Abstract
Objective
To review the literature on integrative care of the patient with head and neck cancer.
Methods
A review of the English language literature for articles relating to integrative care of patients with head and neck cancer, focusing on treatment of sequelae of surgery and chemoradiation.
Results
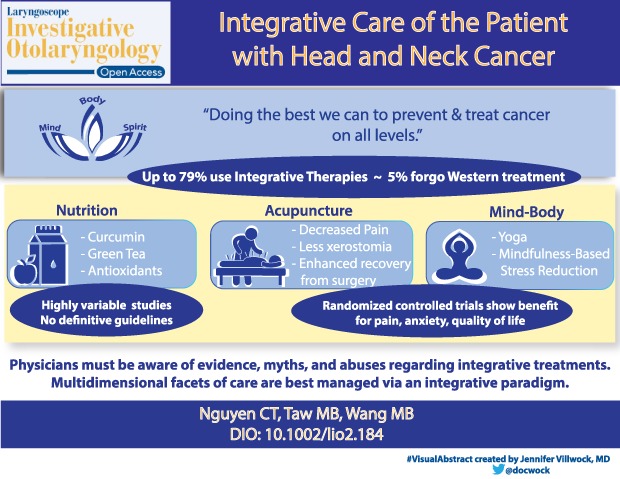
Many studies show a significant utilization of nontraditional (complementary/integrative) treatments by patients in dealing with head and neck cancer. Treatment of head and neck cancer entails potentially debilitating consequences of surgery and/or chemoradiation on cosmesis, speech, swallowing, breathing, and quality of life. While complementary/integrative treatments for head and neck cancer are not established as effective therapies, their use for relieving sequelae of treatment, improving quality of life, and providing potential chemoprevention is well documented.
Conclusion
Given the landscape of increasing use of nontraditional methodologies by patients with head and neck cancer and the complexity of care, the head and neck cancer surgeon should be aware of the uses and abuses of complementary/integrative medicine by patients as they navigate their care.
Level of Evidence
5
Keywords: Integrative, head and neck cancer, patient
A Special Visual Abstract has been developed for this paper. (Visual Abstract 1)
INTRODUCTION
Integrative medicine is a relatively new term for old fashioned medicine: it is medicine that encompasses care of the whole patient, taking into account socioeconomic, spiritual, family, and psychological backgrounds. In terms of cancer care, it is in the words of a leading proponent, Dr. Andrew Weil, “Simply … doing the best we can to prevent and treat cancer and support those affected by it on all levels: body, mind, and spirit.”1 A patient with head and neck cancer (HNC) offers the opportunity for the physician to take a truly integrative approach to care.
Integrative oncology is not a rejection of standard proven treatment modalities, nor is it tacit endorsement of nontraditional cancer therapeutics that have not been scrutinized. Rather, it emphasizes certain tenets: 1) the innate ability of the body to heal itself; 2) the importance of living a healthy lifestyle; 3) using a whole‐person approach; and 4) stressing the sanctity of the doctor–patient relationship.
An older term, alternative medicine, has been abandoned to avoid confusion with the goals as stated above. To illustrate why, a national multi‐center study in Norway in 1992 evaluated the use of alternative medicine (AM) in cancer patients. In a Cox regression model adjusted for demographic, disease, and treatment factors, the hazard ratio of death for use of AM compared with no use was 1.30, (95% confidence interval [CI] 0.99, 1.70; P = .056), suggesting that AM use may predict a shorter survival. AM use had the most detrimental effect in patients with a good ECOG (Eastern Cooperative Oncology Group) performance status (PS) of 0 (hazard ratio for use = 2.32, 95% CI, 1.44, 3.74, P = .001), when compared with an ECOG PS of 1 or higher).2
A minority of patients (< 5%) is believed to forgo traditional cancer therapeutics for truly alternative therapies. These patients typically harbor a deep distrust of the medical establishment, a dissatisfaction with conventional medical practice, and a strong desire for control. In contrast, most patients who turn to integrative oncology do so to either prevent cancer, enhance their standard cancer treatment, or mollify the side effects of cancer or its treatment.3 Recent reviews show anywhere from one‐third to one‐half of patients with cancer use some form of integrative cancer therapy, along with their standard treatments. In HNC, this range is between 6% and 79%. The most commonly used therapies by patients with HNC are herbal medicines, medicinal teas, acupuncture and vitamins/minerals, followed by mind‐body interventions, such as visualization.3
In this paper, we will review the literature on integrative therapies as these pertain to patients with HNC, focusing on dealing with the numerous sequelae they can experience following treatment. With the intense treatment regimens for cancer, which may include surgery, radiation, chemotherapy, or a combination of all three, patients with HNC suffer adverse impacts to their swallowing, speaking, and quality of life (QoL). Integrative treatments can provide considerable relief for many of their symptoms and may offer a valuable component to their care.
Dysphagia Prevention
Dysphagia is one of the most common complications of head and neck radiation treatment and one of the main predictors of poor post‐treatment QoL.4, 5, 6 The incidence of HNC treatment‐related dysphagia is estimated to be 10,000 to 20,000 new cases per year. Posttreatment dysphagia occurs in at least 50 to 60% of patients and results from multiple factors, such as xerostomia, taste loss, stricture, fibrosis, and trismus. The dysphagia will worsen during treatment and for 3 to 6 months afterwards, and usually tends to stabilize by 1 year. The overall sum of these deleterious effects leads to decreased oral caloric intake, weight loss, reliance on feeding tubes, increased morbidity, decreased QoL, and increased use of health care services.4, 5, 6, 7
A comprehensive swallow preservation program has been found to be effective in mitigating post treatment dysphagia in patients with HNC. For maximal efficacy, pretreatment evaluation and education is done by a speech‐language pathologist (SLP) with expertise in swallowing. This includes assessment of swallowing, done with a modified barium swallow study. Patients are given education about their cancer and expected treatment side effects, and instruction in an exercise program using surface electromyography (S‐EMG) for biofeedback during certain exercises (Mendelsohn maneuver, effortful swallow, lingual protrusion, and tongue‐press exercise). Patients are also recommended to obtain a passive jaw motion device (TheraBite, Atos Medical, West Allis, Wisconsin, U.S.A.) for use during radiation therapy to reduce the risk of trismus. For optimal results, they continue to meet at regular intervals with the SLP during and after treatment. Such programs have resulted in preserving swallow function in compliant patients over the course of their cancer treatment and reducing the likelihood of G‐tube dependence.4, 8, 9
Exercise
Decreased physical activity is thought to contribute to 5% of cancer deaths in the US. It is estimated that 60% of all US adults are not regularly physically active, with another 25% nearly completely sedentary. Exercise is believed to contribute to preventing tumorigenesis through its effects on reducing circulating levels of insulin, growth factors, hormones, and by improving immune function. However, the optimal duration, intensity, and frequency of physical activity for cancer prevention is not known.10
Herbal Therapies and Nutritional Strategies
Patients with HNC have a risk of recurrence and development of second primary cancers, making chemoprevention a priority in their care after treatment. While herbal therapies alone are not effective for cancer treatment, they have low risk of toxicity and there is potential to combine them with standard therapies, with the goal of synergistic action. Several herbs have demonstrated promising anti‐cancer and potential chemopreventive activity in patients with HNC.
Curcumin (diferuloylmethane) is a polyphenol and the chief component of the spice turmeric, which is derived from the rhizome of the East Indian plant Curcuma longa. Commonly employed as a flavoring and coloring agent in Asian cuisine, turmeric has also been widely utilized for thousands of years in Ayurvedic medicine for its antioxidant, antiseptic, analgesic, antimalarial and anti‐inflammatory properties.
Curcumin has been shown to suppress the activation of NFκB, an inducible transcription factor that regulates the expression of genes involved in inflammation, as well as the control of cell proliferation and survival.11 Activation of NFκB is increased in many cancers, and is associated with various steps in the development of malignancy, such as expression of anti‐apoptotic genes, angiogenesis, tumor promotion and metastasis.12 Studies have demonstrated constitutive expression of NFκB in HNC.13
Curcumin has been studied in multiple human cancers including melanoma, head and neck, breast, colon, pancreatic, prostate, and ovarian carcinomas. The mechanisms by which curcumin exerts its anti‐cancer effects are diverse, targeting many levels of regulation in the processes of cellular growth and apoptosis. Because of the multiple targets of curcumin on cell growth regulatory processes, it holds much promise as a potential chemotherapeutic agent for many human cancers. Curcumin's inhibitory effect on carcinogenesis has been demonstrated in several animal models of various tumor types including oral cancer, mammary carcinoma and intestinal tumors. A pilot study demonstrated inhibition of IKKβ kinase activity, a component of the NFκB cascade, as well as inhibition of proinflammatory cytokines in the saliva of oral cancer patients after treatment with curcumin.14 Further trials are necessary in patients with HNC to establish the value and feasibility of curcumin as a chemopreventive agent.
Green tea contains several polyphenols, including epigallocatechin‐3‐gallate (EGCG),epicatechin‐3‐gallate (ECT), epigallocatechin (EGC), and epicatechin (EC), which have been shown to function as antioxidants and to mediate signaling transduction pathways which inhibit cell proliferation, angiogenesis, and invasion. A Japanese cohort study found a negative association between green tea consumption and cancer incidence, especially among females drinking more than 10 cups a day.15 Another large cohort study in Japan found a modest inverse association of oral cancer risk with increased green tea consumption in women only, while the same reduced risk was not found in men.16 A pilot study using green tea extracts in doses of 2000 to 2500 mg/day demonstrated reduced smoking‐induced DNA damage and aneuploidy, as well as increased apoptosis, in oral cells of smokers.17 In vivo studies of the combination of EGCG, the major polyphenol in green tea, and the EGFR tyrosine kinase inhibitor erlotinib demonstrated a synergistic inhibition of head and neck tumor growth in an animal model.18 This combination treatment regimen may have potential as a chemopreventive protocol for HNC.
Other promising compounds containing high levels of antioxidants have shown some efficacy in oral cancer chemoprevention. Black raspberry extracts contain ellagic acid, an antioxidant with antiproliferative properties, and have been used in clinical trials for patients at high risk of developing esophageal and colon cancers. A recent multicenter study of a freeze‐dried black raspberry gel used to treat oral premalignant lesions demonstrated statistically significant reduction in lesion sizes, histologic grade, and loss of heterozygosity events, indicating the potential for the use of black raspberry gel as a chemopreventive agent.19 Bowman Birk inhibitor (BBI), a soybean‐derived serine protease inhibitor with chymotrypsin and trypsin inhibitory activity has been studied for its anticancer activity and found to suppress radiation‐induced transformation in cell lines. While a phase IIa chemoprevention trial of patients with oral leukoplakia treated for one month with BBI as a troche demonstrated a 24% decrease in total lesion areas, a follow‐up randomized phase IIb trial comparing a 6‐month treatment course of placebo versus BBI did not demonstrate significant differences in lesion size, clinical response, or histologic change between the study arms.20 Cucurbitacins are tricyclic triterpenoids identified in cucumbers which function as inhibitors of STAT and Janus kinase signaling pathways. They have been found, when combined with radiation, to suppress growth and metastases of HNC stem cells in xenograft studies.21
In contrast, studies have demonstrated either no efficacy or even adverse effects from the use of certain supplements. Bairati et al. conducted a multicenter, double‐blind, placebo‐controlled, randomized chemoprevention trial among 540 patients with stage I or II HNC treated by radiation therapy between October 1, 1994, and June 6, 2000. Supplementation with alpha‐tocopherol (400 IU/day) or placebo began on the first day of radiation therapy and continued for 3 years after the end of radiation therapy. Compared with patients receiving placebo, patients receiving alpha‐tocopherol supplements had a higher rate of second primary cancers during the supplementation period (HR = 2.88, 95% CI = 1.56 to 5.31) but a lower rate after supplementation was discontinued (HR = 0.41, 95% CI = 0.16 to 1.03). Similarly, the rate of having a recurrence or second primary cancer was higher during (HR = 1.86, 95% CI = 1.27 to 2.72) but lower after (HR = 0.71, 95% CI = 0.33 to 1.53) supplementation with alpha‐tocopherol. The authors concluded that this was an unexpected adverse effect on the occurrence of second primary cancers and on cancer‐free survival.22
In the Nutritional Prevention of Cancer Study, selenium, an antioxidant mineral compound, was used to reduce the risk of non‐melanoma skin cancer. However, the authors found no decrease in the incidence of basal cell or squamous cell skin cancer.23 A meta‐analysis of the effects of antioxidants on cancer prevention from randomized controlled studies showed no clinical evidence to support an overall primary and secondary preventive effect of antioxidant supplements on cancer.24
Multivitamins (MVI) have also been studied as cancer preventive agents. In the Physicians' Health Study II, a randomized controlled trial (RCT) was conducted on middle aged male physicians who took either a placebo or a MVI to evaluate the outcome of cancer diagnosis. Men taking a daily multivitamin had a statistically significant reduction in the incidence of total cancer compared with placebo (multivitamin and placebo groups, 17.0 and 18.3 events, respectively, per 1000 person‐years; hazard ratio [HR], 0.92; 95% CI, 0.86–0.998; P = .04).25 In a separate more recent study done in 2017 on the Vitamins and Lifestyle (VITAL) cohort, researchers included 77,118 participants from 50 to 76 years of age. A population‐based cancer registry was used to identify patients as having primary, invasive lung cancer. The exposure of primary interest was the 10‐year average daily dose from individual and multivitamin supplements. Among men using vitamin B6 and B12 from individual supplement sources, but not from multivitamins, there was a 30% to 40% increase in lung cancer risk. Moreover, for men who smoked and took vitamin B6 and B12, the risk was even higher. Curiously, use of supplemental vitamins B6, folate, and B12 was not associated with lung cancer risk among women.26 Given the known risk factor of HNC among smokers, this is an important message to deliver to patients with HNC using these specific vitamins.
Dietary Supplements and Herbal Interactions With Chemotherapy
Patients with cancer frequently use dietary supplements, including herbal therapies, vitamins, Chinese medicine, and homeopathy for both real and desired benefits. Many patients may not tell their doctors about their use of supplements and herbal therapies, possibly from fear or embarrassment. However, it is crucial for physicians to maintain open and nonjudgmental pathways of communication in order to optimize patient care. There are multiple potential harmful interactions of herbal and nontraditional therapies with chemotherapeutic agents. Interactions between these therapies may result in altered pharmacokinetics of the chemotherapeutic agent, including changes in absorption, distribution, metabolism, and excretion. Specific metabolic steps which may be altered include the cytochrome P450 system, as well as drug transporters such as P‐glycoprotein and other transmembrane transporters.27 Herbs which should be avoided while receiving chemotherapy include ginkgo, echinacea, ginseng, St. John's wort, kava, and grapeseed. Other herbs/supplements which do not appear to have adverse interactions with chemotherapeutic agents include saw palmetto, black cohosh, cranberry, and milk thistle. (Table 1)
Table 1.
Dietary Supplements and Herbal Interactions With Chemotherapy.
| Herbs to avoid during chemotherapy | Herbs with no known adverse chemotherapy interactions |
|---|---|
| Ginkgo | Saw palmetto |
| Echinacea | Black cohosh |
| Ginseng | Cranberry |
| St. John's wort | Milk thistle |
| Kava | |
| Grapeseed |
Herbal supplements can have adverse effects through other pharmacokinetic interactions. Some herbals may interact with anesthetic agents, while others have effects on coagulation. Garlic, ginger, ginseng, gingko, green tea, kava, and fish oil have been found to increase the risk of perioperative bleeding,28 The American Society of Anesthesiologists recommends that all herbal supplements be discontinued 2 to 3 weeks prior to elective surgical procedures.29 Herbals and supplements are not regulated by the Food and Drug Administration, and patients and doctors should seek to use those approved by the National Formulary or United States Pharmacopeia or labeled as produced with Good Manufacturing Process.
Nutritional Strategies
Maintenance of adequate nutrition and stable weight is a challenge for patients with HNC, due to the significant problems with chewing, swallowing, and eating. This is often a result of the cancer itself, depending on the location of the primary site, as well as from the sequelae of treatment. Eating requires additional effort and time and may not be enjoyable. In addition, the laborious effort required to chew and swallow adversely affects the pleasurable social component of eating with others or in public. Consultation with a dietician and/or nutritionist is very helpful for patients as they grapple with their limitations in eating. Books, websites, and literature with recipes, tips, and advice are available. A local support group may be available as well. The national organization Support for People with Head and Neck Cancer has over 125 local chapters (http://www.spohnc.org).
Acupuncture for Patients With Head and Neck Cancer
Acupuncture has been used in the management of cancer pain and other symptoms related to HNC, such as post‐neck dissection pain and dysfunction, nausea/vomiting, xerostomia, and chemoradiation therapy–related dysphagia.30
One RCT examined the effect of auricular acupuncture on cancer pain and enrolled 90 patients who had persistent pain with a visual analog score (VAS) of 30 mm or more despite receiving analgesics for at least one month.31 Subjects were randomly divided into 3 groups: 1) true ear acupuncture at acupoints detected via electrodermal signal that was administered over 2 courses; 2) placebo ear acupuncture; and 3) ear seeds at placebo points. The primary outcome was assessment of the VAS after 2 months. Statistically significant reduction of pain was found with true ear acupuncture (36%), while both placebo groups had little change (2%) (P < .0001).
Another RCT was conducted at Memorial Sloan Kettering and investigated the effect of acupuncture upon chronic pain and dysfunction in patients who had undergone neck dissection for HNC.32 Fifty‐eight patients were randomly assigned to receive 1 month of either weekly acupuncture or usual care, which consisted of physical therapy and analgesic/anti‐inflammatory medications. The primary outcome was the Constant‐Murley score, which is a composite measure of pain, function and activities of daily living. A secondary outcome was the Xerostomia Inventory to assess dry mouth. Significant reductions in pain/dysfunction (adjusted difference between groups 11.2; 95% CI, 3.0 to 19.3; P = .008) and xerostomia (adjusted difference −5.8; 95% CI, −0.9 to −10.7; P = .02) were demonstrated in patients who had received acupuncture.
A similar prospective randomized trial was done on patients with chronic shoulder pain and functional impairment experienced after neck dissection for HNC and enrolled 48 patients in weekly acupuncture versus usual care.33 The Constant‐Murley score was the primary outcome measure once again, though the Neck Dissection Impairment Index (NDII) was used as the secondary endpoint to quantify site‐specific, self‐reported QoL. The acupuncture group showed statistically significant improvement in both the Constant‐Murley score (gain difference between groups = 13.6, P < .01) and NDII (gain difference between groups = 11.5, P < .01).
There is also data demonstrating efficacy of acupuncture in patients who have undergone craniotomy with general anesthesia. A systematic review and meta‐analysis, which pooled data from 10 RCTs with a total of 700 patients, found that acupuncture had significantly reduced the need of volatile anesthetics during surgery (P < .001), decreased postoperative nausea and vomiting (P = .017), led to faster extubation time (P = .001), improved postoperative patient recovery (P = .003) and reduced blood levels of the brain tissue injury marker S100β 48 hours after operation (P = .001).34 Such effects of acupuncture may have implications for patients with HNC who undergo skull base surgery.
Radiation‐induced xerostomia is another condition that may be amenable to acupuncture. A randomized feasibility trial, that was conducted in collaboration with MD Anderson Cancer Center, had enrolled 23 patients with nasopharyngeal carcinoma undergoing radiotherapy and compared real acupuncture to sham acupuncture.35 Both groups received treatment 3 times per week. Subjective measures included the Xerostomia Questionnaire (XQ) and the MD Anderson Symptom Inventory for head and neck cancer (MDASI‐HN), while objective measures were the unstimulated whole salivary flow rates (UWSFR) and stimulated salivary flow rates (SSFR). XQ scores for real acupuncture were significantly lower than sham controls (all P values < .001 except for week 3 which was .006) with clinically significant differences found at week 6 (RR 0.28; 95% CI, 0.10, 0.79) and at week 11 (RR 0.17; 95% CI, 0.03, 1.07). Similar findings were seen for MDASI‐HN scores. No differences were found for UWSFR and SSFR.
The same authors conducted a larger RCT that included 86 patients with xerostomia who were followed for 6 months after radiotherapy and compared acupuncture versus standard of care.36 Once again, XQ scores for acupuncture were significantly lower than for control in week 3 and throughout the 6 month follow up period (P = .003 at week 3, P < .0001 during all other weeks) with clinically significant differences found at week 11 (RR 0.63; 95% CI, 0.45–0.87) and at 6 months (RR 0.38; 95% CI, 0.19–0.76). There were similar findings for the MDASI‐HN scores, with group differences found for both UWSFR and SSFR.
Chemoradiation therapy (CRT) related dysphagia has also been studied using acupuncture. A RCT enrolled 42 patients who had stage III–IV squamous cell carcinoma with dysphagia after CRT and randomized them either to active acupuncture or sham acupuncture.37 Swallowing‐related QoL was assessed using the MD Anderson Dysphagia Inventory (MDADI) total and subscale scores. Significant improvement was found in both groups from baseline to 12 months post‐CRT for MDADI total scores (AA: +7.9; SA +13.9; P = .044, P < .001) and MDADI global subscale scores (AA: +25.0; SA +22.7; P = .001, P = .002). In addition, acupuncture was found to be safe. Hence, acupuncture may offer potential for treating CRT‐related dysphagia in patients with HNC, though further large scale pragmatic clinical trials are needed to evaluate the effectiveness of acupuncture versus standard of care. These would involve prospective randomized trials comparing active acupuncture with either sham acupuncture or standard swallowing therapies for patients with CRT‐related dysphagia. Mechanistic research is also needed to elucidate underlying specific versus non‐specific effects of acupuncture.
Suffering
Dr. Eric Cassell writes, “Suffering is experienced by persons, not merely by bodies, and has its source in challenges that threaten the intactness of the person as a complex social and psychological entity…. Suffering can include physical pain but is by no means limited to it…. The relief of suffering and the cure of disease must be seen as twin obligations of a medical profession that is truly dedicated to the care of the sick…. Physicians' failure to understand the nature of suffering can result in medical intervention that (though technically adequate) not only fails to relieve suffering but becomes a source of suffering itself.”38 The pain of cancer is not merely in the physical domain, but in the social and emotional realms as well.
In a March 2016 systematic review, Barber et al. examined depression and survival in patients with HNC. The incidence of depression among patients with HNC may be as high as 40%. The authors found a significant decrease in survival in depressed patients in two of the three studies that met their inclusion criteria.39 Furthermore, patients with HNC have more than 3 times the incidence of suicide compared with the general US population. Suicide rates were highest among those with cancers of the larynx and hypopharynx in a 2015 study.40
A panel from the Society for Integrative Oncology (SIO) published clinical practice guidelines on the use of integrative therapies for specific clinical indications during and after breast cancer treatment, including anxiety/stress, depression/mood disorders, fatigue, QoL/physical functioning, chemotherapy‐induced nausea and vomiting, lymphedema, chemotherapy‐induced peripheral neuropathy, pain, and sleep disturbance based on a systematic literature review from 1990 through 2015.41 Many of their recommendations are broadly applicable, as the data were pulled from cancer patient populations in general as well as breast cancer in particular.
They found good evidence for yoga as a means to reduce anxiety, improve mood and depressive symptoms, and to improve QoL, giving it a B grade (there is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial: offer/provide this modality). The most commonly practiced form of yoga in the United States is Hatha yoga, which emphasizes postures (asanas) and breathing exercises (pranayama).
Among modalities receiving the highest grade of A in the SIO review for depression and mood disturbance were relaxation and meditation. In particular, mindfulness‐based stress reduction (MBSR) was singled out. MBSR, developed by Dr. Jon Kabat Zinn in 1979, is an 8‐week program whose aim is to help people learn how to use their inner resources and abilities to respond more effectively to stress, pain, and illness.42 It focuses on teaching moment to moment non‐judgmental awareness, and emphasizes self‐compassion. A Phase I study explored whether an individualized MBSR program could be successfully used for patients with HNC undergoing curative treatment to impact pre‐ and posttest outcome measures of psychological distress, depression, anxiety and QoL. The authors found an association between higher postintervention mindfulness and lower psychological distress and higher total, social and emotional QoL.43
Along with the attendant stress on the individual patient, cancer also affects caregivers, family, and communities. Funk et al. reviewed the evidence on social support in HNC and found that those caring for patients with HNC experience considerable distress, significant fears of recurrence, and are at increased risk of developing posttraumatic stress disorder.44 The 2016 United Kingdom national multidisciplinary guidelines on QoL considerations in HNC recognized that partnership and marital issues are of significant importance, as well as family. In their directions for future interventions they recommend practitioners be trained in couple therapy and family therapy as well as counselling.45 Studies on social support have shown high levels of perceived support, larger social networks, and marriage to be associated with decreases in relative risk for cancer mortality of 25%, 20%, and 12%, respectively.46
One of the major sources of anxiety for patients with cancer is the fear of cancer recurrence (FCR). In a prospective study of 189 posttreatment patients with HNC, Ghazali et al. (2012) investigated predictors of significant, or extreme, FCR. They found that 35% of patients experienced significant FCR (30% persistently), and reported younger age and mood/anxiety issues to be predictive of significant FCR, but disease‐ and treatment‐related factors were not.47 Paradoxically then, younger patients expected to have good prognoses may actually suffer more from FCR than their older, sicker counterparts in HNC. To help deal with FCR, a group led by Otto et al designed a trial involving 67 women with early stage breast cancer who were randomly assigned to either a 6‐week online gratitude intervention or a 6‐week online control condition. Results showed that patients in the gratitude intervention experienced a significant decrease in death‐related FCR compared to the control group. The gratitude intervention was also observed to prevent declines in positive affect seen in the control group.48
Vanderweele et al. wrote, “For centuries, physicians and other healers have witnessed how illness focuses attention on “ultimate meaning, purpose, and transcendence, and … relationship to self, family, others, community, society, nature, and the significant or sacred.” Patients often discover strength and solace in their spirituality, both informally through deeper connections with family and friends, and formally through religious communities and practices. However, modern day clinicians regularly overlook dimensions of spirituality when considering the health of others—or even themselves.”49 Religious belief, as opposed to none, was linked to feeling better in all categories of side effects at all points of time before, during, and directly after radiotherapy in a study of patients with HNC in Germany.50 One framework that may be useful to begin discussion around spirituality lies in the HOPE acronym: H, for questions on sources of hope; O, for defining any organized religion that patients may belong to; P, for personal spirituality practices; and E, for how this may affect end‐of‐life issues and medical care.51
Fatigue and poor sleep are common complaints for patients with HNC, often working in a positive feedback cycle. Obstructive sleep apnea (OSA) has been shown to be more prevalent in patients with HNC, especially following radiation therapy. A hypopharynx or larynx primary site as well as larger tumor size also appears to correlate with developing OSA. But the severity of fatigue does not necessarily correspond to the severity of OSA. It is felt that pro‐inflammatory cytokines, radiation‐related, may be the key to this discrepancy.52
Qi gong and tai chi, both ancient practices that integrate movement (physical postures), meditation (focused attention), and controlled breathing, have been studied to combat anxiety, fatigue, pain, and to support the immune system in patients with cancer. As part of the core tenet in traditional Chinese medicine, these therapeutic practices aim to enhance vital energy to balance a patient's spiritual, emotional, mental, and physical health. Three months of doing tai chi practice in breast cancer survivors with insomnia reduced cellular inflammatory responses and expression of genes encoding pro‐inflammatory mediators.53
In the SIO report, acupuncture, ginseng, and hypnosis were given grade C recommendations, clinicians may consider their use in specific cases for small literature‐based net benefit in treating fatigue. Importantly, guarana, a native plant in the Amazon basin whose seeds have twice the caffeine of coffee beans, and acetyl‐L‐carnitine, a natural chemical in the body which is broken down to carnitine where it is involved in fatty acid transfer to mitochondria, were not recommended for fatigue related to cancer treatment due to a lack of demonstrated benefit.41
Emphasizing the importance of sleep, a subgroup analysis of the Nurses Health Study looked at the association between sleep and survival among women with breast cancer with 30 years of follow‐up.54 They found that increased sleep duration after diagnosis and regular sleep difficulties were associated with a strong elevated risk of all‐cause mortality and a moderate greater risk of breast cancer mortality. Gentle yoga may be helpful to promote sleep, according to the SIO clinical practice guidelines.41
CONCLUSION
Patients with HNC face an array of distressing issues throughout and after treatment including dysphagia, fear of cancer recurrence, desire for cancer prevention, depression/suicide, fatigue, poor sleep, and altered family dynamics. Given the increasing use of nontraditional therapies, physicians need to be aware of the uses, abuses, myths, and evidence for integrative treatments for patients with HNC. The multidimensional facets of care for the individual patient with HNC are best managed in an integrative paradigm, potentially involving safe and rational use of complementary medicine as well as standard Western care, taking into account not just the prognosis of cancer or its immediate treatment related side effects, but the whole person.
Editor's Note: This Manuscript was accepted for publication 4 June 2018.
No funding source was received for this work. Dr. Nguyen is a consultant for Neurovision Medical in Ventura, CA. The other authors do not have any conflicts of interest to report.
Presented at the American Academy of Otolaryngology–Head & Neck Surgery Foundation Annual Meeting, Chicago, IL, USA, September 10–13, 2017.
BIBLIOGRAPHY
- 1. Abrams D, Weil A. Integrative Oncology. New York: Oxford University Press; 2014. [Google Scholar]
- 2. Rydberg T, Vickers A, Bremnes RM, Wist EA, Kaasa S, Cassileth BR. Does use of alternative medicine predict survival from cancer? Eur J Cancer 2003;39(3):372. [DOI] [PubMed] [Google Scholar]
- 3. Matovina C, Birkeland AC, Zick S, Shuman AG. Integrative medicine in head and neck cancer. Otolaryngol Head Neck Surg 2017;156(2):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carroll WR, Locher JL, Canon CL, Bohannon IA, McColloch NL, Magnuson JS. Pretreatment swallowing exercises improve swallow function after chemoradiation. Laryngoscope 2008;118(1):39–43. [DOI] [PubMed] [Google Scholar]
- 5. Kulbersh BD, Rosenthal EL, McGrew BM, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope 2006;116(6):883–886. [DOI] [PubMed] [Google Scholar]
- 6. Maurer J, Hipp M, Schäfer C, Kolbl O. Dysphagia. Impact on quality of life after radio (chemo) therapy of head and neck cancer. Strahlenther Onkol 2011;187(11):744–749. [DOI] [PubMed] [Google Scholar]
- 7. Thomas L, Moore EJ, Olsen KD, Kasperbauer JL. Long‐term quality of life in young adults treated for oral cavity squamous cell cancer. Ann Otol Rhinol Laryngol 2012;121(6):395–401. [DOI] [PubMed] [Google Scholar]
- 8. Duarte VM, Chhetri DK, Liu YF, Erman AA, Wang MB. Swallow preservation exercises during chemoradiation therapy maintains swallow function. Otolaryngol Head Neck Surg 2013;149(6):878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng KA, Kuan EC, Unger L, Lorentz WC, Wang MB, Long JL. A swallow preservation protocol improves function for veterans receiving chemoradiation for head and neck cancer. Otolaryngol Head Neck Surg 2015;152(5):863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Infographics AICR . American Institute for Cancer Research. http://www.aicr.org/learn-more-about-cancer/infographics/. Accessed October 17, 2017.
- 11. Singh S, Aggarwal BB. Activation of transcription factor NFκB is suppressed by curcumin (diferuloylmethane). J Biol Chem 1995;270(42):24995–25000. [DOI] [PubMed] [Google Scholar]
- 12. Garg A, Aggarwal BB. Nuclear transcription factor‐κB as a target for cancer drug development. Leukemia 2002;16:1053–1068. [DOI] [PubMed] [Google Scholar]
- 13. Wang D, Veena MS, Stevenson K, et al. Liposome‐encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor kappaB by an AKT‐independent pathway. Clin Cancer Res 2008;14(19):6228–6236. [DOI] [PubMed] [Google Scholar]
- 14. Kim SG, Veena MS, Basak SK, et al. Curcumin treatment suppresses IKKβ kinase activity of salivary cells of patients with head and neck cancer: a pilot study. Clin Cancer Res 2011;17(18):5953–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imai K, Suga K, Nakachi K. Cancer‐preventive effects of drinking green tea among a Japanese population. Prev Med 1997;26:769–775 [DOI] [PubMed] [Google Scholar]
- 16. Ide R, Fujino Y, Hoshiyama Y, et al. A prospective study of green tea consumption and oral cancer incidence in Japan. Ann Epidemiol 2007;17:821–826. [DOI] [PubMed] [Google Scholar]
- 17. Schwartz JL, Baker V, Larios E, et al. Molecular and cellular effects of green tea on oral cells of smokers: a pilot study. Mol Nutr Food Res 2005;49:43–51. [DOI] [PubMed] [Google Scholar]
- 18. Zhang X, Zhng H, Tighiouart M, et al. Synergistic inhibition of head and neck tumor growth by green tea (‐)‐epigallocatechin‐3‐gallate and EGFR tyrosine kinase inhibitor Int J Cancer 2008;123:1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mallery SR, Tong M, Shumway BS, et al. Topical application of a mucoadhesive freeze‐dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: results from a multicentered, placebo‐controlled clinical trial. Clin Cancer Res 2014;20(7):1910–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armstrong WB, Taylor TH, Kennedy AR, et al. Bowman birk inhibitor concentrate and oral leukoplakia: a randomized phase IIb trial. Cancer Prev Res (Phila) 2013;6(5):410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen YW, Chen KH, Huang PI, et al. Cucurbitacin I suppressed stem‐like property and enhanced radiation‐induced apoptosis in head and neck squamous carcinoma–derived CD44(+)ALDH1(+) cells. Mol Cancer Ther 2010;9(11):2879–2892. [DOI] [PubMed] [Google Scholar]
- 22. Bairati I, Meyer F, Gélinas M, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst 2005. 6;97(7):481–488. [DOI] [PubMed] [Google Scholar]
- 23. Clark LC, Combs GF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996;276(24):1957–1963. [PubMed] [Google Scholar]
- 24. Myung SK, Kim Y, Ju W, Choi HJ, Bae WK. Effects of antioxidant supplements on cancer prevention: meta‐analysis of randomized controlled trials. Ann Oncol 2010;21(1):166–179. [DOI] [PubMed] [Google Scholar]
- 25. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA 2012;308(18):1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brasky TM, White E, Chen CL. Long‐term, supplemental, one‐carbon metabolism‐related vitamin B use in relation to lung cancer risk in the Vitamins and Lifestyle (VITAL) Cohort. J Clin Oncol 2017;35(30):3440–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sparreboom A, Cox MC, Acharya MR, and Figg WD. Herbal remedies in the united states: potential adverse interactions with anticancer agents. J Clin Oncol 2004;22(12):2489–2503. [DOI] [PubMed] [Google Scholar]
- 28. Wang, CA , Moss J, Yuan CS. Commonly used dietary supplements on coagulation function during surgery. Medicines (Basel) 2015;2(3):157–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaye AD, Kucera I, Sabar R. Perioperative anesthesia clinical considerations of alternative medicines. Anesthesiol Clin North America 2004;22(1):125–139. [DOI] [PubMed] [Google Scholar]
- 30. Lu W, Rosenthal DS. Acupuncture for cancer pain and related symptoms. Curr Pain Headache Rep. 2013;17(3):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alimi D, Rubino C, Pichard‐Léandri E, Fermand‐Brulé S, Dubreuil‐Lemaire ML, Hill C. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol. 2003;21(22):4120–4126. [DOI] [PubMed] [Google Scholar]
- 32. Pfister DG, Cassileth BR, Deng GE, et al. Acupuncture for pain and dysfunction after neck dissection: results of a randomized controlled trial. J Clin Oncol. 2010;28(15):2565–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deganello A, Battat N, Muratori E, et al. Acupuncture in shoulder pain and functional impairment after neck dissection: a prospective randomized pilot study. Laryngoscope 2016;126(8):1790–1795. [DOI] [PubMed] [Google Scholar]
- 34. Asmussen S, Maybauer DM, Chen JD, et al. Effects of acupuncture in anesthesia for craniotomy: a meta‐analysis. J Neurosurg Anesthesiol 2017;29(3):219–227. [DOI] [PubMed] [Google Scholar]
- 35. Meng Z, Kay Garcia M, Hu C, et al. Sham‐controlled, randomised, feasibility trial of acupuncture for prevention of radiation‐induced xerostomia among patients with nasopharyngeal carcinoma. Eur J Cancer. 2012;48(11):1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meng Z, Garcia MK, Hu C, et al. Randomized controlled trial of acupuncture for prevention of radiation‐induced xerostomia among patients with nasopharyngeal carcinoma. Cancer 2012;118(13):3337–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu W, Wayne PM, Davis RB, et al. Acupuncture for chemoradiation therapy‐related dysphagia in head and neck cancer: a pilot randomized sham‐controlled trial. Oncologist 2016;21(12):1522–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cassell E. The nature of suffering and the goals of medicine. New York: Oxford University Press; 2004. [Google Scholar]
- 39. Barber B, Dergousoff J, Slater L, et al. Depression and survival in patients with head and neck cancer: a systematic review. JAMA Otolaryngol Head Neck Surg 2016;142(3):284–288. [DOI] [PubMed] [Google Scholar]
- 40. Kam D, Salib A, Gorgy G, et al. Incidence of suicide in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg 2015;141(12):1075–1081. [DOI] [PubMed] [Google Scholar]
- 41. Greenlee H, DuPont‐Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence‐based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin 2017;67:194–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. History of MBSR . https://www.umassmed.edu/cfm/mindfulness-based-programs/mbsr-courses/about-mbsr/history-of-mbsr/. Accessed October 4, 2017.
- 43. Pollard A, Burchell JL, Castle D, et al. Individualised mindfulness‐based stress reduction for head and neck cancer patients undergoing radiotherapy of curative intent: a descriptive pilot study. Eur J Cancer Care (Engl) 2017. doi: 10.1111/ecc.12474. [DOI] [PubMed] [Google Scholar]
- 44. Howren MB, Christensen AJ, Karnell LH, Funk GF. Psychological factors associated with head and neck cancer treatment and survivorship: evidence and opportunities for behavioral medicine. J Consult Clin Psychol 2013;81(2):299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rogers SN, Semple C, Babb M, Humphris G. Quality of life considerations in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 2016;130(Suppl 2):S49–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: a meta‐analysis. Crit Rev Oncol Hematol 2010;75(2):122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ghazali N, Cadwallader E, Lowe D, Humphris G, Ozakinci G, Rogers SN. Fear of recurrence among head and neck cancer survivors: longitudinal trends. Psychooncology 2013;22(4):807–813. [DOI] [PubMed] [Google Scholar]
- 48. Otto AK, Szczesny EC, Soriano EC, Laurenceau JP, Siegel SD. Effects of a randomized gratitude intervention on death‐related fear of recurrence in breast cancer survivors. Health Psychol 2016;35(12):1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. VanderWeele TJ, Balboni TA, Koh HK. Health and spirituality. JAMA 2017;318(6):519–520. [DOI] [PubMed] [Google Scholar]
- 50. Becker G, Momm F, Xander C, et al. Religious belief as a coping strategy: an explorative trial in patients irradiated for head‐and‐neck cancer. Strahlenther Onkol 2006;182(5):270–276. [DOI] [PubMed] [Google Scholar]
- 51. Saguil A, Phelps K. The spiritual assessment. Am Fam Physician 2012;86(6):546–550. [PubMed] [Google Scholar]
- 52. Zhou J, Jolly S. Obstructive sleep apnea and fatigue in head and neck cancer patients. Am J Clin Oncol 2015;38(4):411–414. [DOI] [PubMed] [Google Scholar]
- 53. Irwin MR, Olmstead R, Breen EC, et al. Tai chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: a randomized controlled trial. J Natl Cancer Inst Monogr 2014;2014(50):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trudel‐Fitzgerald C, Zhou ES, Poole EM, et al. Sleep and survival among women with breast cancer: 30 years of follow‐up within the Nurses' Health Study. Br J Cancer 2017;116(9):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]


