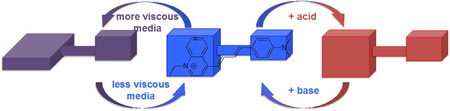
Abstract
Styryl dyes, specifically LDS group dyes, are known solvatochromic and electrochromic probes for monitoring mitochondrial potential in cellular environments. However, the ability of these dyes to respond to fluctuations in viscosity, pH and temperature has not been established. In this study, we demonstrated that LDS 798 (also known as Styryl-11) can sense environmental viscosity (via fluorescence lifetime changes) as well as pH changes (ratiometric intensity change) in the absence of polarity variations. Polarity changes can be probed by spectral changes using LDS 798. Therefore, all properties of the media should be considered, when these types of dyes are used as electrochormic/solvatochromic sensors in cellular environments.
Graphical Abstract
Introduction
Styryl dyes are a class of fluorophores that have found numerous applications in photography1–4 and have been widely used as laser dyes,5 molecular probes for cellular processes, including vesicle trafficking and exocytosis,6,7 and lipid membrane studies.8 A specific subgroup of the family of styryl dyes, known as the LDS dye group, has been renowned for its ability to rapidly respond to variations in cell membranes’ electric potential (electrochromic-response) and polarity (solvatochromic-response) due to the alteration of charge distribution within its molecular frame, producing significant changes in both its emission intensity and its lifetimes.8,9 However, it could be argued that such changes in fluorescence properties of LDS dyes can also be regulated by a number of other parameters of cellular environments, including viscosity and pH. To the best of our knowledge, the effect of these properties on the photo-physical properties of the majority of LDS dyes has not been explored. Therefore, such studies could provide valuable and complementary information for the interpretation of the electronic potential and polarity dependency data.
Styryl-11 or LDS 798 (1-ethyl-4-(4-(p-dimethylamino phenyl)-1,3-butadienyl)n-quinolinium percholate, Figure 1) is a well-known, commercialized pumped-laser dye, with absorption and emission maxima of 575 nm and 765 nm, respectively (a quantum yield of ~ 3% in dioxane).10 LDS 798 has a short lifetime (<50 ps) in water, which has been utilized for measuring the fluorescence instrument response functions (IRF, for color sensitive detectors) needed for the accurate deconvolution of fluorescence lifetimes10 in the red spectral region.
Figure 1.
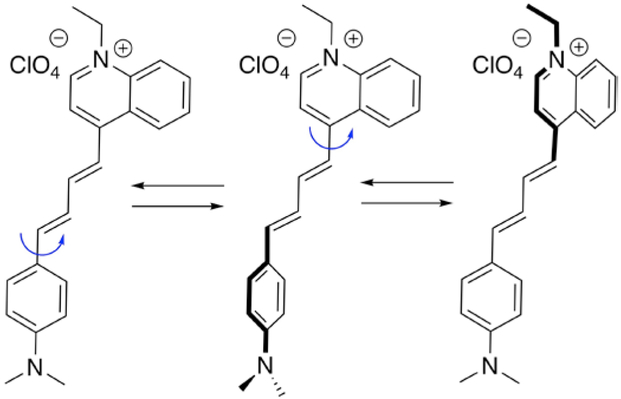
Structure and several conformers of LDS 798
Previously, we showed that the solubility and solvatochromic properties of LDS 798 could be used to determine their diffusion coefficients and particle sizes of small unilamellar vesicles and lipid-based nanoparticles.11 Our recent work on BODIPY-based molecular rotors12–14 prompted us to evaluate the potential of LDS dyes as molecular viscometers. It should be pointed out that a number of small molecule rotors have been recently used as molecular viscometers,15–18 yet the use of LDS-type dyes as probes for media’s viscosity has not been established. It is plausible that the rotation of the C–C bond connecting the quinolinium head region and the dimethylaminophenyl tail (Figure 1) would lead to intramolecular charge transfer, thus turning LDS 798 into a molecular rotor, which might be capable of responding to changes the in microviscosity of the environment. Moreover, the presence of the dimethylamino-group should make LDS 798 sensitive to the pH of the surrounding environment. This is significant because viscosity and pH inside various cell compartments, e.g., cell membrane, cytoplasm, mitochondria, and nucleus, vary significantly.19,20 Therefore, detailed characterization of a probe response to each individual effector (viscosity, pH, potential) is imperative for utilizing this dye as a multi-response sensor for determining and monitoring physical properties of various types of media.
Experimental
Materials and methods
All chemicals and solvents were from commercial sources, they were of the highest grade possible, and were used as received. LDS 798 was purchased from Exciton (Dayton, OH) and stock solution was prepared by dissolving the LDS 798 in dimethyl sulfoxide (DMSO) to yield a 1 mM solution. Methanol:glycerol mixtures were made by mixing appropriate volumes of the two solvents. The viscosities and densities of glycerol, methanol and their mixtures were measured using the Anton-Paar Lovis 4500 M microviscometer. Furthermore, 1H NMR spectra were recorded on a Bruker (400 MHz) spectrometer. The buffers were made using phosphate and acetate salts, and the pH of each buffer was measured using a pH700 benchtop pH-meter (Oakton Instruments). The UV-Vis absorption spectra of LDS 798 were measured with the Cary 60 UV-Vis spectrophotometer (Agilent Technologies). The fluorescence spectra of LDS 798 in various solvents were measured with a Cary Eclipse fluorescence spectrometer (Varian Inc.) in a square geometry setup. Both absorption and fluorescence measurements were performed using 1×1 cm cuvettes with stoppers. All temperature dependent measurements were conducted using the Cary single cell Peltier accessory (Varian Inc.). The quantum yields (QY) of LDS 798 were determined using Cresyl Violet as a reference (using the QY of Cresyl Violet as 0.54 in ethanol17) according to the following equation:
where Is and IR are the integrated fluorescence intensities for the sample and the reference compounds, respectively; ODs and ODR are the measured absorption values for the sample and the reference, respectively; nS and nR are the refractive indexes of the solvents used for the sample and the reference, respectively. The fluorescence lifetime of LDS 798 was measured using the FluoTime-300 high-performance fluorescence lifetime spectrometer with Picoharp 300 TCSPC module (PicoQuant, GmbH). The excitation source for lifetime measurements was the high-power, ultra-broadband white light supercontinuum laser system SC450-PP (Fianium, Inc), which created laser pulses at a 5 MHz repetition rate with a pulse width less than 100 ps. The laser excitation wavelength was selected using a prism based Aviv monochromator. The emission of LDS 798 was detected using an R3809U-50 micro-channel plate photomultiplier tube (MCPPMT, Hamamatsu, Inc). The system IRF was measured to be less than 100 ps. The fluorescence lifetime was measured at the magic angle condition (54.7°) to eliminate the depolarization effects due to molecular rotation. Then, data analysis was done using the FluoFit program (PicoQuant, GmbH, and Version 4.4) with a discrete multi-exponential deconvolution model:
where IRF (t’) is the instrumental response function at time t = t’, αiis the amplitude of the ith component’s decay at time t, and 𝜏i is the lifetime of the ith element. The average lifetime <𝜏ava>was calculated as follows:
where
For amplitude averaged lifetime τAMP, αi is the fractional amplitude. The radiative rates (Kr) and Knr were calculated from measured averaged lifetimes and quantum yield as follows.
Results and Discussion
In LDS 798 (Figure 1), the internal rotation of the two aromatic moieties connected by the s-trans diene unit should produce several rotamers (also referred to as conformers), with two extremes, e.g., planar, where the two aromatic groups and the diene are in conjugation, and twisted, where the two aromatic groups are perpendicular to each other. Thus, if photophysical properties of the dye would depend on the viscosity of the media, LDS 798 might be used as a molecular viscometer in the absence of polarity changes or when the polarity is similar to that of methanol: glycerol mixtures.
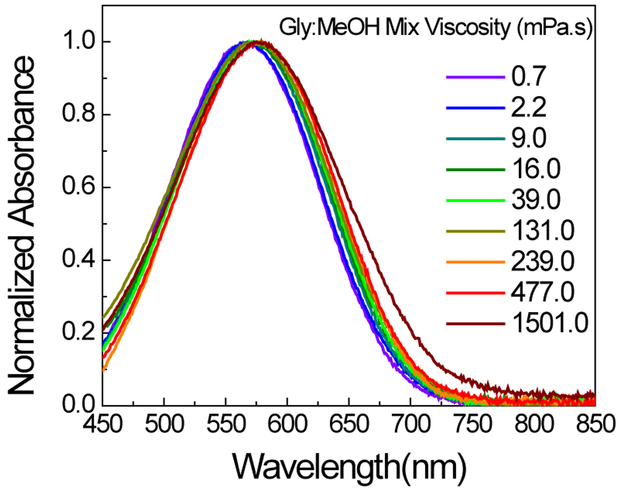
Towards this end, the absorption and fluorescence of LDS in solvents of various viscosities were investigated. In order to minimize the effect of polarity, glycerol and methanol (dielectric constants of 33 and 43, respectively) as well as their respective mixtures were used, which provided a fairly large viscosity range, i.e., from 0.682 mPa•s (methanol) to 1501 mPa•s (glycerol) (Table S1). The absorption maximum (λabmax ca. 570 nm) of LDS 798 did not show a significant variation as the viscosity was varied (Figure 2). Notably, because the full-width half-max (FWHM) remained fairly constant at ca. 145 nm over the range of viscosities, the possibility of self-aggregation of LDS 798 under those conditions could be ruled out.
Figure 2:
Normalized absorption spectra of LDS 798 in glycerol: methanol mixtures of various viscosities.
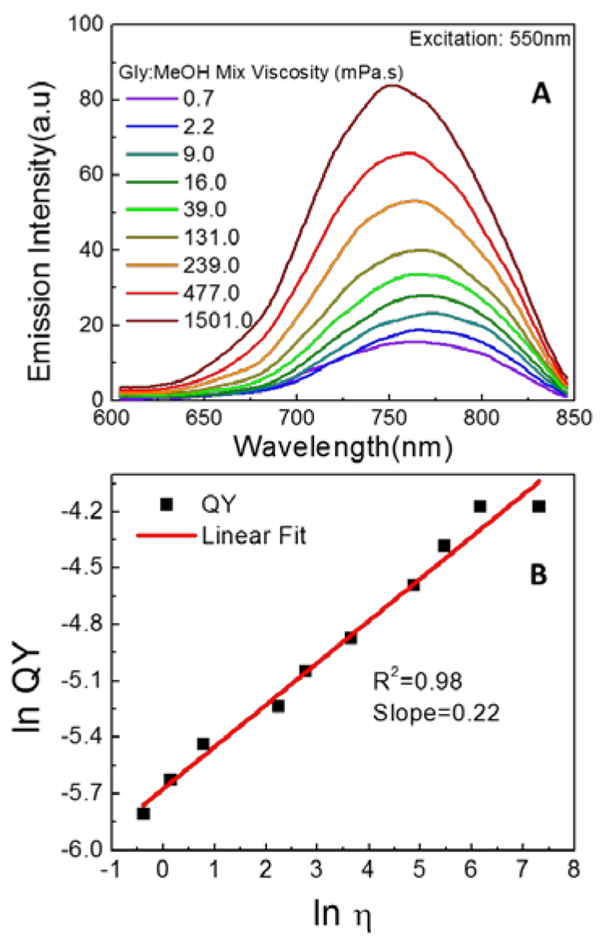
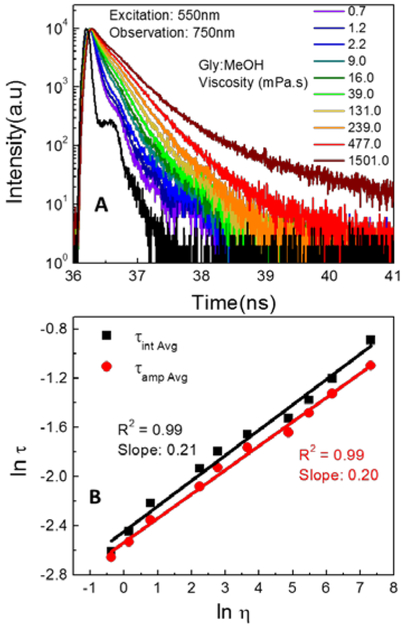
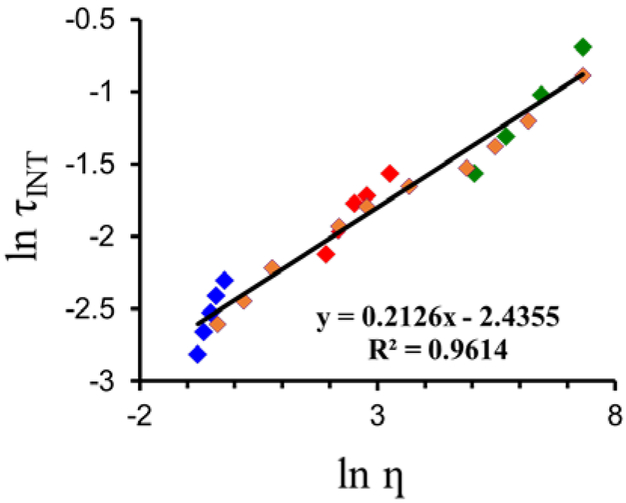
Next, the emission spectra of LDS 798 (Figure 3A) revealed a moderate, viscosity dependent blue-shift of the emission peak: λemmax was 775 nm in low viscosity media (methanol) and 750 nm in high viscosity media (glycerol). This change was associated with a concomitant five-fold change in emission intensity. Furthermore, a five-fold change in QY of the dye showed a good linear correlation as a function of the media’s viscosity (Figure 3B), as expected for a molecular rotor. Because the above experiments showed that viscosity influenced both the emission intensity and consequently the QY of LDS 798, the fluorescence lifetime of LDS 798 was expected to change as well, similar to the situations observed in many fluorescent molecular rotors. Therefore, the fluorescence lifetimes of LDS 798 in the mixtures of glycerol and methanol were measured (Figure 4A), and the linear correlations over the entire viscosity range were noted for average fluorescence lifetimes (Figure 4B). Similar to the QYs, a five-fold change in the lifetime was observed over a range of viscosities, which could be beneficial for using LDS 798 as a viscometer. Notably, the slope (Figure 4B), which could vary from 0.2 to 1.4,(21) appeared to be a good fit for an ideal molecular rotor according to the Förster–Hoffmann theory.(22) Furthermore, it was evident from QY and lifetime measurements that the non-radiative rate (Knr) should change as a function of the media’s viscosity, whereas the radiative rate (Kr) should not change significantly (Figure S1). Due to resistance to internal rotation in the high viscosity environment, the non-radiative rate decreased dramatically (more than 5 times), while the radiative rate did not change much. As such, enhancement in the fluorescence lifetime and the quantum yield of the dye was noted.
Figure 3:
(A) emission spectra of LDS 798 in different glycerol: methanol mixtures as measured without correction for detector sensitivity at 20°C (B) Quantum yield (QY) of LDS 798 as a function of viscosity of the media at 20°C.
Figure 4:
(A) fluorescence intensity decays of LDS 798 in different glycerol: methanol mixtures at room temperature 20°C. (B) Fluorescence lifetime as a function of viscosity of the media at 20°C.
In view of viscosity’s dependence on temperature, it was imperative to evaluate the effect of temperature on the photophysical properties of LDS 798.23 Towards this end, the fluorescence of LDS 798 as a function of temperature in several solvents was measured (Figure S2; Tables S2 – S4). Within this range of temperature (which corresponded to a 20-fold change of viscosity), a 10-fold decrease in intensity, accompanied by a 20 nm red-shift in the emission maximum, was observed (Figure S2).
However, since fluorophores’ intensity variations strongly depend on the concentration of the dye, which could lead to ambiguous results, the fluorescence lifetimes of LDS 798, which are independent of the concentration of the probe, were evaluated in individual solvents as well as their mixtures at various temperatures (Figures 5 and S3). Altering viscosity, either by changing the temperature of the media or by changing the composition of the media, was important in order to establish whether the observed changes in the fluorescence lifetimes were due to viscosity alone or due to specific interactions of the dye with the media. A linear correlation between viscosity and LDS 798’s fluorescence lifetimes was observed (Figures 5, S6). Significantly, similar correlations between the lifetimes of LDS 789 and viscosities, which were tuned either by changing the composition of the media or the temperature (Figure S6), were obtained. This suggested that viscosity was the major factor that affected the fluorescence lifetimes of LDS 798, whereas the effect of temperature might have been insignificant.
Figure 5.
Effect of media’s viscosity on the lifetimes of LDS 798. MeOH, 10–50 °C (blue), ΔT = 10 ° C; glycerol, 20–50 ° C, DT = 10 ° C (green); MeOH/glycerol – 50/50 (v/v), 20–50 °C, DT = 10 ° C (red); MeOH/glycerol – 0/100 to 100/0 (v/v), 20 ° C (orange)
In view of the established ability of LDS 798 to respond to polarity changes in the environment,11 we decided to examine the correlation between the fluorescence lifetimes of LDS 798 and media’s polarity (Figure S7). No significant correlations were noted, which indicated that the fluorescence lifetimes of LDS 798 were not suitable for tracking polarity changes of the media. Spectral shift in LDS 798 can be used to sense polarity.11 This observation provided further support for using fluorescence lifetimes of LDS 798 as a photo-physical property for monitoring viscosity of an environment.
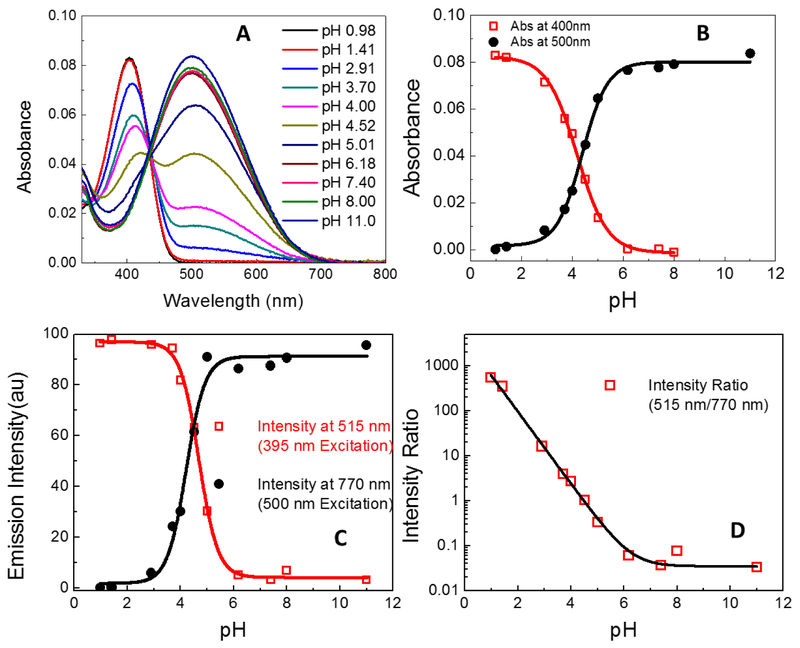
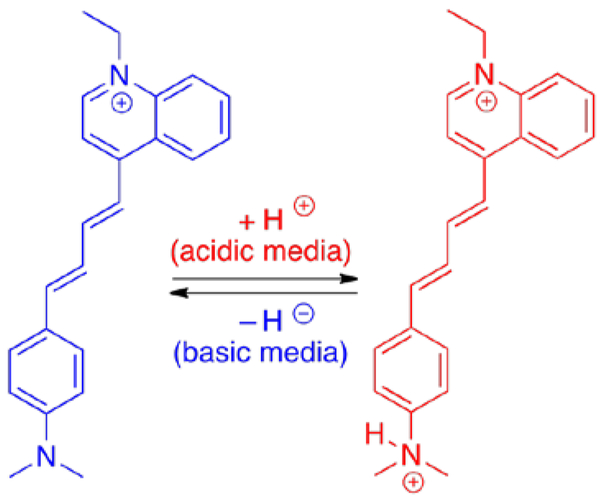
As mentioned previously, different cellular compartments could have different pH ranges, and since LDS 798 has a pH-sensitive dimethylamino-group, this dye could be a potential pH-sensitive probe for various cellular environments. The absorption spectra of LDS 798 in different pH buffers (Figure 7A) exhibited two distinct maxima at 405 nm and 500 nm, which could be attributed to the protonated and neutral forms of LDS 798 (Figure 6). The presence of an isosbestic point (ca. 430 nm) indicated the presence of two distinct forms, with the protonated form exhibiting a distinct absorption peak at 400 nm, and the non-protonated form showing the absoption maximum at 500 nm (Figure 7A).
Figure 7:
(A) Absorbance of LDS 798 at different pH. (B) Deconvoluted absorptions of each species (protonated and non-protonated) as a function of pH. (C) Fluorescence intensity measured at 515 nm and 770nm plotted as a function of pH. (D) Ratio of fluorescence intensity (515 nm to 770 nm) plotted as a function of pH.
Figure 6.
Non-protonated (left) and protonated (right) forms of LDS 798; anions are omitted. The protonation of the neutral form in the acidic environment was confirmed by 1H NMR spectroscopy (Figure S8).
In general, the measured absorption spectra are known to overestimate the contribution from any single species due to spectral overlap. Therefore, each absoption spectrum (Figure 7A) was deconvoluted to measure the net absorption contributed by each species as a function of pH (Figure S4). The corresponding absorption maxima as a function of pH allowed to determine the pKa of LDS 798, which was 4.4 (Figure 7B). It should be noted that the absoprtion spectrum of LDS 798 in pH 4.52 (Figure 7A) exhibited two peaks with about equal absorptions, which indicated that the two species existed in equilibrium.
Considering the significantly higher sensitivity of fluorescence measurements compared to that of absorption measurements, it was important to evaluate how the fluorescence intensity was affected as a function of pH (Figure S4). It appeared that the emission maxima of LDS 798 showed a similar correlation to the absorption maxima in regard to the pH variations, also suggesting the pKa vaule of 4.4 (Figure 7C).
Since ratiometric measurements are less prone to artifacts, and therefore are more reliable than one-wavelength intensity measurements, the ratio of 515/770 nm intensities as a function of pH was evaluated (Figure 7D). It should be noted that two different excitation wavelengths (395 nm and 500 nm) were used to obtain the emission intensities at 515 nm and 770 nm, respectively. It appeared that in acidic media (the most relevant to biological systems) a significant change (> 10,000 fold) of the ratio was noted, whereas in basic media, minimal change of the ratio was observed. Thus, LDS 798 could serve as an extremely sensitive pH probe in acidic environments, such as a lysosomal compartment and outer mitochondrial membrane, and it could also be applied for water quality testings.
Conclusions
In summary, the photophysical properties of LDS 798 dye were found to depend on the viscosity and the pH of the media. Specifically, the lifetimes and the quantum yields of the dye were shown to be directly proportional to viscosity in the absence of polarity changes. Moreover, absorption and fluorescence measurements as a function of pH proved that LDS 798 could be an excellent pH sensor in acidic pH. LDS 798 proved to be a multi-responsive probe. Notably, the changes in the emission and the absorption maxima of LDS 798 should be used to probe the polarity variations of the media. Therefore, a cohort of the photophysical properties of LDS 798 (and potentially other LDS dyes) allows probing various properties of the media. Significantly, since many fluorophores have structural elements that can undergo internal rotation, which might be sensitive to the micro-viscosity variations, a comprehensive evaluation of all properties of the media on the photo-physical responses of the probe must be taken into account.
Supplementary Material
Acknowledgements
This work was supported in part by NSF (CBET 1403226 to S.V.D. and CBET 1264608 to I.G.) and NIH (R21EB017985 and R01EB12003 to Z.G.).
Footnotes
Conflict of interest
There are no conflicts to declare.
Notes and references
- 1.Banerji JC, Mandal AK and Banerjee BK, Dyes Pigments, 1982, 3, 273–280. [Google Scholar]
- 2.Jha BN and Banerji JC, Dyes Pigments, 1985, 6, 213–226. [Google Scholar]
- 3.Jha BN, Jha RK and Banerji JC, Dyes Pigments, 1986, 7, 133–152. [Google Scholar]
- 4.Kawashima Y and Ohtani H, Konica Corporation, European Pat., EP0611109 A1, 1994. [Google Scholar]
- 5.Yang JL, Zhu ZH and Yao ZG, Chem. J. Chinese U, 1990, 11, 286–289. [Google Scholar]
- 6.Betz WJ, Mao F and Bewick GS, J. Neurosci, 1992, 12, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betz WJ, Mao F and Smith CB, Imaging exocytosis and endocytosis. Curr. Opin. Neurobiol, 1996, 6, 365–371. [DOI] [PubMed] [Google Scholar]
- 8.Fluhler E, Burnham VG and Loew LM, Biochemistry, 1985, 24, 5749–5755. [DOI] [PubMed] [Google Scholar]
- 9.Xu C and Loew LM, Biophys. J, 2003, 84, 2768–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luchowski R, Gryczynski Z, Sarkar P, Borejdo J, Szabeldki M, Kapusta P and Gryczynski I, Rev. Sci. Instrum, 2009, 80, 033109. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar P, Luchowski R, Raut S, Sabnis N, Remaley A, Lacko AG, Thamake S, Gryczynski Z and Gryczynski I, Biophys. Chem, 2010, 153, 61–69. [DOI] [PubMed] [Google Scholar]
- 12.Raut S, Kimball J, Fudala R, Doan H, Maliwal B, Sabnis N, Lacko AG, Gryczynski I, Dzyuba SV and Gryczynski Z, Phys. Chem. Chem. Phys, 2014, 16, 27037–27042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimball JD, Raut S, Jameson LP, Smith NW, Gryczynski Z and Dzyuba SV, RSC Adv, 2015, 5, 19508–19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L Raut S, Kimball JD, Fudala R, Bora I, Chib R, Jaafari H, Castillo MK, Smith NW, Gryczynski I, Dzyuba SV and Gryczynski Z, Phys. Chem. Chem. Phys, 2016, 18, 4535–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klymchenko AS, Acc. Chem. Res, 2017, 50, 366–375. [DOI] [PubMed] [Google Scholar]
- 16.Zhu H, Fan J, Du J and Peng X, Acc. Chem. Rsc, 2016, 49, 2115–2126. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z, Cao J, He Y, Yang JH, Kim T, Peng X and Kim JS, Chem. Soc. Rev, 2014, 43, 4563–4601. [DOI] [PubMed] [Google Scholar]
- 18.Kuimova MK, Phys. Chem. Chem. Phys, 2012, 14, 12671–12686. [DOI] [PubMed] [Google Scholar]
- 19.Demaurex N, News Physiology Sci, 2002, 17, 1–5. [DOI] [PubMed] [Google Scholar]
- 20.Paroutis P, Touret N and Grinstein S. Physiology, 2004, 19, 207–215. [DOI] [PubMed] [Google Scholar]
- 21.Magde D, Brannon JH, Cremers TL and Olmsted J, J. Phys. Chem, 1979, 83, 696–699. [Google Scholar]
- 22.Kaschke M, Kleinschmidt J and Wilhelmi B, Laser Chem, 1985, 5, 119–132. [Google Scholar]
- 23.Wu Y, Štefl M, Olzyńska A, Hof M, Yahioglu G, Yip P, Casey DR, Ces O, Humpolíčková J and Kuimova MK, Phys. Chem. Chem. Phys, 2013, 15, 14986–14993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.