate cells (HSCs)[20]. The coexistence of different cell types creates a need for intercellular communication network in order to maintain liver homeostasis[21]. Many pathophysiological events are regulated by EVs which can be transferred from donor cells to recipient cells and can activate or regulate cell functions including protein expression, cell proliferation and differentiation and/or antiviral responses. This intercellular communication might be done through EVs, and for this reason, it is necessary to shed light into the physiology and pathology of hepatic EVs[21].
Primary hepatocytes secrete EVs proteins that include exosomal marker proteins (e.g., Tsg101, CD63 and CD81), hepatic-specific proteins, like the asialoglycoprotein receptor, and different proteins associated with metabolic disorder which need further investigation and identification[22].
EVs in drug-induced liver injury
Nowadays, traditional standard biomarkers for liver injury are based on the measurement of hepatic enzymes in plasma or serum including AST, ALT, alkaline phosphatase (AP) and gamma-glutamil-transpeptidase[23]. However, serum or plasma levels of these enzymes do not always reflect the stage of liver disease, therefore causing significant limitations in the diagnosis and staging of different chronic and acute liver disorders. For this reason, miRNAs have emerged as new potential biomarkers of liver injury.
Liver-derived miRNAs may originate from resident parenchymal and non-parenchymal cells and can be significantly altered in certain liver diseases. It can be found as non-vesicle associated miRNA (free circulating miRNA) or associated with vesicles (EVs miRNA) being the last one, the more stable biomarkers[24].
The use of miRNAs as potential biomarker of liver injury was demonstrated in a mouse model of APAP-induced acute liver injury. It was found a significant increase in miR-122 levels in EVs released from hepatocytes[25]. The same results were observed in a rat model of APAP-induced liver injury with increased levels of circulating EVs. These results correlated with a study in primary human hepatocytes (PHH)[26]. These results strongly support that miRNAs might be used as potential biomarkers of liver diseases, being miR-122 associated with EVs proposed as biomarker in drug-induced liver injury (DILI).
EVs in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
Non-alcoholic fatty liver disease (NAFLD) is characterized by over-accumulation of fat in the liver producing hepatic steatosis triggering an inflammatory reaction which results in the development of non-alcoholic steatohepatitis (NASH). Both diseases are characterized by an increase of circulating EVs. In order to characterize EVs cargo, it was demonstrated that hepatocyte-derived EVs released during lipotoxic fatty acids are enriched in Vanin-1 (VNN1) and miR128-3p. VNN1 is responsible of the internalization of EVs into HSCs and miR128-3p inhibits the expression of PPAR-gamma provoking an activation of stellate cells inducing fibrosis in the liver[27]. Altogether these results indicate that VNN1 and miR128-3p released by hepatocytes associated with EVs during lipotoxicity might be important during HSCs activation in NAFLD/NASH.
EVs in alcoholic liver disease
In an attempt to further characterize the critical role of EVs in vivo during alcoholic liver disease (ALD), Saha and colleagues[28], used an experimental model of ALD. The authors first found a significant increase in the total number of EVs in the serum of mice with an alcohol diet and the effect of serum EVs derived from ALD mice in naïve recipient mice. To characterize the different components in EVs release to ALD mice they found an increase in miR-192 and miR-30a levels compared to control EVs. Moreover, hepatocyte released EVs causing an increase in the percentage of F4/80hi CD11blow (KCs) and TNF-α, suggesting the link between innate immune cell activation and hepatocyte intoxication during the process of alcoholic liver injury.
Hepatic resident macrophages (KCs) and infiltrating macrophages play a pivotal role in ALD pathogenesis whose production of proinflammatory cytokines exhibited the inflammatory process characteristic of alcoholic hepatitis (AH). For this reason, it is necessary to characterize specific proteins implicated in macrophage activation.
In order to evaluate the in vivo role of macrophages, Verma and collaborators[29] described that cultured hepatocytes released CD40L in EVs in response to alcohol exposure which leads to macrophage activation. In contrast, Saha et al[28] showed that Hsp90 as the cause of macrophage activation, demonstrating that there was a significant increase in levels of Hsp90 EVs secreted from hepatocytes in ALD. These studies reveal that Hsp90 and CD40L carried by EVs released from hepatocytes in response to alcohol intake, have an important role in macrophage activation during ALD.
EVs in hepatocellular carcinoma
Several studies suggest that EVs contribute to proliferation and propagation of hepatocellular carcinoma (HCC) cells during HCC[30]. It was demonstrated that EVs released by CD90+ cells provoked an increase in vascular endothelial growth factor 1 in endothelial cells which lead with metastasis. Moreover, it has suggested that EVs collaborate with the microenvironment that promote tumor survival and growth[31]. It was found that EVs released by metastatic HCC cells induce hepatocytes to secrete metalloproteinase-2 and -9 which facilitate the invasion of HCC cells[32].
Kogure et al[33], characterized the cargo of EVs release by HCC cells in vitro identifying several miRNA, such as miR-584, miR-517c, miR-378, miR-520f, miR142-5p, miR-451, miR-518d, miR-215, miR-376a, miR-133b, and miR-367. These studies indicate that oncogenic cargo released by HCC cells modulate tumor environment facilitating the invasion of HCC cells promoting the proliferation and differentiation of tumors.
Viral hepatitis
The role of CCL5 released by HCV-infected macrophages/KCs thereby inducing the activation of HSCs through the phosphorylation of ERK was demonstrated. In fact, the neutralization of CCL5 in HSCs in culture using supernatant from HCV-infected macrophages caused a significant down-regulation of inflammatory and profibrotic genes[34]. Another study demonstrated that liver cells treated with IFN-α induced resistance to HBV replication in infected liver cells by cell-cell communication through EVs[35]. These results provide evidence that EVs have an important role during viral infection and antiviral response.
Extrahepatic EVs
So far, the role of EVs in different pathophysiological events in the liver was discussed. However, several articles revealed the role of EVs in remote organs taking part of different events under pathological conditions, such as pulmonary disease, neurodegenerative disorders, cardiovascular diseases and during immunopathological processes.
EVs in pulmonary disease
The liver takes an important role in maintaining systemic homeostasis[36]. The injured liver can induce different pathogenic processes in remote organs. Indeed, EVs are linked with different pathological conditions inside and outside the liver[37]. For this reason, hepatocyte-derived-EVs are suggested to have an important role in the pathogenesis of pulmonary disease.
To characterize the critical role of hepatic pathogenic processes, and their implications in the lung, Royo et al[37] investigated the role of Arg1 carried by EVs as one of the factors responsible for the lung damage. The study confirms that hepatic EVs and the effect of Arg1 might propagate the injury in the lung inducing pulmonary endothelial dysfunction. It concludes that EVs take an important part in communication between the liver and lung, could be Arg1 the responsible for pulmonary endothelial dysfunction.
EVs in neurodegenerative disorders
On the other hand, we discuss the role of EVs in different neurodegenerative disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD) and multiple sclerosis (MS) as a potential source of information in neurodegenerative disorders[38].
It has been suggested that lipids cargo in EVs released from neurons promoting the formation of β-amyloid (βA) peptides contributing to neuronal damage in AD[39]. Furthermore, it was found that AD patients have lower levels of miR-193b in blood which are correlated with levels in cerebral spinal fluid (CSF)[40]. In addition, miR-132 and miR-212 are downregulated in neurodegenerative disorders including AD[41].
PD is characterized by an accumulation of α-synuclein protein. Therefore the cargo inside EVs was analysed and showed that this protein is present outside and inside of EVs, and their secretion contribute to the development of the disease[42].
In order to understand the role of EVs in MS, researchers showed that EVs are released from brain endothelium and have increased levels of β2-microglobulin, MHC II, CD40 and ICOSL. Moreover, they are involved in the activation of CD4+ and CD8+ lymphocytes[43]. Furthermore, serum EVs were able to decrease the levels of miR-122-5p, miR-196b-5p, miR-301a-3p, miR-532-5p[44].
Considering these results, EVs might contribute to the progression of neurodegenerative diseases and thus be used in the clinical setting as biomarkers or drug delivery tools.
EVs in cardiovascular diseases
Emerging studies reveals that EVs have regulatory effects in cardiovascular diseases being released by endothelial cells, cardiomyocytes, fibroblasts and stem cells and participating in pathophysiological processes contributing to the development of disease[45].
EVs have been involved in the regulation of cardiomyocyte hypertrophy and cardiac fibrosis. It was demonstrated that EVs released from myocytes carry Hsp90 together with IL-6. Both molecules are involved in the activation of cardiac fibroblasts causing increased collagen production and deposition during cardiac hypertrophy[46]. Furthermore, it was found a significantly increase in the levels of miR-21-3p in pericardial fluid in a mice model of transverse aortic constriction-induced hypertrophy. This miR-21-3p associated with EVs was released by fibroblasts and was uptake by cardiomyocytes leading to an activation of intercellular signalling pathways which provoke cellular hypertrophy[47]. Interestingly, EVs play a critical role in intercellular communication between fibroblasts and cardiomyocytes during the hypertrophic process contributing to cardiac fibrosis.
EVs in immunopathology
Another important issue is the role of EVs in antiviral immune response. Torralba and colleagues[48], investigated that EVs released from T cells contained mitochondrial DNA and this genetic material can be transferred unidirectionally from T cells to dendritic cells (DCs) during the formation of antigen-dependent contacts. The possible signalling pathways which are activating in DCs were analysed, finding a significantly increase in the expression of different genes. Most of them were involved in the antiviral response mediated by IFN-I resulting into immune protection effect against virus infection leading a decrease viral infection. Altogether these results indicate that EVs from T cells conferred protection to DCs against virus infection through antigen-driven contacts.
CONCLUSION
In summary, the data show that EVs can be used not only as diagnostic but theranostic tool for the treatment of acute and chronic liver disease (Table 1). EVs can be released by hepatocytes carrying miRNA as potential biomarkers in DILI or triggering macrophage activation in ALD and an activation of HSCs in NAFLD/NASH. Emerging evidences suggests that EVs promotes the proliferation and migrations of tumor cells. Additionally, circulating EVs have an effect outside the liver as seen in the lung taking particularly interest the link between EVs released by hepatocytes and the effect in pulmonary disease. The effect of EVs in the brain as seen in different neurodegenerative disorders contributing to the progress and development of the diseases; in the heart, having regulatory effects in cardiovascular diseases and finally during viral infection for their immune protection effect.
Table 1.
Summary of extracellular vesicles biomarkers in hepatic and extracellular vesicles
| Type of disease | Sample | Species | Biomarker | Ref. | |
| Intrahepatic | Drug-induced liver injury (DILI) | Plasma/serum/cell culture | Mouse/rat/human | miR-122 [↑] | [25,26] |
| Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) | Cell culture | Mouse | miR-128-3p [↑]; VNN1 | [27] | |
| Alcoholic liver disease (ALD) | Serum/cell culture | Mouse/human | miR-192, miR-30a [↑]; CD40L, Hsp90 | [28,29] | |
| Hepatocellular carcinoma (HCC) | Cell culture | Human | Vascular endotelial growth factor 1, MMP2, MMP9; miR-584, miR-517c, miR-378, miR-520f, miR142-5p, miR-451, miR-518d, miR-215, miR376a, miR-133b, and miR-367 | [31-33] | |
| Viral hepatitis (HBV/HCV) | Cell culture | Human | Viral RNA; CCL5 | [34] | |
| Extrahepatic | Pulmonary disease | Serum/cell culture | Rat | Arg 1 | [37] |
| Alzheimer’s disease (AD) | CSF/blood/tissue | Human | β-amiloyd; miR-193b, miR-132 [↓] | [39,41] | |
| Parkinson’s disease (PD) | Cell culture | Mouse | α-sinucleyn | [42] | |
| Multiple Sclerosis (MS) | Serum/cell culture | Mouse/human | Beta-2-microglobulin, MHC-II, CD40,ICOSL; miR-122-5p, miR-196b-5p, miR-301a-3p, miR-5p [↓] | [43,44] | |
| Cardiovascular disease (CVDs) | Cell culture/pericardial fluid | Rat/mouse | Hsp90, IL-6; miR-21-3p [↑] | [46,47] | |
| Immunopathology | Cell culture | Human | mtDNA | [48] |
VNN1: Vanin-1; MMP: Matrix metalloproteinase.
In conclusion, EVs are important intercellular communication mediators during pathology and physiology events. It would be interesting in future studies to investigate the particularly role of EVs in the development of diseases. However, little data support the function of EVs in physiopathological processes suggests the need for further research.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Peer-review started: July 26, 2018
First decision: August 27, 2018
Article in press: October 5, 2018
P- Reviewer: Koizume S, Kanda T, Link A, Marcos R S- Editor: Wang XJ L- Editor: A E- Editor: Bian YN
Contributor Information
Laura Morán, Department of Immunology, Ophthalmology and ORL, Complutense University School of Medicine, Madrid 28040, Spain.
Francisco Javier Cubero, Department of Immunology, Ophthalmology and ORL, Complutense University School of Medicine, Madrid 28040, Spain; 12 de Octubre Health Research Institute (imas12), Madrid 28041, Spain. fcubero@ucm.es.
References
- 1.Cho YE, Song BJ, Akbar M, Baek MC. Extracellular vesicles as potential biomarkers for alcohol- and drug-induced liver injury and their therapeutic applications. Pharmacol Ther. 2018;187:180–194. doi: 10.1016/j.pharmthera.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devhare PB, Ray RB. Extracellular vesicles: Novel mediator for cell to cell communications in liver pathogenesis. Mol Aspects Med. 2018;60:115–122. doi: 10.1016/j.mam.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 4.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 5.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, Gores GJ, Malhi H. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology. 2016;64:2219–2233. doi: 10.1002/hep.28814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemoinne S, Thabut D, Housset C, Moreau R, Valla D, Boulanger CM, Rautou PE. The emerging roles of microvesicles in liver diseases. Nat Rev Gastroenterol Hepatol. 2014;11:350–361. doi: 10.1038/nrgastro.2014.7. [DOI] [PubMed] [Google Scholar]
- 8.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 11.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wendler F, Stamp GW, Giamas G. Tumor-Stromal Cell Communication: Small Vesicles Signal Big Changes. Trends Cancer. 2016;2:326–329. doi: 10.1016/j.trecan.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle W, Miller D, Zhang HG. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65:342–347. doi: 10.1016/j.addr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 15.Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bátiz LF, Castro MA, Burgos PV, Velásquez ZD, Muñoz RI, Lafourcade CA, Troncoso-Escudero P, Wyneken U. Exosomes as Novel Regulators of Adult Neurogenic Niches. Front Cell Neurosci. 2016;9:501. doi: 10.3389/fncel.2015.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol. 2016;65:213–221. doi: 10.1016/j.jhep.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang JK, Young RF, Ashraf H, Canty JM Jr. Inhibiting Extracellular Vesicle Release from Human Cardiosphere Derived Cells with Lentiviral Knockdown of nSMase2 Differentially Effects Proliferation and Apoptosis in Cardiomyocytes, Fibroblasts and Endothelial Cells In Vitro. PLoS One. 2016;11:e0165926. doi: 10.1371/journal.pone.0165926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 21.Cai S, Cheng X, Pan X, Li J. Emerging role of exosomes in liver physiology and pathology. Hepatol Res. 2017;47:194–203. doi: 10.1111/hepr.12794. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Chen R, Kemper S, Brigstock DR. Pathways of production and delivery of hepatocyte exosomes. J Cell Commun Signal. 2018;12:343–357. doi: 10.1007/s12079-017-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 24.Köberle V, Pleli T, Schmithals C, Augusto Alonso E, Haupenthal J, Bönig H, Peveling-Oberhag J, Biondi RM, Zeuzem S, Kronenberger B, et al. Differential stability of cell-free circulating microRNAs: implications for their utilization as biomarkers. PLoS One. 2013;8:e75184. doi: 10.1371/journal.pone.0075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, Kodys K, Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holman NS, Mosedale M, Wolf KK, LeCluyse EL, Watkins PB. Subtoxic Alterations in Hepatocyte-Derived Exosomes: An Early Step in Drug-Induced Liver Injury? Toxicol Sci. 2016;151:365–375. doi: 10.1093/toxsci/kfw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L, Pinatel EM, Alisi A, Nobili V, Feldstein AE. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-γ. Cell Mol Gastroenterol Hepatol. 2015;1:646–663.e4. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha B, Momen-Heravi F, Furi I, Kodys K, Catalano D, Gangopadhyay A, Haraszti R, Satishchandran A, Iracheta-Vellve A, Adejumo A, et al. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology. 2018;67:1986–2000. doi: 10.1002/hep.29732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64:651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santangelo L, Battistelli C, Montaldo C, Citarella F, Strippoli R, Cicchini C. Functional Roles and Therapeutic Applications of Exosomes in Hepatocellular Carcinoma. Biomed Res Int. 2017;2017:2931813. doi: 10.1155/2017/2931813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, Ngai SM, Chan TF, Wong N. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36:1008–1018. doi: 10.1093/carcin/bgv081. [DOI] [PubMed] [Google Scholar]
- 33.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasaki R, Devhare PB, Steele R, Ray R, Ray RB. Hepatitis C virus-induced CCL5 secretion from macrophages activates hepatic stellate cells. Hepatology. 2017;66:746–757. doi: 10.1002/hep.29170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, Liu J, Pan T, Chen J, Wu M, et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 36.Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13:267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royo F, Moreno L, Mleczko J, Palomo L, Gonzalez E, Cabrera D, Cogolludo A, Vizcaino FP, van-Liempd S, Falcon-Perez JM. Hepatocyte-secreted extracellular vesicles modify blood metabolome and endothelial function by an arginase-dependent mechanism. Sci Rep. 2017;7:42798. doi: 10.1038/srep42798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Croese T, Furlan R. Extracellular vesicles in neurodegenerative diseases. Mol Aspects Med. 2018;60:52–61. doi: 10.1016/j.mam.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem. 2012;287:43108–43115. doi: 10.1074/jbc.M112.404467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu CG, Song J, Zhang YQ, Wang PC. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol Med Rep. 2014;10:2395–2400. doi: 10.3892/mmr.2014.2484. [DOI] [PubMed] [Google Scholar]
- 41.Lau P, Frigerio CS, De Strooper B. Variance in the identification of microRNAs deregulated in Alzheimer’s disease and possible role of lincRNAs in the pathology: the need of larger datasets. Ageing Res Rev. 2014;17:43–53. doi: 10.1016/j.arr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Chang C, Lang H, Geng N, Wang J, Li N, Wang X. Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett. 2013;548:190–195. doi: 10.1016/j.neulet.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Wheway J, Latham SL, Combes V, Grau GE. Endothelial microparticles interact with and support the proliferation of T cells. J Immunol. 2014;193:3378–3387. doi: 10.4049/jimmunol.1303431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selmaj I, Cichalewska M, Namiecinska M, Galazka G, Horzelski W, Selmaj KW, Mycko MP. Global exosome transcriptome profiling reveals biomarkers for multiple sclerosis. Ann Neurol. 2017;81:703–717. doi: 10.1002/ana.24931. [DOI] [PubMed] [Google Scholar]
- 45.Bei Y, Das S, Rodosthenous RS, Holvoet P, Vanhaverbeke M, Monteiro MC, Monteiro VVS, Radosinska J, Bartekova M, Jansen F, et al. Extracellular Vesicles in Cardiovascular Theranostics. Theranostics. 2017;7:4168–4182. doi: 10.7150/thno.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Datta R, Bansal T, Rana S, Datta K, Datta Chaudhuri R, Chawla-Sarkar M, Sarkar S. Myocyte-Derived Hsp90 Modulates Collagen Upregulation via Biphasic Activation of STAT-3 in Fibroblasts during Cardiac Hypertrophy. Mol Cell Biol. 2017;37 doi: 10.1128/MCB.00611-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torralba D, Baixauli F, Villarroya-Beltri C, Fernández-Delgado I, Latorre-Pellicer A, Acín-Pérez R, Martín-Cófreces NB, Jaso-Tamame ÁL, Iborra S, Jorge I, González-Aseguinolaza G, Garaude J, Vicente-Manzanares M, Enríquez JA, Mittelbrunn M, Sánchez-Madrid F. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat Commun. 2018;9:2658. doi: 10.1038/s41467-018-05077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


