Abstract
Introduction
Interleukin-17A (IL-17A), a pro-inflammatory cytokine, plays an important role in the pathogenesis of asthma. A number of studies have investigated the relationship between IL-17A rs2275913 polymorphism and risk of asthma. However, the results obtained are inconclusive. The aim of this meta-analysis is to clarify the relationship between IL-17A rs2275913 polymorphism and asthma risk.
Material and methods
Searches were conducted in PubMed, Web of Science, Elsevier, Google Scholar, Wanfang and Chinese National Knowledge Infrastructure (CNKI) databases, and data were extracted from eligible studies by two independent reviewers. The pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. Publication bias, heterogeneity and sensitivity analysis were also assessed.
Results
Ten studies with a total of 5016 subjects were included. Overall, the results indicated a significant association between the IL-17A rs2275913 polymorphism and the risk of asthma (G vs. A: OR = 0.866, 95% CI: 0.789–0.951, p = 0.003; GG+GA vs. AA: OR = 0.752, 95% CI: 0.633–0.895, p = 0.001). In subgroup analysis by age and ethnicity, the G allele of rs2275913 in IL-17A was significantly associated with a reduced risk of asthma in children and Asians.
Conclusions
The results of this meta-analysis indicate that the G allele of rs2275913 in IL-17A is a protective factor for the development of asthma.
Keywords: interleukin-17a, polymorphism, asthma, risk, meta-analysis
Introduction
Asthma is one of the chronic airway inflammatory diseases in children and adults, characterized by airway hyper-responsiveness (ARH), reversible airflow obstruction, airway remodeling and recurrent symptoms of wheezing, chest tightness and cough [1]. Human asthma is heterogeneous in terms of genetics, severity and in all likelihood pathophysiology [2]. An increasing number of studies have demonstrated a significant relationship between genetic polymorphisms of cytokines and asthma susceptibility [3–6].
Interleukin-17A (IL-17A) is produced by some types of inflammatory cell, including T helper type-17 (Th17), activated mast cells, NK cells, eosinophils cells, neutrophils cells and basophils cells [7–9]. Accumulated studies suggest that IL-17A plays a crucial role in the pathogenesis of asthma. The levels of IL-17 mRNA and/or proteins are found to be elevated in the lungs, sputum and bronchoalveolar lavage fluids from patients with asthma [10, 11]. The elevation of plasma IL-17A level has been further shown to be associated with asthma severity [11]. A recent study have demonstrated that IL-17A promotes asthma progression by inducing inflammatory cell migration/infiltration and production of other pro-inflammatory cytokines [12].
A number of studies have investigated the association of IL-17A polymorphisms with susceptibility to asthma. Several studies have reported the relationship between IL-17A rs2275913 polymorphism and asthma risk [12–20]. Maalmi et al. have shown that the G allele of rs2275913 in IL-17A is associated with an increased risk of asthma [12]. However, Wang et al. failed to find an association between IL-17A rs2275913 polymorphism and asthma risk [16]. Due to the contradictory and inconclusive results, we performed this meta-analysis to clarify the correlation between IL-17A rs2275913 polymorphism and asthma risk.
Material and methods
Identification and eligibility of relevant studies
Searches were conducted in PubMed, Web of Science, Elsevier, Google Scholar, Wanfang and Chinese National Knowledge Infrastructure (CNKI) databases to identify available studies published until August 2016. The search terms were: (“interleukin-17” OR “IL-17”) AND (“gene” OR “polymorphism” OR “genetic variant”) AND (“asthma” OR “asthmatic” OR “bronchial asthma”). Furthermore, the reference lists of the eligible studies were identified. No restrictions were placed on language, population, sample size or publication date.
Inclusion and exclusion criteria
Studies included in this meta-analysis were screened according to the following criteria: (1) a case-control study design, (2) evaluation of the association between IL-17A rs2275913 polymorphism and asthma risk, (3) offered sufficient data to calculate the odds ratios (ORs) and 95% confidence intervals (CIs). The main exclusion criteria of the study were: (1) no usable data offered, (2) non-case-control studies, (3) duplicate papers.
Data extraction
Two reviewers reviewed all eligible studies independently. The following information was collected: first author, year of publication, country, ethnicity, age, numbers of eligible cases and controls, and genotype and allele frequency information. Disagreements were resolved by discussion and consensus.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) in controls was measured using the χ2 test. The strength of the association between IL-17A rs2275913 polymorphism and asthma risk was assessed by ORs and 95% CIs in an allele model (G vs. A), a codominant model (GG vs. AA, GG vs. GA), a dominant model (GG+GA vs. AA) and a recessive model (GG vs. GA+AA) respectively. The statistical significance of the combined ORs was determined by the Z-test. The χ2 based Cochrane Q-test and I 2 index were used for the assessment of significant heterogeneity between studies (p < 0.10). A random-effects model was performed when heterogeneity was observed in studies. Stratified analyses were adopted by age and ethnicity. Potential publication bias was assessed through Begg’s rank correlation test and Egger’s linear regression test. The value of p < 0.05 was considered statistically significant, except for tests of heterogeneity (p < 0.10). All statistical analyses were performed with Stata (version 12.0, Stata Corporation, College Station, TX).
Results
Study characteristics
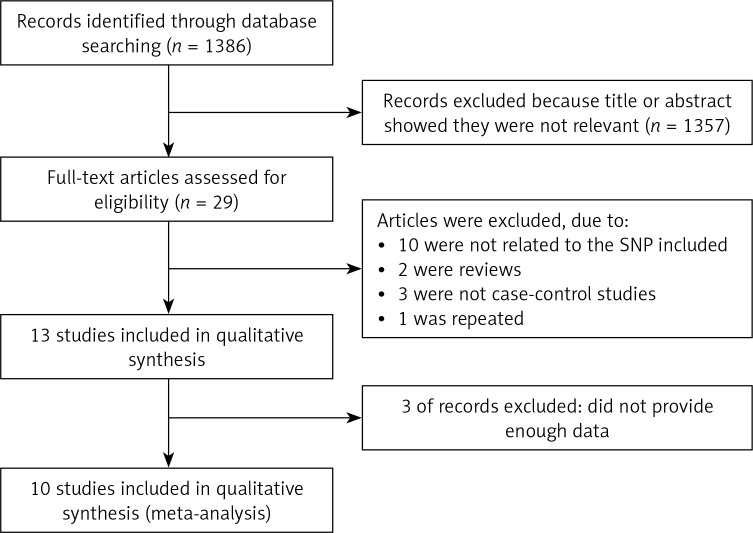
The detailed process of selection is shown in Figure 1. In total, 1,386 studies met the search criteria in the initial search of databases. Following screening, ten case-control studies [12–21] with 2,510 asthma cases and 2,506 controls were included. As for ethnicity, seven studies investigated Asian populations [13–16, 18–20], two studies investigated African populations [12, 21], and one study investigated a Caucasian population [17]. As for age, seven studies investigated children [12–17, 19], and three studies investigated adults [18, 20, 21]. The genotype frequencies of IL-17A rs2275913 polymorphism in each study are shown in Tables I and II.
Figure 1.
Flowchart showing the process of study selection
Table I.
Characteristics of case-control studies included in meta-analysis
| Author | Year | Country | Ethnicity | Age group | Gender | Case (n) | Control (n) | Genotyping method |
|---|---|---|---|---|---|---|---|---|
| Wang | 2009 | China | Asian | Child | Mix | 931 | 1027 | PCR-RFLP |
| Chen | 2010 | China | Asian | Child | Mix | 168 | 205 | PCR-RFLP |
| Wei | 2011 | China | Asian | Child | Mix | 186 | 198 | PCR-RFLP |
| Wang | 2011 | China | Asian | Child | Mix | 287 | 217 | PCR-LDR |
| Luo | 2013 | China | Asian | Adult | Mix | 103 | 48 | RT-PCR |
| Maalmi | 2014 | Tunisia | African | Child | Mix | 171 | 171 | PCR-RFLP |
| Narbutt | 2014 | Poland | Caucasian | Child | Mix | 166 | 166 | PCR-RFLP |
| Zeng | 2015 | China | Asian | Child | Mix | 224 | 150 | PCR-RFLP |
| Du | 2016 | China | Asian | Adult | Mix | 125 | 132 | PCR-RFLP |
| Resende | 2016 | Portugal | African | Adult | Mix | 192 | 149 | RT-PCR |
Table II.
Distribution of IL-17F genotype among cases and controls
| Studies | Case | Control | HWE P-value | ||||
|---|---|---|---|---|---|---|---|
| GG | GA | AA | GG | GA | AA | ||
| Wang | 129 | 234 | 110 | 141 | 251 | 122 | 0.6178 |
| Chen | 53 | 65 | 50 | 68 | 105 | 32 | 0.4145 |
| Wei | 53 | 78 | 55 | 62 | 107 | 29 | 0.1160 |
| Wang | 71 | 151 | 59 | 53 | 110 | 33 | 0.0602 |
| Luo | 29 | 45 | 29 | 14 | 24 | 10 | 0.9614 |
| Maalmi | 132 | 39 | 0 | 110 | 55 | 6 | 0.7847 |
| Narbutt | 28 | 43 | 12 | 69 | 74 | 10 | 0.0918 |
| Zeng | 56 | 120 | 48 | 51 | 82 | 17 | 0.0617 |
| Du | 70 | 60 | 2 | 94 | 21 | 10 | < 0.0001 |
| Resende | 69 | 71 | 8 | 94 | 81 | 17 | 0.9397 |
Quantitative synthesis
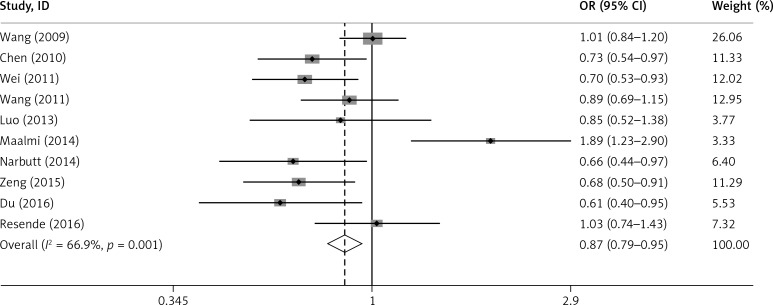
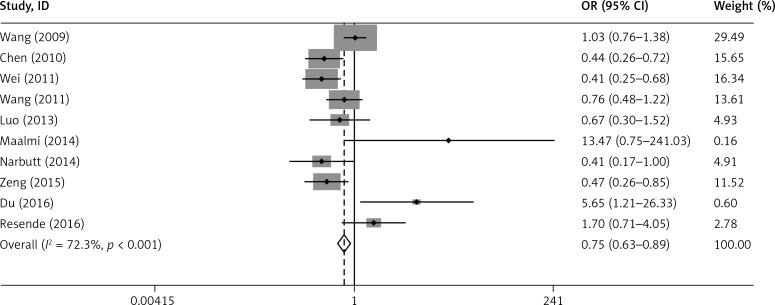
The main results of the relationship between IL-17A rs2275913 polymorphism and asthma risk are listed in Table III. Overall, the results indicated a significant association between IL-17A rs2275913 and asthma (G vs. A: OR = 0.866, 95% CI: 0.789–0.951, p = 0.003; GG+GA vs. AA: OR = 0.752, 95% CI: 0.633–0.895, p = 0.001). However, no significant association was found in the recessive model (GG vs. GA+AA: OR = 0.879, 95% CI: 0.766–1.008, p = 0.065) (Figures 2 and 3).
Table III.
Summary ORs and 95% CIs for IL-17F rs2275913 polymorphism and asthma risk
| Genetic comparisons | Population | Type of model | Heterogeneity | Test of association | |||
|---|---|---|---|---|---|---|---|
| I 2 (%) | P-value | OR | 95% CI | P-value | |||
| G vs. A | Overall | Random | 66.9 | 0.001 | 0.866 | 0.789–0.951 | 0.003 |
| Child | Random | 74.8 | 0.001 | 0.859 | 0.692–1.066 | 0.167 | |
| Adult | Random | 41.0 | 0.183 | 0.831 | 0.692–1.133 | 0.241 | |
| Asian | Random | 43.8 | 0.099 | 0.794 | 0.685–0.921 | 0.002 | |
| African | Random | 79.6 | 0.027 | 1.373 | 0.755–2.498 | 0.299 | |
| GG+GA vs. AA | Overall | Random | 72.3 | < 0.001 | 0.752 | 0.633–0.895 | 0.001 |
| Child | Random | 72.1 | 0.001 | 0.607 | 0.403–0.913 | 0.016 | |
| Adult | Random | 68.8 | 0.040 | 1.594 | 0.552–4.603 | 0.389 | |
| Asian | Random | 74.1 | 0.001 | 0.681 | 0.452–1.026 | 0.066 | |
| African | Random | 48.5 | 0.164 | 3.068 | 0.461–20.410 | 0.246 | |
Figure 2.
Forest plot for the overall association between IL-17A rs2275913 polymorphism and asthma risk under the G vs. A contrast model
Figure 3.
Forest plot for the overall association between IL-17A rs2275913 polymorphism and asthma risk under the GG+GA vs. AA contrast model
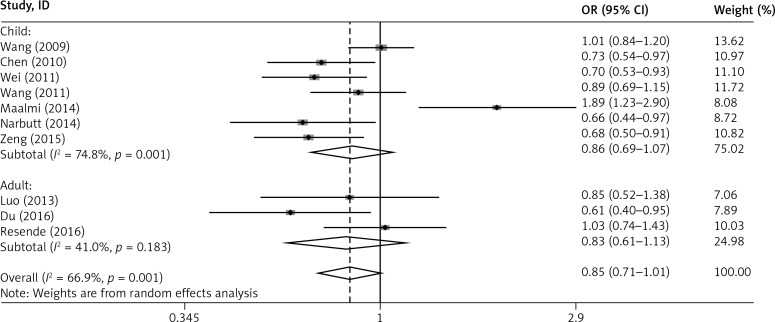
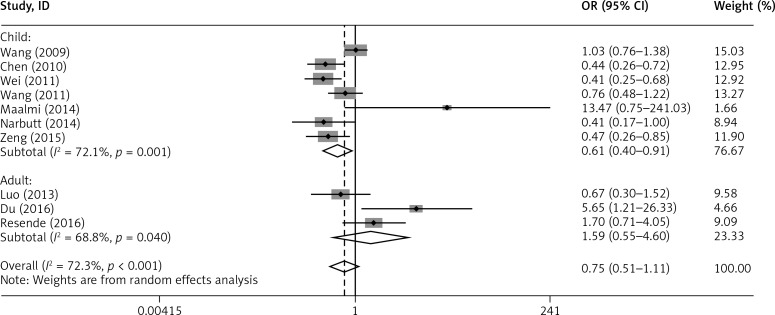
In addition, subgroup analysis by age showed that the G allele of rs2275913 in IL-17A was associated with a reduced risk of asthma in children (G vs. A: OR = 0.859, 95% CI: 0.692–1.066, p = 0.167; GG+GA vs. AA: OR = 0.607, 95% CI: 0.403–0.913, p = 0.016), but not in adults (G vs. A: OR = 0.831, 95% CI: 0.692–1.133, p = 0.241; GG+GA vs. AA: OR = 1.594, 95% CI: 0.552–4.603, p = 0.389) (Figures 4 and 5).
Figure 4.
Forest plot of the association between IL-17A rs2275913 polymorphism and asthma risk stratified by age under the G vs. A contrast model
Figure 5.
Forest plot of the association between IL-17A rs2275913 polymorphism and asthma risk stratified by age under the GG+GA vs. AA contrast model
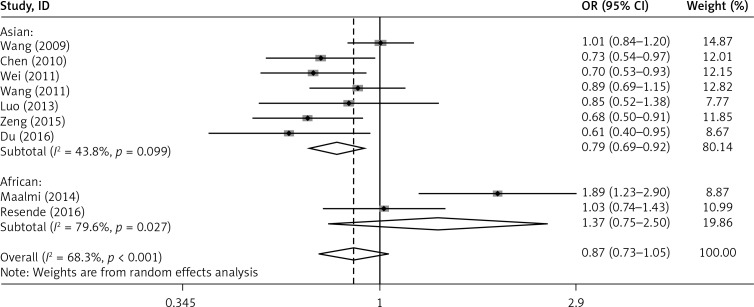
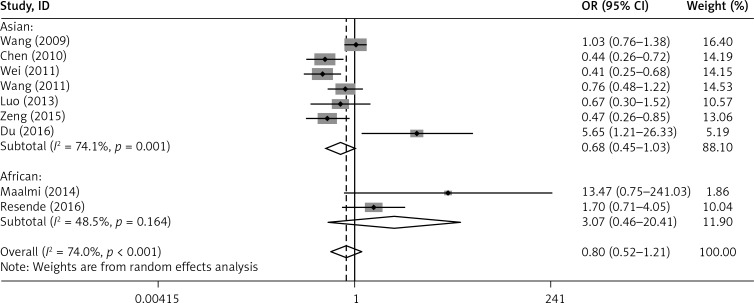
Furthermore, subgroup analysis by ethnicity revealed that the G allele of rs2275913 in IL-17A was associated with a reduced risk of asthma in Asians (G vs. A: OR = 0.794, 95% CI: 0.685–0.921, p = 0.002; GG+GA vs. AA: OR = 0.681, 95% CI: 0.452–1.026, p = 0.066). However, no association was found in Africans (G vs. A: OR = 1.373, 95% CI: 0.755–2.498, p = 0.299; GG+GA vs. AA: OR = 3.068, 95% CI: 0.461–20.410, p = 0.246) (Figures 6 and 7).
Figure 6.
Forest plot of the association between IL-17A rs2275913 polymorphism and asthma risk stratified by ethnicity under the G vs. A contrast model
Figure 7.
Forest plot of the association between IL-17A rs2275913 polymorphism and asthma risk stratified by ethnicity under the GG+GA vs. AA contrast model
Heterogeneity, sensitivity and publication bias analysis
Among the four genetic models, significant heterogeneity was observed in the allele model (G allele vs. A allele) and dominant model (GG+GA vs. AA) with a p < 0.10. After stratifying by age and ethnicity, heterogeneity was still significant in adults, children, Asians and Africans (Table III). Sensitivity analysis was conducted to assess the stability of the results. No material alterations were detected, suggesting the reliability of our results. Furthermore, no significant publication bias was detected by Begg’s test or Egger’s test (all p > 0.05).
Discussion
To the best of our knowledge, this is the first meta-analysis to assess the effect of IL-17A rs2275913 polymorphism on risk of asthma. In the present study, ten studies with a total of 5,016 participants (2,510 asthma cases and 2,506 controls) were ultimately identified. The results of this meta-analysis indicate that the G allele of rs2275913 in IL-17A is a protective factor for the development of asthma.
In subgroup analysis by age and ethnicity, the G allele of rs2275913 in IL-17A is significantly associated with a reduced risk of asthma in children and Asians.
Numerous studies have indicated that IL-17A plays an important role in the development of infections, autoimmune diseases, tumors and allergic disorders, including asthma [22–24]. It has been shown that IL-17A stimulates the production of a variety of inflammatory mediators, including cytokines, chemokines and adhesion molecules, by bronchial epithelial cells and endothelial cells [25]. Meanwhile, IL-17A induces accumulation of multiple inflammatory cells in the airway mucosa and submucosa to promote asthma development [26, 27]. In addition, IL-17A has been demonstrated to be a potent factor causing airway remodeling by inducing the release of remodeling-associated cytokines (including IL-6, IL-8, IL-11, GM-CSF and VEGF) from airway epithelial cells, endothelial cells and fibroblasts, which promote secretion of mucins by goblet cells, enhance migration/proliferation of airway smooth muscle (ASM) cell and inhibit apoptosis of ASM cells [28–33]. Taken together, IL-17A both initiates and aggravates the pathological process of asthma.
The human IL-17A gene is located on chromosome 6p12.1 [34]. The a allele of rs2275913 in the promoter region of the IL-17 gene has been reported to be associated with risk of ulcerative colitis [35], gastric cancer [36] and atopic dermatitis [17]. The a allele of rs2275913 enhances IL-17A promoter activity and promotes its transcription, leading to enhanced inflammation in the airway [37]. However, conflicting research results have been reported on the association between IL-17A rs2275913 polymorphism and IL-17A production. Maalmi et al. [12] have found that patients with GG genotype demonstrate mild-to-moderate asthma and low IL-17 levels. Chen et al. [14] have shown that IL-17A rs2275913 polymorphism does not affect IL-17A expression level in peripheral blood mononuclear cells (PBMCs).
In this meta-analysis, a more precise conclusion was provided by combining all eligible case-control studies. The results showed that the G allele of rs2275913 in IL-17A was significantly associated with a reduced risk of asthma. Furthermore, subgroup analysis by age showed that the G allele of rs2275913 in IL-17A was associated with a reduced risk of asthma in children, but not in adults. Asthma is believed to be a multifactorial disorder affected not only by genetic predisposition but also by environmental factors [38, 39]. The discrepancy between children and adults may be caused by the different duration of exposure of asthma patients to environmental risk factors (including contacting allergens and stimulating factors, air pollution, smoking, and occupational exposure) [40]. Subgroup analysis by ethnicity revealed that the G allele of rs2275913 in IL-17A was a protective factor against asthma in Asians, and no association was found in Africans, suggesting that ethnicity affects asthma susceptibility by cytokine polymorphisms, such as IL-17A rs2275913 polymorphism.
Several potential limitations in this meta-analysis should be considered. Firstly, the limited number of studies that qualified for inclusion may lead to relatively insufficient power. Secondly, the majority of the included studies were conducted in Asians and children, with a lack of data from other ethnicities and adults. Thirdly, significant heterogeneity was found between studies. After subgroup analysis by age and ethnicity, the heterogeneity was dramatically reduced for adults, suggesting that a number of factors including differences in age, gender, environment and lifestyle factors affected heterogeneity.
In conclusion, this meta-analysis indicates that the G allele of rs2275913 in IL-17A acts as a protective factor for the development of asthma. Further large-scale and well-designed studies are still needed to confirm our findings.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 81070045).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Mathew J, Aronow WS, Chandy D. State of the art paper. Therapeutic options for severe asthma. Arch Med Sci. 2012;8:589–97. doi: 10.5114/aoms.2012.30280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan L, Xiao-Ling S, Zheng-Yan C, Guo-Ping L, Sen Z, Zhuang C. HSP70/CD80 DNA vaccine inhibits airway remodeling by regulating the transcription factors T-bet and GATA-3 in a murine model of chronic asthma. Arch Med Sci. 2013;9:906–15. doi: 10.5114/aoms.2013.33180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freidin MB, Puzyrev VP, Ogorodova LM, Kobiakova OS, Kulmanakova IM. [Polymorphism of interleukins and interleukin receptor genes: population distribution and association with atopic bronchial asthma] Genetika. 2002;38:1710–8. [PubMed] [Google Scholar]
- 4.Tam EK, Jourdan-LeSaux C, Stauder S, et al. Polymorphisms in the interleukin-4 receptor alpha chaIn: association with traits of allergy and asthma in an admixed population in Hawaii. Cell Mol Biol. 2003;49:1345–9. [PubMed] [Google Scholar]
- 5.Heinzmann A, Bauer E, Ganter K, Kurz T, Deichmann KA. Polymorphisms of the TGF-beta1 gene are not associated with bronchial asthma in Caucasian children. Pediatr Allergy Immunol. 2005;16:310–4. doi: 10.1111/j.1399-3038.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 6.Kacprzak D, Pawliczak R. Does aspirin-induced oxidative stress cause asthma exacerbation? Arch Med Sci. 2015;11:494–504. doi: 10.5114/aoms.2014.41960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–54. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 8.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–94. doi: 10.1016/j.jaci.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 9.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullens DM, Truyen E, Coteur L, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakir J, Shannon J, Molet S, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–8. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 12.Maalmi H, Beraies A, Charad R, Ammar J, Hamzaoui K, Hamzaoui A. IL-17A and IL-17F genes variants and susceptibility to childhood asthma in Tunisia. J Asthma. 2014;51:348–54. doi: 10.3109/02770903.2013.876647. [DOI] [PubMed] [Google Scholar]
- 13.Wang JY, Shyur SD, Wang WH, et al. The polymorphisms of interleukin 17A (IL17A) gene and its association with pediatric asthma in Taiwanese population. Allergy. 2009;64:1056–60. doi: 10.1111/j.1398-9995.2009.01950.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Deng Y, Zhao J, et al. The polymorphism of IL-17 G-152A was associated with childhood asthma and bacterial colonization of the hypopharynx in bronchiolitis. J Clin Immunol. 2010;30:539–45. doi: 10.1007/s10875-010-9391-8. [DOI] [PubMed] [Google Scholar]
- 15.Wei B, Zhu LL, Deng DF. Association between polymorphisms in promoter region of interleukin-10 and interleukin-17 gene and childhood asthma. Progress Modern Biomed. 2011;11:307–9. [Google Scholar]
- 16.Wang J, Zhou J, Lin LH, Li J, Peng X, Li L. Association of single nucleotide polymorphism of IL-17 gene promoter with childhood asthma. Acad J Second Mil Med Univ. 2011;32:481–4. [Google Scholar]
- 17.Narbutt J, Wojtczak M, Zalinska A, et al. The A/A genotype of an interleukin-17A polymorphism predisposes to increased severity of atopic dermatitis and coexistence with asthma. Clin Exp Dermatol. 2015;40:11–6. doi: 10.1111/ced.12438. [DOI] [PubMed] [Google Scholar]
- 18.Luo S. The polymorphism of interleukin 17-152A and its association with bronchial asthma. 2013: Dalian Medical University; book. [Google Scholar]
- 19.Zeng Y, Li B, Yang J, Xia MQ, Zhu XP. Correlation study of polymorphism of interleukin-17A -152G/A of childhood asthma in Guiyang. J Guiyang Med. 2015;6:588–91. [Google Scholar]
- 20.Du J, Han JC, Zhang YJ, et al. Single-nucleotide polymorphisms of IL-17 gene are associated with asthma susceptibility in an Asian population. Med Sci Monit. 2016;22:780–7. doi: 10.12659/MSM.895494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resende EP, Todo-Bom A, Loureiro C, et al. Asthma and rhinitis have different genetic profiles for IL13, IL17A and GSTP1 polymorphisms. Rev Port Pneumol. 2006;23:10–6. doi: 10.1016/j.rppnen.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Wang L, Zhao H, et al. Th17 cells are associated with the Th1/Th2 cell balance during Echinococcus multilocularis infection. Mol Med Rep. 2014;10:236–40. doi: 10.3892/mmr.2014.2170. [DOI] [PubMed] [Google Scholar]
- 23.Yamada H. Current perspectives on the role of IL-17 in autoimmune disease. J Inflamm Res. 2010;3:33–44. doi: 10.2147/jir.s6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straus DS. TNFalpha and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Mol Cancer. 2013;12:78. doi: 10.1186/1476-4598-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang SK. IL-17 cytokine family. J Allergy Clin Immunol. 2004;114:1265–73. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 27.Newcomb DC, Peebles RS Jr. Th17-mediated inflammation in asthma. Curr Opin Immunol. 2013;25:755–60. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang Y, Al-Alwan L, Risse PA, et al. Th17 cytokines induce human airway smooth muscle cell migration. J Allergy Clin Immunol. 2011;127:1046–53. doi: 10.1016/j.jaci.2010.12.1117. [DOI] [PubMed] [Google Scholar]
- 29.Chang Y, Al-Alwan L, Risse PA, et al. Th17-associated cytokines promote human airway smooth muscle cell proliferation. FASEB. 2012;26:5152–60. doi: 10.1096/fj.12-208033. [DOI] [PubMed] [Google Scholar]
- 30.Laan M, Prause O, Miyamoto M, et al. A role of GM-CSF in the accumulation of neutrophils in the airways caused by IL-17 and TNF-alpha. Eur Respir J. 2003;21:387–93. doi: 10.1183/09031936.03.00303503. [DOI] [PubMed] [Google Scholar]
- 31.Linden A. Interleukin-17 and airway remodelling. Pulm Pharmacol Therap. 2006;19:47–50. doi: 10.1016/j.pupt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol. 2009;183:6236–43. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–43. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 34.Wang JY, Lin CG, Bey MS, et al. Discovery of genetic difference between asthmatic children with high IgE level and normal IgE level by whole genome linkage disequilibrium mapping using 763 autosomal STR markers. J Human Genetics. 2005;50:249–58. doi: 10.1007/s10038-005-0248-6. [DOI] [PubMed] [Google Scholar]
- 35.Arisawa T, Tahara T, Shibata T, et al. The influence of polymorphisms of interleukin-17A and interleukin-17F genes on the susceptibility to ulcerative colitis. J Clin Immunol. 2008;28:44–9. doi: 10.1007/s10875-007-9125-8. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Liu Y, Cao D, Jiang M, Luo F. IL-17A and IL-17F polymorphisms and gastric cancer risk: a meta-analysis. Genet Mol Res. 2015;14:7008–17. doi: 10.4238/2015.June.26.10. [DOI] [PubMed] [Google Scholar]
- 37.Espinoza JL, Takami A, Nakata K, et al. A genetic variant in the IL-17 promoter is functionally associated with acute graft-versus-host disease after unrelated bone marrow transplantation. PloS One. 2011;6:e26229. doi: 10.1371/journal.pone.0026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cookson W. The alliance of genes and environment in asthma and allergy. Nature. 1999;402:B5–11. doi: 10.1038/35037002. [DOI] [PubMed] [Google Scholar]
- 39.Wills-Karp M, Ewart SL. Time to draw breath: asthma-susceptibility genes are identified. Nat Rev Genet. 2004;5:376–87. doi: 10.1038/nrg1326. [DOI] [PubMed] [Google Scholar]
- 40.Stelmach I, Majak P, Jerzyńska J, Bojo M, Cichalewski Ł, Smejda K. Children with severe asthma can start allergen immunotherapy after controlling asthma with omalizumab: a case series from Poland. Arch Med Sci. 2015;11:901–4. doi: 10.5114/aoms.2015.48546. [DOI] [PMC free article] [PubMed] [Google Scholar]