Abstract
Background:
Muscle sampling is often used as a surrogate for staging quality in patients with bladder cancer. We examined the association of staging quality at diagnosis and survival among patients with bladder cancer.
Methods:
We reviewed the clinical records of all individuals within the Los Angeles SEER Registry with an incident diagnosis of non-muscle-invasive bladder cancer in 2004–2005. We recorded patient demographics, tumor characteristics, staging quality (presence of muscle in the specimen and mention of muscle in the pathology report), and vital status. Using mixed-effects and competing-risks regression analyses, we quantified the association of patient and tumor characteristics on staging quality and cancer-specific survival.
Results:
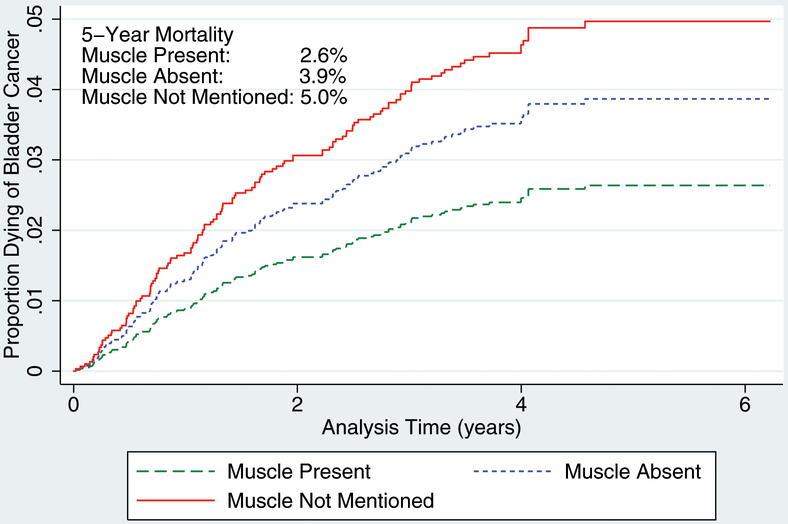
Our sample included 1,865 patients, 335 urologists, and 278 pathologists. Muscle was reported as present in 972 (52.1%), reported as absent in 564 (30.2%), and was not mentioned in 329 (17.7%) of the initial pathology reports. The presence of muscle did not differ according to grade or depth of invasion. Mortality was associated with staging quality (p<0.05). Among patients with high-grade disease, 5-year cancer-specific mortality was 8.0%, 13.0%, and 21.5%, respectively, when muscle was present, absent, or not mentioned.
Conclusions:
The omission of muscle in the specimen or its mention in the pathology report in nearly half of all diagnostic resections is associated with increased mortality, particularly in patients with high-grade disease. Because urologists cannot reliably discern between high- and low-grade or Ta and T1 disease, we contend that patients with bladder cancer should undergo adequate muscle sampling at the time of endoscopic resection.
Keywords: Urinary Bladder Neoplasms, Bladder Cancer Mortality, Quality of Healthcare, Pathology
Precis:
We reviewed the medical records of 1865 patients with bladder cancer in Los Angeles County and found that suboptimal staging in nearly half of all diagnostic resections was associated with increased mortality, particularly in patients with high-grade disease.
INTRODUCTION
Despite the vertiginous pace of technological advancement in medicine, treatment of patients with bladder cancer remains largely dependent on the unique anatomic properties of the bladder—being readily accessible to visual inspection, endoscopic resection, and instillation of intravesical agents. Bladder cancer treatment guidelines emphasize “careful inspection of the whole urothelial lining” and “remov[al] of all visible lesions” by transurethral resection of bladder tumors (TURBT).1, 2 At diagnosis, the resection should include “the underlying bladder wall with the detrusor muscle,” in order to “adequately address the depth of invasion.”1, 2 Muscle invasion is the quintessential biomarker—diagnostic and prognostic. Muscle-invasive bladder cancer portends a much worse prognosis than non-muscle-invasive disease and does not respond to intravesical therapy. Upon the diagnosis of muscle invasion, receiving definitive treatment in a timely fashion is associated with improved survival and less advanced pathological stage.3, 4 This survival benefit is also conferred upon those with high-grade T1 lesions, likely because patients receive surgical attention before muscle invasion occurs.5, 6 Therefore, knowing whether the tumor invades the muscle is crucial to decision making (i.e., conservative vs aggressive treatment).
However, previous studies have found that up to half of diagnostic TURBTs do not contain muscle; further, Dalbagni et al found that 16% of restaging TURBTs still lacked this prognostic indicator.7, 8 While urologists can usually discern malignant from benign lesions on cystoscopy,9, 10 they cannot accurately identify muscle-invasion by cystoscopy;10 and hence, pathologic confirmation is necessary. Additionally, pathologic analysis of urothelial carcinoma is fraught with inter- and intra-observer unreliability;11–15 as many as 55% of pT1 reports are restaged following central histopathological review,12 while pathologists faced twice with the same specimen reach the same conclusion only 62% of the time.11
In order to characterize practice patterns of diagnostic staging at the population level, and to understand whether they affect survival, we analyzed the clinical records of 1,865 patients from the population-based Los Angeles SEER registry. Previous studies characterizing quality of diagnostic resection have been limited to single institutions or small multicenter analyses; we sought to understand whether the proportion of TURBTs containing muscle would be consistent at the population level. Further, we examined pathologic reports to understand whether detrusor muscle would be mentioned if absent. Third, we sought to characterize the sociodemographic and practice characteristics associated with worse outcomes. Finally, in a process-outcomes link, we examined the association of TURBT and pathologic quality—the presence or mention of detrusor—with mortality. We hypothesized that a better quality resection and appropriate pathologic reporting would be associated with improved survival.
MATERIALS AND METHODS
Data Source
The National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) cancer registries collect information on all incident cancers occurring in 17 defined geographic regions. To obtain information on adjuvant treatment or utilization of services that are not necessarily amenable to claims data, NCI annually conducts Patterns of Care (POC) studies on selected cancer sites. In combination with POC, a Rapid Response Surveillance Study was conducted to quantify adequacy of diagnostic staging and quality of pathology reporting—as they too are outside the scope of standard SEER abstraction. The Los Angeles Cancer Surveillance Program, the SEER cancer registry for Los Angeles County, routinely collects pathology reports of all incident cancer cases in Los Angeles County. Using subject and registration identification numbers, collection of operative reports and chart abstraction were then linked with SEER longitudinal data to quantify survival. The Los Angeles Cancer Surveillance Program, California Cancer Registry, and UCLA obtained institutional review board approval before initiating the study. Trained Certified Tumor Registrar (CTR) abstractors were responsible for reviewing outpatient and hospital records to verify urothelial carcinoma characteristics and demographic information. Additionally, each patient’s physician was asked to indicate all treatments including chemotherapy and novel agents. For quality control, 5% of records were re-abstracted.
Study Population
We reviewed the consecutive records of all 1,865 residents of Los Angeles County diagnosed with non-muscle-invasive (stage Ta, Tis and T1) urothelial carcinoma of the bladder (International Classification of Diseases, Oncology, 3rd revision [ICD-O-3] Site code: C67 and histology codes 8120 or 8130) in 2004 and 2005. Patients with a diagnosis of bladder cancer found on autopsy or on death certificate, or who were under age 18 were ineligible for the study. We also restricted our analysis to those patients with known grade, stage, and histology for whom operative and pathologic reports were available. Patients were excluded if their operative or pathologic reports were unavailable.
Study Variables
From chart abstraction, we collected information on the following variables: NCI-designated cancer center, operating urologist, reporting pathologist, quality of staging, and TNM staging. The data fields were selected in an a priori fashion. However, the term and label used to describe each data field was continually revised in an iterative process, as a result of the significant amount of variation in terminology used in the operative and pathology reports. The data were then merged with the SEER database to ascertain patient demographics (age, gender, race/ethnicity, marital status, socioeconomic status, insurance type), tumor characteristics (SEER stage and grade), and follow-up information. Adequacy of staging was stratified by the presence, absence, or mention of detrusor muscle in the pathology report. Trained abstractors searched for alternative nomenclature for level of invasion (e.g., stroma, angiolymphatic/lymphovascular space).
Statistical Analysis
Correlation between categorical variables and the presence or mention of muscle was derived by χ2 analyses. Likelihood of the presence or mention of muscle was generated by multivariate mixed-effects logistic regression analysis. This multivariate model determined the association of staging quality with patient demographics and tumor characteristics. Because receipt of staging quality and pathology reporting may be clustered on the operating urologist and reporting pathologist, respectively, we generated multilevel logistic regression models for each measure (e.g., presence of muscle attributed to the urologist, mention of muscle attributed to the pathologist) to account for both fixed and random effects associated with diagnostic staging. Each model included patient age, gender, race/ethnicity, marital status, socioeconomic status (quintiles), SEER grade and stage, and institution type as fixed terms, while each unique surgeon and pathologist was appended to the random effects part of the multilevel model. With the existing sample size of 1865 patients nested within 335 providers, an alpha of 0.05, and a power of 80%, our mixed-effects logistic regression model will have the capability to detect a 30% difference in the odds ratio.
Because patients may die from non-cancer causes, we utilized a maximum likelihood, competing-risks regression model as described by Fine and Gray to determine bladder cancer-related mortality rates.16 We defined the event of interest as bladder cancer-related death, while the competing event was defined as non-cancer-related death. This model adjusted for patient age, gender, race/ethnicity, marital status, socioeconomic status, SEER grade and stage, institution type, and quality of staging. Estimates are reported as sub-hazard ratios (HR) with corresponding 95% confidence intervals. Additionally, since patients treated by the same provider may have similar outcomes, we accounted for clustering by utilizing the Huber-White sandwich variance estimator to the competing-risks regression analysis to produce more conservative confidence intervals.17, 18 While competing-risks regression analyses do not have a goodness-of-fit statistic, we utilized the Cox model as a proxy. We confirmed non-violation of the proportional hazards assumption using “log-log” plots. A post-estimation function after the competing-risks regression model was utilized to generate cumulative incidence curves of bladder cancer-related mortality rates.
We conducted all analyses with STATA software 13.1 (Stata, College Station, Texas). All statistical tests were 2-tailed, and the probability of a type I error was set at 0.05.
RESULTS
The median age of our cohort was 73 years. The majority was male (76.5%), White (69.8%), and married (64.5%), with a moderately differentiated (47.1%), stage Ta (60.7%) tumor (Table 1). At diagnostic TURBT, detrusor muscle was present in 972 patients (52.1%), absent in 564 (30.2%), and was not mentioned in 329 (17.7%).
Table 1.
Cohort Characteristics (N=1865)
| Variables | Proportion (%) |
|---|---|
| Age | |
| ≤55 | 233 (12.5%) |
| 56–65 | 326 (17.5%) |
| 66–75 | 530 (28.4%) |
| 76–85 | 583 (31.3%) |
| >85 | 193 (10.3%) |
| Gender | |
| Male | 1427 (76.5%) |
| Female | 438 (23.5%) |
| Race | |
| White | 1301 (69.8%) |
| Black | 133 (7.1%) |
| Hispanic | 235 (12.6%) |
| Other | 196 (10.5%) |
| Marital Status | |
| Other | 662 (35.5%) |
| Married | 1203 (64.5%) |
| SES | |
| Highest | 601 (32.2%) |
| High Middle | 475 (25.5%) |
| Middle | 317 (17.0%) |
| Low Middle | 297 (15.9%) |
| Lowest | 175 (9.4%) |
| Insurance | |
| HMO/PPO | 806 (43.2%) |
| Medicare | 716 (38.4%) |
| Medicaid | 73 (3.9%) |
| County | 211 (11.3%) |
| Other | 59 (3.2%) |
| SEER T-Stage | |
| Ta | 1133 (60.7%) |
| Tis | 70 (3.8%) |
| T1 | 662 (35.5%) |
| SEER Grade | |
| Well Differentiated | 302 (16.2%) |
| Moderately Differentiated | 878 (47.1%) |
| Poorly Differentiated | 381 (20.4%) |
| Undifferentiated | 304 (16.3%) |
| NCI-Designated Cancer Center | |
| No | 1719 (92.2%) |
| Yes | 146 (7.8%) |
| Detrusor Muscle | |
| Present | 972 (52.1%) |
| Not Present | 564 (30.2%) |
| Not mentioned | 329 (17.7%) |
Abbreviations: SES=Socioeconomic status; NCI= National Cancer Institute; HMO=Health Maintenance Organization; PPO=Preferred Provider Organization
Bivariable analysis demonstrated that poor staging quality was significantly associated with female gender (p<0.01), Black or Other race (p=0.03), unmarried (p=0.01), lower SES (p<0.01), lower stage (Ta, Tis; p<0.01), lower grade (p<0.01), and treatment at a non-NCI-designated Cancer Center facility (p<0.01) (Table 2).
Table 2:
Bivariable analysis with quality of staging
| Muscle Present | Muscle Absent | Muscle not Mentioned | p-value | |
|---|---|---|---|---|
| Age | 0.24 | |||
| ≤55 | 104 (44.6%) | 85 (36.5%) | 44 (18.9%) | |
| 56–65 | 173 (53.1%) | 102 (31.3%) | 51 (15.6%) | |
| 66–75 | 285 (53.8%) | 146 (27.5%) | 99 (18.7%) | |
| 76–85 | 307 (52.7%) | 169 (29.0%) | 107 (18.3%) | |
| >85 | 103 (53.4%) | 62 (32.1%) | 28 (14.5%) | |
| Gender | <0.01 | |||
| Male | 775 (54.3%) | 411 (28.8%) | 241 (16.9%) | |
| Female | 197 (45.0%) | 153 (34.9%) | 88 (20.1%) | |
| Race/Ethnicity | 0.03 | |||
| White | 706 (54.3%) | 384 (29.5%) | 211 (16.2%) | |
| Black | 62 (46.6%) | 43 (32.3%) | 28 (21.1%) | |
| Hispanic | 120 (51.0%) | 73 (31.1%) | 42 (17.9%) | |
| Other | 84 (42.9%) | 64 (32.6%) | 48 (24.5%) | |
| Marital Status | 0.01 | |||
| Other | 316 (47.7%) | 213 (32.2%) | 133 (20.1%) | |
| Married | 656 (54.5%) | 351 (29.2%) | 196 (16.3%) | |
| SES | <0.01 | |||
| Highest | 336 (55.9%) | 182 (30.3%) | 83 (13.8%) | |
| High Middle | 258 (54.3%) | 126 (26.5%) | 91 (19.2%) | |
| Middle | 147 (46.4%) | 106 (33.4%) | 64 (20.2%) | |
| Low Middle | 156 (52.5%) | 90 (30.3%) | 51 (17.2%) | |
| Lowest | 75 (42.8%) | 60 (34.3%) | 40 (22.9%) | |
| Insurance | 0.55 | |||
| HMO/PPO | 407 (50.5%) | 249 (30.9%) | 150 (18.6%) | |
| Medicare | 374 (52.3%) | 225 (31.4%) | 117 (16.3%) | |
| Medicaid | 38 (52.0%) | 21 (28.8%) | 14 (19.2%) | |
| County | 124 (58.8%) | 51 (24.2%) | 36 (17.0%) | |
| Other | 29 (49.2%) | 18 (30.5%) | 12 (20.3%) | |
| SEER T-Stage | <0.01 | |||
| Ta | 544 (48.0%) | 333 (29.4%) | 256 (22.6%) | |
| Tis | 25 (35.7%) | 26 (37.1%) | 19 (27.2%) | |
| T1 | 403 (60.9%) | 205 (31.0%) | 54 (8.1%) | |
| SEER Grade | <0.01 | |||
| Well Differentiated | 139 (46.0%) | 81 (26.8%) | 82 (27.2%) | |
| Moderately Differentiated | 450 (51.3%) | 269 (30.6%) | 159 (18.1%) | |
| Poorly Differentiated | 215 (56.4%) | 111 (29.1%) | 55 (14.5%) | |
| Undifferentiated | 168 (55.3%) | 103 (33.9%) | 33 (10.8%) | |
| NCI-Designated Cancer Center | <0.01 | |||
| No | 876 (50.9%) | 529 (30.8%) | 314 (18.3%) | |
| Yes | 96 (65.7%) | 35 (24.0%) | 15 (10.3%) |
Abbreviations: SES=Socioeconomic status; NCI=National Cancer Institute; HMO=Health Maintenance Organization; PPO=Preferred Provider Organization
Because patient characteristics may be associated with diagnostic staging or pathology report quality, we performed a mixed-effects logistic regression analysis in order to understand the associations with the presence or mention of muscle in diagnostic TURBT (Table 3). Better staging (i.e., presence of muscle) was associated with advanced age (66–75 years: OR 1.60, 95% CI 1.02–2.50; 76–85 years: OR 1.59, 95% CI 1.03–2.47) and male gender (OR 1.41, 95% CI 1.05–1.89). Compared with patients with non-invasive tumors (i.e., Ta lesions), urologists appropriately sampled muscle less often in patients with Tis (OR 0.46, 95% CI 0.24–0.92). However, T1 tumors, which have a significantly higher rate of being upstaged to muscle-invasive disease, were no more likely to have muscle in the specimen (OR 1.12, 95% CI 0.83–1.50). Pathologists, however, did report on the absence or presence of muscle (i.e., mention of muscle) among patients diagnosed with T1 lesions (OR 3.45, 95% CI 2.19–5.43), moderately differentiated (OR 1.57, 95% CI 1.02–2.42) and poorly differentiated (OR 1.84, 95% CI 1.03–3.29) tumors.
Table 3:
Mixed-effects logistic regression analysis predicting presence or mention of muscle at diagnosis
| Muscle Present | Muscle Mentioned | |
|---|---|---|
| Variables | OR (95% CI) | OR (95% CI) |
| Age | ||
| ≤55 | 1.00 (referent) | 1.00 (referent) |
| 56–65 | 1.40 (0.91–2.18) | 1.19 (0.66–2.14) |
| 66–75 | 1.60 (1.02–2.50)* | 0.73 (0.41–1.29) |
| 76–85 | 1.59 (1.03–2.47)* | 0.76 (0.43–1.33) |
| >85 | 1.67 (0.97–2.86) | 1.35 (0.64–2.85) |
| Gender | ||
| Male | 1.00 (referent) | 1.00 (referent) |
| Female | 0.71 (0.53–0.95)* | 0.97 (0.66–1.42) |
| Race/Ethnicity | ||
| White | 1.00 (referent) | 1.00 (referent) |
| Black | 0.82 (0.50–1.37) | 0.63 (0.33–1.22) |
| Hispanic | 1.04 (0.70–1.53) | 1.08 (0.65–1.81) |
| Other | 0.81 (0.51–1.27) | 0.96 (0.52–1.75) |
| Marital Status | ||
| Other | 1.00 (referent) | 1.00 (referent) |
| Married | 1.12 (0.86–1.46) | 1.38 (0.97–1.96) |
| SES | ||
| Highest | 1.00 (referent) | 1.00 (referent) |
| High Middle | 1.23 (0.89–1.71) | 0.93 (0.60–1.44) |
| Middle | 0.82 (0.57–1.18) | 0.76 (0.46–1.24) |
| Low Middle | 1.01 (0.68–1.49) | 1.10 (0.64–1.88) |
| Lowest | 0.62 (0.38–1.01) | 0.79 (0.43–1.48) |
| Insurance | ||
| HMO/PPO | 1.00 (referent) | 1.00 (referent) |
| Medicare | 0.98 (0.71–1.36) | 1.19 (0.78–1.83) |
| Medicaid | 1.43 (0.73–2.82) | 1.04 (0.43–2.54) |
| County | 1.30 (0.83–2.04) | 1.45 (0.80–2.63) |
| Other | 1.26 (0.61–2.60) | 0.37 (0.14–0.95)* |
| SEER T-Stage | ||
| Ta | 1.00 (referent) | 1.00 (referent) |
| Tis | 0.47 (0.24–0.92)* | 0.75 (0.35–1.62) |
| T1 | 1.12 (0.83–1.50) | 3.45 (2.19–5.43)* |
| SEER Grade | ||
| Well Differentiated | 1.00 (referent) | 1.00 (referent) |
| Moderately Differentiated | 1.04 (0.72–1.51) | 1.57 (1.02–2.42)* |
| Poorly Differentiated | 1.12 (0.71–1.76) | 1.84 (1.03–3.29)* |
| Undifferentiated | 0.97 (0.60–1.55) | 1.49 (0.76–2.92) |
| NCI-Designated Cancer Center | ||
| No | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.53 (0.84–2.79) | 1.16 (0.43–3.13) |
Abbreviations: 95% CI=95% Confidence Interval; SES=Socioeconomic status; NCI=National Cancer Institute; HMO=Health Maintenance Organization; PPO=Preferred Provider Organization
Random intercept for presence of muscle=Operating Urologist
Random intercept for mention of muscle=Reporting Pathologist
denotes statistically significant with p<0.05
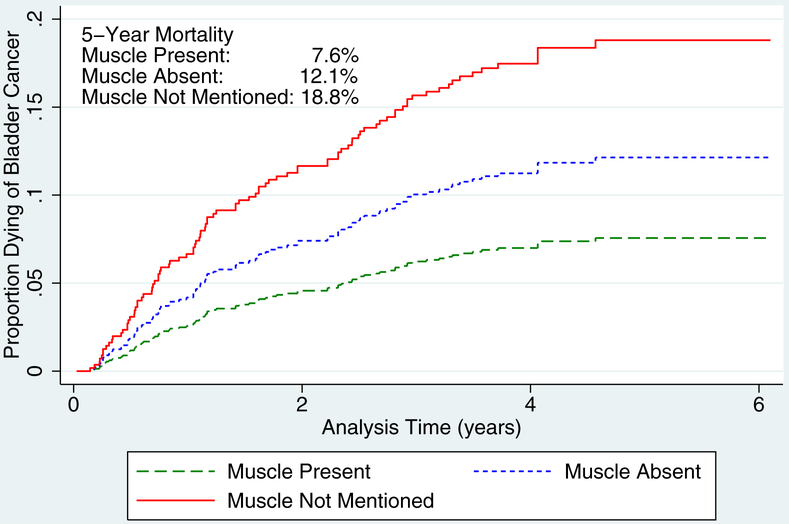
In order to establish a process-outcomes link, we performed a competing-risks regression analysis of bladder cancer death for all patients and for those with high-grade disease (Table 4)., When compared with those patients that were adequately staged, the incidence of bladder cancer mortality is significantly higher when muscle was absent (HR 1.48, 95% CI 1.00–2.18) or when muscle was not mentioned (HR 1.91, 95% CI 1.12–3.24). When limited to those with high-grade disease, the effect size of quality of staging was even more significant—muscle absent (HR 1.64, 95% CI 1.05–2.57) muscle not mentioned (HR 2.65, 95% CI 1.40–5.02). The 5-year mortality for those with high-grade lesions was 7.6% when muscle was present, 12.1% when muscle was absent, and 18.8% when muscle was not mentioned (Figure 1b).
Table 4:
Bladder cancer-specific mortality
| All grades | High-grade | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Variables | ||
| Age | ||
| ≤55 | 1.00 (referent) | 1.00 (referent) |
| 56–65 | 1.63 (0.44–6.00) | 1.70 (0.37–7.75) |
| 66–75 | 3.16 (0.91–11.03) | 2.91 (0.66–12.84) |
| 76–85 | 5.38 (1.56–18.55)* | 5.21 (1.19–22.76)* |
| >85 | 6.63 (1.87–23.50)* | 5.53 (1.15–26.64)* |
| Gender | ||
| Male | 1.00 (referent) | 1.00 (referent) |
| Female | 1.20 (0.77–1.88) | 1.28 (0.76–2.16) |
| Race | ||
| White | 1.00 (referent) | 1.00 (referent) |
| Black | 1.31 (0.65–2.62) | 1.54 (0.65–3.65) |
| Hispanic | 0.79 (0.41–1.53) | 0.58 (0.25–1.31) |
| Other | 0.82 (0.49–1.38) | 0.90 (0.47–1.72) |
| Marital Status | ||
| Other | 1.00 (referent) | 1.00 (referent) |
| Married | 0.79 (0.51–1.22) | 0.74 (0.44–1.24) |
| SES | ||
| Highest | 1.00 (referent) | 1.00 (referent) |
| High Middle | 1.59 (0.97–2.59) | 1.25 (0.70–2.24) |
| Middle | 1.38 (0.73–2.61) | 1.09 (0.49–2.40) |
| Low Middle | 1.43 (0.79–2.58) | 1.32 (0.66–2.67) |
| Lowest | 1.40 (0.68–2.90) | 1.08 (0.42–2.78) |
| Insurance | ||
| HMO/PPO | 1.00 (referent) | 1.00 (referent) |
| Medicare | 1.20 (0.80–1.81) | 1.10 (0.68–1.78) |
| Medicaid | 0.99 (0.28–3.45) | 0.92 (0.19–4.38) |
| County | 0.82 (0.41–1.61) | 0.52 (0.23–1.15) |
| Other | 1.34 (0.44–4.11) | 1.65 (0.50–5.46) |
| SEER T-Stage | ||
| Ta | 1.00 (referent) | 1.00 (referent) |
| Tis | 3.52 (1.44–8.57)* | 3.36 (0.97–11.63) |
| T1 | 5.05 (3.00–8.50)* | 5.70 (2.59–12.71)* |
| SEER Grade | ||
| Well Differentiated | 1.00 (referent) | --- |
| Moderately Differentiated | 2.00 (0.69–5.85) | --- |
| Poorly Differentiated | 4.73 (1.67–13.39)* | 1.00 (referent) |
| Undifferentiated | 4.24 (1.47–12.22)* | 0.96 (0.58–1.60) |
| NCI-Designated Cancer Center | ||
| No | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.22 (0.68–2.19) | 1.13 (0.51–2.49) |
| Quality of Staging | ||
| Muscle present | 1.00 (referent) | 1.00 (referent) |
| Muscle absent | 1.48 (1.00–2.18)* | 1.64 (1.05–2.57)* |
| Muscle not mentioned | 1.91 (1.12–3.24)* | 2.65 (1.40–5.02)* |
Abbreviations: 95% CI=95% Confidence Interval; SES=Socioeconomic status; NCI=National Cancer Institute; HMO=Health Maintenance Organization; PPO=Preferred Provider Organization
denotes statistically significant with p<0.05
Figure 1.
Bladder cancer–specific mortality: (A) all grades and (B) high grade.
DISCUSSION
Our study has three principal findings. First, we found that a significant proportion of TURBTs are of suboptimal quality: only half report the presence of muscle in the pathology report. While other analyses have reported similar results,8, 19 this finding has not been previously corroborated at a population level. Although invasion of detrusor muscle is the most crucial predictor of treatment response and whether aggressive treatments should be offered,3–6 it is impossible for the patient and physician to proceed when muscle is not even sampled. Dalbagni et al and others have associated surgeon experience with improved outcomes; increased attention to resection quality in training programs may reduce this gap and offers one avenue for improvement.
In addition to resection quality, pathology reports are also often remiss: 37.0% of the resections without muscle failed to mention its absence. Given the central role of detrusor invasion in treatment decisions, urologists and pathologists should ensure that the presence or absence of muscle is consistently noted. While analysis is complicated by the unreliability of urothelial carcinoma pathology,11–14 policymakers and reimbursement modeling should implement quality criteria to improve the reliability of these results. This would offer a stronger foundation upon which urologists can construct an appropriate treatment plan.
Second, we showed that staging quality is associated with specific sociodemographic characteristics. Muscle was more likely to be present with advancing age and among men. It is unclear why there was an association between quality of resections in women and among younger patients. This association may be attributed to confounding, interaction, clustering, or chance alone. Mention of muscle was more likely in stage T1 and higher-grade (moderately and poorly differentiated) tumors. Pathologists rightly mention muscle when there is a higher probability of invasion (stage T1, high-grade). Clinicians use these results to inform treatment decisions.
Finally, we demonstrated a process-outcomes link between quality of diagnostic staging and survival. In both general and subgroup analyses, mortality was highest among the elderly with higher-stage lesions, in whom diagnostic staging lacked muscle (absent or not mentioned). Further, analysis of 5-year mortality rates demonstrated a mortality gradient when detrusor was present, absent but mentioned (1.5-fold increase), and absent but not mentioned (1.9-fold increase); this gradient was augmented among those with high-grade disease (1.6-fold and 2.5-fold, respectively). On subset analysis, we found that the increased risk of mortality due to inadequate staging was primarily attributed to patients with lamina propria invasion.
The reasons for the survival disadvantage among those with no mention of muscle are not clearly apparent. In order to address this, we propose a modification to the AJCC staging criteria that will highlight the absence of detrusor from the pathology sample. Where muscle is absent from the biopsy sample, we propose the addition of an “x” to the AJCC criteria: when absent, pTa, pTis, or pT1 would instead be denoted as pTax, pTisx, or pT1x, respectively. This revision would serve as a two-way benchmark: first, for the urologist to aim for sampling of the detrusor, and second, for the pathologist to analyze and document the presence of detrusor in the sample. Therefore, this benchmark would not only highlight the presence or absence of detrusor, but would also serve to enhance the communication between urologist and pathologist.
Despite a large sample size and robust statistical methodology, our study is subject to methodological limitations. As with any observational study, omitted-variable bias may have impacted the pathology results. In particular, some may contend that detrusor muscle may not need to be necessarily for smaller less aggressive lesions. While this may be true in certain circumstances, whereby anatomic differences would preclude more thorough staging, this does not diminish the strength of our findings, namely an association between staging quality and outcomes. Another limitation may be the unmeasured correlation between quality of the institution (including the physician or pathologist) with staging quality. That is, centers of excellence may be more likely to adequately stage a patient, but the improved outcomes may be attributed to the institutional quality and not necessarily the quality of the resection. However, we did perform a mixed-effects model, which accounted for the surgeon, pathologist, and the institution. Our study was also limited by the lack of information on comorbidity or tobacco use. While those factors may contribute to receipt of more complex treatments such as chemotherapy or surgery, they are not likely to influence the diagnostic staging. Although our analysis demonstrates a strong relationship between pathology results and mortality, further analysis is necessary to examine whether restaging TURBTs are performed when indicated. Restaging resections should be performed for each diagnostic TURBT that lacks muscle, whether or not its absence is mentioned. As previously shown by others,20 we suspect that this is not the case for our cohort—thus our proposal to modify the AJCC criteria to denote the absence of detrusor. In fact, we found that the incidence of restaging TURBT was low (12–15%) and not significantly influenced by the presence of muscle (p>0.05; data not shown). Finally, it is entirely possible that among the 17% of pathology reports that did not have muscle mentioned in the pathology report, a proportion in fact did have muscle present. Unfortunately, we do not have access to those slides. Nevertheless, the fact that the risk of bladder cancer mortality is highest in this group suggests that most of the time muscle was absent and the urologist falsely assumed that staging was adequate.
While non-muscle-invasive bladder cancer continues to present diagnostic challenges, we have identified two areas for improvement: (1) that urologist training should emphasize TURBT quality by improving the proportion of diagnostic procedures containing muscle; and (2) that urologists and pathologists should more clearly communicate regarding resection pathology, whether through modified staging criteria, closer partnerships or both.
CONCLUSIONS
Nearly half of all diagnostic TURBTs in our population-based series did not include muscle. This omission is associated with increased mortality, particularly in patients with high-grade disease. While most of the morbidity is attributable to those with high-grade or T1 disease, we found that urologists cannot discern between high- and low-grade or Ta and T1 disease. Against this backdrop, we contend that all patients with bladder cancer should undergo endoscopic resection that includes detrusor muscle sampling followed by accurate pathology reporting.
Acknowledgments
This work was supported by the National Institutes of Health Loan Repayment Program (L30 CA154326 (Principal Investigator: KC)); STOP Cancer Foundation (Principal Investigator: K.C.), and the Rapid Response Surveillance Study through the NCI (Principal Investigator: K.C. and D.D.).
Footnotes
Disclosures: The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862–01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Conflict of Interest: None
REFERENCES
- 1.Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64: 639–653. [DOI] [PubMed] [Google Scholar]
- 2.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178: 2314–2330. [DOI] [PubMed] [Google Scholar]
- 3.Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS, Project UDiA. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer. 2009;115: 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003;169: 110–115; discussion 115. [DOI] [PubMed] [Google Scholar]
- 5.Badalato GM, Gaya JM, Hruby G, et al. Immediate radical cystectomy vs conservative management for high grade cT1 bladder cancer: is there a survival difference? BJU Int. 2012;110: 1471–1477. [DOI] [PubMed] [Google Scholar]
- 6.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol. 2001;166: 1296–1299. [PubMed] [Google Scholar]
- 7.Mariappan P, Finney SM, Head E, et al. Good quality white-light transurethral resection of bladder tumours (GQ-WLTURBT) with experienced surgeons performing complete resections and obtaining detrusor muscle reduces early recurrence in new non-muscle-invasive bladder cancer: validation across time and place and recommendation for benchmarking. BJU Int. 2012;109: 1666–1673. [DOI] [PubMed] [Google Scholar]
- 8.Dalbagni G, Vora K, Kaag M, et al. Clinical outcome in a contemporary series of restaged patients with clinical T1 bladder cancer. Eur Urol. 2009;56: 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herr HW. The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol. 1999;162: 74–76. [DOI] [PubMed] [Google Scholar]
- 10.Cina SJ, Epstein JI, Endrizzi JM, Harmon WJ, Seay TM, Schoenberg MP. Correlation of cystoscopic impression with histologic diagnosis of biopsy specimens of the bladder. Hum Pathol. 2001;32: 630–637. [DOI] [PubMed] [Google Scholar]
- 11.Richards B, Parmar MK, Anderson CK, et al. Interpretation of biopsies of “normal” urothelium in patients with superficial bladder cancer. MRC Superficial Bladder Cancer Sub Group. Br J Urol. 1991;67: 369–375. [DOI] [PubMed] [Google Scholar]
- 12.Compérat E, Egevad L, Lopez-Beltran A, et al. An interobserver reproducibility study on invasiveness of bladder cancer using virtual microscopy and heatmaps. Histopathology. 2013;63: 756–766. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez RE, Keane TE, Hardy HT, Amin MB. pT1 urothelial carcinoma of the bladder: criteria for diagnosis, pitfalls, and clinical implications. Adv Anat Pathol. 2000;7: 13–25. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Beltran A, Cheng L. Stage pT1 bladder carcinoma: diagnostic criteria, pitfalls and prognostic significance. Pathology. 2003;35: 484–491. [DOI] [PubMed] [Google Scholar]
- 15.Maruniak NA, Takezawa K, Murphy WM. Accurate pathological staging of urothelial neoplasms requires better cystoscopic sampling. J Urol. 2002;167: 2404–2407. [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94: 496–509. [Google Scholar]
- 17.White H A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48: 817–830. [Google Scholar]
- 18.Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Berkeley, CA: University of California Press, 1967. [Google Scholar]
- 19.Mariappan P, Zachou A, Grigor KM, Group EU-O. Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur Urol. 2010;57: 843–849. [DOI] [PubMed] [Google Scholar]
- 20.Skolarus TA, Ye Z, Montgomery JS, et al. Use of restaging bladder tumor resection for bladder cancer among Medicare beneficiaries. Urology. 2011;78: 1345–1349. [DOI] [PubMed] [Google Scholar]