Abstract
Background: Mutations in RMRP, encoding a non-coding RNA molecule, underlie cartilage-hair hypoplasia (CHH), a syndromic immunodeficiency with multiple pathogenetic mechanisms and variable phenotype. Allergy and asthma have been reported in the CHH population and some patients suffer from autoimmune (AI) diseases.
Objective: We explored AI and allergic manifestations in a large cohort of Finnish patients with CHH and correlated clinical features with laboratory parameters and autoantibodies.
Methods: We collected clinical and laboratory data from patient interviews and hospital records. Serum samples were tested for a range of autoantibodies including celiac, anti-cytokine, and anti-21-hydroxylase antibodies. Nasal cytology samples were analyzed with microscopy.
Results: The study cohort included 104 patients with genetically confirmed CHH; their median age was 39.2 years (range 0.6–73.6). Clinical autoimmunity was common (11/104, 10.6%) and included conditions previously undescribed in subjects with CHH (narcolepsy, psoriasis, idiopathic thrombocytopenic purpura, and multifocal motor axonal neuropathy). Patients with autoimmunity more often had recurrent pneumonia, sepsis, high immunoglobulin (Ig) E and/or undetectable IgA levels. The mortality rates were higher in subjects with AI diseases ( = 14.056, p = 0.0002). Several patients demonstrated serum autoantibody positivity without compatible symptoms. We confirmed the high prevalence of asthma (23%) and allergic rhinoconjunctivitis (39%). Gastrointestinal complaints, mostly persistent diarrhea, were also frequently reported (32/104, 31%). Despite the history of allergic rhinitis, no eosinophils were observed in nasal cytology in five tested patients.
Conclusions: AI diseases are common in Finnish patients with CHH and are associated with higher mortality, recurrent pneumonia, sepsis, high IgE and/or undetectable IgA levels. Serum positivity for some autoantibodies was not associated with clinical autoimmunity. The high prevalence of persistent diarrhea, asthma, and symptoms of inflammation of nasal mucosa may indicate common pathways of immune dysregulation.
Keywords: allergy, asthma, autoantibodies, enteropathy, RMRP
Introduction
Cartilage-hair hypoplasia (CHH, OMIM #250250) is caused by mutations in RMRP, which encodes the long non-coding RNA component of mitochondrial RNA-processing endoribonuclease. CHH is characterized by metaphyseal chondrodysplasia, hair hypoplasia, combined immunodeficiency, anemia, and increased risk of malignancies. Autoimmune (AI) diseases have occasionally been reported in CHH, such as enteropathy, hemolytic anemia (AIHA), hypoparathyroidism, hypo- or hyperthyroidism, juvenile rheumatoid arthritis and neutropenia (1–4). The prevalence of asthma is increased and has been reported in 16% (4/25) of Amish and 24% (10/34) of Finnish patients (3, 5). Allergic rhinoconjunctivitis is also excessively prevalent (41%, 23/56) in the Finnish CHH population (6).
RMRP mutations induce cell cycle abnormalities resulting in defective proliferation and increased apoptosis of T cells (7, 8). Patients with CHH have markedly reduced numbers of recent thymic emigrants and low naïve CD4+ and CD8+ cells, but normal counts of CD4+CD25highCD127low regulatory T cells and double-negative T cells (6, 8). Eosinophilia and increased immunoglobulin (Ig) E levels are uncommon (6).
The knowledge of the disease mechanisms in CHH has expanded in recent years to include impaired gene regulation (9) and defects in telomere biology (10, 11). In addition, lack of autoimmune regulator (AIRE) expression and absence of Foxp3+ T cells in the thymus of a single patient with CHH has been reported (12). The loss of AIRE function associated with AIRE mutations is responsible for Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy (APECED), a recognized polyendocrine syndrome with defective T and B cell tolerance (13, 14). This could support a possible role of impaired regulatory T cell function in the pathogenesis of CHH.
We have recently demonstrated broad autoantibody reactivity in subjects with CHH using the microarray technique (15). Compared with healthy controls, CHH patients showed significantly higher reactivity for autoantibodies against gliadin, nuclear antigens, thyroglobulin, vitronectin, as well as for myositis-specific antibodies.
In order to further explore the prevalence and characteristics of AI and allergic manifestations in individuals with CHH, we analyzed clinical and laboratory data for a large group of Finnish CHH patients. We also tested serum samples from several of these patients for a variety of autoantibodies. To complement our previous research, we applied different measurement methods and tested mostly for autoantibodies that were not included in our recent study (15).
Methods
We originally invited all known Finnish patients with CHH to a prospective cohort study in 1985–1991. Those who agreed to participate (Cohort 1, n = 80) underwent interview, clinical examination and blood sampling; RMRP mutation testing was performed when the gene was identified in 2001. A follow-up visit was conducted in 2012–2015, when Cohort 1 and all other thus far identified Finnish CHH patients were contacted and invited for a clinical visit and blood tests. This study thus included three groups of patients (in total 104 patients): (1) patients from Cohort 1 who attended the follow-up visit (n = 32), (2) patients from Cohort 1 who were unavailable for the follow-up visit (n = 48), and (3) patients recruited to the study at the follow-up visit (Cohort 2, n = 24). We collected complete data by means of interviews and from hospital records for all patients participating the follow-up visit (n = 56). For those patients from Cohort 1 who did not attend the follow-up visit, we obtained health information from two Finnish National Medical Databases. The Care Register for Health Care has since 1969 recorded the activities of health centers, hospitals and other institutions providing inpatient care, as well as home-nursing care. Outpatient primary health care data was derived from the Register of Primary Health Care Visits, which covers all health centers in Finland since 2011. Database information included health service providers, dates of the visits, diagnoses, as well as diagnostic and therapeutic procedures. We then contacted all identified hospitals and requested all patients' detailed health records for the analysis. Special attention was given to the signs and symptoms attributed to autoimmunity, such as skin rashes, joint symptoms and gastrointestinal complaints and they were systematically discussed during the interviews.
Asthma diagnosis was made by physicians and/or pulmonologists using spirometry and assessment of response to asthma medications. Allergy was similarly diagnosed by physicians based on clinical presentation and in some cases, on skin tests and/or serum specific IgE levels. Sepsis diagnosis was made by physicians and supported by positive findings in blood cultures.
Serum samples for autoantibody measurement were obtained from those who agreed to donate blood at the follow-up visit (n = 33) and stored at −70°C until analyses. The analyzed autoantibodies were related to celiac disease, based on frequent gastrointestinal complaints among the patients, and on autoantibodies that are commonly seen in patients with AIRE mutations (Table 1). One of the eight chosen autoantibodies (deamidated gliadin peptide IgG) has previously been tested with microarray technique in 16 of the available 33 samples (15).
Table 1.
Antibody assays performed on serum samples of patients with cartilage-hair hypoplasia.
| Antibody | Abbreviation | Measurement method | Normal values in adults* |
|---|---|---|---|
| Deamidated gliadin peptide IgA | DGP-IgA | FEIA | <7 U/ml |
| Deamidated gliadin peptide IgG | DGP-IgG | FEIA | <7 U/ml |
| Tissue transglutaminase IgA | tTG-IgA | FEIA | <7 U/ml |
| Tissue transglutaminase IgG | tTG-IgG | FEIA | <10 U/ml |
| Endomysium IgA | EMA-IgA | IIF | Titer <5 |
| Endomysium IgG | EMA-IgG | IIF | Titer <5 |
| Interleukin 17 IgG | IL-17 | Radioligand binding1 | <218 Index |
| Interleukin 22 IgG | IL-22 | Radioligand binding1 | <270 Index |
| Interferon ω IgG | IFN-ω | Radioligand binding2 | <200 Index |
| 21-Hydroxylase IgG | 21-OH | Radioligand binding3 | <57 Index |
To further evaluate the cause for symptoms related to rhinoconjuctivitis we examined nasal samples for eosinophilic, neutrophilic and goblet cells from five patients with physician-diagnosed allergic rhinitis. Subjects had refrained from application of local nasal decongestants, steroids or systemic antihistamines prior to sampling. Samples were taken during spring and summer months (May–August), from both anterior nares beneath the middle turbinates using a standard cotton swab. Samples were let to set on a glass slide at room temperature. Cytology was evaluated by an experienced technician using eosin-methylene blue staining and microscopy.
Statistical analyses were performed with IBM SPSS version 23 software. Fisher's exact test, logistic regression analysis and Kaplan-Meier method were implicated when appropriate.
The study protocol was approved by the Institutional Research Ethics Committee at Helsinki University Hospital, Finland, and informed consents were obtained from all participants and caregivers.
Results
Our study group included 104 patients (61 women, 43 men) with genetically confirmed CHH. Their median age at the last follow-up was 39.2 years (range 0.6–73.6 years). There were 17 children aged <18.0 years, 49 young adults (18.0–45.0 years) and 38 adults aged >45.0 years. The majority of patients (n = 79; 76%) were homozygous for the g.70A>G RMRP mutation, others were compound heterozygous for g.70A>G and either g.262G>T (n = 22; 21%) or duplication TACTCTGTGA at −13 (n = 3; 3%).
Altogether 11 patients (11/104, 10.6%) had been diagnosed with AI conditions (Table 2). Clinical features of all study patients are presented in Table 3. In addition to AI diseases that have been previously reported in patients with CHH (enteropathy, hemolytic anemia, hyperthyroidism, and juvenile rheumatoid arthritis), we report psoriasis, idiopathic thrombocytopenic purpura, narcolepsy, and multifocal motor axonal neuropathy all of which have been regarded as autoimmune diseases (19–22).
Table 2.
Autoimmune conditions diagnosed in 11 out of 104 patients with cartilage-hair hypoplasia.
| No. | AI disease | Age at diagnosis of AI disease, yrs | Age at the latest follow-up, yrs | Treatment | Outcome of AI disease | Other symptoms |
|---|---|---|---|---|---|---|
| 1 | Enteropathy | 3 | Died in young adulthood | None specific | Died from pneumonia following bowel occlusion | Anemia, arthralgia, asthma, pneumonia |
| MMAN | 28 | IVIG | Moderate clinical improvement | |||
| 2 | Seronegative juvenile polyarthritis | 6 | Young adulthood | NSAIDs, i/a and oral steroids, MTX, HCQ, leflunomide | Etanercept is under consideration for relapsing arthritis | None |
| 3 | AIHA | 10 | Young adulthood | IVIG | Remission after 9 yrs of treatment | Arthralgia, Hirschsprung disease, lymphoma, pneumonia, sepsis |
| 4 | Narcolepsy | 11 | Young adulthood | None specific, narcolepsy was not related to influenza vaccine | No remission | Allergy, anemia |
| 5 | AIHA | 11 | Died in adolescence | Prednisolone | Remission after short course of therapy | Anemia, pneumonia, sepsis |
| Psoriasis | 12 | Local therapy | Disease under control until death | |||
| Enteropathy | 14 | None specific | Died from pneumonia following profound diarrhea | |||
| 6 | Graves' disease | 15 | Young adulthood | Carbimazole, thyroidectomy | Cured after thyroidectomy | Allergy, arthralgia |
| 7 | ITP | 29 | Died in adulthood | Prednisolone | Remission after 1.5 yrs of therapy. Died from lymphoma | Allergy, arthralgia, diarrhea, lymphoma, pneumonia, sepsis |
| 8 | Celiac disease* | 30 | Died in adulthood | Unknown | Unknown. Died from pneumonia | Arthralgia, pneumonia |
| 9 | Enteropathy | 35 | Young adulthood | None specific | Investigations ongoing | Anemia, arthralgia, pneumonia |
| 10 | Psoriasis | 41 | Died in adulthood | Local therapy | Disease under control until death from lymphoma | Allergy, arthralgia, Hirschsprung disease, lymphoma, pneumonia |
| 11 | Ulcerative colitis | 68 | Died in mature adulthood | Mesalamine | Disease under control until death from end-stage lung disease | Allergy, asthma, bronchiectasis, meylodysplasia, pneumonia |
AI, autoimmune; AIHA, autoimmune hemolytic anemia; HCQ, hydroxychloroquine; i/a, intra-articular; ITP, idiopathic thrombocytopenic purpura; IVIG, intravenous immunoglobulin; MMAN, multifocal motor axonal neuropathy; MTX, methotrexate; No, patient number; NSAID, nonsteroidal anti-inflammatory drug; yrs, years.
data on histopathology is not available.
Table 3.
Clinical and laboratory characteristics of 104 patients with cartilage-hair hypoplasia.
| Manifestations | No. of patients (%) | Comments |
|---|---|---|
| Recurrent sinopulmonary and/or ear infections Hospitalization and/or surgery required |
84/104 (81) 50/80 (63) |
Acute otitis media, rhinosinusitis and/or pneumonia Tympanostomy and/or sinus surgery |
| Physician-diagnosed asthma | 24/104 (23) | |
| Allergy to pollen and/or animal Positive allergy testing |
40/104 (39) 5/9 (56) |
Skin testing and/or allergen-specific serum IgE |
| Joint symptoms | 56/104 (54) | Arthralgia or morning joint stiffness |
| Arthrosis Juvenile rheumatoid arthritis |
20/56 (36) 1/56 (2) |
Confirmed radiologically Seronegative polyarthritis |
| Gastrointestinal symptoms Prolonged and/or recurrent diarrhea Dyspepsia |
32/104 (31) 23/32(72) 18/32 (56) |
|
| Gastroscopy performed Colonoscopy performed Chronic gastritis Duodenal villous atrophy |
18/32 (56) 8/32 (25) 5/18 (28) 3/18 (17) |
Normal results in eight (8/18, 44%) patients Normal in all except one case of ulcerative colitis Diagnosed on gastroscopy Diagnosed on gastroscopy |
| Anemia Red blood cell transfusion required AIHA |
32/104 (31) 13/32 (41) 2/32 (6) |
|
| Elevated IgE* | 5/69 (7) | |
| Elevated deamidated gliadin peptide IgA | 2/33 (6) | Values of 9 and 20 (normal < 7 U/ml) |
| Elevated deamidated gliadin peptide IgG | 2/33 (6) | Values of 10 and 14 (normal < 7 U/ml) |
| Elevated interferon ω IgG | 1/33 (3) | Value of 206 (normal < 200 Index) |
| Elevated 21-Hydroxylase IgG | 2/33 (6) | Values of 67 and 77 (normal < 57 Index) |
AIHA, autoimmune hemolytic anemia; Ig, immunoglobulin; No, number.
According to local laboratory age-adjusted reference values.
Among those with autoimmunity, recurrent pneumonia, and sepsis were more frequently documented than in those without autoimmunity (6/11, 55% vs. 13/93, 14%, p = 0.001, B coefficient 2.65 and 4/11, 36% vs. 6/93, 6%, p = 0.019, B coefficient 2.14, respectively). Rates of autoimmunity were higher in patients with elevated IgE levels (2/5, 40%; p = 0.011, B coefficient 2.96) and in patients with undetectable IgA levels (3/5, 60%; p = 0.036, B coefficient 3.33), as compared with patients with normal IgE and IgA levels.
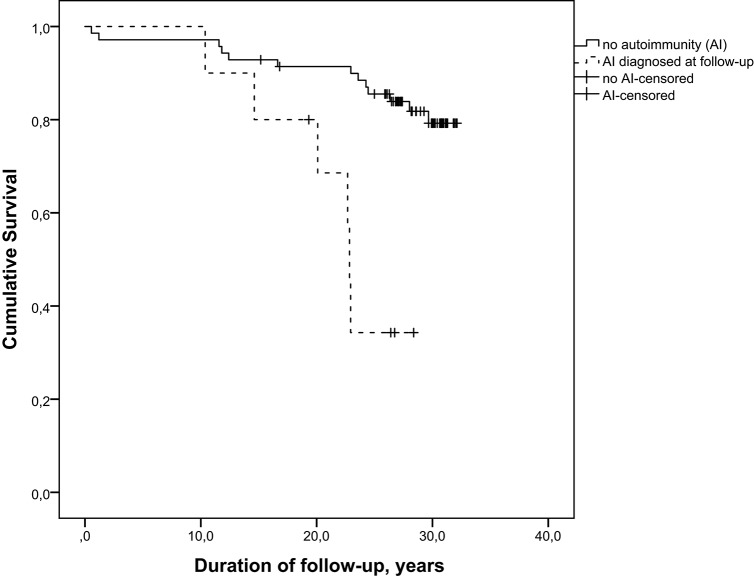
Among patients from Cohort 1, 10/80 individuals have developed AI diseases during follow-up. The Kaplan-Meier analysis (log rank test) of these 80 patients demonstrated that survival distributions for patients with and without AI conditions were significantly different, = 14.056, p = 0.0002 (Figure 1).
Figure 1.
Survival of patients with cartilage-hair hypoplasia differs significantly in subjects with and without autoimmune conditions, log rank = 14.056, p = 0.0002.
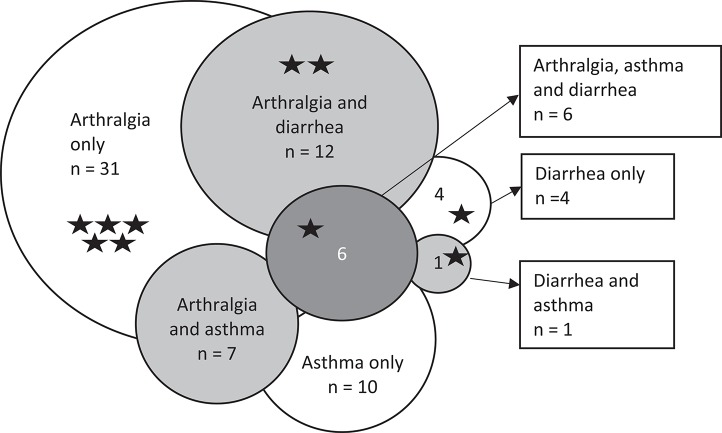
More than half of our patients (56/104, 54%) suffered from arthralgia or morning joint stiffness, but only one was diagnosed with genuine seronegative juvenile polyarthritis. Osteoarthritis was common among patients with arthralgia (20/56, 36%) and was increasingly prevalent with increasing age. Mean age at last follow-up in those with osteoarthritis was 51.7 years, while it was 35.2 years in those without osteoarthritis. Patients with a history of arthralgia reported more often sinusitis (38/56, 68% vs. 18/48, 38%, p = 0.002, B coefficient 1.3) and pollen allergy (27/56, 48% vs. 13/48, 27%, p = 0.035, B coefficient 0.95). When arthralgia, asthma and diarrhea were compiled in a Venn diagram (n = 71), asthma and diarrhea overlapped with arthralgia in 7 and 12 patients, respectively (Figure 2). The overlap, however, did not reach statistical significance. This group of 71 patients contained most of the patients with AI conditions (10/11).
Figure 2.
Modified Venn diagram of arthralgia, asthma, persistent diarrhea and their combinations in 71 patients with cartilage-hair hypoplasia. n, number of patients. Stars indicate patients with autoimmune diseases.
Gastrointestinal symptoms were common in our cohort (32/104, 31%) and the most frequent complaint was prolonged/recurrent diarrhea. In at least three patients with diarrhea, broad screening for bacterial, viral and parasitic infections was negative. Twelve patients followed lactose-free diet, but this did not alleviate diarrhea. Eight patients had undergone colonoscopy, with normal results in all except one subject with ulcerative colitis. Gastroscopy findings were abnormal in 10/18 patients and included gastroesophageal reflux (n = 5), chronic gastritis (n = 5), peptic ulcer (n = 2), and/or duodenal villous atrophy (DVA, n = 3). Another patient had received the diagnosis of celiac disease, but data on histology were unavailable. Significantly more patients with diarrhea reported arthralgia (18/23, 78% vs. 38/81, 47%, p = 0.011, B coefficient 1.41), as compared with those without diarrhea. In the subgroup of patients with osteoarthritis (n = 20), diarrhea was not more common than in those without osteoarthritis.
Anemia was diagnosed in 32 patients (31%), mostly in childhood. Thirteen of these patients (41%) required red blood cell transfusions. Two subjects had AIHA, in others anemia was hypoplastic and attributed to CHH itself and mostly resolved by adulthood. Four of the patients required blood transfusions as adults. There were no cases of AI neutropenia, but one patient had idiopathic thrombocytopenic purpura.
Physician-diagnosed asthma (23%) and allergy (39%) were both highly prevalent. Asthma was diagnosed in 29% (5/17) of children and in 22% (19/87) of adults. In some patients with clinical symptoms of pollen allergy, skin testing and/or serum allergen-specific IgE testing had been negative. Elevated IgE levels were infrequently documented (5/69 patients), also in subjects without allergic symptoms. Eosinophilia was more common in subjects with pollen allergy (5/34, 15% vs. 1/50, 2%, p = 0.038). Hospitalization for pneumonia and/or surgery for sinopulmonary and/or ear infections were more common in patients with asthma [19/24 (79%) vs. 31/80 (39%), p = 0.019, B coefficient 1.13].
Subgroup of patients tested for autoantibodies
Serum samples were available from 33 adult patients (21 women, 12 men) at median age of 45.4 years (range 19.9–69.1 years). One patient was receiving immunoglobulin substitution at the time of blood sampling, he tested negative for all autoantibodies. One had unmeasurable IgA, therefore EMA-IgG and tTG-IgG were measured in serum with normal results. In a few patients, we detected slightly elevated autoantibodies including anti-interferon-ω IgG (IFN-ω, n = 1), anti-21-hydroxylase IgG (21-OH, n = 2), anti-gliadin-peptide IgA (n = 2) and anti-gliadin-peptide IgG (n = 2; Tables 3, 4). These patients had no celiac disease and none of the typical manifestations of APECED, including adrenal insufficiency, hypoparathyroidism and mucocutaneous candidiasis. All other autoantibodies tested negative in all patients.
Table 4.
Results of autoantibody measurements in 33 patients with cartilage-hair hypoplasia.
| Pt No. | AI | Arthralgia | G/i | Pollen allergy | BA | DGP-IgA, U/ml | DGP-IgG, U/ml | tTG-IgA, U/ml | EMA-IgA, titer | IL-17, index | IL-22, index | IFN-ω, index | 21-OH, index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | + | – | + | + | <7 | <7 | <7 | <5 | 211 | −52 | 34 | 23 |
| 2 | – | – | – | – | – | <7 | 14 | <7 | <5 | −5 | 81 | 64 | 55 |
| 3 | – | + | – | + | – | 20 | 10 | 7 | <5 | −2 | −9 | 62 | −27 |
| 4 | – | + | – | + | – | <7 | <7 | <7 | <5 | 64 | 2 | −50 | −34 |
| 5 | – | – | – | + | – | <7 | <7 | <7 | <5 | −49 | −25 | 0 | 6 |
| 6 | – | + | + | – | – | 7 | 7 | <7 | <5 | −37 | 30 | 13 | 40 |
| 7 | – | + | + | + | – | <7 | <7 | <7 | <5 | −30 | −70 | 111 | 11 |
| 8 | – | + | + | + | – | <7 | <7 | <7 | <5 | 31 | 101 | 155 | 35 |
| 9 | – | + | + | + | – | <7 | <7 | <7 | <5 | −28 | −7 | 49 | −33 |
| 10 | – | + | + | + | + | <7 | <7 | <7 | <5 | −53 | 84 | 49 | 47 |
| 11 | – | + | + | – | – | <7 | <7 | <7 | <5 | −62 | 23 | −39 | −21 |
| 12 | – | – | + | + | + | <7 | <7 | <7 | <5 | −40 | 90 | 136 | 67 |
| 13 | – | + | – | + | + | <7 | <7 | <7 | <5 | −84 | −95 | −48 | −30 |
| 14 | – | + | – | + | – | <7 | <7 | <7 | <5 | −27 | 99 | 22 | 15 |
| 15 | – | – | – | – | – | <7 | <7 | <7 | <5 | −10 | −60 | 171 | 46 |
| 16 | – | – | – | – | – | <7 | <7 | <7 | <5 | −32 | 39 | 29 | 11 |
| 17 | – | + | – | + | – | <7 | <7 | <7 | <5 | −32 | 73 | 48 | 77 |
| 18 | – | – | + | + | – | <7 | <7 | <7 | <5 | −62 | −11 | −46 | 7 |
| 19 | – | + | – | + | + | <7 | <7 | <7 | <5 | −52 | −110 | −110 | −4 |
| 20 | – | + | + | – | – | 9 | <7 | <7 | <5 | −42 | −27 | −86 | −21 |
| 21 | – | – | – | – | – | <7 | <7 | <7 | <5 | −26 | 15 | −36 | 46 |
| 22 | – | + | – | – | – | <7 | <7 | <7 | <5 | 16 | 105 | 130 | 77 |
| 23 | – | – | + | – | – | <7 | <7 | <7 | <5 | −59 | 50 | 79 | 54 |
| 24 | – | – | – | + | – | <7 | <7 | <7 | <5 | −66 | 3 | 54 | 16 |
| 25 | – | – | – | – | – | <7 | <7 | <7* | <5* | 51 | 37 | 68 | 18 |
| 26 | – | + | – | – | – | <7 | <7 | <7 | <5 | −38 | −10 | 42 | 21 |
| 27 | – | – | – | – | – | <7 | <7 | <7 | <5 | 48 | 183 | −194 | −4 |
| 28 | – | – | – | – | – | <7 | <7 | <7 | <5 | −98 | −71 | −71 | −1 |
| 29 | – | + | + | – | – | <7 | <7 | <7 | <5 | −69 | −18 | −45 | 4 |
| 30 | – | – | + | + | + | <7 | <7 | <7 | <5 | −69 | 19 | −202 | −30 |
| 31 | – | – | – | – | – | <7 | <7 | <7 | <5 | −8 | 66 | 206 | 38 |
| 32 | – | – | + | + | – | <7 | <7 | <7 | <5 | −32 | 90 | 69 | 30 |
| 33 | – | + | + | + | – | <7 | <7 | <7 | <5 | −51 | −17 | 40 | 40 |
Numbers in bold represent elevated values. For reference values please see Table 1.
Because of immunoglobulin A deficiency diagnosed in this patient, tTG-IgG and EMA-IgG were measured, both were negative. 21-OH, 21-hydroxylase; AI, autoimmune disease; BA, bronchial asthma; DGP, deamidated gliadin peptide; EMA, endomysium; G/i, gastrointestinal symptoms; IFN, interferon; Ig, immunoglobulin; IL, interleukin; No, number; Pt, patient; tTG, tissue transglutaminase.
Subgroup of patients tested for nasal cytology
Nasal samples were available from five patients. Although they all reported physician-diagnosed allergic rhinitis, eosinophils were almost absent in all subjects, only two out of five subjects had single cells visible unilaterally (Table 5). Further, neutrophils were detected in moderate amounts in only one patient, and goblet cells in another. Lymphocytes were not found in any samples.
Table 5.
Cytology of nasal cells from five subjects with cartilage-hair hypoplasia.
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms |
Pollen allergy, rhinoconjuctivitis |
Pollen allergy |
Pollen allergy, nasal congestion, shortness of breath and wheezing |
Chronic nasal congestion, runny nose |
Periodic nasal congestion |
|||||
| Nasal side | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left |
| Lymphocytes | – | – | – | – | – | – | – | – | – | – |
| Eosinophils | – | (+) | – | – | – | – | (+) | – | – | – |
| Neutrophils | – | + | – | + | – | – | (+) | (+) | ++ | ++ |
| Goblet cells | ++ | ++ | (+) | (+) | – | – | – | – | (+) | (+) |
Grading of eosinophilic cells (per microscopic view): –, 0 cells; +/-, 1-3 cells; +, 3–5 cells; ++, 20–30 cells; +++, cells predominate. Grading of neutrophilic cells (per microscopic view): –, no cells; +, cells clearly visible; ++, moderate number of cells visible.
Discussion
We demonstrate high prevalence (11/104, 10.6%) of AI conditions in a large cohort of Finnish patients with CHH compared with the prevalence of 5.4% in general Finnish population (23). Autoimmunity, allergy, diarrhea, arthralgia, and asthma in our patients may reflect common pathways of immune dysregulation behind these symptoms.
With microarray technique, multiple autoantibodies have been demonstrated in all (n = 16) tested serum samples from patients with CHH (15). The absence of compatible symptoms may reflect the benign nature of these antibodies, consistent with reported presence of autoantibodies in healthy individuals (24). RMRP mutations may contribute to AI phenomena by various mechanisms, such as impaired Th17 cell functions or altered expression of AIRE. Autoantibodies typically observed in APECED patients (IFN-ω, IL-17, IL-22, 21-OH) have not been included in our previous study. Here, we describe positivity for these antibodies in three of our patients, but the indices were close to the limit of positivity and the patients had no compatible clinical manifestations. Thus, the significance of these findings is uncertain.
The data on the CD4+CD25highCD127low regulatory T cells measured in 11 patients from our cohort has previously been published (6). The numbers of regulatory T cells were normal in 10/11 patients, including two patients with autoimmunity (measured after the development of AIHA and juvenile idiopathic arthritis) and low in a single 67-year-old patient without AI manifestations. However, functional abnormalities in regulatory T cells cannot be excluded and the role of CTLA-4 deficiency behind the immune dysregulation should be further explored.
Many of our study patients described gastrointestinal problems, most commonly recurrent and/or prolonged diarrhea. Eleven of our patients avoided lactose, which did not improve diarrhea. Endoscopy was not systematically performed in patients with CHH and thus AI enteropathy cannot be excluded. The presence of DVA in three subjects with negative celiac screen underscores the necessity for systematic endoscopic evaluation of CHH individuals with gastrointestinal complaints. Apart from celiac disease, DVA is frequent in patients with common variable immunodeficiency and numerous other disorders, and DVA etiology should always be identified and treated to prevent malnutrition and malignant transformation (25, 26).
Serum tissue transglutaminase (tTG) IgA was negative in all tested patients and tTG IgG was negative in a single patient with IgA deficiency. However, in our previous microarray study (15), five out of 16 patients tested positive for tTG IgG and two of them reported diarrhea, therefore the possible link between tTG antibodies and gastrointestinal symptoms in patients with CHH deserves further studies. Isolated positive tTG IgG is uncommon in celiac disease, but can be present in other AI conditions, including inflammatory bowel disease (27). The discordance of positivity for tTG IgG in our current and previous study may be explained by higher sensitivity of microarray technique.
Asthma was more common in individuals with CHH who required hospitalization and/or surgery for sinopulmonary and/or ear infections. This may suggest asthma as a marker for a more severe immunodeficiency in CHH but may also reflect the retrospective nature of our study and the influence of age, with accumulation of infectious episodes and asthma diagnoses in older patients. Asthma was not associated with pollen allergy in this cohort, raising the question of possible misdiagnosis of one or both of these disorders in some patients. Asthma diagnosis is challenging in CHH due to the absence of height-age-adjusted reference values for spirometry.
Autoimmunity has been implied as a mechanism for lung disease in some patients with APECED and asthma-like symptoms, airway hyperresponsiveness and bronchiectasis (28, 29). We have previously demonstrated high prevalence of bronchiectasis in individuals with CHH, also in those without clinical signs of immunodeficiency (5). Thus, some patients with CHH designated to have asthma, may instead suffer from an underlying autoimmune lung disease and pulmonary problems in subjects with CHH require further investigations.
Four of the eleven patients with AI diseases died of pneumonia or end-stage lung disease. Although in three of them lung manifestations preceded the onset of autoimmunity, we suggest that patients with CHH and AI conditions should be carefully evaluated for pulmonary symptoms and consideration given for prophylactic antibiotics and/or immunoglobulin substitutions to prevent lung damage from recurrent infections.
In allergic rhinitis and asthma, eosinophils are recruited by pro-inflammatory cytokines and mediators and are the predominant cells in later phases of an allergic response (30, 31). The role of neutrophils in allergic inflammation is debated, but some studies report increasing numbers in nasal cytology in patients with allergic rhinitis (32–34). While all five tested subjects reported recurrent or chronic symptoms of allergic rhinitis and nasal samples were collected during the peak pollen season, none had increased numbers of eosinophilic, neutrophilic or lymphocytic nasal cells. This points to the presence of a chronic mucous inflammation, congruent with the systemic immune dysregulation, also supported by the fact that some patients with symptoms of pollen allergy tested negative for allergens.
In conclusion, we report here a high prevalence and a wide spectrum of AI diseases in a large cohort of patients with CHH. We report mild positivity for some autoantibodies, not associated with clinical autoimmunity. The high prevalence of arthralgia, persistent diarrhea, asthma and symptoms of inflammation of nasal mucosa may indicate common pathways of immune dysregulation. Gastrointestinal, pulmonary and joint symptoms in subjects with CHH should be thoroughly evaluated to exclude underlying autoimmunity.
Ethics statement
This study was carried out in accordance with the recommendations of Institutional Research Ethics Committee at Helsinki University Hospital, Finland, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Research Ethics Committee at Helsinki University Hospital, Finland.
Author contributions
OM, SV, and EH designed the study; SV analyzed the data and drafted the manuscript; RM performed nasal sampling; EH performed autoantibody analysis. All authors contributed to the manuscript writing and approved the final version.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- AI
autoimmune
- AIHA
autoimmune hemolytic anemia
- APECED
Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy
- CHH
cartilage-hair hypoplasia
- DVA
duodenal villous atrophy
- Ig
immunoglobulin
- IL
interleukin
- RMRP
RNA component of mitochondrial RNA-processing endoribonuclease.
Footnotes
Funding. The study was funded by the Sigrid Jusélius Foundation (OM), the Academy of Finland (OM), the Folkhälsan Research Foundation (OM), the Helsinki University Hospital Research Funds (HV, MT, and OM), the Swedish Childhood Cancer Foundation (OM), the Foundation for Pediatric Research (MT and OM), University of Helsinki and Helsinki University Hospital through the Doctoral Programme in Clinical Research (RM), The Jalmari and Rauha Ahokas Foundation (RM) and the Doctoral School in Health Sciences at the University of Helsinki (SV), The Regional Health Authorities of Western Norway and The Norwegian Research Council (EH).
References
- 1.Bonafé L, Dermitzakis ET, Unger S, Greenberg CR, Campos-Xavier BA, Zankl A, et al. Evolutionary comparison provides evidence for pathogenicity of RMRP mutations. PLoS Genet. (2005) 1:e47. 10.1371/journal.pgen.0010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacchetta J, Ranchin B, Brunet AS, Bouvier R, Duquesne A, Edery P, et al. Autoimmune hypoparathyroidism in a 12-year-old girl with McKusick cartilage hair hypoplasia. Pediatr Nephrol. (2009) 24:2449–53. 10.1007/s00467-009-1256-0 [DOI] [PubMed] [Google Scholar]
- 3.Rider NL, Morton DH, Puffenberger E, Hendrickson CL, Robinson DL, Strauss KA. Immunologic and clinical features of 25 Amish patients with RMRP 70 A–>G cartilage hair hypoplasia. Clin Immunol. (2009) 131:119–28. 10.1016/j.clim.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 4.Bordon V, Gennery AR, Slatter MA, Vandecruys E, Laureys G, Veys P, et al. Clinical and immunologic outcome of patients with cartilage hair hypoplasia after hematopoietic stem cell transplantation. Blood (2010) 116:27–35. 10.1182/blood-2010-01-259168 [DOI] [PubMed] [Google Scholar]
- 5.Kostjukovits S, Klemetti P, Föhr A, Kajosaari M, Valta H, Taskinen M, et al. High prevalence of bronchiectasis in patients with cartilage-hair hypoplasia. J Allergy Clin Immunol. (2017) 139:375–8. 10.1016/j.jaci.2016.07.023 [DOI] [PubMed] [Google Scholar]
- 6.Kostjukovits S, Klemetti P, Valta H, Martelius T, Notarangelo LD, Seppänen M, et al. Analysis of clinical and immunologic phenotype in a large cohort of children and adults with cartilage-hair hypoplasia. J Allergy Clin Immunol. (2017) 140:612–4.e5. 10.1016/j.jaci.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiel CT, Mortier G, Kaitila I, Reis A, Rauch A. Type and level of RMRP functional impairment predicts phenotype in the cartilage hair hypoplasia-anauxetic dysplasia spectrum. Am J Hum Genet. (2007) 81:519–29. 10.1086/521034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Fuente MA, Recher M, Rider NL, Strauss KA, Morton DH, Adair M, et al. Reduced thymic output, cell cycle abnormalities, and increased apoptosis of T lymphocytes in patients swith cartilage-hair hypoplasia. J Allergy Clin Immunol. (2011) 128:139–46. 10.1016/j.jaci.2011.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogler LE, Kosmyna B, Moskowitz D, Bebawee R, Rahimzadeh J, Kutchko K, et al. Small RNAs derived from lncRNA RNase MRP have gene-silencing activity relevant to human cartilage-hair hypoplasia. Hum Mol Genet. (2014) 23:368–82. 10.1093/hmg/ddt427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kostjukovits S, Degerman S, Pekkinen M, Klemetti P, Landfors M, Roos G, et al. Decreased telomere length in children with cartilage-hair hypoplasia. J Med Genet. (2016) 54:365–70. 10.1136/jmedgenet-2016-104279 [DOI] [PubMed] [Google Scholar]
- 11.Aubert G, Strauss KA, Lansdorp PM, Rider NL. Defects in lymphocyte telomere homeostasis contribute to cellular immune phenotype in cartilage-hair hypoplasia. J Allergy Clin Immunol. (2017) 140:1120–9.e1. 10.1016/j.jaci.2016.11.051 [DOI] [PubMed] [Google Scholar]
- 12.Poliani PL, Facchetti F, Ravanini M, Gennery AR, Villa A, Roifman CM, et al. Early defects in human T-cell development severely affect distribution and maturation of thymic stromal cells: possible implications for the pathophysiology of Omenn syndrome. Blood (2009) 114:105–8. 10.1182/blood-2009-03-211029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer S, Woodward M, Hertel C, Vlaicu P, Haque Y, Kärner J, et al. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell (2016) 166:582–95. 10.1016/j.cell.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Husebye ES, Anderson MS, Kämpe O. Autoimmune polyendocrine syndromes. N Engl J Med. (2018) 378:1132–41. 10.1056/NEJMra1713301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biggs CM, Kostjukovits S, Dobbs K, Laakso S, Klemetti P, Valta H, et al. Diverse autoantibody reactivity in cartilage-hair hypoplasia. J Clin Immunol. (2017) 37:508–10. 10.1007/s10875-017-0408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oftedal BE, Kämpe O, Meager A, Ahlgren KM, Lobell A, Husebye ES, et al. Measuring autoantibodies against IL-17F and IL-22 in autoimmune polyendocrine syndrome type I by radioligand binding assay using fusion proteins. Scand J Immunol. (2011) 74:327–33. 10.1111/j.1365-3083.2011.02573.x [DOI] [PubMed] [Google Scholar]
- 17.Oftedal BE, Wolff AS, Bratland E, Kämpe O, Perheentupa J, Myhre AG, et al. Radioimmunoassay for autoantibodies against interferon omega; its use in the diagnosis of autoimmune polyendocrine syndrome type I. Clin Immunol. (2008) 129:163–9. 10.1016/j.clim.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 18.Falorni A, Bini V, Betterle C, Brozzetti A, Castaño L, Fichna M, et al. Determination of 21-hydroxylase autoantibodies: inter-laboratory concordance in the Euradrenal International Serum Exchange Program. Clin Chem Lab Med. (2015) 53:1761–70. 10.1515/cclm-2014-1106 [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. (2017) 49:1–8. 10.1016/j.coi.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imbach P. Development and research in idiopathic thrombocytopenic purpura: an inflammatory and autoimmune disorder. Pediatr Blood Cancer. (2006) 47(Suppl. 5):685–6. 10.1002/pbc.20969 [DOI] [PubMed] [Google Scholar]
- 21.Partinen M, Kornum BR, Plazzi G, Jennum P, Julkunen I, Vaarala O. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol. (2014) 13:600–13. 10.1016/S1474-4422(14)70075-4 [DOI] [PubMed] [Google Scholar]
- 22.Nowacek DG1, Teener JW. Multifocal motor neuropathy. Semin Neurol. (2012) 32:500–5. 10.1055/s-0033-1334468 [DOI] [PubMed] [Google Scholar]
- 23.Raevuori A, Haukka J, Vaarala O, Suvisaari JM, Gissler M, Grainger M, et al. The increased risk for autoimmune diseases in patients with eating disorders. PLoS ONE (2014) 9:e104845. 10.1371/journal.pone.0104845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, et al. Mechanisms of autoantibody-induced pathology. Front Immunol. (2017) 8:603. 10.3389/fimmu.2017.00603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khodadad A, Aghamohammadi A, Parvaneh N, Rezaei N, Mahjoob F, Bashashati M, et al. Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci. (2007) 52:2977–83. 10.1007/s10620-006-9736-6 [DOI] [PubMed] [Google Scholar]
- 26.Pallav K, Leffler DA, Tariq S, Kabbani T, Hansen J, Peer A, et al. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther. (2012) 35:380–90. 10.1111/j.1365-2036.2011.04938.x [DOI] [PubMed] [Google Scholar]
- 27.Shor DB, Orbach H, Boaz M, Altman A, Anaya JM, Bizzaro N, et al. Gastrointestinal-associated autoantibodies in different autoimmune diseases. Am J Clin Exp Immunol. (2012) 1:49–55. [PMC free article] [PubMed] [Google Scholar]
- 28.Alimohammadi M, Dubois N, Sköldberg F, Hallgren A, Tardivel I, Hedstrand H, et al. Pulmonary autoimmunity as a feature of autoimmune polyendocrine syndrome type 1 and identification of KCNRG as a bronchial autoantigen. Proc Natl Acad Sci USA. (2009) 106:4396–401. 10.1073/pnas.0809986106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shum AK, Alimohammadi M, Tan CL, Cheng MH, Metzger TC, Law CS, et al. BPIFB1 is a lung-specific autoantigen associated with interstitial lung disease. Sci Transl Med. (2013) 5:206ra139. 10.1126/scitranslmed.3006998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelfand EW. Inflammatory mediators in allergic rhinitis. J Allergy Clin Immunol. (2004) 114:S135–8. 10.1016/j.jaci.2004.08.043 [DOI] [PubMed] [Google Scholar]
- 31.Naclerio RM, Bachert C, Baraniuk JN. Pathophysiology of nasal congestion. Int J Gen Med. (2010) 3:47–57. 10.2147/IJGM.S8088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelardi M, Iannuzzi L, Quaranta N, Landi M, Passalacqua G. NASAL cytology: practical aspects and clinical relevance. Clin Exp Allergy (2016) 46:785–92. 10.1111/cea.12730 [DOI] [PubMed] [Google Scholar]
- 33.Canakcioglu S, Tahamiler R, Saritzali G, Alimoglu Y, Isildak H, Guvenc MG, et al. Evaluation of nasal cytology in subjects with chronic rhinitis: a 7-year study. Am J Otolaryngol. (2009) 30:312–7. 10.1016/j.amjoto.2008.06.015 [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Zhou Y, Zhang L, Wang Y, Pepper AN, Cho SH, et al. Individualized treatment of allergic rhinitis according to nasal cytology. Allergy Asthma Immunol Res. (2017) 9:403–9. 10.4168/aair.2017.9.5.403 [DOI] [PMC free article] [PubMed] [Google Scholar]