Abstract
Objectives
We sought to determine how the pathology altered electrically evoked auditory brainstem responses (EABRs) in patients with hearing loss by evaluating EABRs in auditory neuropathy patients with OTOF mutations comparing with various types of congenital deafness.
Methods
We included 15 patients with congenital hearing loss, grouped according to pathology: OTOF mutations (n = 4), GJB2 mutations (n = 4), SLC26A4 mutations (n = 4), or cytomegalovirus infections (n = 3). EABRs were recorded when patients underwent cochlear implantation surgery. We evaluated the latencies and amplitudes of the recorded EABRs and compared them statistically between four groups.
Results
The EABR latencies of Wave III and Wave V, and of the interval between them, were significantly longer in the OTOF mutation group than in the GJB2 and SLC26A4 mutation groups (Wave III) and in all three other groups (Wave V and Wave III‐V latency); amplitudes were not significantly different between groups.
Conclusions
Our results suggest OTOF mutations cause delayed (or slowed) postsynaptic neurotransmission, although the presumed mechanism involved reduced presynaptic transmission between hair cells and spiral ganglion neurons.
Level of Evidence
Mainly a case report
Keywords: Auditory neuropathy, OTOF, electrically evoked auditory brainstem responses
INTRODUCTION
Auditory neuropathy (AN) is a disease characterized by absent or abnormal auditory nerve function with normal outer hair cell function. Clinically, AN presents as sensorineural hearing loss with accompanying impaired speech discrimination; diagnostic data are characterized by preserved otoacoustic emissions (OAE) or cochlear microphonic (CM) and a disturbed auditory brain stem response (ABR).1, 2
A part of AN is known to be caused by genetic mutations including OTOF,3 OPA1,4 and PJVK mutations.5 In fact, an OTOF mutation was first reported as a genetic cause of DFNB9.6 OTOF mutations account for 1.4% to 5% of cases of autosomal recessive non‐syndromic hearing impairment.7, 8, 9, 10, 11, 12, 13, 14 The majority of patients carrying two mutant alleles of OTOF show severe‐to‐profound congenital hearing loss. In Japanese patients with AN, OTOF mutations accounted approximately 60% of the cases.15 Within at least the first one or two years after birth they show preserved OAE or CM without an ABR response,16 so they are diagnosed with AN. The trans‐membrane protein OTOFERLIN, encoded by the OTOF gene, is expressed in the inner and outer hair cells of the rodent cochlea.6, 17 This protein is a critical regulator of vesicle fusion with the plasma membrane following glutamate release or during the need for vesicle replenishment at the afferent ribbon synapses between inner hair cells and spiral ganglion neurons.18 Thus, AN caused by OTOF mutation is thought to be caused by disrupted synaptic function (an auditory synaptopathy) at synapses between the inner hair cells and spiral ganglion neuron.19
Treatment of profound‐to‐severe hearing loss in patients with AN requires cochlear implantation (CI); however, the efficacy of CI in such cases is controversial.20, 21, 22, 23, 24 On the other hand, AN caused by OTOF mutations is thought to be a better candidate for cochlear implantation because the electrode can stimulate the auditory nerves directly, thus bypassing impaired synapses. Several reports have demonstrated an adequate level of cochlear implant performance in patients with OTOF mutations.25, 26, 27 However, precise evaluations of postsynaptic functions in this disease are lacking.
Electrically evoked auditory brainstem responses (EABRs) can be used for measuring neuronal activity in the cochlear nerve after CI implantation; in addition, the clinical usefulness of EABR analysis has been reported.28, 29 Thus far, electrophysiological investigations were reported using EABR analysis in AN patient.30, 31, 32, 33 Runge et al. showed reduced wave V supra‐threshold amplitude with AN and indicated residual dys‐synchronous neuronal activity in the central auditory pathway.31
Given the heterogeneity of potential cause of AN, grouping the patient by the cause is needed. In this report we focused on AN caused by OTOF mutations. To clarify the postsynaptic neuronal physiological changes in auditory neuropathy caused by OTOF mutations, we compared the EABR responses of patients who underwent CI to treat one of several forms of congenital hearing loss. We also compared the physiological characteristics of EABRs after long‐term or short‐term cochlear implantation for patients with OTOF mutations.
MATERIALS AND METHODS
Enrolled Patients
We retrospectively analyzed the EABR results of patients who had undergone CI from December 2008 to November 2016, with implants manufactured by MED‐EL or Advanced Bionics; we had to exclude implants manufactured by Cochlear for technical reasons. We included patients who were diagnosed with OTOF, GJB2, or SLC26A4 mutations by genetic testing, and who were diagnosed with maternal CMV infections by umbilical cord inspection. We enrolled 15 patients, including four each with OTOF, GJB2, or SLC26A4 mutations; and three patients with previous maternal CMV infections. Among these patients, four patients had undergone bilateral CI. We analyzed EABR wave forms obtained from six ears with OTOF mutations, six with GJB2 mutations, four with SLC26A4 mutations, and four from patients with CMV infections (Table 1).
Table 1.
Enrolled Patients, Their Demographic Characteristics, and Their Pathological Findings
| Patient | Cause of Deafness | Sex | Operation Age | Operation side | Implanted CI model | Imaging Findings | Wave III Latency (mSec) | Wave V Latency (mSec) |
|---|---|---|---|---|---|---|---|---|
| #1 | OTOF | Male | 3Y4M | rt | MED‐EL PULSAR FLEX soft | no inner ear malformation | 2.24 | 4.65 |
| #2 | OTOF | Female | 1Y9M | rt | MED‐EL CONCERTO flex28 | no inner ear malformation | 2.66 | 5.60 |
| #2‐2nd | OTOF | Female | 3Y0M | lt | MED‐EL CONCERTO flex28 | no inner ear malformation | 2.70 | 5.49 |
| #3 | OTOF | Female | 2Y3M | rt | Advanced Bionics Hifocus MS | no inner ear malformation | 2.34 | 5.28 |
| #3‐2nd | OTOF | Female | 2Y10M | lt | Advanced Bionics Hifocus MS | no inner ear malformation | 2.34 | 5.28 |
| #4 | OTOF | Female | 1Y11M | rt | Advanced Bionics Hifocus MS | no inner ear malformation | 2.44 | 4.46 |
| #5 | GJB2 | Male | 2Y1M | rt | MED‐EL PULSAR FLEX soft | no inner ear malformation | 2.36 | 4.19 |
| #5‐2nd | GJB2 | Male | 5Y5M | lt | MED‐EL CONCERTO flex28 | no inner ear malformation | 2.00 | 4.30 |
| #6 | GJB2 | Male | 1Y6M | rt | MED‐EL CONCERTO MI1000PIN flex soft | no inner ear malformation | 2.13 | 4.34 |
| #6‐2nd | GJB2 | Male | 2Y1M | lt | MED‐EL CONCERTO flex28 | no inner ear malformation | 2.20 | 4.06 |
| #7 | GJB2 | Male | 2Y7M | rt | MED‐EL CONCERTO flex28 | no inner ear malformation | 2.02 | 4.39 |
| #8 | GJB2 | Male | 3Y6M | rt | MED‐EL CONCERTO Flex28 | no inner ear malformation | 2.22 | 4.08 |
| #9 | SLC26A4 | Male | 1Y11M | rt | MED‐EL CONCERTO Mi100 Flex soft | large vestibular aqueduct | 2.12 | 3.97 |
| #10 | SLC26A4 | Female | 3Y5M | rt | MED‐EL CONCERTO Mi100 Flex soft | large vestibular aqueduct | 2.31 | 4.07 |
| #11 | SLC26A4 | Female | 4Y0M | rt | Advanced Bionics Mid Scala | large vestibular aqueduct | 2.20 | 4.00 |
| #12 | SLC26A4 | Female | 3Y10M | rt | MED‐EL CONCERTO flex28 | large vestibular aqueduct | 2.08 | 3.86 |
| #13 | CMV | Female | 3Y8M | lt | MED‐EL CONCERTO flex28 | no inner ear malformation | 2.38 | 4.26 |
| #14 | CMV | Male | 3Y8M | lt | MED‐EL CONCERTO flex28 | no inner ear malformation | 2.06 | 4.17 |
| #15 | CMV | Female | 1Y6M | lt | MED‐EL CONCERTO flex28 | no inner ear malformation | 2.21 | 4.15 |
| #15‐2nd | CMV | Female | 2Y1M | rt | MED‐EL CONCERTO flex28 | no inner ear malformation | 2.21 | 4.37 |
Notes: * The note “2nd” in the Patient column represents the second operation undergone by that patient. CI = cochlear implant; CMV = cytomegalovirus.
Deafness genes were tested at the Laboratory of Auditory Disorders, National Institute of Sensory Organs, National Tokyo Medical Center by the Sanger method using the genomic DNA extracted from patients' peripheral blood cells when their deafness genes have not been clarified by previous genetic testing performed by BML supported by National Health Insurance. We performed the Sanger methods as previously reported.15, 34, 35 Genes and mutations of the enrolled patients are shown in Supplementary Table 1.
While c.3256G>A (p.G1086R) mutation of OTOF gene has not been reported as a pathogenic mutation, according to the ACMG guideline,36 we concluded it as a likely pathogenic mutation because this variant fulfilled PM2, PM3, PP3, and PP4. Similarly, c.1264‐2A>G mutation of SLC26A4 gene also has not been reported as a pathogenic mutation, but we concluded it as a pathogenic mutation because this variant fulfilled PVS1, PM2, PM3, and PP4.
All procedures were approved by the Ethics Review Committee of National Hospital Organization Tokyo Medical Center, Japan and other participating institutions, and were conducted only after written informed consent had been obtained from each subject or from the parents of the subjects.
Measurement of EABR
EABRs were recorded as described previously.28 In brief, they were recorded by stimulating each electrode of cochlea implant in the cochlea using the Neuropack Σ (Nihon Koden Co., Tokyo, Japan) electrodiagnostic system, which was triggered externally by the stimulus output of the proprietary MED‐EL or Advanced Bionics software and interface unit. The interface unit was also connected to a stock speech processor, which transmitted the stimulus signal across the skin to the implanted device. The electrically evoked brainstem potentials were recorded by using needle electrodes placed on the forehead (different electrode) and nape (indifferent electrode), and the reference electrode was placed on the contralateral shoulder. The recording of electrical activity included two or three replications of 1000 sweeps at each stimulus level, with a time window of 10 ms for each stimulus condition. Frequency cut‐offs of 100 and 1000 Hz were used. The pulse duration was set to 30 μs and the stimulation amplitude for a single recording fell from 1200 current unit (cu) to 200 cu at 200 cu intervals for MED‐EL and 600 cu to 200 cu at 100 cu intervals for Advanced Bionics. If no response was detected, pulse duration was increased up to 100 μsec.
Categories of Auditory Performance (CAP) Scale
For accessing speech perception, we used CAP scale, which comprises a hierarchical scale of auditory perceptive ability. In this scale, the lowest level (0) describe no awareness of environmental sounds and the level (7) is presented by the ability to use a telephone with a known speaker.37, 38
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 23.0 (Armonk, New York, USA). For multiple comparisons, we used the Tukey–Kramer method. The results of multiple experiments are presented as the mean ± standard deviation. All tests used a P value of .05 as the threshold for significance.
RESULTS
Representative EABR waveforms obtained from each group, and the aspects of the waveforms that were analyzed, are shown in Figure 1. We compared latencies and amplitudes of Wave III and Wave V in this study. The Wave V latencies were significantly longer in the group with OTOF mutations than in any other group (Fig. 2A); however, no difference was observed in amplitudes (Fig. 2B). When we compared Wave III latencies and amplitudes, the latencies were significantly longer in the OTOF mutation group relative to the GJB2 and SLC26A4 mutation groups, but not the CMV group (Fig. 3A). No significant differences were observed in Wave III amplitudes (Fig. 3B). Amplitudes in all groups were found to have very high standard deviations. We also analyzed the latency difference between Wave III and Wave V across groups. The Wave III–Wave V latencies were also significantly longer in the OTOF group than in all other groups (Fig. 4).
Figure 1.
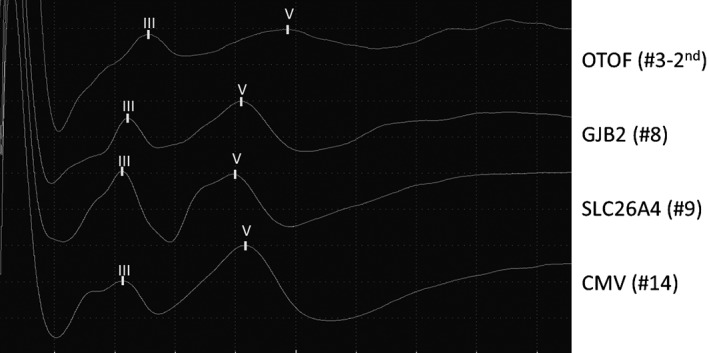
Representative evoked auditory brainstem response wave forms for each group. Groups were composed according to the identified pathology (OTOF, GJB2, or SLC26A4 mutations or cytomegalovirus [CMV] infection).
Figure 2.
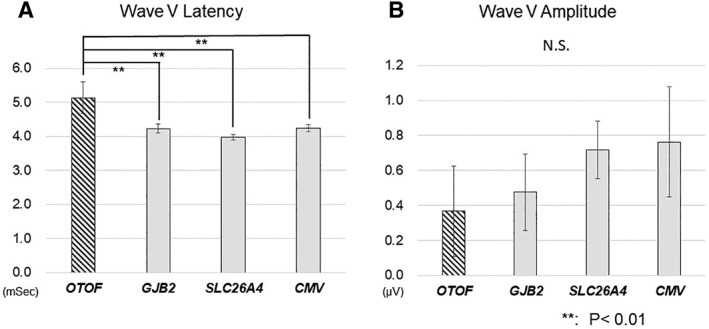
Comparison of evoked auditory brainstem response (EABR) Wave V latencies (A) and amplitudes (B) between pathology groups.
EABR Wave V latency was significantly longer in patients with OTOF mutations than those in all other groups; no significant changes were observed in Wave V amplitudes between the groups. ** P < .01
Figure 3.
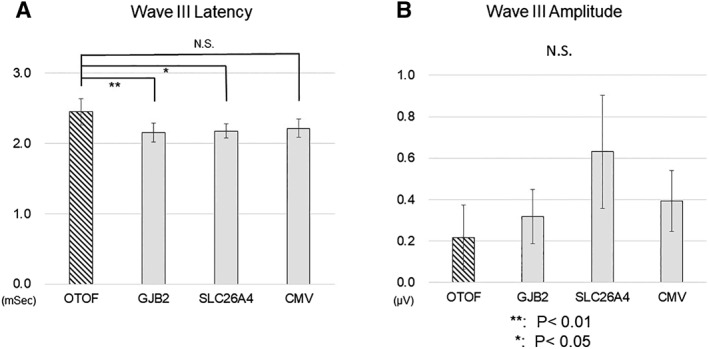
Comparison of Wave III latencies (A) and amplitudes (B) between groups.
The evoked auditory brainstem response Wave III latency was significantly longer in patients with OTOF mutations than in those with GJB2 and SLC26A4 mutations, but not in those with CMV infection; no significant changes were observed in wave III amplitudes between the groups. ** P < .01, * P < .05
Figure 4.
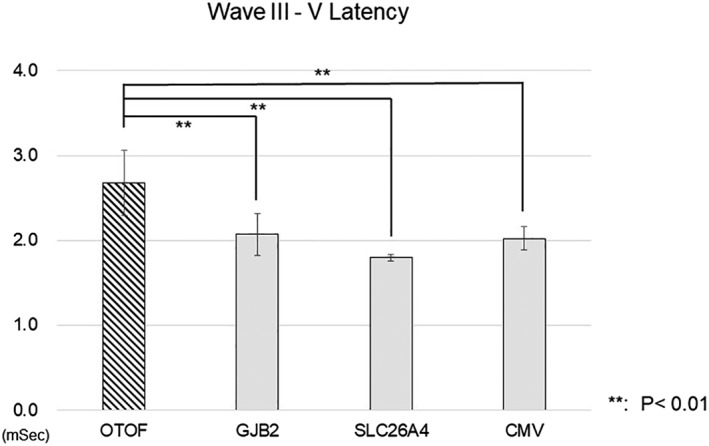
Comparison of the latencies from Wave III to Wave V between groups.
The latency between evoked auditory brainstem response Wave III and Wave V was significantly longer in patients with OTOF mutations than in all other groups. ** P < .01, * P < .05
Finally, in order to evaluate the clinical relevant of this elongation of EABR wave form, we compare the patients' speech perception between the groups evaluated by CAP score. There was no significant difference between the groups (Fig. 5A). Moreover, there was no significant relationship between CAP score and EABR latency (Fig. 5B).
Figure 5.
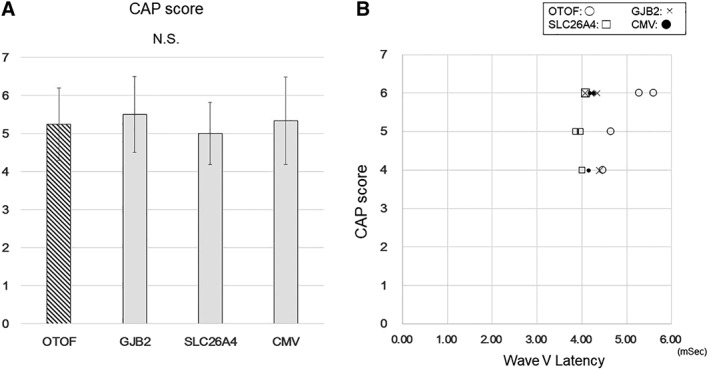
Comparison of CAP score between the groups.
There was no significant difference between the groups (A). No significant relationship was detected between CAP score and EABR latency (B).
DISCUSSION
Our EABR analyses revealed what appears to be delayed postsynaptic neurotransmission in AN caused by OTOF mutations. We also noted that these results indicated that postsynaptic activity was still disturbed after CI implantation. However, OTOF mutations induce presynaptic insufficiency at the synaptic junctions of the hair cells and spiral ganglion neurons. Given these observations, we suggest that cochlear nerve synchronies were reduced in the OTOF group, whereas primarily neuronal conduction was preserved. Nerve development and nervous system maturation resulting in firing synchrony develops through increasing electrical pre‐ and postsynaptic stimulation.39, 40 The disturbed synchronies also observed with OTOF mutations could be caused by insufficient presynaptic stimulation, and/or the delay of nervous system maturation including the pre‐ and postsynaptic neural network.
It is also possible that the OTOF mutation disturbs neurotransmission in not only hair cell‐spiral ganglion synapses, but also in the cochlear nucleus or other more central aspects of the auditory pathway. Thus far, OTOF expression in the central auditory pathway includes spiral ganglion neurons has not been reported in the adult rodent. However, its expression was reported in other parts of the central nervous system, including the cerebellum, in the rat.17 Our results suggest that otoferlin is important in normal neurotransmission in the human central auditory pathway, although there are no reports on otoferlin expression in primate or human. A more detailed expression study of the auditory pathway in primates will need to be carried out in near future.
Our results showed that the latencies of Wave III and Wave V in patients with AN due to OTOF mutations were longer than those in patients with AN due to other mutations or CMV. Thus far, Runge et al. documented poor post‐synaptic ECAP response in one of two patients with an OTOF mutation.41 Our observation with EABR analysis is compatible with their report. Some reports have mentioned that postoperative speech and hearing ability is affected by the EABR latencies, and that longer latencies (greater delays) might predict poorer outcomes.42, 43 Whereas patients with OTOF mutations generally respond well to CI and actually we could not point out clinical relevant with this elongation of EABR wave form by CAP score (Fig. 5A,B), our result suggest there is a hidden negative effect on neurotransmission between the cochlear implant and the brain which cannot be detected by CAP score. Our results suggest the need for more careful follow‐up of the effects of CI implantation in patients with AN caused by OTOF mutations. Moreover, it is possible that neuronal maturation mediated by CI would be observed. A larger‐scale study with follow‐up analysis will need to be carried out in near future.
In conclusion, we unveiled a novel pathophysiology of auditory neuropathies caused by OTOF mutations which affect more central auditory pathway beyond the synapse between the hair cells and spiral ganglion neurons. We also found that EABRs are useful for clarifying the pathophysiology of congenital hearing loss with cochlear implants.
This manuscript has not been published and is not under consideration for publication elsewhere. All the authors have read the manuscript and have approved this submission.
Conflict of Interest: The authors declare that they have no competing interests.
Financial Disclosure: This work was supported by JSPS KAKENHI (Grant Number 16K11205), and research grants from Pfizer health Research Foundation and GSK Japan
Author Contributions: MH, SM, and KK designed the study and wrote the manuscript. MH, SM, CE, TM, and KK analyzed the data.
BIBLIOGRAPHY
- 1. Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain 1996;119:741–753. [DOI] [PubMed] [Google Scholar]
- 2. Kaga K, Nakamura M, Shinogami M, Tsuzuku T, Yamada K, Shindo M. Auditory nerve disease of both ears revealed by auditory brainstem responses, electrocochleography and otoacoustic emissions. Scand Audiol 1996;25:233–238. [DOI] [PubMed] [Google Scholar]
- 3. Varga R, Kelley PM, Keats BJ, et al. Non‐syndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J Med Genet 2003;40:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delettre C, Lenaers G, Griffoin JM, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin‐related protein, is mutated in dominant optic atrophy. Nat Genet 2000;26:207–210. [DOI] [PubMed] [Google Scholar]
- 5. Delmaghani S, del Castillo FJ, Michel V, et al. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat Genet 2006;38:770–778. [DOI] [PubMed] [Google Scholar]
- 6. Yasunaga S, Grati M, Cohen‐Salmon M, et al. A mutation in OTOF, encoding otoferlin, a FER‐1‐like protein, causes DFNB9, a nonsyndromic form of deafness. Nat Genet 1999;21:363–369. [DOI] [PubMed] [Google Scholar]
- 7. Choi BY, Ahmed ZM, Riazuddin S, et al. Identities and frequencies of mutations of the otoferlin gene (OTOF) causing DFNB9 deafness in Pakistan. Clin Genet 2009;75:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duman D, Sirmaci A, Cengiz FB, Ozdag H, Tekin M. Screening of 38 genes identifies mutations in 62% of families with nonsyndromic deafness in Turkey. Genet Test Mol Biomarkers 2011;15:29–33. [DOI] [PubMed] [Google Scholar]
- 9. Hutchin T, Coy NN, Conlon H, et al. Assessment of the genetic causes of recessive childhood non‐syndromic deafness in the UK—implications for genetic testing. Clin Genet 2005;68:506–512. [DOI] [PubMed] [Google Scholar]
- 10. Iwasa Y, Nishio SY, Yoshimura H, et al. OTOF mutation screening in Japanese severe to profound recessive hearing loss patients. BMC Med Genet 2013;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin YJ, Park J, Kim AR, Rah YC, Choi BY. Identification of a novel splice site variant of OTOF in the Korean nonsyndromic hearing loss population with low prevalence of the OTOF mutations. Int J Pediatr Otorhinolaryngol 2014;78:1030–1035. [DOI] [PubMed] [Google Scholar]
- 12. Mahdieh N, Shirkavand A, Rabbani B, et al. Screening of OTOF mutations in Iran: A novel mutation and review. Int J Pediatr Otorhinolaryngol 2012;76:1610–1615. [DOI] [PubMed] [Google Scholar]
- 13. Rodriguez‐Ballesteros M, Reynoso R, Olarte M, et al. A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy. Hum Mutat 2008;29:823–831. [DOI] [PubMed] [Google Scholar]
- 14. Romanos J, Kimura L, Favero ML, et al. Novel OTOF mutations in Brazilian patients with auditory neuropathy. J Hum Genet 2009;54:382–385. [DOI] [PubMed] [Google Scholar]
- 15. Matsunaga T, Mutai H, Kunishima S, et al. A prevalent founder mutation and genotype‐phenotype correlations of OTOF in Japanese patients with auditory neuropathy. Clin Genet 2012;82:425–432. [DOI] [PubMed] [Google Scholar]
- 16. Kaga K. Auditory nerve disease and auditory neuropathy spectrum disorders. Auris Nasus Larynx 2016;43:10–20. [DOI] [PubMed] [Google Scholar]
- 17. Schug N, Braig C, Zimmermann U, et al. Differential expression of otoferlin in brain, vestibular system, immature and mature cochlea of the rat. Eur J Neurosci 2006;24:3372–3380. [DOI] [PubMed] [Google Scholar]
- 18. Roux I, Safieddine S, Nouvian R, et al. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell 2006;127:277–289. [DOI] [PubMed] [Google Scholar]
- 19. Moser T, Starr A. Auditory neuropathy—neural and synaptic mechanisms. Nat Rev Neurol 2016;12:135–149. [DOI] [PubMed] [Google Scholar]
- 20. Teagle HF, Roush PA, Woodard JS, et al. Cochlear implantation in children with auditory neuropathy spectrum disorder. Ear Hear 2010;31:325–335. [DOI] [PubMed] [Google Scholar]
- 21. Kontorinis G, Lloyd SK, Henderson L, et al. Cochlear implantation in children with auditory neuropathy spectrum disorders. Cochlear Implants Int 2014;15(Suppl 1):S51–S54. [DOI] [PubMed] [Google Scholar]
- 22. Humphriss R, Hall A, Maddocks J, Macleod J, Sawaya K, Midgley E. Does cochlear implantation improve speech recognition in children with auditory neuropathy spectrum disorder? A systematic review. Int J Audiol 2013;52:442–454. [DOI] [PubMed] [Google Scholar]
- 23. Ji F, Li J, Hong M, et al. Determination of benefits of cochlear implantation in children with auditory neuropathy. PLoS One 2015;10:e0127566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrison RV, Gordon KA, Papsin BC, Negandhi J, James AL. Auditory neuropathy spectrum disorder (ANSD) and cochlear implantation. Int J Pediatr Otorhinolaryngol 2015;79:1980–1987. [DOI] [PubMed] [Google Scholar]
- 25. Rouillon I, Marcolla A, Roux I, et al. Results of cochlear implantation in two children with mutations in the OTOF gene. Int J Pediatr Otorhinolaryngol 2006;70:689–696. [DOI] [PubMed] [Google Scholar]
- 26. Wu CC, Hsu CJ, Huang FL, et al. Timing of cochlear implantation in auditory neuropathy patients with OTOF mutations: Our experience with 10 patients. Clin Otolaryngol 2018;43:352–357. [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez‐Ballesteros M, del Castillo FJ, Martin Y, et al. Auditory neuropathy in patients carrying mutations in the otoferlin gene (OTOF). Hum Mutat 2003;22:451–456. [DOI] [PubMed] [Google Scholar]
- 28. Minami SB, Takegoshi H, Shinjo Y, Enomoto C, Kaga K. Usefulness of measuring electrically evoked auditory brainstem responses in children with inner ear malformations during cochlear implantation. Acta Otolaryngol 2015;135:1007–1015. [DOI] [PubMed] [Google Scholar]
- 29. Bierer JA, Faulkner KF, Tremblay KL. Identifying cochlear implant channels with poor electrode‐neuron interfaces: Electrically evoked auditory brain stem responses measured with the partial tripolar configuration. Ear Hear 2011;32:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buss E, Labadie RF, Brown CJ, Gross AJ, Grose JH, Pillsbury HC. Outcome of cochlear implantation in pediatric auditory neuropathy. Otol Neurotol 2002;23:328–332. [DOI] [PubMed] [Google Scholar]
- 31. Runge‐Samuelson CL, Drake S, Wackym PA. Quantitative analysis of electrically evoked auditory brainstem responses in implanted children with auditory neuropathy/dyssynchrony. Otol Neurotol 2008;29:174–178. [DOI] [PubMed] [Google Scholar]
- 32. Shallop JK, Peterson A, Facer GW, Fabry LB, Driscoll CL. Cochlear implants in five cases of auditory neuropathy: Postoperative findings and progress. Laryngoscope 2001;111:555–562. [DOI] [PubMed] [Google Scholar]
- 33. Jeong SW, Kim LS, Kim BY, Bae WY, Kim JR. Cochlear implantation in children with auditory neuropathy: Outcomes and rationale. Acta Otolaryngol 2007;127:36–43. [DOI] [PubMed] [Google Scholar]
- 34. Okamoto Y, Mutai H, Nakano A, et al. Subgroups of enlarged vestibular aqueduct in relation to SLC26A4 mutations and hearing loss. Laryngoscope 2014;124:E134–E140. [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto N, Mutai H, Namba K, et al. Prevalence of TECTA mutation in patients with mid‐frequency sensorineural hearing loss. Orphanet J Rare Dis 2017;12:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Archbold S, Lutman ME, Marshall DH. Categories of auditory performance. Ann Otol Rhinol Laryngol Suppl 1995;166:312–314. [PubMed] [Google Scholar]
- 38. Archbold S, Lutman ME, Nikolopoulos T. Categories of auditory performance: Inter‐user reliability. Br J Audiol 1998;32:7–12. [DOI] [PubMed] [Google Scholar]
- 39. Tessier‐Lavigne M, Goodman CS. The molecular biology of axon guidance. Science 1996;274:1123–1133. [DOI] [PubMed] [Google Scholar]
- 40. Yamada A, Uesaka N, Hayano Y, Tabata T, Kano M, Yamamoto N. Role of pre‐ and postsynaptic activity in thalamocortical axon branching. Proc Natl Acad Sci U S A 2010;107:7562–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Runge CL, Erbe CB, McNally MT, et al. A novel otoferlin splice‐site mutation in siblings with auditory neuropathy spectrum disorder. Audiol Neurootol 2013;18:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Groenen PA, Makhdoum M, van den Brink JL, Stollman MH, Snik AF, van den Broek P. The relation between electric auditory brain stem and cognitive responses and speech perception in cochlear implant users. Acta Otolaryngol 1996;116:785–790. [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Pan T, Deshpande SB, Ma F. The relationship between EABR and auditory performance and speech intelligibility outcomes in pediatric cochlear implant recipients. Am J Audiol 2015;24:226–234. [DOI] [PubMed] [Google Scholar]


