Abstract
Background
In the view of the epidemic growth of sensitization to indoor allergens in Southern Vietnam, there is a requirement to screen large population.
Objective
To evaluate skin prick tests (SPTs) as predictors of positive specific IgE (sIgE) to dust allergens, among patients with chronic respiratory diseases (CRDs).
Methods
The sensitization to Blomia tropicalis (Blo t), Dermatophagoides pteronissinus (Der p), and Blattella germanica allergens (Bla g) were evaluated among 610 CRD, both SPT (≥4 mm) and sIgE by immuno-CAP (≥0.7 kUA/L).
Results
Based on sIgE, 45%, 32%, and 33% of patients with CRD were sensitized to Blo t, Der p, and Bla g, respectively, compared to 19%, 18%, and 13% by SPT. The association between SPT and sIgE was statistically significant, though the Kappa factor was fair (i.e., 0.39 to 0.23). While the specificity of SPT to detect sensitization (compared to sIgE) was >90% among the whole population, the sensitivity was only 34%, 41%, and 24% for Bo t, Der p, and Bla g, suggesting that SPT was not enough sensitive to screen the indoor allergen sensitization. Though, among the <10 pack-year (PY) smokers, the sensitivity was 43% for Blo t, 52% for Der p, and 61% for Blo t and/or Der p, compared to 27%, 30%, and 35% among the ≥10 PY smokers. The sensitivity/specificity was not associated with the diagnosis of asthma compared to chronic obstructive pulmonary disease.
Conclusion
In the present circumstance, SPT to dust mites allergens can be used to detect a sensitization among CRD population in Southern Vietnam.
Keywords: Skin test, Immunoglobulin E, Allergen, Chronic respiratory diseases, Vietnam
INTRODUCTION
The chronic respiratory diseases (CRDs) include asthma, chronic obstructive pulmonary disease (COPD), occupational lung diseases, lung fibrosis, and pulmonary hypertension [1]. The prevalence of patients with CRD is increasing particularly in low-middle income countries [2,3]. Their main risk factors are tobacco smoking, indoor and outdoor pollution, occupational risk, vulnerability, and allergens sensitization [1]. Recent studies suggested that indoor allergen sensitization is quickly increasing among several Asian countries [4].
Sensitization to house dust mite is the most frequent allergen among asthmatics in China [5], Thailand [6], Singapore [7], and the North of Vietnam [8]. In the South of Vietnam, we recently reported that, dust mites and cockroaches were the most frequent allergens sensitizers among patients with CRD and that the migration from rural to urban area increased the risk of dust mite sensitization [9]. However, due to the frequent consumption of shrimp, as well as the possible known cross-reactivity between dust mite and shrimp, its sensitization has to be considered in this population.
In view of this epidemic, there is a need to screen sensitization among large population to prevent exposure and, if required, implement desensitization. In vitro specific IgE (sIgE) assays are currently available only in a limited number of reference centers and it is a financial burden for low income large population while the cheap and unsophisticated skin prick tests (SPTs) can be widely used in most of the healthcare centers. Though, commercial allergen extracts have been validated for western countries but not in other parts of the World where dust mite and cockroach species could be different on an allergenic point of view. Thus, the aim of this study was to validate the SPT, compared to sIgE, to screen dust mite sensitization among patients with CRD in Southern Vietnam.
MATERIALS AND METHODS
General design and population selection
Outpatients were randomly included at Pham Ngoc Thach (PNT) hospital, the reference center for respiratory diseases in Southern Vietnam. Both active and suspected tuberculosis patients were excluded by routine chest X-ray and microbial sputum analysis. After obtained informed written consent, a lung function test (LFT) was measured [9] while blood sampling and SPT were performed after stopping antihistamines at least 5 days.
The main inclusion criteria were the presence of chronic respiratory symptoms lasting 3 months or more with a significant defect of lung function [9]. After measurement of the LFT before and after bronchodilator, asthma and COPD were defined as recommended by Global Initiative for Asthma and the Global Initiative for Chronic Obstructive Lung Disease guidelines [10,11].
The study was approved by the Committee of PNT hospital (CS/PT/13/12) and was registered with ClinicalTrials.gov (NCT02517983).
Skin prick test
SPT was performed by pricking the Stallerpoint on the back region with a panel of commercial airborne allergens (Stallergenes, France): Blomia tropicalis (Blo t), Dermatophagoides pteronissinus (Der p), and cockroach (Blattella germanica, Bla g). A SPT was also performed with native fresh shrimp in view of the frequent consumption and the cross-reactivity with dust mite. All tests included a positive (histamine) and a negative control.
Wheals and flares were recorded at 15 minutes after allergen testing. The wheal diameter was measured as the average of the largest wheal diameter and its perpendicular. Although there was a large consensus to define a wheal of 3 mm as positive, in our particular Vietnamese population, a cutoff 4 mm was used because some authors recommended this threshold in presence of CRDs [12], and it was less dependent on the race [13] and/or the type of tested allergen [14]. Patients with a histamine reaction < 4 mm were excluded.
A patient “sensitized to dust mite” was defined as positive for at least Der p and/or Blo t.
Specific IgE
Specific IgE to native house dust mite (Der p), storage mite (Blo t), German/Oriental/American cockroach and shrimp, were measured by using ImmunoCAP (respectively codes: d1, d201, i6/i207/i206, and f24 from Phadia IDM 1000, Uppsala, Sweden). Based on the calculation of the best sensitivity and specificity in regard of positive SPT (see below), a significant level of sIgE ≥ 0.70 kUA/L was considered positive. A concentration of sIgE ≥ 0.35 kUA/L was defined as detectable threshold.
A patient with sIgE ≥ 0.70 kUA/L for Der p and/or Blo t was defined as sensitization to dust mite.
Statistical analysis
IBM SPSS Statistics version 23.0 (IBM Co., Armonk, NY, USA) was used for data analysis. The sensitivity and specificity were calculated to validate SPT in regard to sIgE as the “reference standard.” The Mantel – Haenzsel method and logistic regression were used to test the interaction of the age with the smoking effect, on the SPT response as the predictor of sIgE. The agreement between the SPT and sIgE was analysed by using Kappa statistics. Kappa values < 0.001 indicate poor agreement: 0.001 to 0.20, slight agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, substantial agreement; and 0.81 to 1.00, almost perfect agreement [15]. The optimal sIgE cutoff level was defined by the Youden index J (sensitivity + specificity – 1) from receiver operating characteristic (ROC) curve computing by positive SPT and sIgE against Der p (had the maximal sum of sensitivity and specificity). The interest of this ROC curve approach, to establish the clinical useful of in vitro IgE tests was reported previously by Plebani et al. [16]. A p value < 0.05 was considered statistically significant.
RESULTS
The demographic characteristics of the studied population (610 patients with CRD, mean age 54 years) with their positivity to SPT, were shown in Table 1. The prevalence of sensitization to Der p, Blo t, Bla g was not different in regard with the sex, while it was more frequent among asthmatics compared to COPD. In both asthmatics and COPD, it was more frequent among < 10 pack-year (PY) smokers compared to heavy smokers.
Table 1. Demographics and clinical characteristics of the whole population and their specific sensitization.
| Variable | All | Der p (+) | Blo t (+) | Bla g (+) | ||
|---|---|---|---|---|---|---|
| No. of patients | 610 | 109 (17.9) | 114 (18.7) | 77 (12.6) | ||
| Sex | ||||||
| Male | 489 | 81 (16.6) | 86 (17.6) | 59 (12.1) | ||
| Female | 121 | 28 (23.1) | 28 (23.1) | 18 (14.9) | ||
| Age (yr) | 54 ± 13 | 47 ± 13 | 50 ± 14 | 50 ± 14 | ||
| Smoke habits | a | |||||
| Nonsmoker | 167 | 46 (27.5) | 40 (24.0) | 24 (14.4) | ||
| Ex-smoker | 232 | 31 (13.4) | 37 (15.9) | 20 (8.6) | ||
| Current smoker | 211 | 32 (15.2) | 37 (17.5) | 33 (15.6) | ||
| Cumulative smoke | ||||||
| <10 PY | 243 | 69 (28.4) | 64 (26.3) | 38 (15.6) | ||
| ≥10 PY | 367 | 40 (10.9)* | 50 (13.6)* | 39 (10.6) | ||
| Diagnosis | ||||||
| COPD | ||||||
| All | 340 | 45 (13.2) | 43 (12.7) | 36 (10.6) | ||
| <10 PY | 103 | 24 (23.3) | 19 (18.4) | 13 (12.6) | ||
| ≥10 PY | 237 | 21 (8.9)∥ | 24 (10.1) | 23 (9.7) | ||
| Asthma | ||||||
| All | 186 | 42 (22.6)† | 51 (27.4)† | 30 (16.1) | ||
| <10 PY | 89 | 30 (33.7) | 30 (33.7) | 17 (19.1) | ||
| ≥10 PY | 97 | 12 (12.4)‡ | 21 (21.6)§ | 13 (13.4) | ||
| Others, all | 84 | 21 (25.0) | 20 (23.8) | 11 (13.1) | ||
Values are presented as number (%) or mean ± standard deviation.
(+), positive skin prick tests for Dermatophagoides pteronissinus (Der p), Blomia tropicalis (Blo t) or Blattella germanica (Bla g); PY, pack-year.
*p < 0.001 comparing smokers < and ≥ 10 PY. †p < 0.001 comparing COPD and asthmatics. ‡p < 0.01 comparing smokers < and ≥ 10 PY among asthmatics. §p < 0.05 comparing smokers < and ≥ 10 PY among asthmatics. ∥p < 0.01 comparing smokers < and ≥ 10 PY among COPD. a: p trend.
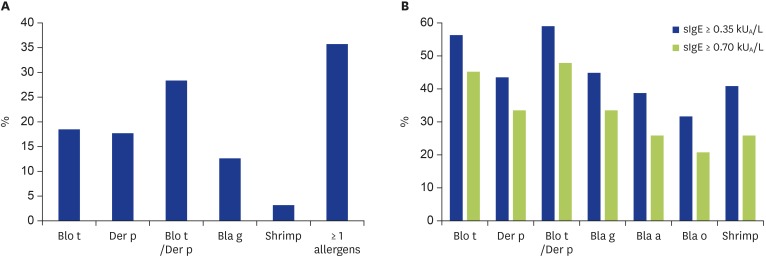
Based on SPT, more than 45% patients were sensitized to at least 1 allergen (Fig. 1A). The most frequent sensitizers were dust mite allergens (28%) whose Der p 18%, Blo t 19%, and Bla 13%. Less than 5% of the patients were sensitized to shrimp.
Fig. 1. Prevalence of allergen sensitization. (A) Prevalence of positive skin prick test (SPT). (B) Prevalence of positive sIgE. The black bars indicate a cutoff of 0.35 kUA/L and the white bares show a cutoff of 0.70 kUA/L specific IgE (sIgE). Blo t, Blomia tropicalis; Der p, Dermatophagoides pteronyssinus; Bla g, Blattella germanica; Bla a, Blattella americana; Bla o, Blattella orientalis.
The ROC curve was computed by positive SPT and sIgE against Der p. A 76% sensitivity and 76% specificity (1-specificity = 0.24), the Youden index J point had the maximal sum of sensitivity and specificity corresponding to the optimal sIgE cutoff level of 0.70 kUA/L. This level was defined as “significant” sIgE in regard with the threshold of the SPT reactivity.
The prevalence of positive sIgE to allergens was displayed in Fig. 1B for 2 different levels defined as “detectable” (≥0.35 kUA/L) or “significant” (≥0.70 kUA/L). Among the 610 patients, 291 (48%) were significantly sensitized to 1 dust mite allergen (Der p and/or Blo t). Among the mite sensitized patients, 187 (64%) were positive to both allergens, while 87 (30%) and 17 (6%) were monosensitized to Blo t and Der p, respectively.
Table 2 shows the sensitivity and specificity of SPT to predict positive sIgE (at concentrations of ≥0.35 or ≥0.7 kUA/L). For the whole population, the specificity was >90% for all allergens while the sensitivity was <50%. The SPT for Blo t and Der p had a sensitivity of only 34% and 41%, while the sensitivity for Bla g was less than 30%; the specificity was more than 92%. Therefore a positive SPT can only detect approximately 30% to 40% of sensitized patients to cockroach or mites and a positive SPT was rarely observed among nonsensitized patients.
Table 2. SPT as predictors of specific IgE.
| sIgE | No. | Sensitivity (%) | Specificity (%) | Kappa* | p value | Sensitivity (%) | Specificity (%) | Kappa* | p value | |
|---|---|---|---|---|---|---|---|---|---|---|
| ≥0.35 kUA/L | ≥0.70 kUA/L | |||||||||
| Blo t, all | 610 | 27 | 93 | 0.18 | <0.001 | 34 | 94 | 0.29 | <0.001 | |
| Der p, all | 610 | 33 | 94 | 0.29 | <0.001 | 41 | 94 | 0.39 | <0.001 | |
| German cockroach, all | 610 | 20 | 93 | 0.14 | <0.001 | 24 | 93 | 0.20 | <0.001 | |
| Oriental cockroach, all | 608 | 22 | 92 | 0.17 | <0.001 | 30 | 92 | 0.25 | <0.001 | |
| American cockroach, all | 610 | 21 | 93 | 0.16 | <0.001 | 28 | 93 | 0.24 | <0.001 | |
| Shrimp, all | 519 | 5 | 97 | 0.02 | NS | 6 | 97 | 0.04 | NS | |
| Blo t and/or Der p, all | 610 | 39 | 87 | 0.31 | <0.001 | 46 | 88 | 0.35 | <0.001 | |
| Blo t | ||||||||||
| Asthma | 186 | 34 | 86 | 0.05 | <0.01 | 38 | 88 | 0.24 | <0.001 | |
| COPD | 340 | 20 | 95 | 0.04 | <0.001 | 26 | 95 | 0.25 | <0.001 | |
| Der p | ||||||||||
| Asthma | 186 | 34 | 92 | 0.05 | <0.001 | 37 | 91 | 0.28 | <0.001 | |
| COPD | 340 | 29 | 96 | 0.05 | <0.001 | 41 | 95 | 0.43 | <0.001 | |
| Blo t and/or Der p | ||||||||||
| Asthma | 186 | 44 | 80 | 0.06 | <0.01 | 48 | 83 | 0.27 | <0.001 | |
| COPD | 340 | 31 | 90 | 0.04 | <0.001 | 40 | 91 | 0.33 | <0.001 | |
| Blo t | ||||||||||
| Smoking <10 PA | 243 | 39 | 89 | 0.05 | <0.001 | 43 | 90 | 0.33 | <0.001 | |
| Smoking ≥10 PA | 367 | 20 | 95 | 0.03 | <0.001 | 27 | 96 | 0.24 | <0.001 | |
| Der p | ||||||||||
| Smoking <10 PA | 243 | 47 | 89 | 0.06 | <0.001 | 52 | 88 | 0.41 | <0.001 | |
| Smoking ≥10 PA | 367 | 22 | 97 | 0.04 | <0.001 | 30 | 97 | 0.33 | <0.001 | |
| Blo T and/or Der p | ||||||||||
| Smoking <10 PA | 243 | 55 | 79 | 0.06 | <0.001 | 61 | 81 | 0.42 | <0.001 | |
| Smoking ≥10 PA | 367 | 28 | 92 | 0.04 | <0.001 | 35 | 92 | 0.28 | <0.001 | |
SPT, skin prick test; sIgE, specific IgE; Der p, Dermatophagoides pteronissinus; Blo t, Blomia tropicalis; Bla g, Blattella germanica; NS, nonsignificant; COPD, chronic obstructive pulmonary disease; PY, pack-year.
*Kappa association between positive sIgE and SPT.
Based on Kappa coefficient, the agreement between positive SPT and positive sIgE were statistically significant (p < 0.001). Though, among the allergens with a statistical relationship between SPT and sIgE, the level of agreement ranged from slight to fair.
The sensitivity/specificity of the SPT was similar among asthmatics and COPD. The sensitivity of the SPT to Blo t, Der p and combined Der p/Blo t was higher among <10 PY smokers; 61% of the patients with a positive SPT to Blo t and/or Der p, had a positive sIgE to at least one of the 2 allergens, compared to 35% among the heavy smokers. One can argue that the age and gender could be confounding factors to explain the smoking effect on the sensitivity of the SPT to detect sIgE. Though, among the <10 PY smokers, the sensitivity of SPT to Blo t and/or Der p was not age-related (chi-square; p = nonsignificant [NS]) and there was no association between sex and the prevalence of sensitization to Der p (38 of 120 males compared 27 of 111 females, (chi-square; p = NS).
DISCUSSION
In southern Vietnam, Blo t and Der p were frequent sensitizers in patients with CRD. Compared to SPT, sIgE was twice frequently positive to most of allergens. A positive SPT to Der p and/or Blo t extracts was a significant predictor for a positive sIgE with a sensitivity > 60% and specificity > 80%, among non- or light-smokers (i.e., < 10 PY).
The high prevalence of dust mite sensitization was consistent with previous studies in Vietnam [8,9,17]. Consistently with our data, a recent study in northern Vietnam reported a prevalence of sensitization by SPT of 23%, 13%, and 13% to Blo t, Der p, and cockroach, respectively, while sensitization was not associated with asthma [8].
Among the patients with positive sIgE, the SPT detected only 41% of Dermatophagoides and 34% of Blomia sensitized patients, respectively. There are several explanations for this discrepancy between SPT and sIgE. The allergen commercial extracts used for SPT, contains mainly Der p1 and Der p2, while in Vietnam the dust mite allergic patients could be sensitized to other allergens, such as Der p5 and/or Der p23. These lasts have been recently reported as frequent or major allergens in tropical countries such as Gabon [18] and Thailand [19]. Blo t5 is a major allergen of Blomia and has some homologies of Der p5. Since more than 64% of our mite sensitized patients cross-reacted to both dust mite and Blomia, Der p5 could also be a significant allergen in our population.
An analysis of commercial European extracts of dust mite indicated that certain natural Der p extracts lack important allergens, on one side, the extracts showed a considerable variability in allergen composition and content as well as in allergenic activity in SPT, on the other side [20]. We used extracts from Stallergenes that were characterized by high concentration of Der p1 and less Der p2 (ratio Der p1/Der p2 = 2.4) while Der p5, Der p10, Der p21, and Der p7 were absent. The lack of these last allergens in the extract from Stallergenes could explain the low frequency of positive SPT to dust mite compared with sIgE against Der p.
The positive sIgE to German or Oriental or American cockroach was more frequent compared to SPT, suggesting that the commercial extract for SPT did not contain all the relevant allergenic components and/or among some patients and/or that positive sIgE were due to sensitization to Cross-reactive Carbohydrate Determinant (CCD). Similarly, the inconsistence between positive SPT (<4%) and sIgE (26%) to shrimp could be also explained by a sensitization to CCD. A high frequency of patients with IgE for shrimp and negative skin test was also consistent with the epidemiological data characterized by a low frequency of clinical allergy in several Asian countries (<1% in China, Thailand) [21] compared to the rapid increasing prevalence of dust mite allergies.
The agreement between the sIgE level and SPT was statistically significant among airborne allergens, but the relation was defined as slight or fair. This result was consistent with a recent European study that showed a moderate agreement between both tests to HDM allergen. However, the SPT was more specific for the diagnosis of asthma [22]. One Brazilian study also showed the agreement, although weak, between SPT to Blo t with its sIgE level [23]. The good agreement between SPT and sIgE against cockroach was concluded in a Thai research on allergic respiratory volunteers [24]. A recent study in Korea also concluded a discrepancy between SPT and sIgE to diagnose allergic sensitization among inhalant allergens and this discrepancy was related to the age [25]. One can argue that our population was a mixture of diagnosis. Though the sensitivity/specificity of SPT was similar among asthmatics and COPD, while it was mainly associated with the smoking habits of the patients. Our current data showed that the sensitivity was not related to age, among the group of <10 PY smokers, suggesting that age was not confounding with “smoking history” on the level of sensitivity of SPT.
One limitation of the study was the 4-mm threshold of positive SPT instead of 3 mm; we cannot exclude a certain loss of sensitivity to screen the sensitized patients. Therefore the conclusions of this study were limited to the Vietnamese population based on the used criteria of sensitization.
We concluded that, based on the present data, SPT to dust mites allergens can be recommended as a first line approach to detect a sensitization among CRD population. With the cost effectiveness and availability of SPT in Vietnam, this method could be extended in the healthcare center in Vietnam.
ACKNOWLEDGEMENTS
We thank the participants and field workers for their time and cooperation. Ha Thi Chu and Nguyễn Thanh Phương were supported by a Partenariat Interuniversitaire Ciblé (ref http://www.cud.be/content/view/1013/504/lang,/) granted from the ARES (Académie de Recherche et d'Enseignement Supérieur) of Belgium.
Footnotes
Author Contributions: Conceptualization: Ha Thi Chu, Isabelle Godin, Olivier Michel. Data curation: Ha Thi Chu, Nguyễn Thanh Phương, Francis Corazza. Formal analysis: Ha Thi Chu, Isabelle Godin, Olivier Michel. Funding acquisition: Ha Thi Chu, Nguyễn Thanh Phương, Tran Thi Mong Hiep, Olivier Michel. Investigation: Ha Thi Chu, Isabelle Godin, Olivier Michel. Project administration: Nguyễn Thanh Phương, Lan Huu Nguyen, Ngo Minh Xuan, Francis Corazza. Resources: Nguyễn Thanh Phương, Lan Huu Nguyen, Ngo Minh Xuan, Francis Corazza. Supervision: Lan Huu Nguyen, Tran Thi Mong Hiep, Ngo Minh Xuan, Olivier Michel. Validation: Ha Thi Chu, Isabelle Godin, Nguyễn Thanh Phương, Lan Huu Nguyen, Tran Thi Mong Hiep, Ngo Minh Xuan, Francis Corazza, Olivier Michel. Writing - original draft: Ha Thi Chu, Isabelle Godin, Olivier Michel. Writing - review & editing: Ha Thi Chu, Isabelle Godin, Nguyễn Thanh Phương, Lan Huu Nguyen, Tran Thi Mong Hiep, Ngo Minh Xuan, Francis Corazza, Olivier Michel.
References
- 1.World Health Organization. Programmes: chronic respiratory diseases [Internet] Geneva (Switzerland): World health organization; c2017. [cited 2017 Jul 4]. Available from: http://www.who.int/respiratory/about_topic/en/ [Google Scholar]
- 2.Bousquet J, Kiley J, Bateman ED, Viegi G, Cruz AA, Khaltaev N, Aït Khaled N, Baena-Cagnani CE, Barreto ML, Billo N, Canonica GW, Carlsen KH, Chavannes N, Chuchalin A, Drazen J, Fabbri LM, Gerbase MW, Humbert M, Joos G, Masjedi MR, Makino S, Rabe K, To T, Zhi L. Prioritised research agenda for prevention and control of chronic respiratory diseases. Eur Respir J. 2010;36:995–1001. doi: 10.1183/09031936.00012610. [DOI] [PubMed] [Google Scholar]
- 3.Burney P, Jarvis D, Perez-Padilla R. The global burden of chronic respiratory disease in adults. Int J Tuberc Lung Dis. 2015;19:10–20. doi: 10.5588/ijtld.14.0446. [DOI] [PubMed] [Google Scholar]
- 4.Wong GW, Leung TF, Ko FW. Changing prevalence of allergic diseases in the Asia-pacific region. Allergy Asthma Immunol Res. 2013;5:251–257. doi: 10.4168/aair.2013.5.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Sun B, Huang Y, Lin X, Zhao D, Tan G, Wu J, Zhao H, Cao L, Zhong N China Alliance of Research on Respiratory Allergic Disease. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy. 2009;64:1083–1092. doi: 10.1111/j.1398-9995.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 6.Yuenyongviwat A, Koonrangsesomboon D, Sangsupawanich P. Recent 5-year trends of asthma severity and allergen sensitization among children in southern Thailand. Asian Pac J Allergy Immunol. 2013;31:242–246. doi: 10.12932/AP0289.31.3.2013. [DOI] [PubMed] [Google Scholar]
- 7.Andiappan AK, Puan KJ, Lee B, Nardin A, Poidinger M, Connolly J, Chew FT, Wang DY, Rotzschke O. Allergic airway diseases in a tropical urban environment are driven by dominant mono-specific sensitization against house dust mites. Allergy. 2014;69:501–509. doi: 10.1111/all.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lâm HT, Ekerljung L, Bjerg A, Văn T Tng N, Lundbäck B, Rönmark E. Sensitization to airborne allergens among adults and its impact on allergic symptoms: a population survey in northern Vietnam. Clin Transl Allergy. 2014;4:6. doi: 10.1186/2045-7022-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu HT, Godin I, Phuong NT, Nguyen LH, Hiep TT, Michel O. Allergen sensitization among chronic respiratory diseases in urban and rural areas of the South of VietNam. Int J Tuberc Lung Dis. 2018;22:221–229. doi: 10.5588/ijtld.17.0069. [DOI] [PubMed] [Google Scholar]
- 10.Global Initiative for Asthma [Internet] place unknown: Global Initiative for Asthma; c2016. [cited 2017 Jul 4]. Available from: http://www.ginasthma.org/ [Google Scholar]
- 11.Global Initiative for Chronic Obstructive Lung Disease [Internet] place unknown: Global Initiative for Chronic Obstructive Lung Disease; c2016. [cited 2017 Jul 4]. Available from: http://goldcopd.org/ [Google Scholar]
- 12.Brand PL, Kerstjens HA, Jansen HM, Kauffman HF, de Monchy JG. Interpretation of skin tests to house dust mite and relationship to other allergy parameters in patients with asthma and chronic obstructive pulmonary disease. The Dutch CNSLD Study Group. J Allergy Clin Immunol. 1993;91:560–570. doi: 10.1016/0091-6749(93)90262-e. [DOI] [PubMed] [Google Scholar]
- 13.Lee-Wong M, Chou V, Silverberg JI. A study of IgE sensitization and skin response to histamine in Asian-Pacific American adults. Allergy Asthma Proc. 2012;33:341–347. doi: 10.2500/aap.2012.33.3575. [DOI] [PubMed] [Google Scholar]
- 14.Kanceljak-Macan B, Macan J, Plavec D, Klepac T, Milković-Kraus S. The 3 mm skin prick test (SPT) threshold criterion is not reliable for Tyrophagus putrescentiae: the re-evaluation of SPT criterion to dust mites. Allergy. 2002;57:1187–1190. doi: 10.1034/j.1398-9995.2002.23730.x. [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 16.Plebani M, Borghesan F, Basso D, Faggian D. Receiver-operating characteristic (ROC) curves: a fundamental tool for improving the clinical usefulness of in vitro IgE tests. Allergy. 1996;51:407–411. [PubMed] [Google Scholar]
- 17.Lâm HT, Tu'ò'ng NV, Lundbäck B, Rönmark E. Storage mites are the main sensitizers among adults in northern Vietnam: results from a population survey. Allergy. 2011;66:1620–1621. doi: 10.1111/j.1398-9995.2011.02730.x. [DOI] [PubMed] [Google Scholar]
- 18.Roos A, Wurmser C, Kokou C, Vrtala S, Valenta R, Pauli G. Reconnaissance des allergènes moléculaires des acariens en Afrique équatoriale. Rev Fr Allergio. 2017;57:236. [Google Scholar]
- 19.Soh WT, Le Mignon M, Suratannon N, Satitsuksanoa P, Chatchatee P, Wongpiyaboron J, Vangveravong M, Rerkpattanapipat T, Sangasapaviliya A, Nony E, Piboonpocanun S, Ruxrungtham K, Jacquet A Mite Allergy Research Cohort (MARC) Study Team. The house dust mite major allergen Der p 23 displays O-Glycan-Independent IgE reactivities but no chitin-binding activity. Int Arch Allergy Immunol. 2015;168:150–160. doi: 10.1159/000442176. [DOI] [PubMed] [Google Scholar]
- 20.Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, Thomas WR, Alessandri C, Chen KW, de Blay F, Valenta R, Vrtala S. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol. 2012;159:253–262. doi: 10.1159/000337654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013;3:3–14. doi: 10.5415/apallergy.2013.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauveau A, Dalphin ML, Mauny F, Kaulek V, Schmausser-Hechfellner E, Renz H, Riedler J, Pekkanen J, Karvonen AM, Lauener R, Roduit C, Vuitton DA, von Mutius E, Dalphin JC PASTURE Study Group. Skin prick tests and specific IgE in 10-year-old children: Agreement and association with allergic diseases. Allergy. 2017;72:1365–1373. doi: 10.1111/all.13148. [DOI] [PubMed] [Google Scholar]
- 23.Ponte JC, Junqueira SB, Veiga RV, Barreto ML, Pontes-de-Carvalho LC, Alcântara-Neves NM. A study on the immunological basis of the dissociation between type I-hypersensitivity skin reactions to Blomia tropicalis antigens and serum anti-B. tropicalis IgE antibodies. BMC Immunol. 2011;12:34. doi: 10.1186/1471-2172-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visitsunthorn N, Sripramong C, Bunnag C, Jirapongsananuruk O. Comparison between specific IgE levels and skin prick test results of local and imported American cockroach, dog, cat, dust mites and mold allergen extracts. Asian Pac J Allergy Immunol. 2017;35:60–65. doi: 10.12932/AP0745. [DOI] [PubMed] [Google Scholar]
- 25.Nam YH, Lee SK. Comparison between skin prick test and serum immunoglobulin E by CAP system to inhalant allergens. Ann Allergy Asthma Immunol. 2017;118:608–613. doi: 10.1016/j.anai.2017.03.005. [DOI] [PubMed] [Google Scholar]