Abstract
Objectives
The robotic retroauricular approach and transoral endoscopic thyroidectomy vestibular approach (TOETVA) have been employed to avoid anterior neck scarring in thyroidectomy with good success. However, outcomes have yet to be compared between techniques. We compare our initial clinical experience with these approaches for thyroid lobectomy at our institution.
Methods
A review of initial consecutive patients who underwent robotic facelift thyroidectomy (RFT) (August 2011–August 2016) at our institution was conducted. This was compared with the same number of initial consecutive patients who underwent TOETVA (September 2016–September 2017) at our institution. Demographics, operative time, pathology, complications, and learning curve were compared between cohorts. Learning curve was defined based on the slope of linear regression models of operative time versus case number.
Results
There were 20 patients in each cohort. There was no statistically significant difference in demographic data between cohorts. One hundred percent of RFT cases versus 95% TOETVA cases (P = .999) were completed without conversion to standard open technique with median operative times of 201 (124–293) minutes versus 188 (89–343) minutes with RFT and TOETVA, respectively (P = .36). There was no incidence of permanent recurrent laryngeal nerve injury in either cohort. The slopes of the regression models were 0.29 versus −8.32 (P = .005) for RFT and TOETVA, respectively.
Conclusion
RFT and TOETVA are safe and feasible options for patients motivated to avoid an anterior neck scar. However, the quicker learning curve without the need for a costly robotic system may make TOETVA the preferred technique for institutions wishing to perform anterior cervical incision‐sparing thyroidectomy.
Level of Evidence
4
Keywords: Retroauricular, transoral thyroidectomy, robotic thyroidectomy, minimally invasive, TOETVA, RFT, remote‐access thyroidectomy
INTRODUCTION
The transcervical approach (TCA) established by Emil Kocher is the most widely used for thyroidectomy.1 Advancements in operative technique have led to decreased morbidity, mortality, and surgery through ever‐shrinking cervical incisions.2 Despite these improvements, studies have demonstrated that there can be a significant negative impact on patient quality of life (QOL) as a result of a visible cervical scar.3 Moreover, it is not only the severity or length of the scar but the mere presence of one that leads to this finding.4 International communities, most notably in Asia, have made significant strides in remote access and minimally invasive thyroidectomy while demonstrating safety profiles similar to those through a traditional anterior cervical incision. These techniques include endoscopic or robotic breast, bilateral axillobreast, and axillary incisions, as well as the retroauricular approach.5 More recently, transoral thyroidectomy has become a favored approach as it provides midline access to the thyroid with minimal extracervical tissue dissection and no cutaneous scar.2, 6, 7, 8, 9, 10, 11, 12
Despite this experience, the North American experience has been decidedly different.5 Early excitement for remote access thyroidectomy was tempered when novel complications were reported via the transaxillary approach.13 Some of this was thought to be due to the differing patient demographics and larger body mass indices (BMI) of most patients in the US cohort relative to the Korean patient population. Moreover, approaches utilizing unfamiliar dissection planes may have also contributed to the comparatively negative North American outcomes. Given these concerns, Terris et al. described the robotic facelift thyroidectomy (RFT) via a retroauricular approach.14 RFT aimed to use dissection planes familiar to head and neck surgeons, approaching the thyroid from a superior and lateral dissection. More recently, Anuwong described the transoral endoscopic thyroidectomy vestibular approach (TOETVA), which similarly, utilizes a familiar dissection plane but provides a midline surgical field with equal access to both thyroid lobes.10, 12, 15, 16, 17
As early North American adopters of both techniques, our institution wished to compare our initial experience with RFT and TOETVA in terms of outcomes, complications, and learning curve. To our knowledge, this is the first manuscript to compare the experience with TOETVA and RFT at a single institution.
MATERIALS AND METHODS
Data Collection & Analysis
A retrospective review of all initial consecutive patients who underwent robotic facelift thyroidectomy (RFT) between August 2011 and August 2016 was conducted and compared with the same number of the initial consecutive patients whom underwent TOETVA for thyroid lobectomy between September 2016 and September 2017 at our institution. All patients met indications for hemithyroidectomy according to the current American Thyroid Association (ATA) Guidelines at the time of the procedure.18, 19 All patients were counseled on available surgical options and the possible conversion to TCA, and written informed consent was obtained. A single surgeon completed all RFT cases, while a separate surgeon completed all TOETVA cases. The surgeon completing the RFT cases was an experienced robotic surgeon with fellowship training in head and neck surgery and a high‐volume transoral robotic surgery practice. For the TOETVA cohort, the surgeon was a high‐volume endocrine surgeon, with fellowship training in head and neck endocrine surgery. He did not, however, have prior laparoscopic surgical experience.
Indications for RFT included: 1) motivation to avoid an anterior cervical neck scar with or without a history of a hypertrophic scar or a keloid, 2) nodule size less than 6 cm in maximum dimension, and 3) thyroid lobe less than 7 cm. Exclusion criteria included: 1) cancer with extrathyroidal extension or lymph node metastasis, 2) Graves’ disease or radiographic evidence of thyroiditis, 3) substernal extension, or 4) previous neck surgery or irradiation. Indications for TOETVA were in accordance with our published recommendations and included motivation to avoid a cervical incision with dominant nodule ≤ 6 cm in largest dimension if preoperative cytopathology was indeterminate or benign, or nodule ≤ 2 cm if cytopathology was suspicious for malignancy or consistent with well‐differentiated thyroid cancer. Those with a history of head and neck surgery or irradiation were excluded as were patients with lymph node metastasis or extrathyroidal disease extension.20 Demographics, imaging findings, nodule characteristics, preoperative and postoperative diagnosis, and other characteristic variables were obtained from the electronic medical record. Outcome measures collected for each technique included the following: completion of the intended procedure, presence of persistent (symptoms present for >3 months) recurrent laryngeal nerve (RLN) or mental nerve injury, operative time (incision to closure), and postoperative day of discharge. Secondary outcome measures included use of a surgical drain, incidence of hematoma/seroma, and inadvertent presence of parathyroid glands within the specimen (incidental parathyroidectomy). Differences in means of parametric demographic and characteristic data were compared between the two cohorts with an unpaired t‐test. Nonparametric data including operative time, largest specimen dimension, and postoperative day of discharge were compared with the Mann‐Whitney U test. The Fisher's exact test was used to determine differences between cohorts in categorical data. A linear regression model was used to define a learning curve for each cohort based on the slope of the operative time vs. case number trend line calculated through the least squares method. Statistical analysis was completed in Stata Statistical Software: Release 15 (StataCorp LLC, College Station, Texas, U.S.A.) with an alpha of 0.05 used as a cutoff for statistical significance. Johns Hopkins Hospital Institutional Review Board approval was obtained for this study.
Surgical Procedure RFT
Our technique is similar to what was described by Terris et al.14 After intubating the patient with a neural integrity monitor electromyogram endotracheal tube, a retroauricular incision extending to the hairline is marked.
Skin and subplatysmal flaps are elevated with preservation of the great auricular nerve. The sternocleidomastoid muscle is distracted laterally and retracted. The strap muscles are elevated and a retractor is placed underneath them. The retractor is suspended from the table and the robot is docked. At this point the thyroid lobe is easily identifiable. The superior pole of the thyroid is divided using the Harmonic scalpel (Ethicon Corp, Somerville, New Jersey, U.S.A.). This is usually done under direct vision after which the remainder of the dissection is done robotically. The RLN is identified and preserved with the assistance of a nerve stimulator. The parathyroid glands are identified and preserved. Again, using the ultrasonic energy device, the isthmus is divided, and the gland is removed. The recurrent and superior laryngeal nerves are stimulated and confirmed to be functionally intact. At the surgeon's judgment, a drain is placed posterior to the hairline and removed prior to discharge (usually within 24 hours). The wound is closed in the standard fashion. The patients were seen during their follow up appointments and their incisions were evaluated (Fig. 1).
Figure 1.

Postoperative appearance of the retroauricular incision for RFT.
RFT = robotic facelift thyroidectomy.
Surgical Procedure TOETVA
Our technique for TOETVA was adapted from that of Anuwong and has been previously described by our group.10, 15, 17 In brief, the patient is positioned supine and intubated with a 6.0 or 7.0 nerve monitoring tube orotracheally. Three incisions are then made in the oral vestibule to accommodate central and lateral trochars. The central incision is 1.5 cm in length and is placed beyond the cranial aspect of the buccal‐mandibular frenulum, while two stab incisions are made at the lateral most aspect of the oral commissure in the mucosal border to avoid mental nerve injury and instrument collision (Fig. 2). A subplatysmal pocket is then developed with use of mechanical dilators. An endoscope is then inserted through the central trochar, and the subplatysmal flap is then fully elevated to the level of the sternal notch inferiorly and sternocleidomastoid muscles laterally with use of endoscopic instrumentation. CO2 insufflation is utilized to maintain this working space and the midline raphe is identified, divided, and the ipsilateral strap muscles are elevated. The thyroid isthmus is then divided and lobectomy is performed in a top‐down fashion utilizing the trachea as a landmark for the RLN, which is most often identified at 2 o'clock and 3 o'clock from the anterior trachea on the right side, and between 9 o'clock and 10 o'clock on the left. Once the lobectomy has been completed the specimen is placed in an endocatch bag and removed via the central incision. Oral incisions are closed with dissolvable suture. (Fig. 3)
Figure 2.
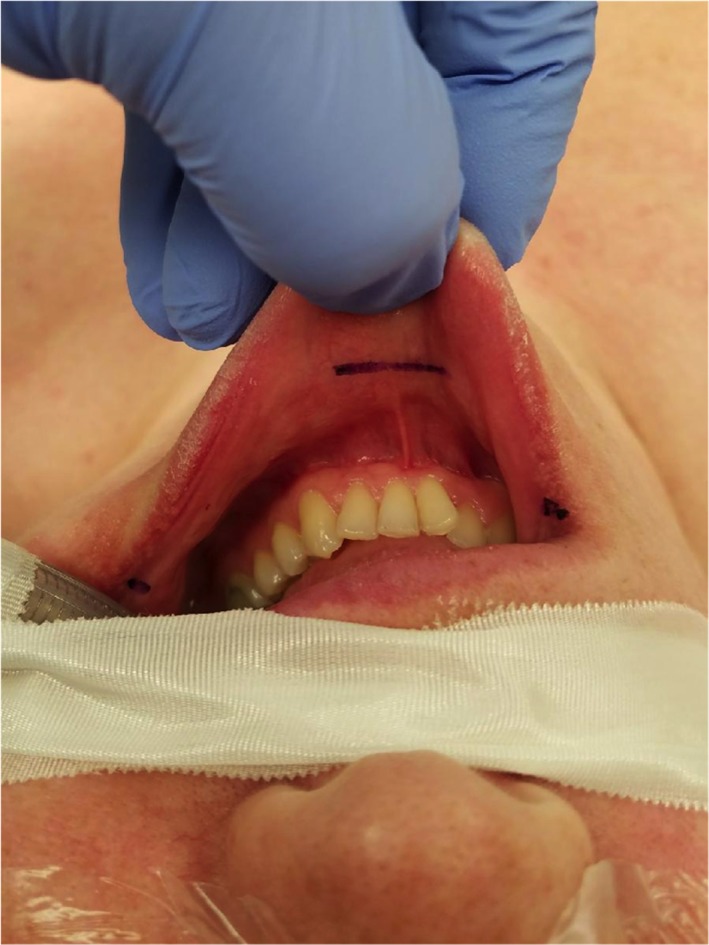
Location of the intraoral incisions for TOETVA.
TOETVA = transoral endoscopic thyroidectomy vestibular approach.
Figure 3.

Postoperative appearance of the intraoral incisions (A) and appearance of the neck (B) following TOETVA.
TOETVA = transoral endoscopic thyroidectomy vestibular approach.
RESULTS
Forty total patients were identified and selected for thyroid lobectomy using the above criteria, with 20 patients each in the RFT and TOETVA cohorts (Table 1). Cases in each cohort were completed consecutively and were the initial such cases completed at our institution. All patients underwent fine needle aspiration biopsy (FNA). Demographic and characteristic data for both cohorts are found in Table 2, while surgical outcomes are highlighted in Table 3. The intended procedure was completed in 100% (20/20) of cases in RFT and 95% (19/20) of cases in the TOETVA cohort (P = .999). One case in the TOETVA cohort was converted to open due to superior pole bleeding and was ultimately completed without complication via a transcervical approach. Median operative time for the RFT and TOETVA (excluding the case converted to TCA) cohorts were 201 (124–293) and 188 (89–343) minutes respectively (P = .36). Within the respective cohorts, there was no difference in operative time between right‐ and left‐sided procedures. In the RFT cohort the median operative time for right‐sided procedures was 205.5 minutes versus 180 minutes for left‐sided procedures (P = .29). Similarly in the TOETVA cohort, median operative time for right‐ and left‐sided procedures were 176 and 238 minutes (P = .40), respectively.
Table 1.
Patient Selection.
| Cohort: | RFT | TOETVA |
|---|---|---|
| Laterality: (n) | ||
| Left | 10 | 8 |
| Right | 10 | 12 |
| Nodule Size: (median, cm, range) | ||
| Dominant nodule size | 3.2 (0.9–5.7) | 3.6 (1.2–7.0) |
| Preoperative pathology | ||
| Bethesda I | 2 | 0 |
| Bethesda II | 8 | 7 |
| Bethesda III | 7 | 8 |
| Bethesda IV | 3 | 2 |
| Besthesda V | 0 | 2 |
| Besthesda VI | 0 | 1 |
RFT = robotic facelift thyroidectomy; TOETVA = transoral endoscopic thyroidectomy vestibular approach.
Table 2.
Characteristic Cohort Data.
| Approach | RFT | TOETVA | P Value |
|---|---|---|---|
| N | 20 | 20 | |
| Age (mean, years) | 37.7 ± 10.1 | 42.6 ± 12.2 | .17 |
| Male (%) | 0 | 20 | .10 |
| Female (%) | 100 | 80 | |
| BMI (mean, kg/m2) | 28.5 ± 7.7 | 27.6 ± 7.5 | .72 |
| Largest specimen dimension (median, cm, range) | 4.5 (2.2–8.5) | 5.0 (3.0–7.8) | .30 |
BMI = body mass index; RFT = robotic facelift thyroidectomy; TOETVA = transoral endoscopic thyroidectomy vestibular approach.
Table 3.
Surgical Outcomes.
| Approach | RFT | TOETVA | P Value |
|---|---|---|---|
| N | 20 | 20 | |
| Operative time* (median, range, minutes) | 201 (124–293) | 188 (89–343) | .36 |
| Permanent RLN injury (%) | 0 | 0 | NA |
| Permanent MN injury (%) | 0 | 0 | NA |
| Extrathyroidal parathyroids within specimen (%)† | 10 | 0 | .49 |
| Placement of drain (%) | 70 | 10 | .0002 |
| Completion of intended approach (%) | 100 | 95 | .999 |
| Postoperative day of discharge (median, range) | 1 (1–2) | 0.5 (0–1) | .002 |
| Ultimate avoidance of cervical incision (%) | 85 | 95 | .6 |
| Hematoma/seroma (%) | 10 | 0 | .49 |
| Hypertrophic scarring (%) | 15 | 0 | .23 |
Converted cases were not included in median operative time for any cohort.
Excluding planned parathyroidectomy.
MN = mental nerve; RFT = robotic facelift thyroidectomy; RLN = recurrent laryngeal nerve; TOETVA = transoral endoscopic thyroidectomy vestibular approach.
The bold P value indicates statistically significant.
Figure 4 demonstrates the trend in operative time as a function of case number for each technique. No patients in either cohort had permanent (symptoms lasting > 3 months) RLN, marginal mandibular nerve (RFT cohort) or mental nerve (TOETVA cohort) injury. One patient in the RFT cohort had sacrifice of a small branch of the great auricular nerve during elevation of the subplatysmal flap. Fourteen patients (70%) in the RFT cohort had drain placement at the time of surgery compared to 2 (10%) in the TOETVA cohort (P = .0002). Median postoperative day (POD) of discharge for the RFT and TOETVA cohorts were 1.0 (1–2) and 0.5 (0–1) days, respectively (P = .002), with 50% of patients in the TOETVA cohort being discharged on POD 0 compared to 0% in the RFT cohort. Two patients in the RFT cohort developed postoperative fluid collections. One RFT patient had persistent nausea and vomiting postanesthesia and subsequently developed a seroma, which was drained and controlled with a pressure dressing at that time. The other RFT patient developed a hematoma that required evacuation in the operating theatre on postoperative day 7. One patient in each cohort (5%) had temporary vocal fold palsy that recovered spontaneously within 3 months. Three patients in the RFT cohort developed postoperative hypertrophic scarring, along the postauricular limb of their incision compared to zero in the TOETVA cohort (P = .23).
Figure 4.
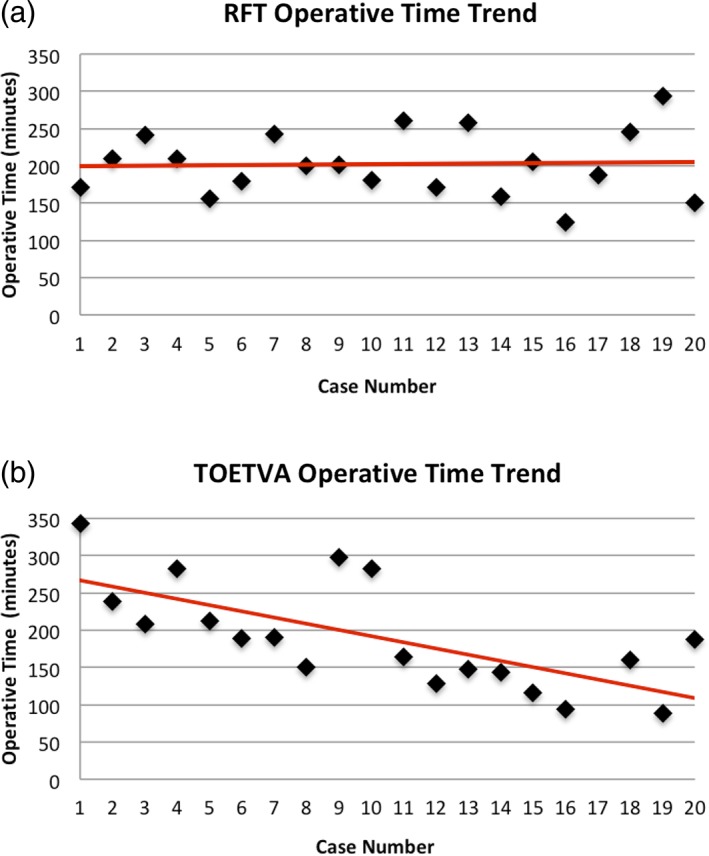
Graphical representation of the learning curves for RFT (A) and TOETVA (B). The slopes of the learning curves are 0.29 and −8.32, respectively.
RFT = robotic facelift thyroidectomy; TOETVA = transoral endoscopic thyroidectomy vestibular approach.
On final pathology, 6 patients had papillary thyroid cancer (PTC) in the RFT cohort, while in the TOETVA cohort 2 patients were found to have PTC, 1 NIFT‐P (noninvasive thyroid neoplasm with papillary‐like nuclear features), and 1 patient with minimally invasive Hurthle cell carcinoma. The remainder of patients in both cohorts were found to have benign pathology. As per the ATA guidelines18 at the time of surgery, completion thyroidectomy was recommended in four patients in the RFT cohort, of which three patients elected to undergo completion thyroidectomy via TCA while the remaining patient did not elect to pursue further surgery. Conversely, no patients required completion thyroidectomy per current ATA guidelines19 in the TOETVA cohort, however, one patient with Hurthle cell carcinoma elected to have completion thyroidectomy via the transoral approach. She underwent uncomplicated completion thyroidectomy via TOETVA 9 days following her initial procedure. As a result of completion thyroidectomies and/or conversions to TCA a total of 17 of 20 and 19 of 20 patients ultimately avoided a cervical incision in the RFT and TOETVA cohorts respectively (P = .60). The slope of the learning curve for the RFT cohort was 0.29 (95% CI, −3.58 to 4.16), which was not significantly different from a slope of 0 (P = .88), while the slope of the learning curve for TOETVA was −8.32 (95% CI, −12.69 to −3.95), which was both significantly different from a slope of 0 (P < .001) and from the slope of the RFT learning curve (P = .005) (Fig. 4). Additionally, when examining the median operative time of the final 10 cases in each cohort there was significant difference with a median of 187 minutes in the RFT cohort compared to 145.5 minutes in the TOETVA cohort (P = .04).
DISCUSSION
Both RFT and TOETVA are safe and effective techniques for performing thyroid lobectomy. There were no incidences of permanent RLN injury in either cohort. One patient in the RFT cohort had intraoperative sacrifice of a branch of the great auricular nerve to facilitate skin and subplatysmal flap dissection. Based on the surgical approach, the marginal mandibular nerve (RFT) and the mental nerve (TOETVA) are also potentially at risk with these remote‐access techniques. However, there were no permanent injuries to these nerves in either cohort in our experience. The intended procedure was completed in 100 and 95% of the cases in the RFT and TOETVA cohorts, respectively. Moreover there was no significant difference in median operative times between the techniques. There were, however, significant differences between the groups in postoperative day of discharge, proportion of patients undergoing drain placement, and learning curve (Table 3). Of note, we found no significant difference in patient clinical characteristics that may have affected the difficulty of the procedure, including BMI or maximum surgical specimen dimension. As such, any resultant differing outcomes are likely not due to these factors, and are more likely reflective of inherent qualities of the techniques themselves (Table 2). Moreover, though the RFT series preceded our TOETVA experience, two separate high volume thyroid surgeons performed cases independently for each cohort. As such, techniques learned from RFT were less likely to translate to improved outcomes in the later series. Further evidence can be seen when examining the incidences of postoperative seroma and hematoma that occurred in the RFT cohort with no such events in the TOETVA group despite the lower rate of drain placement.
Although there was no significant difference in median operative time between techniques, the slopes of the linear regressions of the trends in operative time for each approach were different (Fig. 4). The learning curve for any given procedure can be defined by two facets, the rate of skill acquisition and the case number after which proficiency has been reached. Previous studies examining the learning curve for remote‐access approaches to the thyroid have defined the case number after which operative time vs. case number plot plateaus as the proficiency case.21 Although the case volume of both cohorts did not provide an adequate sample size to identify a plateau point and henceforth proficiency case in either series, there was a difference in the slopes of the linear regressions as described above. The slope of the linear regression of the operative time versus case number plot was 0.29 for the RFT cohort, which was not significantly different from 0, suggesting no significant change in procedural proficiency. Conversely, the slope of the TOETVA regression line was −8.3, which was both significantly different from 0 and from the RFT cohort trendline. This corresponds to a difference in the rate of skill acquisition, and as such, a difference in learning curves between TOETVA and RFT.
One factor that may have contributed to the differences in learning curves between procedures is the frequency with which they were performed. Our 20 RFT cases were completed over a 4.5‐year period, while the same number of cases were completed in 1 year in the TOETVA cohort. That being said, our institutional learning curves are consistent with previous literature citing the learning curve for robotic thyroidectomy techniques to be on the order of 35 to 50 cases, while an estimate of 7 to 10 cases has been previously cited as the learning curve for TOETVA.11, 21, 22, 23 Likewise, the difference in baseline experience between surgeons in the respective cohorts may have also contributed to the difference in rate of skill acquisition, as the surgeon in the TOETVA cohort had no prior laparoscopic experience, while the surgeon in the RFT cohort was an experienced transoral robotic surgeon. As such, it is possible that the steep slope of the learning curve seen in the TOETVA cohort was a function of laparoscopic skill acquisition and may not be specific to the procedure itself. If so, this may suggest that after this initial steep portion of the learning curve where laparoscopic skills are quickly developed, TOETVA may be able to be performed significantly faster than RFT. This is supported by the TOETVA operative time trend and the fact that there was a significant difference in the operative time of the final 10 TOETVA cases in comparison to the final 10 RFT cases (145.5 minutes vs. 187 minutes, P = .04). To understand this further, the learning curves of various surgeons with differing levels of respective laparoscopic and robotic skill will need to be analyzed for each technique.
In the United States, there may be less motivation to adopt these longer surgical techniques in the absence of commensurate increases in reimbursement.5 Transcervical thyroid surgery has an excellent safety profile in experienced hands, and can also be done efficiently. When opting for TCA, patients and surgeons expect excellent outcomes with a cervical incision that heals well in most patients, and they increasingly expect such surgery on an outpatient basis. In a system in which “time is money,” the safety, efficacy and efficiency of traditional techniques have established a high threshold to further innovation. In such a setting, slower remote access surgeries, especially those with the added expense of the robot, may be difficult to justify for all patients. Despite this, however, some patients would be willing to pay more and accept a higher risk of complications if they were able to avoid a cervical incision.24
There is a learning curve with the various remote access techniques, and indeed with any new technique. Despite this, early outcomes with TOETVA show significant promise. Unlike other remote access techniques, TOETVA appears to have a relatively short learning curve and operative times that are trending towards that of TCA as more cases are completed. While this has been demonstrated in a large volume Asian cohort, it is not clear that this generalizes to the Western population.11 Our early experience and results suggest that TOETVA's international success can be replicated in North America. Moreover, 50% of patients in our TOETVA cohort were discharged to home the same day as surgery, including the final 6 cases. If these trends continue, the difference in cost between TOETVA and TCA may become negligible, particularly because TOETVA does not require the added cost of a surgical robot. Furthermore, given some patients’ willingness to pay more to avoid a neck scar and the cited negative impact on quality of life that neck scarring can have, we may find that TOETVA in fact provides greater health utility than TCA in select cases.4, 24 Further studies are needed to better quantify both the cost of TOETVA in comparison to TCA and the societal value in avoiding a cervical incision.
CONCLUSION
RFT and TOETVA can both be safely utilized to perform thyroid lobectomy in the Western patient population, but the learning curve with TOETVA appears to be shorter. Additionally, the ability to discharge patients on the same timeline as TCA, with operative times trending towards that of the open approach provide great promise for TOETVA. Moreover, the use of instrumentation widely available at most hospitals lends TOETVA to broader adoption and implementation for many institutions. This series adds to the existing literature on anterior cervical incision‐sparing thyroid surgery in the United States, and offers patients and surgeons further perspective in regards to two potential techniques. Further studies are needed to quantify the cost of each technique in comparison to TCA and to determine the true value each may provide.
Editor's Note: This Manuscript was accepted for publication 18 June 2018.
Conflicts of Interest: RPT is a consultant for Medtronics and Hemostatix.
Funding: None
BIBLIOGRAPHY
- 1. Halsted WS. IV. (I) The Excision of Both Lobes of the Thyroid Gland for the Cure of Graves's Disease. (II) The Preliminary Ligation of the Thyroid Arteries and of the Inferior in Preference to the Superior Artery. Ann Surg 1913;58:178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: II. Clinical feasibility and safety. Laryngoscope 2011;121:1636–1641. [DOI] [PubMed] [Google Scholar]
- 3. Best AR, Shipchandler TZ, Cordes SR. Midcervical scar satisfaction in thyroidectomy patients. Laryngoscope 2017;127:1247–1252. [DOI] [PubMed] [Google Scholar]
- 4. Choi Y, Lee JH, Kim YH, et al. Impact of postthyroidectomy scar on the quality of life of thyroid cancer patients. Ann Dermatol 2014;26:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berber E, Bernet V, Fahey TJ, 3rd , et al. American Thyroid Association Statement on Remote‐Access Thyroid Surgery. Thyroid 2016;26:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kandil E, Saeed A, Mohamed SE, Alsaleh N, Aslam R, Moulthrop T. Modified robotic‐assisted thyroidectomy: an initial experience with the retroauricular approach. Laryngoscope 2015;125:767–771. [DOI] [PubMed] [Google Scholar]
- 7. Anuwong A. Transoral endoscopic thyroidectomy vestibular approach: a series of the first 60 human cases. World J Surg 2016;40:491–497. [DOI] [PubMed] [Google Scholar]
- 8. Kang SW, Lee SC, Lee SH, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery 2009;146:1048–1055. [DOI] [PubMed] [Google Scholar]
- 9. Russell JO, Noureldine SI, Al Khadem MG, Tufano RP. Minimally invasive and remote‐access thyroid surgery in the era of the 2015 American Thyroid Association guidelines. Laryngoscope Investig Otolaryngol 2016;1:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell JO, Clark J, Noureldine SI, et al. Transoral thyroidectomy and parathyroidectomy – A North American series of robotic and endoscopic transoral approaches to the central neck. Oral Oncol 2017;71:75–80. [DOI] [PubMed] [Google Scholar]
- 11. Anuwong A, Ketwong K, Jitpratoom P, Sasanakietkul T, Duh QY. Safety and outcomes of the transoral endoscopic thyroidectomy vestibular approach. JAMA Surg 2017;153:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richmon JD, Kim HY. Transoral robotic thyroidectomy (TORT): procedures and outcomes. Gland Surg 2017;6:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hinson AM, Kandil E, O'Brien S, et al. Trends in robotic thyroid surgery in the United States from 2009 through 2013. Thyroid 2015;25:919–926. [DOI] [PubMed] [Google Scholar]
- 14. Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: patient selection and technical considerations. Surg Laparosc Endosc Percutan Tech 2011;21:237–242. [DOI] [PubMed] [Google Scholar]
- 15. Anuwong A. Transoral endoscopic thyroidectomy vestibular approach: a series of the first 60 human cases. World J Surg 2016;40:491–497. [DOI] [PubMed] [Google Scholar]
- 16. Anuwong A, Kim HY, Dionigi G. Transoral endoscopic thyroidectomy using vestibular approach: updates and evidences. Gland Surg 2017;6:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Razavi CR, Fondong A, Tufano RP, Russell JO. Central neck dissection via the transoral approach. Ann Thyroid 2017;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, Cooper DS, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 19. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Razavi CR, Russell JO. Indications and contraindications to transoral thyroidectomy. Ann Thyroid 2017;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee J, Yun JH, Nam KH, Soh EY, Chung WY. The learning curve for robotic thyroidectomy: a multicenter study. Ann Surg Oncol 2011;18:226–232. [DOI] [PubMed] [Google Scholar]
- 22. Park JH, Lee J, Hakim NA, et al. Robotic thyroidectomy learning curve for beginning surgeons with little or no experience of endoscopic surgery. Head Neck 2015;37:1705–1711. [DOI] [PubMed] [Google Scholar]
- 23. Lee J, Lee JH, Nah KY, Soh EY, Chung WY. Comparison of endoscopic and robotic thyroidectomy. Ann Surg Oncol 2011;18:1439–1446. [DOI] [PubMed] [Google Scholar]
- 24. Coorough NE, Schneider DF, Rosen MW, et al. A survey of preferences regarding surgical approach to thyroid surgery. World J Surg 2014;38:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]


