Abstract
Introduction
Colorectal cancer (CRC) is common, with a worldwide incidence estimated at more than 1 million cases annually. Therefore, the search for agents for CRC treatment is highly warranted. Inositol-6 phosphate (IP6) is enriched in rice bran and possesses many beneficial effects. In the present study the effect of IP6 on autophagy-mediated death by modulating the mTOR pathway in HT-29 colon cancer cells was studied.
Material and methods
Autophagy was assessed by acridine orange (AO) staining, transmission electron microscopy, and western blotting to detect LC3-II and Beclin 1. Akt/mTOR signaling protein expression was also analyzed by western blotting. Apoptosis was analyzed by annexin V staining.
Results
Incubation of cells with IP6 resulted in downregulation of the p-Akt at 3h. Along with that confocal microscopic analysis of p-AKT, IP6 administration resulted that a diminished expression of p-Akt. mTOR pathway regulates autophagy and incubation with IP6 to HT-29 cells showed decreased expression of p-70S6Kinase, 4-EBP-1 in a time-dependent manner. Inositol-6 phosphate (10 μg/ml, 24 and 48 h) induced autophagic vesicles, as confirmed by AO staining and transmission electron microscopy. We also found increased expression of LC3-II and Beclin 1 in a time-dependent manner after incubation with IP6. Furthermore, IP6 induced apoptosis, as revealed by annexin V staining.
Conclusions
Our results clearly indicate that IP6 induces autophagy by inhibiting the Akt/mTOR pathway.
Keywords: inositol-6-phosphate, colorectal cancer, autophagy, mammalian target of rapamycin, apoptosis
Introduction
Colorectal cancer (CRC) is common, with a worldwide incidence estimated at more than 1 million cases annually. Surgery remains the most effective curative treatment for CRC, but the risk of recurrence is high. Only 70% of cases are resectable, of which 75% are curable [1, 2]. Thus, many patients receive adjuvant chemotherapy. Therefore, the search for chemotherapeutic agents for the treatment of CRC is highly warranted [3, 4]. Many natural products can act as strong chemopreventive/therapeutic agents against CRC [5–8].
Inositol-6 phosphate (IP6) is a simple ringed carbohydrate with six phosphate groups attached to each carbon. It is a major form of phosphorylated inositol present in food, constituting 1–5% by weight of most cereals, nuts, oilseed, legumes, and grains. Inositol 6 phosphate, which occurs at 9.5–14.5% by weight in rice bran, possesses various health benefits [9]. Both in vivo and in vitro experiments provide convincing evidence for the anti-carcinogenic effects of IP6 [10]. Our own studies have shown that IP6 is effective against azoxymethane-induced CRC. It reduces the incidence of aberrant crypt foci and tumor formation [11]. It inhibits cell proliferation, arrests the cell cycle at the G2M phase, and induces apoptosis in hepatocellular carcinoma [12, 13].
Mammalian target of rapamycin (mTOR) is a vital signaling pathway involved in many cellular processes. It regulates many intracellular signaling molecules to provide energy resources to the cell. The involvement of mTOR signaling in CRC is well known. Similarly, the inhibition of mTOR signaling leads to the inhibition of CRC cell growth in vitro and adenoma formation in vivo [14–16]. Autophagy is a physiological process that allows the degradation of cytoplasmic contents, including unfolded proteins and membranous organelles, under certain stress conditions. This serves as a temporary survival mechanism [17]. mTOR1 is oncogenic and facilitates intracellular signaling for the translation of proteins required by the cell; it is also used by cancer cells for survival and growth. mTOR1 is a negative regulator of autophagy via binding to the Atg13-ULK1/2-FIP200 complex, phosphorylation of the complex components, and inhibition of autophagy. Nutrient deprivation results in the release of mTOR1 and dephosphorylation of ULK1/2 and Atg13 and hence facilitates the activation of autophagy [16]. Inositol 6 phosphate is considered a potent chemotherapeutic agent, and the mechanism of killing HT-29 human colon cancer cells is still not clear. We hypothesized that IP6 inhibits mTOR signaling, leading to the activation of autophagy-mediated death in HT-29 colon cancer cells.
Material and methods
Cell culture
The human colorectal cancer cell line HT-29 was purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and the cells were grown in Dulbecco’s Modified Eagle Medium with the following supplements: 10% (v/v) fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells were grown in a sterile cell culture flask at 37°C in the presence of 5% carbon dioxide (CO2).
Cell viability assay
Cells were seeded and cultured with various doses of IP6 (2.5–20 μg/ml) for 24 h. Culture media were replaced with 0.05% MTT solution and incubated at 37°C for 2 h. Dimethyl sulfoxide was added after the removal of the MTT solution. After a brief incubation period, the absorbance of the solution was measured using a microplate reader.
Immunofluorescence
Cells were grown to 60–70% confluence on glass coverslips in 6-well plates and treated with IP6 for 16 h. After treatment, cells were washed with phosphate-buffered saline (PBS), fixed with acetone:methanol (–20°C, 10 min), and blocked in 10% nonimmune goat serum (Sigma-Aldrich, St. Louis, MO, USA) for 1 h. LC3-II (NOVUS Biologicals, Littleton, CO, USA) antibody was applied (1 h), followed by incubation (1 h) with Alexa Fluor 594 F(ab’)2 fragment of goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA). Nuclei were stained with Hoechst. Then, the slides were assessed using a fluorescent microscope.
Transmission electron microscopy
Samples were prepared for transmission electron microscopy according to a published protocol [18]. Briefly, the cells were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 30 min, post-fixed in 1% osmium tetroxide in the same buffer for 30 min, dehydrated in graded ethanol, washed with propylene oxide, embedded in Epon, and then sectioned using an ultra-microtome at a thickness of 90 nm. Thin sections were stained with 5% uranyl acetate and 5% lead citrate and then examined using a transmission electron microscope at 75 kV.
Autophagy detection
Autophagy is a lysosomal degradation pathway for cytoplasmic material. The acidic intracellular compartments were visualized by acridine orange (AO) staining. After incubation, cells were washed with PBS and stained with 1 mg/ml AO for 15 min at 37ºC. Subsequently, cells were washed and analyzed under an inverted fluorescent microscope. Fluorescence microscopy was performed using 490-nm band-pass blue excitation filters and a 515-nm long-pass barrier filter. Owing to differences in acidity, autophagic lysosomes appeared as orange/red fluorescent cytoplasmic vesicles, while the cytoplasm and nucleolus were green.
Determination of apoptosis by annexin V-FITC
HT-29 colon cancer cells were seeded in 96-well plates at 2 × 105 cells per well for 16 h, treated with 10 μg/ml IP6 or control, and incubated at 37°C for 24 and 48 h. The harvested cells were labeled with a mixture of 1 : 1 ratio of annexin V-FITC (20 μg/ml) and propidium iodide (50 μg/ml) at 37°C for 30 min in darkness. Then, the cells were analyzed for the expression of annexin V, using a high-throughput screening machine (Thermo Scientific, Waltham, MA, USA).
Western blot analysis
Western blot analysis was performed to detect mTOR pathway components (p70-S6K, Total p-70-S6 Kinase, p-4EBP1, and Total-4-EBP1) and autophagy markers (Beclin 1 and LC3-II) [19]. Cells were lysed in ice-cold, whole-cell lysis buffer. For cytoplasmic and nuclear extraction, cells were lysed in cytoplasmic lysis buffer and nuclei were pelleted and lysed in hypotonic buffer (30 min). The protein concentration was measured by the Bradford method. Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane and blocked in 4% nonfat milk with 0.3% Tween 20. Antigen-antibody complexes were visualized by chemiluminescence.
Transfection of LC-3 siRNA
The LC3 SiRNA Kit was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and used according to the instructions provided in the kit. HT-29 cells were seeded at a concentration of 2 × 105 cells 24 h prior to transfection in a 6-well plate. After 24 h, the medium was discarded and cells were washed three times with PBS. Fetal bovine serum-free medium with 5% penicillin/streptomycin was added to the cells. Three transfection complexes were prepared (resulting in final concentrations of LC3 siRNA/control siRNA of 0.5 μg/well) 10 min before they were applied to the medium. The cells were treated with 100 μl of a transfection complex. Cells were incubated for 6 h and supplemented with 1 ml of normal growth medium containing 2 times the normal concentration of serum and antibiotics (2× normal growth medium), without removing the transfection mixture. The cells were incubated for an additional 18–24 h and the medium was aspirated and replaced with fresh 1× normal growth medium. These cells were used to identify the cell viability and to analyze the expression of LC3-II. Each experimental condition was repeated in triplicate.
Statistical analysis
At least 3 independent biological replicates were performed for all experiments. For western blots, one representative blot is shown in the Figures. Quantification was performed from 3 independent experiments and the results are expressed as means ± SD. Statistical evaluations were performed by Student’s t-test for pairwise comparisons. P < 0.05 was considered statistically significant.
Results
Inositol-6 phosphate is cytotoxic to HT-29 colon cancer cells
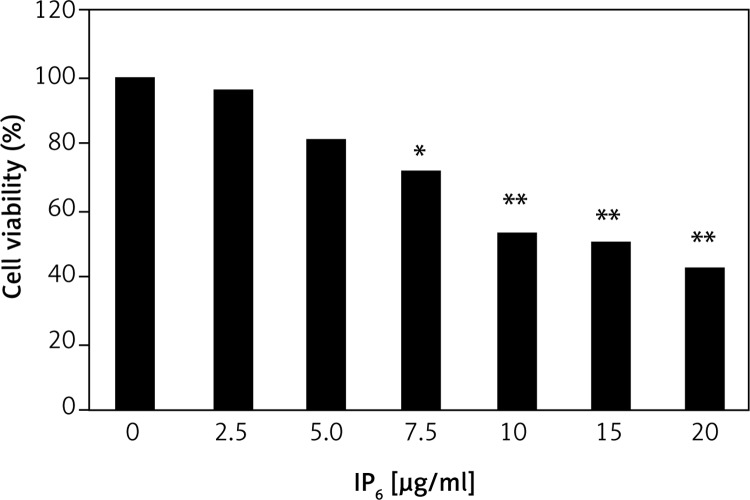
Cell viability was analyzed by MTT assay. HT-29 colon cancer cells were treated with IP6 at various concentrations, i.e., 0, 2.5, 5, 7.5, 10, 15, and 20 μg/ml, for 24 h. We found that the IC50 was 10 μg/ml (Figure 1). Accordingly, subsequent experiments were performed using a concentration of 10 μg/ml.
Figure 1.
Inositol-6 phosphate (IP6) is cytotoxic to HT-29 colon cancer cells. MTT cell viability assay. Inositol-6 phosphate showed IC50 at the dose of 10 μg/ml. The detailed methods are described in the Material and methods section
Statistical significance compared with the untreated group. The statistical significance at *p < 0.05 and **p < 0.01
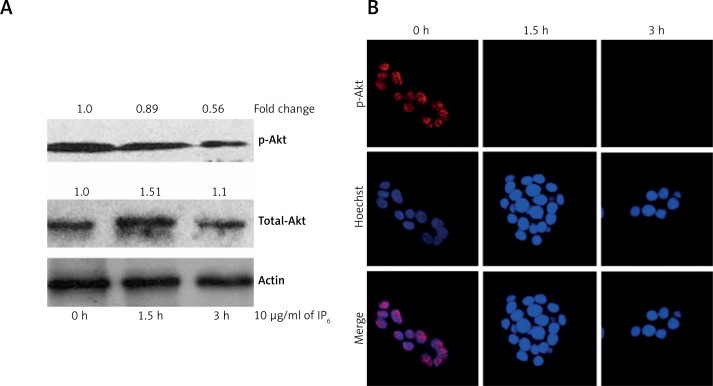
Inositol-6 phosphate inhibits Akt signaling in HT-29 colon cancer cells
The results of western blot analysis of Akt signaling are summarized in Figure 2. Administration of IP6 to HT-29 colon cancer cells at doses of 10 and 15 μg/ml for 3 h resulted in decreased expression of p-Akt compared to the control cells. We did not detect alterations in the expression of total-Akt. We further confirmed the expression of p-Akt in HT-29 colon cancer cells by immunofluorescence. We obtained results similar to those in the western blot analysis. Accordingly, we confirmed that IP6 inhibits the expression of p-Akt in HT-29 colon cancer cells.
Figure 2.
Inositol-6 phosphate (IP6) inhibits expression of p-Akt. A – Western blot analysis of p-Akt. Incubation of HT-29 cells with IP6 tends to suppress the expression of p-Akt in less than 3 h compared with control cells. The quantification of western blots using ImageJ software was illustrated as fold change. B – Immunofluorescent analysis of p-Akt. The detailed methodology is described in the Material and Methods section (60× magnification). Similar results were obtained as those in the western blot analysis
Statistical significance compared with the untreated group. The statistical significance at *p < 0.05 and **p < 0.01
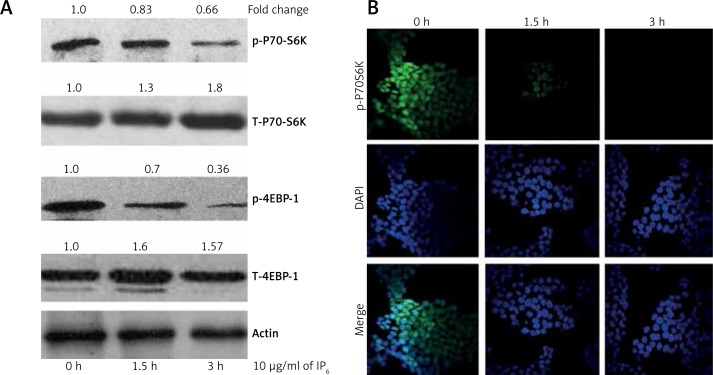
Inositol-6 phosphate inhibits mTOR signaling in HT-29 colon cancer cells
We next assessed the effect of IP6 on downstream targets of mTOR. It is well established that activated mTOR transmits its signal to activate P70-S6 kinase (S6K70) and negatively regulates eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1), which binds to and inhibits eukaryotic translation initiation factor (eIF) 4E [20]. Among several phosphorylation sites of P70-S6K, phosphorylation at T421/S424 is necessary for its activation and phosphorylation at T389 is required for full kinase function [21]. The activation of S6K70 was significantly decreased after IP6 treatment (Figure 3 A). The total P70-S6K and total 4EBP1 levels were unaltered in all groups. We thus found that IP6 significantly inhibits the mTOR pathway in HT-29 colon cancer cells.
Figure 3.
Inositol-6 phosphate (IP6) suppresses expression of downstream targets of mTOR signaling. A – Western blot analysis of p-Akt. Incubation of HT-29 cells with IP6 tends to suppress the expression of downstream targets of mTOR signaling, such as p70-S6K and p-4EBP1, in less than 3 h. We also noted that the total forms of both p70-S6K and 4EBP1 were unaltered by treatment with IP6. The quantification of western blots using ImageJ software was illustrated as fold change. B – Immunofluorescent analysis of p-p70S6K. The detailed methodology is described in the Material and methods section (40× magnification). Similar results were obtained as those in the western blot analysis
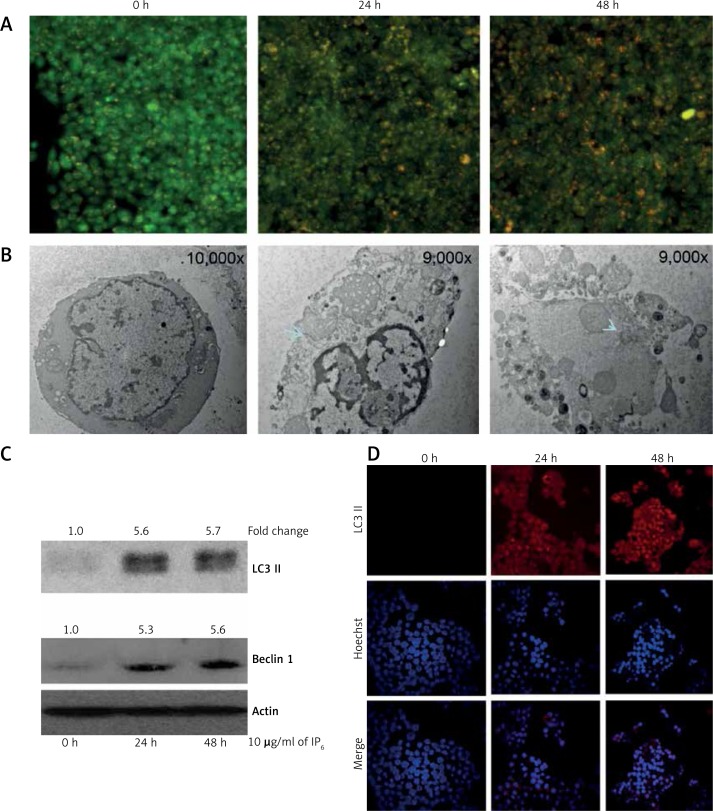
Inositol-6 phosphate induces autophagy in HT-29 colon cancer cells
We determined the effect of IP6 on the formation of acidic vesicular organelles in HT-29 cells by fluorescent microscopy after staining with AO. As shown in Figure 4 A, IP6 treatment increased AO staining in acidic vesicular organelles in HT-29 in a time-dependent manner. At 10 μg/ml IP6, the number of AO-stained cells increased dramatically when compared to that of control cells. When these IP6-treated cells were examined under a transmission electron microscope, double- or multi-membrane structures containing high-electron-density substances characteristic of autophagosomes and autolysosomes were present (Figure 3 B). Many autolysosomes were degraded in cells treated with 10 μg/ml IP6 for 24 and 48 h (Figure 3 B).
Inositol-6 phosphate activates LC3-II and Beclin 1 in HT-29 colon cancer cells
It is known that the Beclin 1 level and LC3 conversion (LC3-I to LC3-II) are selective markers of autophagy [22]. As shown in Figure 4 C, IP6 treatment markedly increased the expression of Beclin 1 and LC3-II, suggestive of autophagy induction. To further confirm the induction of autophagy by IP6, we utilized fluorescence microscopy to examine autophagosome formation in HT-29 cells. The conversion of LC3-I (a soluble form) to LC3-II (a lipidized form) is associated with the membranes of autophagosomes [23] and can be detected by the formation of punctate structures. Treatment of cells for 24 h and 48 h with IP6 resulted in increased expression of LC3-II in HT-29 colon cancer cells (Figure 4 D). These findings clearly indicated that IP6 induces autophagy by activating the expression of LC3-II in HT-29 colon cancer cells.
Figure 4.
Inositol-6 phosphate (IP6) induces autophagy in HT-29 cells. A – Autophagy is a lysosomal degradation pathway for cytoplasmic material. The acidic intracellular compartments were visualized by acridine orange (AO) staining. Cells were treated with 10 μg/ml IP6 for 24 and 48 h. After incubation, cells were washed with phosphate-buffered saline and stained with 1 mg/ml AO for 15 min at 37ºC. Subsequently, cells were washed and analyzed under an inverted fluorescent microscope. B – Transmission electron microscopic analysis of HT-29 cells after treatment with IP6. When IP6-treated cells were examined under a transmission electron microscope, doubleor multimembrane structures containing high-electron-density substances characteristic of autophagosomes and autolysosomes were present. Many autolysosomes were degraded in cells treated with 10 μg/ml IP6 for 24 and 48 h. C – Western blot analysis of LC3 and Beclin 1. Incubation of IP6 with HT-29 cells resulted in increased expression of LC3-II and Beclin 1. The quantification of western blots using ImageJ software was illustrated as fold change. D – Immunofluorescent analysis of LC3-II. The detailed methodology is described in the Material and methods section (40× magnification). Similar results were obtained as those observed in the western blot analysis
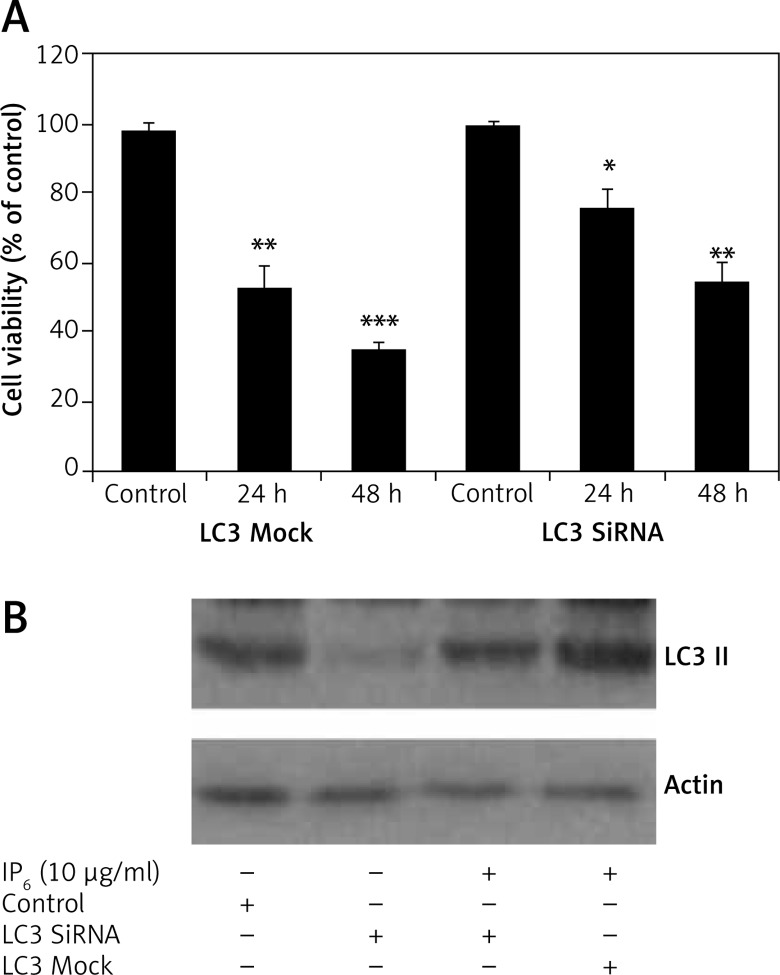
Inositol-6 phosphate-induced cell death is mediated by LC3-II
IP6-mediated cell viability was observed in HT-29 Mock (Mock SiRNA control) and siRNA-LC3 cells. LC3 silencing prevented IP6-induced HT-29 cell death (Figure 5 A). In HT-29 SiRNA mock cells, IP6-induced LC3A expression was blocked (Figure 5 B). These results confirmed that IP6-induced cell death in HT-29 was mediated by the activation of LC3-II.
Figure 5.
Inositol-6 phosphate (IP6)-induced cell death mediated by LC3-II. A – IP6-mediated cytotoxicity was analyzed in HT-29 siRNA-mock (siRNA control) and siRNA-LC3 cells by an MTT assay. B – The expression of LC3-II was analyzed in HT-29 siRNA mock and siRNA-LC3 cells by western blotting
The statistical significance at *p < 0.05, **p < 0.01 and ***p < 0.0001.
Inositol-6 phosphateinduces apoptosis in HT-29 colon cancer cells
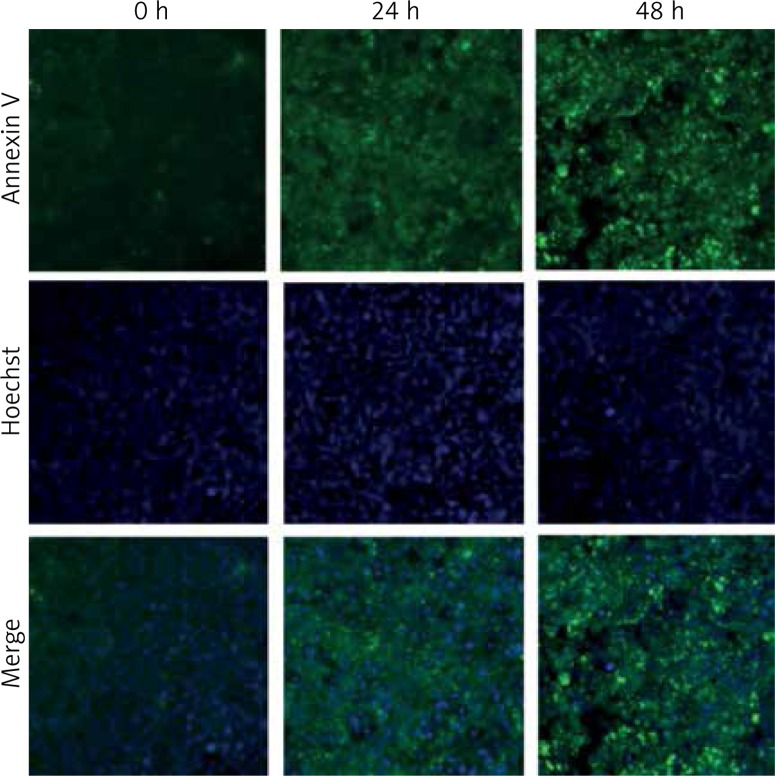
If a natural/synthetic drug source can induce apoptosis, it can be developed as a chemotherapeutic agent [5, 24, 25]. As shown in Figure 6, treatment with IP6 resulted in an increase in the cell population that was positive for annexin V-FITC staining. Starting at 24–48 h, HT-29 cells underwent apoptosis, as detected by the green fluorescence of annexin V-FITC when compared with that of control cells (Figure 6). Hence, we confirmed that IP6 induces apoptosis, as evidenced by the increased amount of exposed phosphatidylserine.
Figure 6.
Inositol-6 phosphate (IP6) increased the expression of annexin V-FITC. Cells were inoculated in a 96-well plate and treated with IP6 for 24 and 48 h. The detailed methodology is described in the Material and methods section. Inositol-6 phosphate increased the expression of annexin V in a time-dependent manner. Annexin V was attached to FITC (green) and the cells were counter-stained with Hoechst (blue) to detect nuclei (20× magnification)
Discussion
Abundant evidence indicates that autophagy plays a vital role in determining cell fate. When subjected to miscellaneous stresses, tumor cells initiate autophagy; nevertheless, the excessive activation of autophagy appears to result in cell death. In our study, we first observed that IP6 at a concentration of 2.5 to 20 μg/ml dose-dependently inhibits the viability of HT-29 colon cancer cells (Figure 1). It is well known that most chemotherapeutic agents are toxic not only to tumor cells, but also to normal cells; thus, these agents produce major side effects. The selective cytotoxicity of IP6 against colon cancer cells makes it an attractive candidate for drug development.
mTORC1 plays a key role in protein synthesis regulation via its effectors S6K1 and 4E-BP1. Constitutively activated mTOR signaling has been shown previously in CRC [26]. Indeed, several ribosomal proteins are up-regulated in CRC, including the S6K1 target S6 [27]. Targeted mTOR inhibition decreases adenoma formation in a mouse familial adenomatous polyposis model [28] and also inhibits CRC cell growth. It is also well known that the inhibition of mTOR signaling in CRC activates autophagy [16]. In the present study, we showed that IP6 inhibits downstream effectors of mTORC1, i.e., P70-S6K1 and 4E-BP1 (Figure 3). Deletion of P70-S6K1 in mice results in defective ribosomal biogenesis and the disruption of a single ribosomal protein shuts down ribosomal synthesis [29]. We confirmed that IP6 inhibits Akt/mTOR signaling in HT-29 colon cancer cells.
mTOR negatively regulates autophagy, and therefore we assessed the effects of IP6 on autophagy [29, 30]. Autophagy is a physiological process that allows the degradation of cytoplasmic contents, including unfolded proteins and membranous organelles, under certain stress conditions. This functions as a temporary survival mechanism for cells. Certain cellular stresses, such as oxidative stress, nutrient starvation, misfolded protein accumulation, and irradiation, could induce autophagy. Once initiated, the process delivers cytoplasmic materials to lysosomes via double membrane organelles termed autophagosomes, which enclose a portion of the cytoplasm and intracellular organelles [31]. However, recent studies have shown that autophagy is also a cell death mechanism and is a response to various anticancer therapies in many kinds of cancer cells [32, 33]. Our results showed that IP6 induces autophagy, as confirmed by the increased expression of LC3-II and Beclin 1 (Figure 4). LC3 is a commonly used autophagy marker, and its processed form, LC3-I, resides in the cytoplasm. After autophagy induction, LC3-II, the conjugated form of LC3, associates with autophagosomes. However, an increase in autophagosomes alone, suggested by increased LC3-II levels, does not necessarily indicate increased autophagy [34].
We also confirmed that IP6 induces apoptosis, based on the increased expression of annexin V (Figure 6). Apoptosis is characterized by cell shrinkage, chromatin condensation, DNA fragmentation, and the activation of specific cysteine proteases known as caspases. We previously showed that rice bran IP6 induces apoptosis by regulating pro- and anti-apoptotic Bax and Bcl-xl, as well as via the activation of caspase-3 and -8 [35, 36]. Inositol-6 phosphate can also induce mitochondrial-mediated apoptosis in HT-29 cells.
In conclusion, the Akt/mTOR pathway plays an essential role in the survival of colon cancer cells. We demonstrated that IP6 inhibits the Akt/mTOR signaling pathway in HT-29 cells. We previously showed that IP6 induces apoptosis in colon cancer cells. In this study, we further elucidated the mechanism by which IP6 causes autophagy, i.e., by activating the expression of LC3-II and Beclin 1. We therefore suggest that IP6 has the potential to be developed as an anti-cancer remedy.
Acknowledgments
Dr. Norhaizan greatly acknowledges the Fundamental Research Grant Scheme (FRGS), Malaysia (Grant Number: 5524455) for financial assistance. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this work (Research Group No. RG-1435-057).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Huerta S, Goulet EJ, Livingston EH. Colon cancer and apoptosis. Am J Surg. 2006;191:517–26. doi: 10.1016/j.amjsurg.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Sheng WY, Yong Z, Yun Z, Hong H, Hai LL. Toll-like receptor 4 gene polymorphisms and susceptibility to colorectal cancer: a meta-analysis and review. Arch Med Sci. 2015;11:699–707. doi: 10.5114/aoms.2015.53288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandurangan AK, Esa NM. Dietary non-nutritive factors in targeting of regulatory molecules in colorectal cancer: an update. Asian Pac J Cancer Prev. 2013;14:5543–52. doi: 10.7314/apjcp.2013.14.10.5543. [DOI] [PubMed] [Google Scholar]
- 4.Pandurangan AK, Dharmalingam P, Sadagopan SK, Ganapasam S. Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Hum Exp Toxicol. 2014;33:1176–85. doi: 10.1177/0960327114522502. [DOI] [PubMed] [Google Scholar]
- 5.Saadatdoust Z, Pandurangan AK, Ananda Sadagopan SK, Mohd Esa N, Ismail A, Mustafa MR. Dietary cocoa inhibits colitis associated cancer: a crucial involvement of the IL-6/STAT3 pathway. J Nutr Biochem. 2015;26:1547–58. doi: 10.1016/j.jnutbio.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Ananda Sadagopan SK, Mohebali N, Looi CY, et al. Forkhead Box Transcription Factor (FOXO3a) mediates the cytotoxic effect of vernodalin in vitro and inhibits the breast tumor growth in vivo. J Exp Clin Cancer Res. 2015;34:147. doi: 10.1186/s13046-015-0266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu D, Yao Q, Chen Y, Hu X, Qing C, Qiu M. The in vitro and in vivo antitumor activities of tetracyclic triterpenoids compounds actein and 26-deoxyactein isolated from rhizome of cimicifuga foetida L. Molecules. 2016;21:E1001. doi: 10.3390/molecules21081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai YJ, Tai CJ, Wang CW, et al. Anti-cancer activity of solanum nigrum (AESN) through suppression of mitochondrial function and epithelial-mesenchymal transition (EMT) in breast cancer cells. Molecules. 2016;21:E553. doi: 10.3390/molecules21050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jariwalla RJ. Rice-bran products: phytonutrients with potential applications in preventive and clinical medicine. Drugs Exp Clin Res. 2001;27:17–26. [PubMed] [Google Scholar]
- 10.Shamsuddin AM. Anti-cancer function of phytic acid. Int J Food Sci Technol. 2002;37:769–82. [Google Scholar]
- 11.Norazalina S, Norhaizan ME, Hairuszah I, Norashareena MS. Anticarcinogenic efficacy of phytic acid extracted from rice bran on azoxymethane-induced colon carcinogenesis in rats. Exp Toxicol Pathol. 2010;62:259–68. doi: 10.1016/j.etp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Nurul-Husna S, Norhaizan ME, Hairuszah I, Abdah MA, Norazalina S, Norsharina I. Rice bran phytic acid (IP6) induces growth inhibition, cell cycle arrest and apoptosis on human colorectal adenocarcinoma cells. J Med Plants Res. 2010;4:2283–9. [Google Scholar]
- 13.Norazalina S, Norhaizan ME, Hairuszah I, Sabariah AR, Husna SN, Norsharina I. Antiproliferation and apoptosis induction of phytic acid in hepatocellular carcinoma (HEPG 2) cell lines. Afr J Biotechnol. 2011;10:16646–53. [Google Scholar]
- 14.Wani ZA, Guru SK, Rao AV, et al. A novel quinazolinone chalcone derivative induces mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR signaling pathway in human colon cancer HCT-116 cells. Food Chem Toxicol. 2016;87:1–11. doi: 10.1016/j.fct.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Wee LH, Morad NA, Aan GJ, Makpol S, Wan Ngah WZ, Mohd Yusof YA. Mechanism of chemoprevention against colon cancer cells using combined gelam honey and ginger extract via mTOR and Wnt/beta-catenin pathways. Asian Pac J Cancer Prev. 2015;16:6549–56. doi: 10.7314/apjcp.2015.16.15.6549. [DOI] [PubMed] [Google Scholar]
- 16.Din FV, Valanciute A, Houde VP, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–15.e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin L, Baehrecke EH. Autophagy, cell death, and cancer. Mol Cell Oncol. 2015;2:e985913. doi: 10.4161/23723556.2014.985913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan WY, Huang H, Tam SC. Receptor-mediated endocytosis of trichosanthin in choriocarcinoma cells. Toxicology. 2003;186:191–203. doi: 10.1016/s0300-483x(02)00746-1. [DOI] [PubMed] [Google Scholar]
- 19.Pandurangan AK, Dharmalingam P, Sadagopan SK, Ramar M, Munusamy A, Ganapasam S. Luteolin induces growth arrest in colon cancer cells through involvement of Wnt/beta-catenin/GSK-3beta signaling. J Environ Pathol Toxicol Oncol. 2013;32:131–9. doi: 10.1615/jenvironpatholtoxicoloncol.2013007522. [DOI] [PubMed] [Google Scholar]
- 20.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 22.Towers CG, Thorburn A. Therapeutic targeting of autophagy. EBioMedicine. 2016;14:15–23. doi: 10.1016/j.ebiom.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Yu H, Shen Y, Ni X, Shen S, Das UN. Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch Med Sci. 2015;11:1081–94. doi: 10.5114/aoms.2015.54865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irani S, Shahmirani Z, Atyabi SM, Mirpoor S. Induction of growth arrest in colorectal cancer cells by cold plasma and gold nanoparticles. Arch Med Sci. 2015;11:1286–95. doi: 10.5114/aoms.2015.48221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai MD, Xu J. Ribosomal proteins and colorectal cancer. Curr Genomics. 2007;8:43–9. doi: 10.2174/138920207780076938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandurangan AK. Potential targets for prevention of colorectal cancer: a focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac J Cancer Prev. 2013;14:2201–5. doi: 10.7314/apjcp.2013.14.4.2201. [DOI] [PubMed] [Google Scholar]
- 28.Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci U S A. 2008;105:13544–9. doi: 10.1073/pnas.0800041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Din FV, Valanciute A, Houde VP, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–15.e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Hu G, Dong Y, et al. Matrine induces Akt/mTOR signalling inhibition-mediated autophagy and apoptosis in acute myeloid leukaemia cells. J Cell Mol Med. 2017;21:1171–81. doi: 10.1111/jcmm.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkhitko AA, Favorova OO, Henske EP. Autophagy: mechanisms, regulation, and its role in tumorigenesis. Biochemistry (Mosc) 2013;78:355–67. doi: 10.1134/S0006297913040044. [DOI] [PubMed] [Google Scholar]
- 32.Suh Y, Afaq F, Khan N, Johnson JJ, Khusro FH, Mukhtar H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis. 2010;31:1424–33. doi: 10.1093/carcin/bgq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y, Sung B, Kang YJ, et al. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int J Oncol. 2014;44:1599–606. doi: 10.3892/ijo.2014.2339. [DOI] [PubMed] [Google Scholar]
- 34.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafie NH, Esa NM, Ithnin H, Saad N, Pandurangan AK. Pro-apoptotic effect of rice bran inositol hexaphosphate (IP6) on HT-29 colorectal cancer cells. Int J Mol Sci. 2013;14:23545–58. doi: 10.3390/ijms141223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafie NH, Mohd Esa N, Ithnin H, Md Akim A, Saad N, Pandurangan AK. Preventive inositol hexaphosphate extracted from rice bran inhibits colorectal cancer through involvement of Wnt/beta-catenin and COX-2 pathways. Biomed Res Int. 2013;2013:681027. doi: 10.1155/2013/681027. [DOI] [PMC free article] [PubMed] [Google Scholar]