Abstract
Introduction
The aim of the study was to confirm whether higher levels of urinary neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1) and N-acetyl-β-D-glucosaminidase (NAG) are associated with mortality risk scores in severe septic patients with acute kidney injury (AKI).
Material and methods
A prospective observational study was performed in an adult critical care unit. A total of 135 patients were included. The levels of urinary NGAL, KIM-1 and NAG were compared between patients with acute physiology and chronic health evaluation (APACHE II) score > 25 (group A, n = 31) and APACHE II score ≤ 25 (group B, n = 104).
Results
Median level of NGAL was 105.1 ng/ml (77.6–132.5) in group A versus 40.0 ng/ml (18.6–60.5) in group B (p < 0.001), KIM-1 was 16.2 ng/ml (10.2–22.3) versus 3.3 ng/ml (1.8–4.6) (p < 0.001), and NAG was 32.0 U/l (17.5–46.4) versus 15.0 U/l (7.7–22.3) (p < 0.001). The area under the receiver operating characteristic curve for NGAL was 0.70 (95% CI: 0.60–0.79), KIM-1 was 0.75 (95% CI: 0.66–0.83), and NAG was 0.69 (95% CI: 0.60–0.79). A NGAL level > 102.5 ng/ml had 95% sensitivity and 76% specificity, KIM-1 > 7.3 ng/ml had 96% sensitivity and 61% specificity, and NAG > 15.4 U/l had 86% sensitivity and 74% specificity.
Conclusions
In severe septic AKI patients, high levels of NGAL, KIM-1 and NAG are associated with mortality risk scores. Urinary NGAL, KIM-1 and NAG concentrations higher than 102.5 ng/ml, 7.3 ng/ml and 15.4 U/l respectively may be used to predict increased of death risk scores.
Keywords: sepsis, acute kidney injury, neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, N-acetyl-β-D-glucosaminidase
Introduction
To predict the prognosis of severe septic acute kidney injury (AKI) in the first 24 h after admission, acute physiology and chronic health evaluation II (APACHE II) is one of the most commonly used tools [1–3]. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) were used to confirm the diagnosis of septic AKI initially, and afterwards to help in classification of severity and guide treatment [4–7]. Studies have also shown that higher levels of NGAL and KIM-1 in adult patients might be used to predict an increased mortality risk [7–9].
Recently, new measurable biomarkers were used as additional tools for septic patients with AKI. N-acetyl-β-D-glucosaminidase (NAG) is included among these new biomarkers. It is a lysosomal enzyme located at the proximal tubule [10]. The increase of NAG was reported in diabetic nephropathy, chronic glomerular disease, delayed renal allograft function, and nephrotoxic drug exposure, and was also more sensitive to diagnose AKI in critically ill patients and preceded an increased level of serum creatinine by 12 h to 4 days. A study showed that higher levels of urinary NAG in patients with AKI were associated with higher probability of receiving hemodialysis and increased mortality risk [11]. At present, NAG concentrations assays are becoming available, and more studies have also shown that increased KIM-1 was correlated with severity of septic AKI [12].
So far, there have been limited available data for patients with severe septic AKI. Therefore, the primary outcome of this study was to investigate whether increased urinary NGAL, KIM-1 and NAG levels were associated with higher mortality risk scores in patients with severe septic AKI. For the secondary outcome of this study, we would also confirm whether higher urinary biomarker concentrations were associated with number of organ failures.
Material and methods
A prospective cohort study was performed in an adult intensive care unit (ICU) of a University Hospital (63-bed ICU of Ningbo First Hospital in China) from November 2013 to July 2016. The protocol of this study was approved by the Ethics Committee of Ningbo No. 1 Hospital, Ningbo University. Inclusion criteria: 1) > 18 years old; 2) admitted to adult ICU; 3) evidence of sepsis [13]. Exclusion criteria: patients with post-renal etiology, renal vasculitis, acute interstitial nephritis, suspected acute glomerulonephritis, chronic dialysis therapy, end-stage kidney disease, prior kidney transplant and having undergone renal transplantation. The following parameters were recorded on admission: gender, age, height, weight, medical history, cause(s) of admission, APACHE II score and admission diagnosis.
Study definition and groups divided
The AKI was defined on the basis of the classification scheme of RIFLE (Risk, injury, Failure, Loss and End-stage of Kidney function) [14]. The diagnosis and severity classification for included patients were according to high levels of serum creatinine (Scr) and decreased urinary output. Baseline Scr was defined as the lowest Scr of outpatients at 6 months preceding hospitalization. Sepsis was defined on the basis of consensus guidelines [13]. According to the mortality risk scores, included patients were divided into group A and group B: the higher mortality risk score group included patients with an APACHE II score > 25 (group A) [15]; the lower mortality risk score group included patients with an APACHE II score ≤ 25 (group B). Moreover, according to the number of organ failures (respiratory, neurological, hepatic, renal, hematological and cardiovascular) following consensus criteria [16], the patients were divided into group A1 and group B1: group A1 included the patients with the number of organ failures ≥ 2; group B1 with number of organ failures < 2.
NGAL, KIM-1 and NAG assay
All samples were collected and reserved in an identical way, and reviewed by two trained investigators. The time between collection and analysis was generally < 12 h. Urinary NGAL and NAG were measured by an enzyme-linked immunosorbent assay (ELISA) kit (Co. Ltd., Tokyo, Japan) following the manufacturer’s instructions. The levels of KIM-1 were determined using a commercially available ELISA kit (Co.USCN, Wuhan, China).
Statistical analysis
The clinical parameters were described by medians and ranges, percentages and frequencies. Groups A and B (A1 and B1) were compared by the Mann-Whitney U test for continuous variables and the exact χ2 test for categorical data. The receiver operating characteristic (ROC) curves and the areas under the curves (AUC) with 95% confidence intervals (CIs) were used to assess the accuracy of NGAL, KIM-1 and NAG to predict the outcomes. A cut-off (threshold) value was established by the Youden criterion. P-value < 0.05 was defined as statistically significant.
Results
Baseline characteristics
In the validation cohort, 135 patients were included in this prospective study (71 male and 64 female). More than half of patients were older than fifty years old. The main ICU admission causes of these patients were infection, respiratory and postoperative disease. A total of 12 (8.76%) patients died during the ICU hospitalization. The levels of urinary NGAL, KIM-1 and NAG were significantly higher in group A and group A1 (Table I).
Table I.
Demographic, clinical, and markers data
| Characteristics | Group A (n = 31) | Group B (n = 104) | P-value |
|---|---|---|---|
| Age (IQR) [years] | 57 (34–76) | 49 (35–79) | 0.048 |
| Male gender, n (%) | 19 (61.3) | 52 (49.1) | 0.331 |
| Weight [kg] | 65 (44–83) | 61 (40–92) | 0.126 |
| Admission diagnosis: | |||
| Postoperative | 6 (2.3) | 43 (58.5) | < 0.001 |
| Cardiac surgery | 3 (1.7) | 6 (9.6) | 0.126 |
| Respiratory | 13 (41.2) | 51 (71.3) | < 0.001 |
| Traumatic | 2 (1.3) | 11 (24.5) | < 0.001 |
| Renal | 20 (68.5) | 33 (38.6) | < 0.001 |
| Neurologic | 9 (26.1) | 31 (41.7) | < 0.001 |
| Others | 2 (1.2) | 17 (21.6) | < 0.001 |
| APACHE II | 30.9 (31.8) | 19.6 (20.3) | < 0.001 |
| Markers: | |||
| NGAL [ng/ml] | 108.9 (19.6) | 49.8 (4.9) | < 0.001 |
| KIM-1 [ng/ml] | 20.3 (3.7) | 13.0 (1.3) | < 0.001 |
| NAG [U/l] | 45.0 (8.1) | 32.5 (3.2) | 0.004 |
| Markers: | Group A1 (n = 46) | Group B1 (n = 89) | P-value |
| NGAL [ng/ml] | 65.2 (9.6) | 54.3(5.8) | 0.029 |
| KIM-1 [ng/ml] | 4.4 (0.6) | 3.37 (0.4) | 0.015 |
| NAG [U/l] | 25.0 (3.7) | 17.6 (1.9) | < 0.001 |
Group A – higher score risk mortality group, group B – lower score risk mortality group; group A1 – number of organ failures ≥ 2, group B1 – numbers of organ failures < 2; admission diagnosis is described by the absolute and relative values (%); the rest of the variables are described by median and interquartile range. NGAL – neutrophil gelatinase-associated lipocalin, KIM-1 – kidney injury molecule-1, NAG – N-acetyl-β-D-glucosaminidase.
Primary outcome
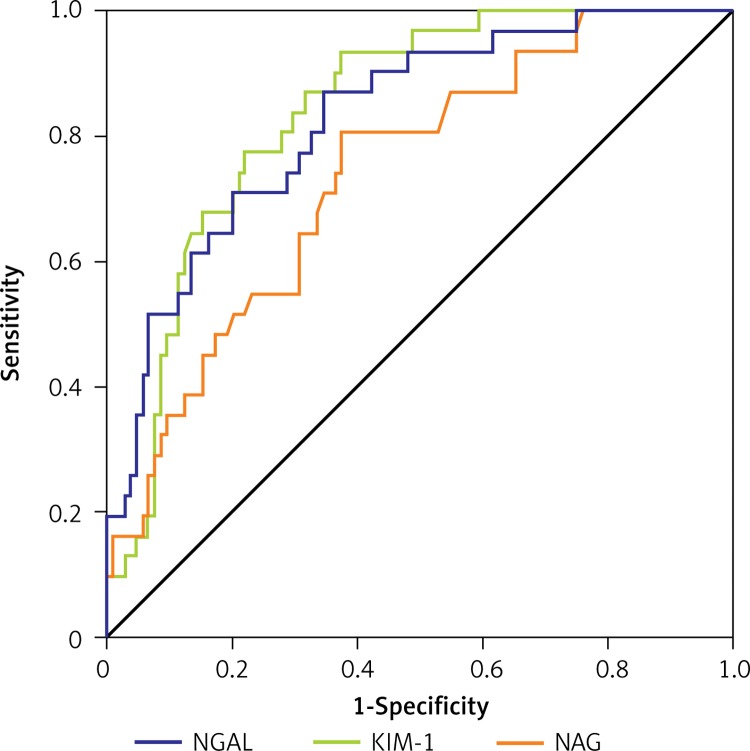
The ROC curve analysis was used to evaluate the values of NGAL, KIM-1 and NAG in prediction of mortality risk scores (Figure 1, Table II). The optimal cut-off value of NGAL in predicting the mortality risk scores was 102.5 ng/ml, and shows a 95% sensitivity and 76% specificity. The levels of KIM-1 higher than 7.3 ng/ml had 96% sensitivity and 61% specificity, and NAG concentration higher than 15.4 U/l had 86% sensitivity and 74% specificity.
Figure 1.
ROC curves for NGAL, KIM-1 and NAG in prediction of risk of mortality scores
NGAL – neutrophil gelatinase-associated lipocalin, KIM-1 – kidney injury molecule-1, NAG – N-acetyl-β-D-glucosaminidase.
Table II.
NGAL, KIM-1, and NAG measurements in prediction of mortality risk scores and organ failure
| Parameters | AUC (95% interval confidence) | |
|---|---|---|
| Risk mortality score | Organ failure (+1) | |
| NGAL | 0.695 (0.603–0.786) | 0.841 (0.764–0.917) |
| KIM-1 | 0.745 (0.661–0.829) | 0.839 (0.769–0.909) |
| NAG | 0.694 (0.597–0.791) | 0.739 (0.644–0.833) |
| Risk mortality score | ||
| With septic non-AKI | With septic AKI | |
| NGAL | 0.811 (0.688–0.933) | 0.839 (0.731–0.946) |
| KIM-1 | 0.951 (0.907–0.995) | 0.553 (0.379–0.727) |
| NAG | 0.707 (0.557–0.857) | 0.634 (0.472–0.796) |
| Organ failure (+1) | ||
| With septic non-AKI | With septic AKI | |
| NGAL | 0.669 (0.537–0.802) | 0.578 (0.420–0.737) |
| KIM-1 | 0.763 (0.649–0.877) | 0.620 (0.465–0.775) |
| NAG | 0.641 (0.498–0.785) | 0.659 (0.505–0.813) |
Results are presented for the whole sample and for subgroups of septic patients with and without AKI. ROC – curve analysis, NGAL – neutrophil gelatinase-associated lipocalin [ng/ml], KIM-1 – kidney injury molecule-1 [ng/ml], NAG – N-acetyl-β-D-glucosaminidase [U/l].
All the included patients were divided into septic AKI and septic non-AKI subgroups in the first 24 h after admission. A subgroup analysis of the mortality risk scores was also performed (Table II). In the septic AKI subgroup, the levels of NGAL, KIM-1 and NAG were higher than 203.8 ng/ml with 89% sensitivity and 75% specificity, 8.9 ng/ml with 89% sensitivity and 93% specificity, 28.9 U/l with 95% sensitivity and 81% specificity, respectively. In the septic non-AKI subgroup, the levels of NGAL, KIM-1 and NAG were higher than 102.5 ng/ml with a sensitivity of 91% and specificity of 63%, 5.1 ng/ml with 91% sensitivity and 76% specificity, 15.3 U/l with 83% sensitivity and 64% specificity, respectively.
Secondary outcome
The ROC curve analysis was used for each biomarker to predict the numbers of organ failures (Figure 2, Table II). The level of 103.1 ng/ml for NGAL gave 91% sensitivity and 72% specificity, the KIM-1 level of 6.2 ng/ml showed 91% sensitivity and 73% specificity, and NAG level of 13.4 U/l had 89% sensitivity and 81% specificity.
Figure 2.
ROC curves for NGAL, KIM-1 and NAG in prediction of number of organ failures
NGAL – neutrophil gelatinase-associated lipocalin, KIM-1 – kidney injury molecule-1, NAG – N-acetyl-β-D-glucosaminidase.
An analysis of septic AKI versus septic non-AKI subgroups was also performed to distinguish the numbers of organ failures (Table II). In the septic AKI subgroup, the levels of NGAL, KIM-1 and NAG were higher than 167.4 ng/ml with 80% sensitivity and 70% specificity, 10.2 ng/ml with 81% sensitivity and 77% specificity, 32.6 U/l with 85% sensitivity and 73% specificity, respectively. In the septic non-AKI subgroup, the levels of NGAL, KIM-1 and NAG were higher than 101.3 ng/ml with 80% sensitivity and 75% specificity, 5.6 ng/ml with 91% sensitivity and 76% specificity, 13.2 U/l with 85% sensitivity and 75% specificity, respectively.
Discussion
It is very important to improve the prognostic assessment for critical ill patients in the ICU. Biomarkers are usually used to classify the severity of these patients, and also help to make treatment plans. In our study, we found that high levels of NGAL, KIM-1 and NAG in urinary concentrations were associated with higher mortality risk scores and increased number of organ failures in patients with severe septic AKI. Because the numbers of ICU deaths in our sample were relatively low, mortality cannot be used as a gold standard to differentiate the prognosis of these patients. Therefore, we used the risk of mortality scores as an agent of ICU prognosis and the numbers of organ failures as the severity classification.
In this prospective study, we found that more than 80% of our samples had a high mortality risk and two or more organ failures. Patients with higher mortality risk scores were older. Increased levels of NGAL were indirectly associated with the severity of disease [4], and a cut-off value of 102.5 ng/ml for NGAL predicted the mortality risk scores with 95% sensitivity and 76% specificity. Further subgroups analysis results showed that NGAL was also a better biomarker of the mortality risk scores in the septic AKI subgroup (Table II), and the cut-off value higher than 203.8 ng/ml with sensitivity of 89% and specificity of 75%. To explain this increased cut-off value, we considered that increased urinary NGAL in septic AKI patients depends on both the severity of renal disease and inflammatory syndrome, whereas in septic non-AKI patients, increased NGAL might due to acute inflammation from trauma or shock. Therefore, the increased level of NGAL in septic AKI might be directly associated with the severity of the disease.
To date, few studies have used KIM-1 as a severity biomarker in adult septic AKI patients [17–19]. High levels of KIM-1 at admission in septic patients were correlated with the high mortality [6]. In our study, we found that KIM-1 could be used to determine septic AKI patients with different mortality risk scores. AUCs under the ROC (AU-ROC) curve range from 0.71 in the Torregrosa et al. [20] study to 0.80 in the study of Wasilewska et al. [8], and with a median of 0.79 in the Zhao et al. [21] study. We obtained a value of 0.83. Our KIM-1 cut-off value of 7.3 ng/ml was similar to the value of 7.6 ng/ml described to predict mortality in septic AKI patients [22] but higher than the value of 6.4 ng/ml obtained by Wasilewska et al. [8] in patients with septic AKI. The reason for this phenomenon might due to different races, specimen detection methods and selection of reagents. Thus, all of these values should be cautiously analyzed due to other factors that might increase the value of KIM-1 in septic patients.
The NAG is also a useful biomarker for the mortality risk with an AU-ROC curve similar to NGAL. Sinha et al. [23] reported a relation between high levels of NAG and mortality risk in patients with sepsis and septic shock. Park et al. [24] found that urinary NAG better distinguished patients with decreased renal function, and reported that the largest AU-ROC curve value was 0.794. Urinary NAG could be used to predict the number of organ failure and the risk of mortality in patient with autosomal dominant polycystic kidney disease. In our study, we found that the NGAL cut-off value of 103.1 ng/ml was similar to the previous studies’ mortality risk values, whereas the KIM-1 cut-off value of 6.2 ng/ml was lower and the NAG cut-off value of 13.4 U/l was similar.
Biomarkers’ additional value should be further evaluated in order to apply to clinical practice. The future study should focus on whether increased urinary NGAL, KIM-1 and NAG in patients with high mortality risk were due to increased biomarker secretion from failure of different organs. Our study still has several limitations. Firstly, this is a prospective observation study, and the sample size is relatively small. Secondly, our study does not involve any therapeutic interventions. Thirdly, this is a single-center study, group A and B (group A1 and B1) may have different diagnosis, and we could not completely rule out that these differences had influenced the level of biomarkers.
In conclusion, in patients with severe septic AKI, high levels of NGAL, KIM-1 and NAG are associated with the mortality risk scores. NGAL, KIM-1 and NAG urinary concentrations higher than 102.5 ng/ml, 7.3 ng/ml and 15.4 U/l respectively may be used to predict increased death risk scores.
Acknowledgments
Our study is supported by the “Medical science and technology fund of Zhejiang province (2017KY134, 2018KY667, 2018KY669, 2018KY685)”, “The clinical scientific research fund of Zhejiang medical association (2015ZYC-A54)”, the Ningbo Natural Science Fund (2017A610188) and the Zhejiang Natural Science Fund (LQ18H150001).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23:905–14. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–85. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 4.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Fan H, Zhao Y, Zhu JH, Song FC. Urine neutrophil gelatinase-associated lipocalin in septic patients with and without acute kidney injury. Ren Fail. 2014;36:1399–403. doi: 10.3109/0886022X.2014.945184. [DOI] [PubMed] [Google Scholar]
- 6.Fadel FI, Abdel Rahman AM, Mohamed MF, et al. Plasma neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury after cardio-pulmonary bypass in pediatric cardiac surgery. Arch Med Sci. 2012;8:250–5. doi: 10.5114/aoms.2012.28552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4:265–80. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasilewska A, Taranta-Janusz K, Dębek W, et al. KIM-1 and NGAL: new markers of obstructive nephropathy. Pediatr Nephrol. 2011;26:579–86. doi: 10.1007/s00467-011-1773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Q, Li HY, Li YM, et al. Compliance with severe sepsis bundles and its effect on patient outcomes of severe community-acquired pneumonia in a limited resources country. Arch Med Sci. 2014;10:970–8. doi: 10.5114/aoms.2014.46216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tucker SM, Pierce RJ, Price RG. Characterisation of human N-acetyl-beta-D-glucosaminidase isoenzymes as an indicator of tissue damage in disease. Clin Chim Acta. 1980;102:29–40. doi: 10.1016/0009-8981(80)90430-1. [DOI] [PubMed] [Google Scholar]
- 11.Fujita H, Narita T, Morii T, et al. Increased urinary excretion of N-acetylglucosaminidase in subjects with impaired glucose tolerance. Ren Fail. 2002;24:69–75. doi: 10.1081/jdi-120002662. [DOI] [PubMed] [Google Scholar]
- 12.Liangos O, Perianayagam MC, Vaidya VS, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–12. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 13.Lipińska-Gediga M, Mierzchała-Pasierb M, Durek G, et al. Procalcitonin kinetics – prognostic and diagnostic significance in septic patients. Arch Med Sci. 2016;12:112–9. doi: 10.5114/aoms.2016.57587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10:251–7. doi: 10.5114/aoms.2014.42577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 17.Ichimura T, Hung CC, Yang SA, et al. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 18.Vaidya VS, Ramirez V, Ichimura T, et al. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–29. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 19.Van Timmeren MM, van den Heuvel MC, Bailly V, et al. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–17. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 20.Torregrosa I, Montoliu C, Urios A, et al. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessels. 2015;30:703–11. doi: 10.1007/s00380-014-0538-z. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Vaidya VS, Brown RP, et al. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101:159–70. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinaseassociated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 23.Sinha V, Vence LM, Salahudeen AK. Urinary tubular proteinbased biomarkers in the rodent model of cisplatin nephrotoxicity: a comparative analysis of serum creatinine, renal histology, and urinary Kim-1, NGAL, and NAG in the initiation, maintenance, and recovery phases of acute kidney injury. J Investig Med. 2013;61:564–8. doi: 10.2310/JIM.0b013e31828233a8. [DOI] [PubMed] [Google Scholar]
- 24.Park HC, Hwang JH, Kang AY, et al. Urinary N-acetyl-β -D glucosaminidase as a surrogate marker for renal function in autosomal dominant polycystic kidney disease: 1 year prospective cohort study. BMC Nephrology. 2012;13:93. doi: 10.1186/1471-2369-13-93. [DOI] [PMC free article] [PubMed] [Google Scholar]