Abstract
Blood glucose homeostasis is well maintained by coordinated control of various hormones including insulin and glucagon as well as cytokines under normal conditions. However, chronic exposure to diabetic environment with high fat/high sugar diets and physical/mental stress can cause hyperglycemia, one of main characteristics of insulin resistance, metabolic syndrome, and diabetes. Hyperglycemia impairs organogenesis and induces organ abnormalities such as cardiac defect in utero. It is a risk factor for the development of metabolic diseases in adults. Resulting glucotoxicity affects peripheral tissues and vessels, causing pathological complications including diabetic neuropathy, nephropathy, vessel damage, and cardiovascular diseases. Moreover, chronic exposure to hyperglycemia can deteriorate cognitive function and other aspects of mental health. Recent reports have demonstrated that hyperglycemia is closely related to the development of cognitive impairment and dementia, suggesting that there may be a cause-effect relationship between hyperglycemia and dementia. With increasing interests in aging-related diseases and mental health, diabetes-related cognitive impairment is attracting great attention. It has been speculated that glucotoxicity can result in structural damage and functional impairment of brain cells and nerves, hemorrhage of cerebral blood vessel, and increased accumulation of amyloid beta. These are potential mechanisms underlying diabetes-related dementia. Nutrients and natural food components have been investigated as preventive and/or intervention strategy. Among candidate components, resveratrol, curcumin, and their analogues might be beneficial for the prevention of diabetes-related cognitive impairment. The purposes of this review are to discuss recent experimental evidence regarding diabetes and cognitive impairment and to suggest potential nutritional intervention strategies for the prevention and/or treatment of diabetes-related dementia.
Keywords: Cognitive function, Dementia, Diabetes Mellitus, Hyperglycemia, Resveratrol
INTRODUCTION
Glycemic status in human body is dynamic in response to internal and external stimuli in a sensitive way. It is well maintained within a normal range by antagonistic feedback regulation of insulin and glucagon. Hormonal regulation of glucose metabolism is also affected by cytokines, adiponectin, interleukin (IL)-1, IL-6, and tumor necrosis factor alpha [1,2,3]. Obesity, physical inactivity, smoking, excessive physical/mental stress, and unhealthy diet consisting of high sugar and high fat foods are risk factors for impaired glucose tolerance (IGT) and/or diabetes [4]. Hyperglycemia is a main characteristic of IGT or diabetes. It affects peripheral tissues and blood vessel, causing diabetic complications. Exposure to high glucose in utero impairs organogenesis of embryos and fetus, leading to organ abnormalities such as cardiac defects [5]. Glucotoxicity induces cell injury of hepatocytes and pancreatic cells through molecular mechanisms of endoplasmic reticulum stress, oxidative stress, and mitochondrial impairment [6,7]. Moreover, chronic exposure to hyperglycemia can deteriorate cognitive function [8]. Hyperglycemia-induced impairment of cognitive function is considered a brain complication of diabetes [9].
Prevalence of diabetes-related dementia has not been reported or estimated yet. However, it cannot be neglected. The prevalence of diabetes and Alzheimer's disease (AD) is getting higher [10,11,12]. There is accumulating evidence demonstrating that hyperglycemia is a potential risk factor for the development of mild cognitive impairment (MCI) or AD [13,14,15,16]. Hyperglycemia increases amyloid beta accumulation on brain lesions, exacerbates oxidative stress, neuroinflammation, and mitochondrial dysfunction, impairs neuronal integrity, and causes neurodegeneration [15,17,18,19,20]. These are potential working mechanisms underlying diabetes-related dementia. In addition to the identification of working mechanisms and major therapeutic targets, extensive efforts have also focused on suggesting nutritional strategies to prevent impairment of cognitive function induced by hyperglycemia. Among many suggested nutrients and functional food components, niacin, folate, vitamin B6, vitamin B12, resveratrol, and their analogues exert potentials to prevent or treat hyperglycemia, diabetes, MCI and AD [21,22,23,24].
In this review, we will discuss current evidence regarding diabetes and cognitive impairment and demonstrate potential working mechanisms and therapeutic targets. We will also summarize the role of nutrients and functional food components that are beneficially associated with the prevention or treatment of diabetes-related dementia. Finally, nutritional intervention strategies for preventing or delaying diabetes-related cognitive impairment are suggested.
HYPERGLYCEMIA AND COGNITIVE FUNCTION
Hyperglycemia is a main characteristic of diabetes. It is a risk factor for the development of diabetic complications. Although several preventive strategies against diabetes have been developed based on better understanding of etiologies of diabetes and related health-promoting policies, the prevalence of diabetes is increasing. It has been estimated that its prevalence could reach more than 10% of world population by 2025 [25]. Diabetic conditions, especially, hyperglycemia, affect blood vessels, bone, and nerve. As people live longer, aging-related diseases become more prevalent. In addition to diabetes and cardiovascular diseases, the possibility of having neurodegenerative diseases is also increasing. Dementia is a disease that cognitive function is significantly impaired, subsequently making it difficult to maintain daily life for an individual. Globally, 46.8 million people are affected by dementia. The number of people with dementia is estimated to increase 1.6-fold by 2030 and 2.8-fold by 2050 worldwide [26]. One paper has demonstrated the decreasing occurrence of dementia over time in fourteen population-based studies and explained that the decreasing prevalence and incidence of dementia is possibly due to improvements in living conditions, better access to education, and improved healthcare systems [27].
Diabetic subjects have increased risk for developing dementia in later life [28,29,30,31]. Because diabetes and dementia have long-term progression in general, long-term follow-up examination is needed to investigate the association between diabetes and dementia. So far, no estimate of diabetes-related dementia is available. However, previous epidemiological analysis and systemic reviews have consistently demonstrated increased risk of dementia in diabetes. Prospective analysis of the Cardiovascular Health Study Cognition Study revealed that incidence of dementia was higher in subjects with diabetes (14.9%) than that in non-diabetic subjects (10.3%) during examination period between 1992 and 1999, with hazard ratio of 1.62 for AD in subjects with diabetes [28]. The increased risk for dementia was additive with the presence of APOE ε4 allele in diabetic subjects [28]. Another prospective analysis of Uppsala Longitudinal Study of Adult Men had median follow-up of about 32 years in 2,322 participants. It demonstrated that low insulin response was associated with a higher risk for AD, with hazard ratio of 1.31 [30]. In a systematic review to investigate the effect of type 2 diabetes on AD by analyzing 15 epidemiologic studies, the pooled adjusted risk ratio of analyzed studies was 1.57 (range, 0.83 to 2.45) [29]. These findings were derived from separate regional cohorts. Evidence on large-scale nationwide investigation is needed to estimate the prevalence and risk of diabetes-related dementia more precisely.
HYPERGLYCEMIA-INDUCED IMPAIRMENT OF COGNITIVE FUNCTION: MECHANISMS
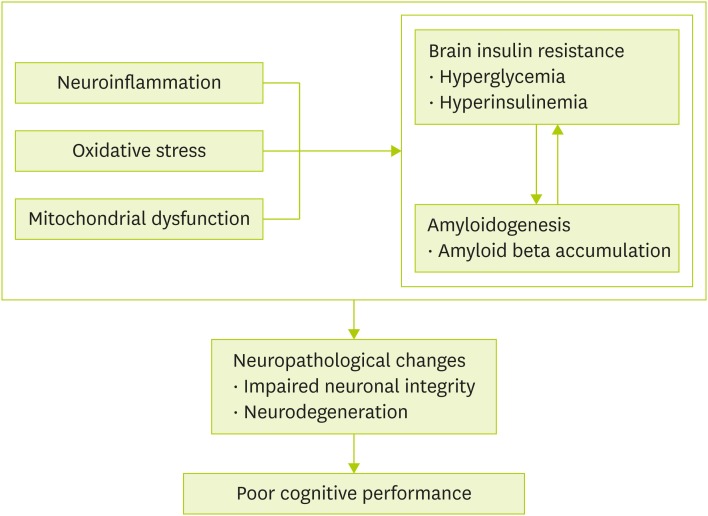
Etiology of diabetes and dementia is quite complex. Risk factors for these two diseases somewhat tend to overlap. Examples of overlapping mechanisms of diabetes and dementia are inflammation, oxidative stress, and mitochondrial dysfunction. Based on previous reports, we will summarize potential mechanisms underlying hyperglycemia-induced impairment of cognitive function (Figure 1). Among suggested mechanisms, brain insulin resistance and amyloidogenesis are central for hyperglycemia-induced impairment of cognitive function. Neuroinflammation, oxidative stress, and mitochondrial dysfunction are known to aggravate brain insulin resistance and amyloid beta accumulation in brain lesion. Prolonged exposure of hyperglycemia and hyperinsulinemia as well as high levels of amyloid beta in brain can lead to deterioration of neuronal structure and function, resulting in poor cognitive performance.
Figure 1. Suggested mechanisms underlying hyperglycemia-induced impairment of cognitive function. Brain insulin resistance and amyloidogenesis are considered as main factors for hyperglycemia-induced impairment of cognitive function, and affected by neuroinflammation, oxidative stress, and mitochondrial dysfunction. Chronic progression of these two main factors causes neuropathological changes disrupting neuronal integrity and function (neurodegeneration), which eventually leads to cognitive disability and dementia.
Brain insulin resistance
Insulin is a major polypeptide hormone to maintain glucose homeostasis by lowering increased blood glucose to normal range. It acts mainly on muscle and adipose tissue because glucose transporter 4 (GLUT4), an insulin-dependent transporter, is highly expressed on these tissues. Later, GLUT4 was found in brain, suggesting possible metabolic impact of brain GLUT4, although the most abundant glucose transporters in brain were insulin-independent transporters such as GLUT1 and GLUT3 [32]. Mice with neuron-specific deletion of insulin receptor had impaired glycemic response to hypoglycemia by inhibiting hypothalamic counter-regulatory response to low levels of blood glucose [33]. This demonstrates that insulin can act as a glucose sensor in hypothalamus involving brain glucose homeostasis. Another study using brain-specific GLUT4 knockout mice showed that GLUT4 deletion in brain caused glucose intolerance and hepatic insulin resistance with reduced glucose uptake in the brain [34]. Similar to mouse model with neuron-specific deletion of insulin receptor, brain-specific GLUT4 knockout mice also showed impaired glucose sensing and counter-regulatory responses to hypoglycemia by inhibiting epinephrine and glucagon responses in hypothalamic paraventricular nucleus [34].
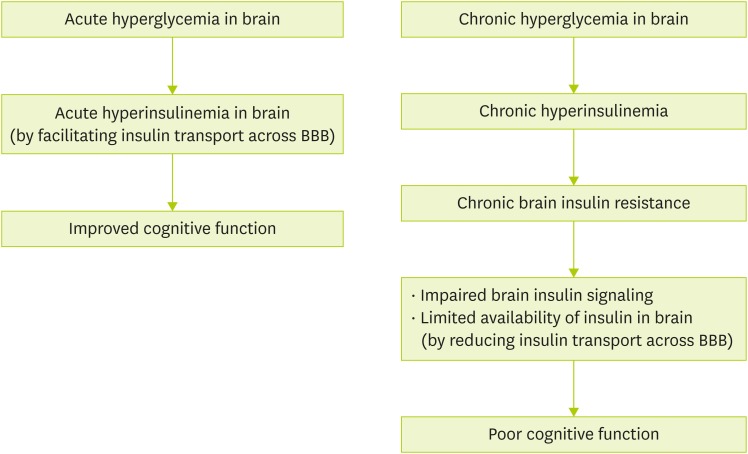
Brain insulin resistance is considered a pathophysiological factor for developing dementia [35]. Figure 2 demonstrates how acute/chronic hyperglycemia affects cognitive function. Acute hyperinsulinemia in response to increases in blood glucose facilitates insulin transport across blood-brain barrier into the brain [36,37]. Insulin receptor is present in hippocampus [38]. It enables insulin to be involved in memory and cognitive function because hippocampus is the main area that is responsible for memory. Intracerebroventricular or intravenous administration of insulin can improve spatial or verbal memory in rats and subjects with AD [39,40,41]. However, chronic hyperinsulinemia in brain insulin resistance leads to impaired insulin signaling and limits available insulin by reducing insulin transporters of blood-brain barrier [42]. Therefore, impaired insulin signaling and limited availability of insulin by chronic hyperinsulinemia are associated with impairment of cognitive performance and the development of neurodegenerative diseases [42]. Unallowed substances in normal condition can also get into the brain because blood-brain barrier transporter is disrupted under the condition of chronic brain insulin resistance [43].
Figure 2. Acute/chronic hyperglycemia and cognitive function. Acute hyperglycemia facilitates insulin transport into brain resulting in acute hyperinsulinemia. Presence of high levels of insulin for a short time improves spatial and verbal memory. On the other hand, chronic exposure to high blood glucose in brain induces chronic brain insulin resistance. Uncontrolled brain insulin resistance accompanied with impaired brain insulin signaling and limited availability of insulin may cause poor cognitive function.
BBB, blood-brain barrier.
Amyloidogenesis
Major pathological characteristics of AD are the presence of senile plaques and neurofibrillary tangles in the brain that cause neurodegeneration in AD [44]. Major components of senile plaques and neurofibrillary tangles are amyloid beta and tau, respectively. Amyloid beta is generated from amyloid precursor protein (APP) through cleavage by beta- and gamma-secretases. Presenilin 1 is a core component of gamma-secretase complex. Hyperphosphorylation of tau also stimulates its aggregation, forming neurofibrillary tangles.
High glucose condition increases amyloid beta production by inhibiting APP degradation, not by increasing APP synthesis in neuronal-like and non-neuronal cells [45]. Abnormal insulin signaling in brain insulin resistance increases amyloid beta accumulation and tau phosphorylation in rodent models of type 1 and type 2 diabetes [46,47,48,49,50,51,52,53,54]. Streptozotocin (STZ)-induced type 1 diabetes shows increased levels of amyloid beta, APP, and tau phosphorylation in hippocampus of senescence-accelerated mice [47]. In other transgenic mouse models of AD, STZ injection-induced type 1 diabetes can aggravate amyloid beta accumulation in brain accompanied by upregulation of beta-site APP cleaving enzyme 1 and full-length APP [48,50]. Similarly, STZ-induced diabetic rats show atrophy of hippocampus, amyloid beta aggregation, synapse loss in brain, and impaired performance of memory and learning [54].
High fat diet-induced type 2 diabetes can induce amyloidogenesis of amyloid beta 1–40 and 1–42 peptides in brain of a transgenic mouse model of AD [49]. High fat diet can also increase amyloid beta synthesis and tau phosphorylation and reduce synaptophysin immunoreactivity, resulting in impaired memory function in a mouse model of AD [52]. High sucrose intake is another dietary factor that can induce insulin resistance. For example, 10% sucrose-sweetened water can result in weight gain and insulin resistance in mice. They also demonstrate features of glucose intolerance and hyperinsulinemia. Mice treated with sucrose-sweetened water also showed more amyloid beta deposit in the brain with memory deficits [46].
NUTRITIONAL INTERVENTION FOR HYPERGLYCEMIA-INDUCED IMPAIRMENT OF COGNITIVE FUNCTION
It is generally considered that anti-diabetic strategy can protect against the development of diabetes-related dementia. This seems to be a reasonable concept. However, few strategies have been proved to be protective. The most successful nutritional component is resveratrol that has been shown to be effective in reducing the risk for diabetes-related dementia.
Resveratrol
Resveratrol is a brain-permeable stilbenoid found in grapes, blueberries, raspberries, and mulberries. It is well known to exert cardio-protective, anti-cancer, anti-inflammatory, and anti-oxidative activities [55]. Table 1 summarizes reports regarding effects of resveratrol on diabetes-related dementia in rodents and human. Recent evidence has demonstrated that the neuroprotective effects of resveratrol is by stimulating amyloid beta clearance and protecting the integrity of blood-brain barrier and neuronal structure [56,57,58]. Resveratrol treatment (10 and 20 mg/kg of body weights) for eight weeks can improve cognitive function assessed by Morris water maze test and increase acetylcholinesterase activity in STZ-injected diabetic rats [59]. Resveratrol-mediated improvement in cognitive performance is related to inhibition of oxidative stress, inflammation, and synaptic loss [59]. In STZ-injected diabetic rats, resveratrol administration for four weeks improved cognitive deficit assessed by Morris water maze test. It also restored resveratrol-mediated alterations of caspase-3, Bax, Bcl-2, N-Methyl-D-aspartate receptor, and brain derived neurotrophic factor in the hippocampus [60]. In similar STZ-treated diabetic rats, resveratrol decreased acetylcholinesterase activity in blood and prevented memory impairment by modulating cholinergic neurotransmission [61]. In a rat model of brain insulin resistance with intracerebroventricular injection of STZ (ICV-STZ), phosphorylation levels of tau and extracellular signal-regulated kinases 1 and 2 (ERK1/2) were increased while activity of sirtuin 1 was decreased [62]. Impaired cognitive capability shown as reduced capacity of spatial memory was also observed in ICV-STZ rats. Resveratrol treatment (30 mg/kg) for eight weeks reversed phosphorylation levels of tau and ERK1/2 and activity of sirtuin 1 in the hippocampus, and cognitive performance in ICV-STZ treated rats [62]. Evidence from clinical trials regarding resveratrol and diabetes-related dementia is limited. In a clinical trial of subjects with type 2 diabetes, a single dose of resveratrol (75, 150, or 300 mg) improved neurovascular coupling capacity and multi-tasking performance [63].
Table 1. The effects of RSV treatment on diabetes-related dementia.
| Ref. No. | Study design | Treatment | Main findings |
|---|---|---|---|
| Tian et al. [59] | Male Sprague-Dawley rats (10–12 weeks old, 200–250 g), single dose of 60 mg/kg STZ (n = 15/group) | RSV (10 mg, 20 mg/kg), 8 weeks | • Improve cognitive performance (Morris water maze test) |
| • Attenuate oxidative stress and inflammation | |||
| • Inhibit synapse loss | |||
| Tian et al. [60] | Male Sprague-Dawley rats (2 months old, 180–210 g), single dose of 60 mg/kg STZ (n = 15/group) | RSV (80 mg/kg), 4 weeks | • Improve cognitive deficit (Morris water maze test) |
| • Reverse alterations in the protein expression of caspase-3, Bax, Bcl-2, NMDAR1 and BDNF | |||
| Du et al. [62] | Male Sprague-Dawley rats (3 months old, 250 ± 20 g), ICV-STZ of 3 mg/kg, twice with an interval of 48 hours (n = 10/group) | RSV (30 mg/kg), 8 weeks | • Improve cognitive capability (Morris water maze test) |
| • Reverse alterations ERK1/2 phosphorylation, tau phosphorylation, sirtuin 1 activity in hippocampus | |||
| Schmatz et al. [61] | Male Wistar rats (70–90 days old, 250–270 g), single dose of 55 mg/kg STZ (n = 6–13/group) | RSV (10 mg/kg), 30 days | • Decrease acetylcholinesterase activity in blood and prevent memory impairment |
| Wong et al. [63] | Thirty-six type 2 diabetes adults (40–80 years old) | Single doses of RSV (0, 75, 150, and 300 mg) | • Improve neurovascular coupling capacity and multi-tasking performance |
RSV, resveratrol; STZ, streptozotocin; NMDAR1, N-Methyl-D-aspartate receptor; BDNF, brain-derived neurotrophic factor; ICV, intracerebroventricular; ERK1/2, extracellular signal-regulated kinases 1 and 2.
Curcumin and other nutritional factors
Curcumin is a main functional compound in Curcuma longa L. rhizomes (turmeric). Due to its anti-inflammatory and anti-oxidative activities, it is effective in improving insulin resistance and diabetes [64,65]. It can also cross the blood-brain barrier and reduce toxicity of amyloid beta aggregates in human neuronal SH-SY5Y cells [66]. Curcumin treatment (10 mg/kg of body weight) for 7 weeks has shown neuroprotective effects with improved performance of active avoidance and locomotor activity in ICV-STZ-induced diabetic rats [67]. Current evidence regarding the effects of curcumin on diabetes-related dementia in rodents was summarized in Table 2. In addition to curcumin, natural compounds such as palinurin, ginsenosides, and flavonoids have been found to be potentially effective for preventing diabetes-related dementia [68]. However, direct evidence that demonstrates their preventive or treatment effects on diabetes-related dementia is lacking.
Table 2. The effects of curcumin on diabetes-related dementia.
| Ref. No. | Study design | Treatment | Main findings |
|---|---|---|---|
| Maithilikarpagaselvi et al. [64] | Male Wistar rats (5 months old, 250–300 g), fructose (60% (w/w) feeding for 10 weeks (n = 10/group) | Curcumin (200 mg/kg), 10 weeks | • Attenuate insulin resistance by decreasing the activation of stress sensitive kinase (IRS-1) in skeletal muscle and inhibiting inflammatory cascades and oxidative stress |
| • No direct evidence on cognitive function | |||
| Naijil et al. [65] | Male Wistar rats (90–110 g), multiple low-dose STZ (n = 6–8/group) | Curcumin pre-treatment (7.5 mg/kg), 60 days | • Decrease α2-adrenergic receptor (sympathetic inhibition of insulin release) and increase β-adrenergic receptor (neuronal stimulation of hyperglycemia-induced β-cell compensatory response) in pancreas |
| • Up-regulate CREB, phospholipase C, insulin receptor, and glucose transporter 2 in pancreas | |||
| • No direct evidence on cognitive function | |||
| Huang et al. [67] | Rat, single dose of 3.0 mg/kg ICV-STZ and subcutaneous D-galactose daily (125 mg/kg) for 7 weeks | Curcumin (10 mg/kg), 7 weeks | • Decrease oxidative stress |
| • Improve the abilities of active avoidance and locomotor activity | |||
| • Attenuate neurodegeneration |
IRS-1, insulin receptor substrate-1; CREB, cyclic AMP response element-binding protein; ICV, intracerebroventricular; STZ, streptozotocin.
Moreover, Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) diet, and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet have been suggested to be effective for preventing dementia [69,70]. However, meaningful findings from clinical trials have not been reported yet regarding effects of these dietary patterns on diabetes-related dementia.
CONCLUSION
Cognitive impairment is often accompanied by progression of diabetes. The association between diabetes and dementia is supported by findings from several epidemiological studies. Based on previous epidemiological and experimental studies, diabetes-related dementia could be considered as type 3 diabetes [71,72]. Existing evidence utilizing rodent models of type 1 and type 2 diabetes have identified two main working mechanisms underlying diabetes-related dementia: brain insulin resistance and amyloidogenesis. Other mechanisms including neuroinflammation, oxidative stress, and mitochondrial dysfunction have also been suggested. As a result, neurodegeneration progresses in structure and functions of neurons, eventually causing impaired cognitive function. As main mechanisms and molecular targets of diabetes-related dementia have been identified, more efforts have been put on the development of interventional strategy to prevent diabetes-related dementia. Because research on dementia needs long-term intervention and extensive follow-up processes, few clinical trials have been registered to investigate nutritional intervention strategy for diabetes-related dementia. So far, antioxidants, phospholipids, omega-3 fatty acids, and polyphenols are known to be beneficial for brain function. Among these potential nutritional components, resveratrol is well identified to be able to improve cognitive function in diabetes. In addition to single nutrient supplementations, approaches of multi-components supplementations or healthy dietary patterns are also applied. In general, multi-components supplementations or dietary patterns rather than single nutritional components seem to be more effective for preventing chronic diseases. Therefore, clinical trials in a long-term and large-scale setting considering multiple dietary components or dietary patterns are needed. Once dementia is diagnosed and pathological changes become evident, it is difficult to reverse its progression. The most important goal for diabetes-related dementia is to identify its individual risk factors and prevent the occurrence of diabetes and dementia. Effective intervention strategies including nutritional approaches should be developed based on reliable scientific evidence.
Footnotes
Funding: This work was supported by a research grants of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03030617) and Seoul Women's University (2018).
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18:1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fève B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 3.Akash MS, Rehman K, Liaqat A. Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2018;119:105–110. doi: 10.1002/jcb.26174. [DOI] [PubMed] [Google Scholar]
- 4.Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017:CD003054. doi: 10.1002/14651858.CD003054.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawson TB, Scott-Drechsel DE, Chivukula VK, Rugonyi S, Thornburg KL, Hinds MT. Hyperglycemia Alters the Structure and Hemodynamics of the Developing Embryonic Heart. J Cardiovasc Dev Dis. 2018;5:E13. doi: 10.3390/jcdd5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049–1061. doi: 10.1016/j.metabol.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EM, Lee YE, Lee E, Ryu GR, Ko SH, Moon SD, Song KH, Ahn YB. Protective effect of heme oxygenase-1 on high glucose-induced pancreatic β-cell injury. Diabetes Metab J. 2011;35:469–479. doi: 10.4093/dmj.2011.35.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overman MJ, Pendleton N, O'Neill TW, Bartfai G, Casanueva FF, Forti G, Rastrelli G, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Lean ME, Punab M, Lee DM, Correa ES, Ahern T, Laurent MR, Verschueren SM, Antonio L, Gielen E, Rutter MK, Vanderschueren D, Wu FC, Tournoy J EMAS study group. Glycemia but not the metabolic syndrome is associated with cognitive decline: findings from the European Male Ageing Study. Am J Geriatr Psychiatry. 2017;25:662–671. doi: 10.1016/j.jagp.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Dolan C, Glynn R, Griffin S, Conroy C, Loftus C, Wiehe PC, Healy ML, Lawlor B. Brain complications of diabetes mellitus: a cross-sectional study of awareness among individuals with diabetes and the general population in Ireland. Diabet Med. 2018;35:871–879. doi: 10.1111/dme.13639. [DOI] [PubMed] [Google Scholar]
- 10.Wang CY, Neil DL, Home P. 2020 vision - an overview of prospects for diabetes management and prevention in the next decade. Diabetes Res Clin Pract. 2018;143:101–112. doi: 10.1016/j.diabres.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer's disease in the United States. Alzheimers Dement. 2018;14:121–129. doi: 10.1016/j.jalz.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weuve J, Hebert LE, Scherr PA, Evans DA. Prevalence of Alzheimer disease in US states. Epidemiology. 2015;26:e4–e6. doi: 10.1097/EDE.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 13.Rojas-Carranza CA, Bustos-Cruz RH, Pino-Pinzon CJ, Ariza-Marquez YV, Gomez-Bello RM, Canadas-Garre M. Diabetes-related neurological implications and pharmacogenomics. Curr Pharm Des. 2018;24:1695–1710. doi: 10.2174/1381612823666170317165350. [DOI] [PubMed] [Google Scholar]
- 14.Pruzin JJ, Nelson PT, Abner EL, Arvanitakis Z. Review: relationship of type 2 diabetes to human brain pathology. Neuropathol Appl Neurobiol. 2018;44:347–362. doi: 10.1111/nan.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaspar JM, Baptista FI, Macedo MP, Ambrósio AF. Inside the diabetic brain: role of different players involved in cognitive decline. ACS Chem Neurosci. 2016;7:131–142. doi: 10.1021/acschemneuro.5b00240. [DOI] [PubMed] [Google Scholar]
- 16.González-Reyes RE, Aliev G, Ávila-Rodrigues M, Barreto GE. Alterations in glucose metabolism on cognition: a possible link between diabetes and dementia. Curr Pharm Des. 2016;22:812–818. doi: 10.2174/1381612822666151209152013. [DOI] [PubMed] [Google Scholar]
- 17.Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, Mahan TE, Sutphen CL, Holtzman DM. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J Clin Invest. 2015;125:2463–2467. doi: 10.1172/JCI79742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DJ, Yu JH, Shin MS, Shin YW, Kim MS. Hyperglycemia reduces efficiency of brain networks in subjects with type 2 diabetes. PLoS One. 2016;11:e0157268. doi: 10.1371/journal.pone.0157268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rom S, Zuluaga-Ramirez V, Gajghate S, Seliga A, Winfield M, Heldt NA, Kolpakov MA, Bashkirova YV, Sabri AK, Persidsky Y. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-1195-5. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silzer TK, Phillips NR. Etiology of type 2 diabetes and Alzheimer's disease: exploring the mitochondria. Mitochondrion. 2018 doi: 10.1016/j.mito.2018.04.004. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 21.Reddy BR, Maitra S, Jhelum P, Kumar KP, Bagul PK, Kaur G, Banerjee SK, Kumar A, Chakravarty S. Sirtuin 1 and 7 mediate resveratrol-induced recovery from hyper-anxiety in high-fructose-fed prediabetic rats. J Biosci. 2016;41:407–417. doi: 10.1007/s12038-016-9627-8. [DOI] [PubMed] [Google Scholar]
- 22.Qin B, Xun P, Jacobs DR, Jr, Zhu N, Daviglus ML, Reis JP, Steffen LM, Van Horn L, Sidney S, He K. Intake of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr. 2017;106:1032–1040. doi: 10.3945/ajcn.117.157834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim EJ, Yang SJ. Nicotinamide reduces amyloid precursor protein and presenilin 1 in brain tissues of amyloid beta-tail vein injected mice. Clin Nutr Res. 2017;6:130–135. doi: 10.7762/cnr.2017.6.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawda C, Moussa C, Turner RS. Resveratrol for Alzheimer's disease. Ann N Y Acad Sci. 2017;1403:142–149. doi: 10.1111/nyas.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer report 2015. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International; 2015. [cited 2018 Sep 23]. Available from https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. [Google Scholar]
- 27.Wu YT, Beiser AS, Breteler MM, Fratiglioni L, Helmer C, Hendrie HC, Honda H, Ikram MA, Langa KM, Lobo A, Matthews FE, Ohara T, Pérès K, Qiu C, Seshadri S, Sjölund BM, Skoog I, Brayne C. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327–339. doi: 10.1038/nrneurol.2017.63. [DOI] [PubMed] [Google Scholar]
- 28.Irie F, Fitzpatrick AL, Lopez OL, Kuller LH, Peila R, Newman AB, Launer LJ. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE ε4: the Cardiovascular Health Study Cognition Study. Arch Neurol. 2008;65:89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer's disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35:152–160. doi: 10.1093/epirev/mxs012. [DOI] [PubMed] [Google Scholar]
- 30.Rönnemaa E, Zethelius B, Sundelöf J, Sundström J, Degerman-Gunnarsson M, Berne C, Lannfelt L, Kilander L. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology. 2008;71:1065–1071. doi: 10.1212/01.wnl.0000310646.32212.3a. [DOI] [PubMed] [Google Scholar]
- 31.Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 32.Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Res. 1998;797:1–11. doi: 10.1016/s0006-8993(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 33.Diggs-Andrews KA, Zhang X, Song Z, Daphna-Iken D, Routh VH, Fisher SJ. Brain insulin action regulates hypothalamic glucose sensing and the counterregulatory response to hypoglycemia. Diabetes. 2010;59:2271–2280. doi: 10.2337/db10-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reno CM, Puente EC, Sheng Z, Daphna-Iken D, Bree AJ, Routh VH, Kahn BB, Fisher SJ. Brain GLUT4 knockout mice have impaired glucose tolerance, decreased insulin sensitivity, and impaired hypoglycemic counterregulation. Diabetes. 2017;66:587–597. doi: 10.2337/db16-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al Haj Ahmad RM, Al-Domi HA. Thinking about brain insulin resistance. Diabetes Metab Syndr. 2018;12:1091–1094. doi: 10.1016/j.dsx.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- 37.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232–2243. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- 39.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 40.Haj-ali V, Mohaddes G, Babri SH. Intracerebroventricular insulin improves spatial learning and memory in male Wistar rats. Behav Neurosci. 2009;123:1309–1314. doi: 10.1037/a0017722. [DOI] [PubMed] [Google Scholar]
- 41.Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 42.Neth BJ, Craft S. Insulin resistance and Alzheimer's disease: bioenergetic linkages. Front Aging Neurosci. 2017;9:345. doi: 10.3389/fnagi.2017.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo DY, Yim HS, Jung HY, Nam SM, Kim JW, Choi JH, Seong JK, Yoon YS, Kim DW, Hwang IK. Chronic type 2 diabetes reduces the integrity of the blood-brain barrier by reducing tight junction proteins in the hippocampus. J Vet Med Sci. 2016;78:957–962. doi: 10.1292/jvms.15-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armstrong RA. The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer's disease. Folia Neuropathol. 2009;47:289–299. [PubMed] [Google Scholar]
- 45.Yang Y, Wu Y, Zhang S, Song W. High glucose promotes Aβ production by inhibiting APP degradation. PLoS One. 2013;8:e69824. doi: 10.1371/journal.pone.0069824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 47.Currais A, Prior M, Lo D, Jolivalt C, Schubert D, Maher P. Diabetes exacerbates amyloid and neurovascular pathology in aging-accelerated mice. Aging Cell. 2012;11:1017–1026. doi: 10.1111/acel.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devi L, Alldred MJ, Ginsberg SD, Ohno M. Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer's disease. PLoS One. 2012;7:e32792. doi: 10.1371/journal.pone.0032792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 50.Jolivalt CG, Hurford R, Lee CA, Dumaop W, Rockenstein E, Masliah E. Type 1 diabetes exaggerates features of Alzheimer's disease in APP transgenic mice. Exp Neurol. 2010;223:422–431. doi: 10.1016/j.expneurol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Liu H, Yang J, Liu X, Lu S, Wen T, Xie L, Wang G. Increased amyloid beta-peptide (1–40) level in brain of streptozotocin-induced diabetic rats. Neuroscience. 2008;153:796–802. doi: 10.1016/j.neuroscience.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Mehla J, Chauhan BC, Chauhan NB. Experimental induction of type 2 diabetes in aging-accelerated mice triggered Alzheimer-like pathology and memory deficits. J Alzheimers Dis. 2014;39:145–162. doi: 10.3233/JAD-131238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, Lefrançois D, Virgili J, Planel E, Giguere Y, Marette A, Calon F. Insulin reverses the high-fat diet-induced increase in brain Aβ and improves memory in an animal model of Alzheimer disease. Diabetes. 2014;63:4291–4301. doi: 10.2337/db14-0375. [DOI] [PubMed] [Google Scholar]
- 54.Wang JQ, Yin J, Song YF, Zhang L, Ren YX, Wang DG, Gao LP, Jing YH. Brain aging and AD-like pathology in streptozotocin-induced diabetic rats. J Diabetes Res. 2014;2014:796840. doi: 10.1155/2014/796840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuršvietienė L, Stanevičienė I, Mongirdienė A, Bernatonienė J. Multiplicity of effects and health benefits of resveratrol. Medicina (Kaunas) 2016;52:148–155. doi: 10.1016/j.medici.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 57.Zhao HF, Li N, Wang Q, Cheng XJ, Li XM, Liu TT. Resveratrol decreases the insoluble Aβ1-42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience. 2015;310:641–649. doi: 10.1016/j.neuroscience.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Hong JH, Lee H, Lee SR. Protective effect of resveratrol against neuronal damage following transient global cerebral ischemia in mice. J Nutr Biochem. 2016;27:146–152. doi: 10.1016/j.jnutbio.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 59.Tian X, Liu Y, Ren G, Yin L, Liang X, Geng T, Dang H, An R. Resveratrol limits diabetes-associated cognitive decline in rats by preventing oxidative stress and inflammation and modulating hippocampal structural synaptic plasticity. Brain Res. 2016;1650:1–9. doi: 10.1016/j.brainres.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 60.Tian Z, Wang J, Xu M, Wang Y, Zhang M, Zhou Y. Resveratrol improves cognitive impairment by regulating apoptosis and synaptic plasticity in streptozotocin-induced diabetic rats. Cell Physiol Biochem. 2016;40:1670–1677. doi: 10.1159/000453216. [DOI] [PubMed] [Google Scholar]
- 61.Schmatz R, Mazzanti CM, Spanevello R, Stefanello N, Gutierres J, Corrêa M, da Rosa MM, Rubin MA, Chitolina Schetinger MR, Morsch VM. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2009;610:42–48. doi: 10.1016/j.ejphar.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 62.Du LL, Xie JZ, Cheng XS, Li XH, Kong FL, Jiang X, Ma ZW, Wang JZ, Chen C, Zhou XW. Activation of sirtuin 1 attenuates cerebral ventricular streptozotocin-induced tau hyperphosphorylation and cognitive injuries in rat hippocampi. Age (Dordr) 2014;36:613–623. doi: 10.1007/s11357-013-9592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong RH, Raederstorff D, Howe PR. Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients. 2016;8:E425. doi: 10.3390/nu8070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Zachariah B. Curcumin prevents inflammatory response, oxidative stress and insulin resistance in high fructose fed male Wistar rats: potential role of serine kinases. Chem Biol Interact. 2016;244:187–194. doi: 10.1016/j.cbi.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 65.Naijil G, Anju TR, Jayanarayanan S, Paulose CS. Curcumin pretreatment mediates antidiabetogenesis via functional regulation of adrenergic receptor subtypes in the pancreas of multiple low-dose streptozotocin-induced diabetic rats. Nutr Res. 2015;35:823–833. doi: 10.1016/j.nutres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Thapa A, Jett SD, Chi EY. Curcumin attenuates amyloid-β aggregate toxicity and modulates amyloid-β aggregation pathway. ACS Chem Neurosci. 2016;7:56–68. doi: 10.1021/acschemneuro.5b00214. [DOI] [PubMed] [Google Scholar]
- 67.Huang HC, Zheng BW, Guo Y, Zhao J, Zhao JY, Ma XW, Jiang ZF. Antioxidative and neuroprotective effects of curcumin in an Alzheimer's disease rat model co-treated with intracerebroventricular streptozotocin and subcutaneous D-galactose. J Alzheimers Dis. 2016;52:899–911. doi: 10.3233/JAD-150872. [DOI] [PubMed] [Google Scholar]
- 68.de Matos AM, de Macedo MP, Rauter AP. Bridging type 2 diabetes and Alzheimer's disease: assembling the puzzle pieces in the quest for the molecules with therapeutic and preventive potential. Med Res Rev. 2018;38:261–324. doi: 10.1002/med.21440. [DOI] [PubMed] [Google Scholar]
- 69.Pistollato F, Iglesias RC, Ruiz R, Aparicio S, Crespo J, Lopez LD, Manna PP, Giampieri F, Battino M. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer's disease: a focus on human studies. Pharmacol Res. 2018;131:32–43. doi: 10.1016/j.phrs.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Marcason W. What are the components to the MIND diet? J Acad Nutr Diet. 2015;115:1744. doi: 10.1016/j.jand.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 71.Bae CS, Song J. The role of glucagon-like peptide 1 (GLP1) in type 3 diabetes: GLP-1 controls insulin resistance, neuroinflammation and neurogenesis in the brain. Int J Mol Sci. 2017;18:E2493. doi: 10.3390/ijms18112493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de la Monte SM, Tong M, Wands JR. The 20-year voyage aboard the journal of Alzheimer's disease: docking at ‘Type 3 Diabetes’, environmental/exposure factors, pathogenic mechanisms, and potential treatments. J Alzheimers Dis. 2018;62:1381–1390. doi: 10.3233/JAD-170829. [DOI] [PMC free article] [PubMed] [Google Scholar]