Abstract
Background
It is not known how cardiac functions are affected during anaphylaxis.
Objective
Our aim was to measure the cardiac functions shortly after an anaphylaxis attack using a new technique that detects subclinical left ventricular dysfunction.
Methods
Patients in our hospital who experienced anaphylaxis and urticaria (control group) due to any cause were included in the study. Tryptase levels were measured on the third hour of the reaction and 6 weeks later. Left ventricular systolic functions were evaluated with global strain measurement using echocardiography, approximately 4 hours and 6-week post reaction.
Results
Twelve patients were included in the anaphylaxis group (83.3% female; mean age, 43.25 ± 9.9 years). The causes of anaphylaxis were drug ingestion (n = 11) and venom immunotherapy. Eight of the anaphylactic reactions (66.7%) were severe and in 9 reactions (75%) tryptase levels increased. In the anaphylaxis group, strain values measured shortly after anaphylaxis were significantly lower than those calculated 6 weeks later (p < 0.001) and tryptase levels significantly increased (p = 0.002). The strain values measured both shortly after anaphylaxis and 6 weeks later did not differ according to severity of anaphylaxis. In severe anaphylaxis, tryptase levels during anaphylaxis and 6 weeks later were significantly higher (p = 0.019, p = 0.035). The control group evidenced no differences regarding strain and tryptase levels measured at reaction and 6 weeks later. At reaction, in the anaphylaxis group, the tryptase levels were higher and the strain values were lower than those in the urticaria group (p = 0.007, p = 0.003).
Conclusion
Cardiac dysfunction may develop during an anaphylaxis independent of severity of reaction.
Keywords: Anaphylaxis, Cardiac function, Speckle tracking echocardiography, Global longitudinal strain, Tryptase
INTRODUCTION
Anaphylaxis is a life-threatening systemic allergic disease with sudden onset and rapid progression [1]. The estimated prevalence of anaphylaxis was reported in 2006 as 50 to 2000 attacks per 100,000 persons or a lifetime prevalence between 0.05% and 2% by the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group [2]. A recent United States study reported that 1.6% of the population manifested a history of anaphylaxis [3]. Studies from Europe find the incidence of anaphylaxis at 1.5–7.9/100,000 person-years [4]. Further research indicates that the incidence of anaphylaxis has increased in United States, Australia, and the United Kingdom [5,6,7]. The mortality rate ranges from 2% to 20% [8]. An anaphylaxis episode can be induced by drug, food, latex, venom or insect bites, but in some cases, the cause may be undetermined [1]. Most studies point to the leading causes of anaphylaxis as medications [9,10,11,12]. The diagnosis of anaphylaxis depends on clinical signs and symptoms; furthermore, accurate diagnosis criteria have been developed [1]. Management basically includes intramuscular epinephrine, intravenous fluid replacement, corticosteroids, and antihistamine [1]. Delayed or improper treatment, especially retarded epinephrine usage, increases mortality risk and can result in death [1,13]. Uncontrolled asthma is another risk factor defined for fatal anaphylaxis [14].
Anaphylaxis can be mild and spontaneously resolved by a rise in the endogen epinephrine level as compensatory but can also be very severe, resulting in respiratory or cardiovascular arrest, even death [15]. The major causes of mortality due to anaphylaxis are the asphyxia caused by obstruction of the upper and/or lower respiratory airways and cardiovascular collapse. Cardiovascular manifestations include hypotension, shock, arrhythmia, ventricular dysfunction, and cardiac arrest [16]. During anaphylaxis, impairment of the coronary blood flow can lead to acute ischemic vascular events such as angina and myocardial infarction.
Although cardiovascular involvement is the one of the major causes of mortality in anaphylaxis, no research to date examines the occurrence of cardiac dysfunction in every attack. The most frequently used method for the evaluation of the left ventricular function is to measure the left ventricular ejection fraction (leftV EF) with echocardiography (ECHO). This method provides an easy way to learn cardiac functions and offers prognostic information. However, the method does possess disadvantages including reproducibility, load dependency, technical problems, the influence of heart rate and translational motion [17]. Furthermore, the conditions leading to subclinical cardiac dysfunctions with limited structural changes in the heart cannot be recognized so the prognosis in such diseases could be poor [18].
In the early stages of myocardial diseases, longitudinal functions decrease but by radial and circumferential compensation, the leftV EF is preserved. Therefore, it is important to evaluate longitudinal functions in conditions that have led to cardiac dysfunction with preserved leftV EF. In this line of inquiry, a new technique evaluating myocardial deformation, namely global longitudinal strain (GLS) measurement, is a promising option which is not load dependent and may be used to detect early left ventricular dysfunction [19].
Strain denotes the percentage of the dimensional deformation occurring in the object (measure of the deformation). Myocardial deformation is the structural changes resulting from a combination of external and internal forces. Myocardial strain is regionally and globally calculated with ECHO and yields valuable information regarding systolic and diastolic functions [20]. The strain is only very slightly affected from passive myocardial movements. This sensitive method measures the cardiac functions in the conditions leading to subclinical dysfunction and detects myocardial involvement in noncardiac diseases which could not be recognized by the standard echocardiographic techniques [21].
The aim of the study is to define the impairment of the cardiac functions during anaphylaxis and assess the possible factors influencing the development of dysfunction.
MATERIALS AND METHODS
Patients who experienced anaphylaxis due to any cause in our hospital were included in the anaphylaxis group and patients who experienced early urticaria without any cardiac complaints were included in the study's control group. Tryptase levels were measured on the third hour of the reaction and 6 weeks later. Left ventricular systolic functions were evaluated with the GLS measurement approximately 4 hours after reaction and repeated 6 weeks later.
Anaphylaxis was diagnosed according to the presence of one of the clinical criteria; (1) rapid onset (minutes to several hours) of an attack with involvement of the mucocutaneous tissue and respiratory compromise or reduced blood pressure or associated symptoms of end-organ dysfunction or both, (2) two or more of the clinical features that occur rapidly after exposure to a potential allergen including involvement of the mucocutaneous tissue, reduced blood pressure or associated symptoms, respiratory compromise, and persistent gastrointestinal symptoms, (3) reduced blood pressure after exposure to known allergen (for adults systolic blood pressure of less than 90 mmHg or greater than 30% decrease from that person's baseline) [22]. The reactions were graded according to severity which were mild (involvement of skin and subcutaneous tissues only), moderate (features suggesting respiratory, cardiovascular, or gastrointestinal involvement) and severe (hypoxia, hypotension, or neurologic compromise) as in the literature [23].
Standard transthoracic ECHO and Speckle tracking ECHO
Mechanical myocardial functions were assessed by ECHO (2-dimensional, M-mod, Philips IE33, X5-1 transthoracic probe) at rest in all patients in the Cardiology Department of the Istanbul Faculty of Medicine. Using standard techniques described in the guidelines of the American Society of Echocardiography, essential images were attained [24]. The values of left ventricular ejection fraction were calculated by the Teicholz method [25]. In all patients, electrocardiographs were performed concurrently.
Additionally, the value of the GLS was measured by Speckle tracking echocardiography (Philips IE35) using the QLAB-CMQ software program. The measurements were obtained from apical chambers (4 chambers, 3 chambers, 2 chambers) through at least 4 consecutive cardiac cycles. At first, borders of the left ventricular endocardium were described from these records; peak systolic strain values were measured based on a seventeen segments model [26]. GLS value below-20 (minus stand for the shortening, thinning, or counterclockwise rotation) was accepted as pathologic, as ECHO guidelines [27].
Blood samples for tryptase measurement were obtained at the second–third hour of reaction and 6 weeks later.
The study was approved by the Ethical Committee of Istanbul Faculty of Medicine (approval number:2015/1931) and written informed consent was obtained from all patients.
Statistical analysis
The results were shown as percentages and mean ± standard deviation. The tryptase and strain values measured during reaction and 6 weeks later were compared with the Wilcoxon T test in the same group and with the independent sample t test between the anaphylaxis and control group and between severe and nonsevere anaphylaxis subgroups. Categorical data were analyzed with the chi-square or Fisher exact test and the correlation between tryptase and strain values was tested with the Pearson correlation. All tests were considered significant when the p value was lower than 0.05.
RESULTS
Anaphylaxis group
Twelve patients were included in the study, and 10 (83.3%) were female. The mean age of the patients was 43.25 ± 9.9 years. Demographic and clinical features of the patients were shown in Table 1. The causes of anaphylaxis were drug allergy in 11 patients and venom immunotherapy in 1 patient. The drugs which were the most common culprits were beta-lactams in 5 patients and quinolone in 3 patients. Only 1 patient had hypertension and coronary artery disease and she was receiving angiotensin receptor blocker, beta-receptor blocker, and aspirin. Eight of the anaphylactic reactions (66.7%) were severe; the others were moderate. The most commonly involved organ system was cardiovascular system (n = 12) and followed by cutaneous and gastrointestinal systems (n = 11). In 8 patients, respiratory system involvement and in 5 patients neurologic signs and symptoms were observed. In 9 reactions (75%) tryptase levels increased. Ten patients needed 0.5-mg epinephrine for treatment, and the remaining patients recovered after 0.25-mg epinephrine injection. All cases were additionally treated with methylprednisolone, pheniramine, and intravenous fluid replacement.
Table 1. Demographic and clinical features of the patients in the anaphylaxis group.
| Patient No. | Sex | Age (yr) | Criteria | Known CAD | Other chronicdisease | Concomitant drugusage | Cause of anaphylaxis | Organ/system involvement | Cardiac complaints | Stage | Adrenaline dose (mg) | Tryptase (µg/dL) | Strain | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 Weeks later | Shortly after anaphylaxis | Shortly after anaphylaxis | 6 Weeks later | ||||||||||||
| 1 | F | 42 | 2 | - | Prolactinoma | Dostinex | NSAID | Skin, GIS, CVS | Tachycardia | 2 | 0.25 | 0.01 | 2 | −21 | −22 |
| 2 | F | 20 | 2 | - | - | - | Ciprofloxacin | Skin, GIS, CVS, | Tachycardia | 2 | 0.5 | 1.50 | 2.56 | −19 | −24 |
| 3 | F | 51 | 2 | - | - | - | Amoxicilln | Skin, GIS, CVS, | Tachycardia | 2 | 0.25 | 0.5 | 0.6 | −25 | −25 |
| 4 | F | 27 | 2 | - | - | - | Amoxicilln | Skin, GIS, CVS, RS | Tachycardia | 2 | 0.5 | 6.37 | 21.6 | −22 | −24 |
| 5 | F | 46 | 2 | - | - | - | Pregabalin | Skin, GIS, CVS, RS and neurologic systems | Tachycardia, hypotension | 3 | 0.5 | 4.12 | 38.4 | −21 | −25 |
| 6 | M | 54 | 2 | - | - | - | Metronidazole | Skin, GIS, CVS, RS , neurologic system | Tachycardia, hypotension | 3 | 0.5 | 9.3 | 44.5 | −17 | −22 |
| 7 | F | 49 | 2 | + | Hypertension | ARB, B Bloker, Aspirin | Cefditoren | Skin, CVS, RS , GIS | Tachycardia, hypotension, chest and back pain | 3 | 0.5 | 4.30 | 52 | −18 | −21 |
| 8 | F | 48 | 2 | - | - | - | Ciprofloxacin | Skin, CVS, RS , GIS, neurologic system | Tachycardia, hypotension | 3 | 0.5 | 7.34 | 28.3 | −21 | −23 |
| 9 | F | 42 | 3 | - | - | - | Venom IT | CVS | Tachycardia, hypotension | 3 | 0.5 | 8.20 | 15.40 | −20 | −22 |
| 10 | M | 50 | 2 | - | - | - | Ampicillin | Skin, CVS, RS, GIS, neurologic | Tachycardia, hypotension | 3 | 0.5 | 6.2 | 36 | −19 | −22 |
| 11 | F | 45 | 2 | - | Hypertension | ARB | Levofloxacin | Skin, CVS, RS , GIS, neurologic system | Tachycardia, hypotension | 3 | 0.5 | 4.55 | 12.2 | −19 | −25 |
| 12 | F | 45 | 2 | - | - | - | Ampisilin | Skin, CVS, RS , GIS | Tachycardia, hypotension | 3 | 0.5 | 2.64 | 14.2 | −21 | −24 |
CAD, coronary artery disease; ARB, angiotensin receptor bloker; NSAID, nonsteroidal anti-inflammatory drug; IT, immunotherapy; GIS, gastrointestinal system; CVS, cardiovascualr system; RS, respiratory system.
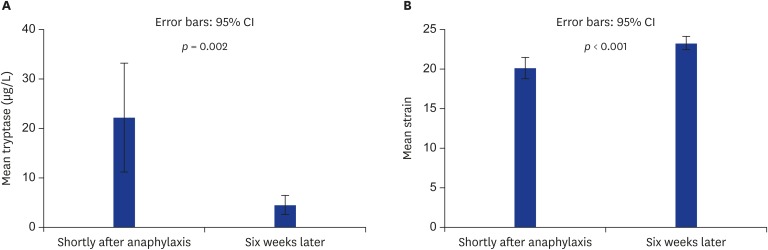
Echocardiographic features were in the normal range in all patients and the findings measured shortly after anaphylaxis and 6 weeks later were similar (Table 2). During anaphylaxis, only 1 patient (patient 7) who had known nonocclusive atherosclerotic plaques in her coronary arteries and hypertension and was using an angiotensin receptor blocker, beta blocker, and aspirin experienced severe chest and back pain. ST depressions were observed on the ECG. Troponin I level did not increase during the follow-up period. Approximately 1 hour later, recovered from the chest and back pain and ST depression was elevated to normal levels. The mean strain value was -20.25 ± 2.09 shortly after anaphylaxis and the strain value significantly increased 6 weeks later to -23.33 ± 1.3 (p < 0.001). The mean baseline (measured 6 weeks later) tryptase level was 4.58 ± 3.01 μg/L and shortly after anaphylaxis tryptase level increased to 22.31 ± 17.44 μg/L (p = 0,002) (Fig. 1).
Table 2. Echocardiographic features of patients measured shortly after anaphylaxis and 6 weeks later.
| Echocardiographic features | Shortly after anaphylaxis | Six weeks later | p value |
|---|---|---|---|
| Left ventricular ejection fraction (%) | 70.75 ± 3.6 | 70.1 ± 2.47 | NS |
| Left atrium diameter (cm) | 3.2 ± 0.4 | 3.3 ± 0.13 | NS |
| Right atrium diameter (cm) | 2.86 ± 0.34 | 2.95 ± 0.53 | NS |
| Left ventricular diastolic diameter (cm) | 4.74 ± 0.38 | 4.8 ± 0.4 | NS |
| Left ventricular systolic diameter (cm) | 2.8 ± 0.3 | 2.9 ± 0.24 | NS |
| Interventricular septal dimension (cm) | 1.05 ± 0.1 | 1.01 ± 0.08 | NS |
| Posterior wall dimension (cm) | 0.9 ± 0.1 | 0.95 ± 0.1 | NS |
| E/A (Mitral flow early (E) and late (A) phase ratio) | 0.98 ± 0.33 | 1.1 ± 0.37 | NS |
Values are presented as mean ± standard deviation.
NS, nonsignificant.
Fig. 1. Comparison of the strain and tryptase level after anaphylaxis and 6 weeks later in the anaphylaxis group. Error bars show the significant difference of mean tryptase (A) and mean strain (B) levels measured shortly after anaphylaxis and 6 weeks later in the anaphylaxis group. CI, confidence interval.
In severe anaphylaxis, tryptase levels both shortly after anaphylaxis and 6 weeks later were higher than those found in the nonsevere anaphylaxis (30.12 ± 15.04 μg/L vs. 6.7 ± 9.7 μg/L, p = 0.019; 5.83 ± 2.3 μg/L vs. 2.1 ± 2.9 μg/L, p = 0.035).
The strain values measured shortly after anaphylaxis were not correlated to the severity of anaphylaxis and the number of anaphylaxis reactions in the history. No association was detected between the doses of adrenaline used for anaphylaxis and the strain values. Also, the decrease of strain values was not correlated with the increase of tryptase levels during anaphylaxis.
Control (urticaria) group
Ten patients who experienced urticaria without any cardiac complaints were included in the control group. Seven (66.7%) were female, with mean age 41.33 ± 8.81 years: the causes of the reaction were drugs in 9 patients (3 beta lactam, 3 nonsteroidal anti-inflammatory drugs, 2 quinolones, and 1 metronidazol) and venom immunotherapy injection in 1 patient. No patient had known coronary artery disease; One had hypertension and was concomitantly using a beta blocker. The mean strain values calculated shortly after reaction and 6 weeks later were -23.44 ± 2.24 and -23.9 ± 2.08. The mean tryptase levels measured shortly after reaction and 6 weeks later were 4.69 ± 1.77 and 4.33 ± 1.75 μg/L.
Comparison of the anaphylaxis and urticaria group
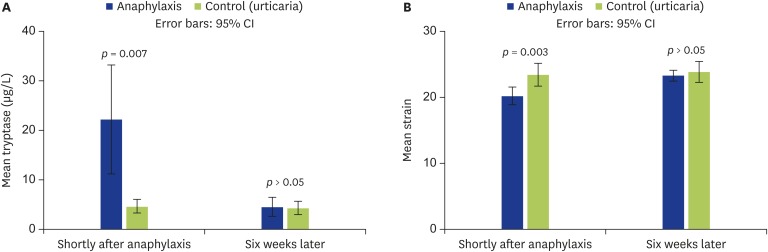
The mean tryptase level measured during reaction was significantly higher in the anaphylaxis group than that of the urticaria group (22.31 ± 17.44 μg/L vs. 4.69 ± 1.77 μg/L, p = 0.007) and the mean tryptase levels were similar in both anaphylaxis and urticaria groups 6 weeks later (4.58 ± 3.01 μg/L vs. 4.33 ± 1.75 μg/L). The mean strain value measured after reaction was lower in the anaphylaxis group than in the urticaria group (-20.25 ± 2.1 vs. -23.44 ± 2.24, p = 0.003). However, the mean strain values calculated 6 weeks later were similar (-23.33 ± 1.3 vs. -23.9 ± 2.08) (Fig. 2).
Fig. 2. The comparison of the anaphylaxis and urticaria groups regarding tryptase and strain values. Error bars show the differences between anaphylaxis and control (urticaria) groups. (A) Mean tryptase levels. (B) Mean strain levels. Blue and green bars represent the anaphylaxis and control group, respectively.
DISCUSSION
The present study is the first investigation which evaluated the cardiac function shortly after an anaphylaxis. The results of this study demonstrate that ventricular functions are affected due to an anaphylaxis attack independent of severity.
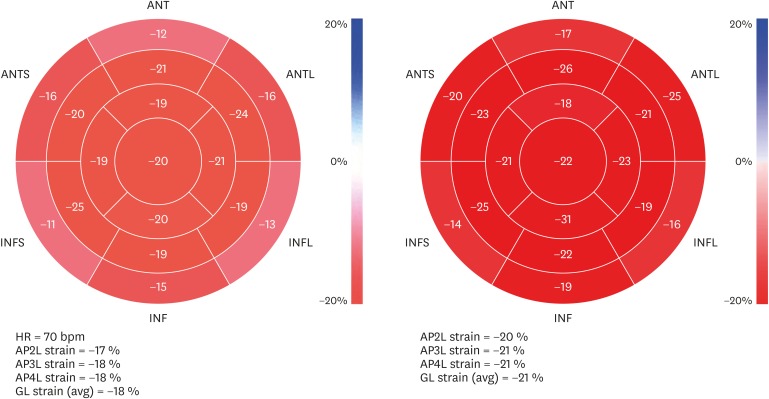
We used Speckle tracking ECHO to obtain a valuable parameter, namely GLS to evaluate cardiac function. Although a new method, GLS has been used in many published reports and routinely in many ECHO laboratories due to its superiority to other parameters [28]. In the setting of normal leftV EF, systolic dysfunction can be detected by Speckle tracking ECHO, a process/an application which assists in identifying rare causes of cardiac pathologies with normal EF including cardiac hypertrophy and amyloidosis as well as evaluating the future cardiac risk as a prognostic marker [27,29,30,31]. In our study, shortly after anaphylaxis, the leftV EF values were measured in the normal ranges in all patients. The major finding of our study is that the mean strain values were significantly lower shortly after the anaphylaxis than those measured 6 weeks later. The strain measures below -20 in 5 patients indicate myocardial dysfunction according to the cardiac guidelines [27] (Fig. 3). The pathologic strain values in those 5 patients were increased to normal levels after 6 weeks. In the other 5 patients, strain values were in the normal ranges during the postanaphylaxis period; however, strain values increased by at least 2 units. In another 2 patients no differences existed in strain measures during anaphylaxis and 6 weeks later. Furthermore, the mean strain value was lower during the reaction in the anaphylaxis group than in the urticaria group. These results demonstrate cardiac involvement in the systemic hypersensitivity reaction.
Fig. 3. Pictures indicate the strain measurement of a patient in the anaphylaxis group. The pictures on the left and on the right were taken shortly after anaphylaxis and 6 weeks later respectively. ANT, anterior; ANTS, anterior septum; ANTL, anterolateral; INF: inferior; INFS, inferior septum; INFL: inferolateral; HR, heart rate; AP2L, apical 2 chamber; AP3L, apical 3 chamber; AP4L, apical 4 chamber; GL, global longitudinal.
The effect of epinephrine to treat anaphylaxis on cardiac function can be considered as a matter of importance. It can be assumed that epinephrine may negatively influence cardiac function through the drug's vasospastic effect on coronary arteries. In our study no correlation was detected between the doses of epinephrine used to treat anaphylaxis and the strain values. A study evaluating left ventricular function and contractile reserve in a small animal model for type 1 diabetes showed that a positive inotropic agent dobutamine led to an increase in strain level [32]. Epinephrine as a positive inotropic agent is assumed to lead to an increase in strain measures as well. However, in the current study, the strain values were lower than those of the control levels (measured 6 weeks later) in most of the patients. We can hypothesize that the strain values probably would have been less if the patients had not been given epinephrine.
Although cardiac involvement in anaphylaxis patients was recovered 6 weeks later, it is not possible to predict the cardiac functions of these patients in the long term. Lower strain values have been recently associated with a higher long-term risk of cardiovascular morbidity and mortality in a low-risk general population [33]. Accordingly, it may be assumed that the patients for whom myocardial strains were affected due to an anaphylaxis and particularly those with recurrent anaphylaxis both require long term follow-up care in terms of cardiac function.
Another main finding of the study is that the cardiac dysfunction developed during anaphylaxis did not depend on the severity of the reaction or the baseline tryptase levels.
The studies evaluating the risk factors for the severe and fatal anaphylaxis demonstrate that the severity of the reaction can be associated with the type of the culprit drug, the personal level of sensitivity, genetic background, age, exercise, comorbidities including coinfections, asthma, cardiovascular diseases, and mastocytosis and also coadministration of some drugs, such as beta blockers and angiotensin converting enzyme inhibitors [8,22,34,35,36]. Unfortunately, we could not analyze the effects of these variables in our patient group because of the small sample size. However, our data indicated that basal tryptase levels and the increment of the tryptase during anaphylaxis were higher in severe anaphylaxis than in non-severe anaphylaxis. Other studies also report that severe anaphylaxis was associated with the increment of tryptase levels during anaphylaxis [37,38,39]. Mirone et al. pointed out that basal tryptase levels were higher in severe anaphylaxis while some authors did not observe such an association [37,39,40,41].
Our study had an important limitation which was the small sample size made difficult to draw an exact conclusion. However, it gave a very crucial opinion about the cardiac functions during anaphylaxis providing a basis for the future studies in this field.
In conclusion, this study demonstrates that cardiac dysfunction may develop during anaphylaxis independent of the severity of the reaction and with proper treatment, may resolve after recovery from anaphylaxis. Higher baseline tryptase levels, even in normal ranges, may be associated with severe anaphylaxis episode, a finding which requires more attention and corroboration with a larger series. Lastly, the long-term cardiac effects of anaphylaxis should be evaluated as well.
ACKNOWLEDGEMENTS
We would like to thank to Prof. Dr. Berrin Umman for expert opinions and supports.
References
- 1.Simons FE, Ardusso LR, Bilò MB, Cardona V, Ebisawa M, El-Gamal YM, Lieberman P, Lockey RF, Muraro A, Roberts G, Sanchez-Borges M, Sheikh A, Shek LP, Wallace DV, Worm M. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014;7:9. doi: 10.1186/1939-4551-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberman P, Camargo CA, Jr, Bohlke K, Jick H, Miller RL, Sheikh A, Simons FE. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97:596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 3.Wood RA, Camargo CA, Jr, Lieberman P, Sampson HA, Schwartz LB, Zitt M, Collins C, Tringale M, Wilkinson M, Boyle J, Simons FE. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:461–467. doi: 10.1016/j.jaci.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Panesar SS, Javad S, de Silva D, Nwaru BI, Hickstein L, Muraro A, Roberts G, Worm M, Bilò MB, Cardona V, Dubois AE, Dunn Galvin A, Eigenmann P, Fernandez-Rivas M, Halken S, Lack G, Niggemann B, Santos AF, Vlieg-Boerstra BJ, Zolkipli ZQ, Sheikh A EAACI Food Allergy and Anaphylaxis Group. The epidemiology of anaphylaxis in Europe: a systematic review. Allergy. 2013;68:1353–1361. doi: 10.1111/all.12272. [DOI] [PubMed] [Google Scholar]
- 5.Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, Weaver A, Bellolio MF, Bergstralh EJ, Stead LG, Li JT. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122:1161–1165. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. J Allergy Clin Immunol. 2007;120:878–884. doi: 10.1016/j.jaci.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007;62:91–96. doi: 10.1136/thx.2004.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pumphrey R. Anaphylaxis: can we tell who is at risk of a fatal reaction? Curr Opin Allergy Clin Immunol. 2004;4:285–290. doi: 10.1097/01.all.0000136762.89313.0b. [DOI] [PubMed] [Google Scholar]
- 9.Brown AF, McKinnon D, Chu K. Emergency department anaphylaxis: A review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108:861–866. doi: 10.1067/mai.2001.119028. [DOI] [PubMed] [Google Scholar]
- 10.Gelincik A, Demirtürk M, Yılmaz E, Ertek B, Erdogdu D, Çolakoğlu B, Büyüköztürk S. Anaphylaxis in a tertiary adult allergy clinic: a retrospective review of 516 patients. Ann Allergy Asthma Immunol. 2013;110:96–100. doi: 10.1016/j.anai.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Sheikh A, Alves B. Hospital admissions for acute anaphylaxis: time trend study. BMJ. 2000;320:1441. doi: 10.1136/bmj.320.7247.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123:434–442. doi: 10.1016/j.jaci.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 13.Beyer K, Eckermann O, Hompes S, Grabenhenrich L, Worm M. Anaphylaxis in an emergency setting - elicitors, therapy and incidence of severe allergic reactions. Allergy. 2012;67:1451–1456. doi: 10.1111/all.12012. [DOI] [PubMed] [Google Scholar]
- 14.Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:1144–1150. doi: 10.1046/j.1365-2222.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 15.Simons FE. Anaphylaxis, killer allergy: long-term management in the community. J Allergy Clin Immunol. 2006;117:367–377. doi: 10.1016/j.jaci.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Triggiani M, Montagni M, Parente R, Ridolo E. Anaphylaxis and cardiovascular diseases: a dangerous liaison. Curr Opin Allergy Clin Immunol. 2014;14:309–315. doi: 10.1097/ACI.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 17.Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: methods and clinical applications. Int J Cardiovasc Imaging. 2009;25(Suppl 1):9–22. doi: 10.1007/s10554-008-9414-1. [DOI] [PubMed] [Google Scholar]
- 18.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF. Tissue Doppler imaging for the pre-clinical diagnosis of cardiomyopathy. Eur Heart J. 2004;25:1865–1866. doi: 10.1016/j.ehj.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol. 2006;47:1313–1327. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 21.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196–1207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons FE, Ardusso LR, Bilò MB, Dimov V, Ebisawa M, El-Gamal YM, Ledford DK, Lockey RF, Ring J, Sanchez-Borges M, Senna GE, Sheikh A, Thong BY, Worm M World Allergy Organization. 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol. 2012;12:389–399. doi: 10.1097/ACI.0b013e328355b7e4. [DOI] [PubMed] [Google Scholar]
- 23.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 25.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 26.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol. 2017;69:1043–1056. doi: 10.1016/j.jacc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Sun JP, Stewart WJ, Yang XS, Donnell RO, Leon AR, Felner JM, Thomas JD, Merlino JD. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103:411–415. doi: 10.1016/j.amjcard.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 30.Pagourelias ED, Duchenne J, Mirea O, Vovas G, Van Cleemput J, Delforge M, Kuznetsova T, Bogaert J, Voigt JU. The relation of ejection fraction and global longitudinal strain in amyloidosis: implications for differential diagnosis. JACC Cardiovasc Imaging. 2016;9:1358–1359. doi: 10.1016/j.jcmg.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, Fang ZY, Prins JB, Stanton T. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. 2015;101:1061–1066. doi: 10.1136/heartjnl-2014-307391. [DOI] [PubMed] [Google Scholar]
- 32.Weytjens C, Cosyns B, D'hooge J, Droogmans S, Lahoutte T, Garbar C, Roossens B, Van Camp G. Evaluation of contractile function and inotropic reserve with tissue velocity, strain and strain rate imaging in streptozotocin-induced diabetes. Eur J Echocardiogr. 2010;11:622–629. doi: 10.1093/ejechocard/jeq032. [DOI] [PubMed] [Google Scholar]
- 33.Biering-Sørensen T, Biering-Sørensen SR, Olsen FJ, Sengeløv M, Jørgensen PG, Mogelvang R, Shah AM, Jensen JS. Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbidity and mortality in a low-risk general population: the copenhagen city heart study. Circ Cardiovasc Imaging. 2017;10:pii: e005521. doi: 10.1161/CIRCIMAGING.116.005521.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieberman P, Nicklas RA, Oppenheimer J, Kemp SF, Lang DM, Bernstein DI, Bernstein JA, Burks AW, Feldweg AM, Fink JN, Greenberger PA, Golden DB, James JM, Kemp SF, Ledford DK, Lieberman P, Sheffer AL, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, Lang D, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph C, Schuller DE, Spector SL, Tilles S, Wallace D. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477–480.e1-42. doi: 10.1016/j.jaci.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Silva R, Gomes E, Cunha L, Falcão H. Anaphylaxis in children: a nine years retrospective study (2001-2009) Allergol Immunopathol (Madr) 2012;40:31–36. doi: 10.1016/j.aller.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Moneret-Vautrin DA, Morisset M, Flabbee J, Beaudouin E, Kanny G. Epidemiology of life-threatening and lethal anaphylaxis: a review. Allergy. 2005;60:443–451. doi: 10.1111/j.1398-9995.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 37.Sala-Cunill A, Cardona V, Labrador-Horrillo M, Luengo O, Esteso O, Garriga T, Vicario M, Guilarte M. Usefulness and limitations of sequential serum tryptase for the diagnosis of anaphylaxis in 102 patients. Int Arch Allergy Immunol. 2013;160:192–199. doi: 10.1159/000339749. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava S, Huissoon AP, Barrett V, Hackett S, Dorrian S, Cooke MW, Krishna MT. Systemic reactions and anaphylaxis with an acute serum tryptase ≥14 μg/L: retrospective characterisation of aetiology, severity and adherence to National Institute of Health and Care Excellence (NICE) guidelines for serial tryptase measurements and specialist referral. J Clin Pathol. 2014;67:614–619. doi: 10.1136/jclinpath-2013-202005. [DOI] [PubMed] [Google Scholar]
- 39.De Schryver S, Halbrich M, Clarke A, La Vieille S, Eisman H, Alizadehfar R, Joseph L, Morris J, Ben-Shoshan M. Tryptase levels in children presenting with anaphylaxis: Temporal trends and associated factors. J Allergy Clin Immunol. 2016;137:1138–1142. doi: 10.1016/j.jaci.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Cavkaytar O, Karaatmaca B, Arik Yilmaz E, Sahiner UM, Sackesen C, Sekerel BE, Soyer O. Basal serum tryptase is not a risk factor for immediate-type drug hypersensitivity during childhood. Pediatr Allergy Immunol. 2016;27:736–742. doi: 10.1111/pai.12604. [DOI] [PubMed] [Google Scholar]
- 41.Mirone C, Preziosi D, Mascheri A, Micarelli G, Farioli L, Balossi LG, Scibilia J, Schroeder J, Losappio LM, Aversano MG, Stafylaraki C, Nichelatti M, Pastorello EA. Identification of risk factors of severe hypersensitivity reactions in general anaesthesia. Clin Mol Allergy. 2015;13:11. doi: 10.1186/s12948-015-0017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]