Abstract
Key points
Some cortical areas are believed to transmit a descending signal in association with motor intention and/or effort that regulates the cardiovascular system during exercise (termed central command). However, there was no evidence for the specific cortical area responding prior to arbitrary motor execution and in proportion to the motor effort.
Using a multichannel near‐infrared spectroscopy system, we found that the oxygenation of the dorsolateral and ventrolateral prefrontal cortices on the right side increases in a feedforward‐ and motor effort‐dependent manner during voluntary one‐armed cranking with the right arm.
This finding may suggest a role of the dorsolateral and ventrolateral prefrontal cortices in triggering off central command and may help us to understand impaired regulation of the cardiovascular system in association with lesion of the prefrontal cortex.
Abstract
Output from higher brain centres (termed central command) regulates the cardiovascular system during exercise in a feedforward‐ and motor effort‐dependent manner. This study aimed to determine a cortical area responding prior to arbitrarily started exercise and in proportion to the effort during exercise. The oxygenation responses in the frontal and frontoparietal areas during one‐armed cranking with the right arm were measured using multichannel near‐infrared spectroscopy, as indexes of regional blood flow responses, in 20 subjects. The intensity of voluntary exercise was 30% and 60% of the maximal voluntary effort (MVE). At the start period of both voluntary cranking tasks, the oxygenation increased (P < 0.05) only in the lateral and dorsal part of the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC) and sensorimotor cortices. Then, the oxygenation increased gradually in all cortical areas during cranking at 60% MVE, while oxygenation increased only in the frontoparietal area and some of the frontal area during cranking at 30% MVE. The rating of perceived exertion to the cranking tasks correlated (P < 0.05) with the oxygenation responses on the right side of the lateral‐DLPFC (r = 0.46) and VLPFC (r = 0.48) and the frontopolar areas (r = 0.47–0.49). Motor‐driven passive one‐armed cranking decreased the oxygenation in most cortical areas, except the contralateral frontoparietal areas. Accordingly, the lateral‐DLPFC and VLPFC on the right side would respond in a feedforward‐ and motor effort‐dependent manner during voluntary exercise with the right arm. Afferent inputs from mechanosensitive afferents may decrease the cortical oxygenation.
Keywords: central command, dorsolateral and ventrolateral prefrontal cortices, motor effort
Key points
Some cortical areas are believed to transmit a descending signal in association with motor intention and/or effort that regulates the cardiovascular system during exercise (termed central command). However, there was no evidence for the specific cortical area responding prior to arbitrary motor execution and in proportion to the motor effort.
Using a multichannel near‐infrared spectroscopy system, we found that the oxygenation of the dorsolateral and ventrolateral prefrontal cortices on the right side increases in a feedforward‐ and motor effort‐dependent manner during voluntary one‐armed cranking with the right arm.
This finding may suggest a role of the dorsolateral and ventrolateral prefrontal cortices in triggering off central command and may help us to understand impaired regulation of the cardiovascular system in association with lesion of the prefrontal cortex.
Introduction
Higher brain centres transmit a feedforward descending signal in association with motor intention independently of the actual exercise intensity and in proportion to motor effort (defined as one's perception of intensity during exercise), which may in turn regulate the cardiovascular system during exercise (Krogh and Lindhard, 1913; Gandevia et al. 1993; Green and Paterson, 2008; Williamson, 2010; Matsukawa, 2012). Such feedforward mechanism is termed central command. Sympathetic and cardiovascular adjustment occurs just before or at the onset of movement not only in conscious animals and humans but also in unanaesthetised ‘decorticate’ animals (Eldridge et al. 1981; Matsukawa et al. 1998; Kadowaki et al. 2011). This suggests that the caudal neural circuits are responsible for generating central command, but does not necessarily mean that a forebrain structure is unrelated to generation of central command. Some cortical areas, such as the anterior cingulate, insular and ventromedial prefrontal cortices, are considered as a candidate involved in the central command neurocircuits (King et al. 1999; Williamson et al. 2002; Nowak et al. 2005; Wong et al. 2007). For instance, the insular cortex has a projection to the lateral hypothalamus and could cause the cardiovascular adjustment (Yasui et al. 1991; Oppenheimer et al. 1992) and activation of the insular cortex occurs when increasing motor effort (Williamson et al. 2001). This background information leads us to the idea that a forebrain structure may transmit a signal for triggering the caudal neural circuits responsible for generating central command (Matsukawa, 2012). If so, a certain cortical area would respond in a feedforward‐ and motor effort‐dependent manner and might trigger off central command prior to and during exercise. However, such cortical area(s) are still unclear.
Our laboratory (Matsukawa et al. 2015; Asahara et al. 2016, 2018) found using near‐infrared spectroscopy (NIRS) that oxygenation in the prefrontal cortex (PFC) increases independently of the actual exercise intensity prior to the onset of arbitrarily started exercise, suggesting increased regional cerebral blood flow (CBF) following feedforward neural activation in the PFC. In addition, the PFC oxygenation increased during exercise in proportion to the force output or electromyogram (EMG) amplitude (Dai et al. 2001; Derosière et al. 2014), suggesting a relationship between PFC oxygenation and motor effort. These findings led us to expect that some cortical area within the PFC may trigger off central command in a feedforward‐ and motor effort‐dependent manner prior to and during exercise. The dorsolateral PFC (DLPFC) in primates has direct projections to caudal autonomic nuclei such as the locus coeruleus and raphe nuclei (Arnsten, 1997; Uylings et al. 2003). The DLPFC may be activated in association with central command, because Mizuguchi et al. (2014) found that the blood oxygenation level‐dependent (BOLD) signal in the DLPFC increased during motor imagery, which is supposed to simulate central control of the cardiovascular system during exercise. Thornton et al. (2001) found that regional CBF of the DLPFC, as well as the premotor and supplementary motor areas (PM‐SMA), increased more during imagery of cycling uphill (i.e. greater motor effort) as compared to imagery of cycling downhill (i.e. lesser motor effort), suggesting DLPFC activation in proportion to the motor effort. However, it remained unknown whether the DLPFC itself behaves in a feedforward‐ and motor effort‐dependent manner during actual exercise.
Along this line, we hypothesised that the DLPFC is one of the specific cortical area activated independently of the actual exercise intensity prior to arbitrary motor execution and responding in proportion to the motor effort (i.e. perceived intensity) during exercise. To test the hypothesis, we examined the oxygenation responses in the DLPFC and other PFC areas, as control, to voluntary one‐armed cranking at low and moderate intensities using a multichannel NIRS. The oxygenation responses in the PM‐SMA and sensorimotor areas were expected to increase in association with exercise intensity (Dai et al. 2001; Derosière et al. 2014). A partial correlation analysis was used to examine the relationship between the subjective degree of motor effort and the oxygenation responses after statistically reducing the effects of systemic haemodynamic changes on the oxygenation responses (Sakakibara et al. 2016). Furthermore, to isolate an influence of mechanical afferents by rhythmic arm movement on the cortical oxygenation, we examined the oxygenation responses of the frontal and frontoparietal areas during motor‐driven passive one‐armed cranking. A part of this study has been published in a preliminary form (Ishii et al. 2017 b).
Methods
Ethical approval
The experimental procedures and protocols conformed to the standards set by the Declaration of Helsinki (except for registration in a database) and were approved (reference number, 1017) by the Institutional Ethical Committee of Hiroshima University. The subjects gave their informed written consent prior to the experiments. Twenty‐two subjects (11 males and 11 females; age, 25 ± 8 years; height, 170 ± 8 cm; body weight, 61 ± 10 kg) participated in this study and were right‐handed as evaluated by the Edinburgh Handedness Inventory (Oldfield, 1971). None of the subjects suffered from any known cardiovascular and neuromuscular disease or took any medication. All experiments were performed in a soundproof room, in which temperature was maintained at 24–26°C.
One‐armed cranking exercise
One‐armed cranking exercise with the right arm was performed for 1 min at 60 rpm in the upright sitting posture on the seat of a specially designed cycle ergometer (Strength Ergo 240 BK‐ERG‐003, Mitsubishi Electric Engineering, Tokyo, Japan). The positions of the crank and seat were adjusted so that the subjects remained in a comfortable and certain posture. Torque against the wheel shaft and angular displacement of the ergometer crank were continuously measured. On a separate day prior to the main experiments, the subjects were familiarised to one‐armed cranking in the laboratory environment and performed an incremental one‐armed exercise test to determine the maximal voluntary effort (MVE) as previously reported (Ishii et al. 2017 a).
Measurements of cortical oxygenation
A 44‐channel NIRS system with 30 probes (FOIRE‐3000, Shimadzu Corp., Kyoto, Japan) was utilised to measure relative concentrations of oxygenated and deoxygenated haemoglobins (Oxy‐ and Deoxy‐Hb). A specially designed plastic helmet was held by adjustable screws and straps over the subject's scalp based on the Cz location. Then the probes, which consisted of 16 light emitters and 14 light detectors, were mounted on the plastic helmet so that they covered the frontal and frontoparietal surface of the head as shown in Fig. 1. The interprobe distance was 3 cm. When near‐infrared light with three different wavelengths (780, 805 and 830 nm) from emitter probes penetrated brain tissue, some of the light was absorbed by Hb and the remaining light scattered by brain tissue was picked up with neighbouring detector probes. The scattered near‐infrared light was measured at a sampling frequency of 7.7 Hz and converted to optical densities with a near‐infrared spectrometer (FOIRE‐3000). The absolute concentrations of the Oxy‐ and Deoxy‐Hb could not be obtained, because the path length of near‐infrared light within brain tissue was unknown. So, the relative changes of NIRS signals against the baseline values were assessed during each cranking intervention.
Figure 1. Anatomical probe and channel locations identified by three‐dimensional digitiser and transcranial magnetic stimulation.

A, anatomical location of the probes on the normalised brain surface. B, schema for anatomical channel location. The correspondence between colour‐filled channels and the cortical areas is as follows: the frontopolar area (Brodmann area (BA) 10) in blue; the lateral site of the dorsolateral prefrontal cortex (lateral‐DLPFC; BA 46) in red; the ventrolateral prefrontal cortex (i.e. the pars triangularis Broca's area, BA 45) in green; the dorsal site of the dorsolateral prefrontal cortex (dorsal‐DLPFC, BA 9) in yellow; the premotor and supplementary motor areas (PM‐SMA, BA 6) in dark blue; the primary motor cortex (BA 4) in wine red; and the somatosensory cortex (BA 1–3, 5 and 7) in orange. The data of NIRS channels in white were excluded from the data analysis because of low probability of any BA (channel 1, 4, 29 and 38) or low signal quality (channel 10 and 13). In 10 subjects, the anatomical locations of channel 23 and 26 were regarded as the arm area of the primary motor cortex, while those were regarded as the PM‐SMA in the other 10 subjects. C, representative recordings of motor evoked potentials (MEPs) of the extensor carpi radialis muscle caused by transcranial magnetic stimulation (TMS) in a subject. TMS was applied five times per site at the same intensity (×1.2 of the motor threshold) around an estimated primary motor cortex, where the greatest Oxy‐Hb response occurred during voluntary one‐armed cranking at 60% of the maximal voluntary effort. The MEPs were superimposed in each site. The MEPs were recorded at the estimated primary motor cortical site (channel 28) and the surrounding sites (1 cm apart from channel 28).
NIRS does not directly measure blood flow but provides information for a balance of oxygen supply and utilisation in the microcirculation within the illuminated tissue. Nevertheless, Hoshi et al. (2001) demonstrated that, in a perfused rat brain model, mechanically increasing blood flow always caused a rise in Oxy‐Hb and a reduction in Deoxy‐Hb, while decreasing blood flow was accompanied by a decrease in Oxy‐Hb with various changes in Deoxy‐Hb. Furubayashi et al. (2013) reported that transcranial magnetic stimulation (TMS) over the primary motor cortex increased Oxy‐Hb of the stimulated area within 3–6 s, suggesting increased oxygenation owing to an increase in CBF following cortical activation. Based on these findings, we regarded the Oxy‐Hb response as an index of regional tissue blood flow response as reported previously (Suzuki et al. 2004; Derosière et al. 2014; Matsukawa et al. 2015; Asahara et al. 2016; Ishii et al. 2017 b). To examine a possible contribution of extracranial skin blood flow to the Oxy‐Hb responses, a laser‐Doppler flow probe was placed on the right side of the forehead (i.e. near channel 9 as shown in Fig. 1) in eight subjects. Forehead skin blood flow was measured with a time constant of 0.1 s by a laser‐Doppler instrument (ALF21, Advance, Tokyo, Japan).
Cardiovascular and electromyogram recordings
An electrocardiogram (ECG) was monitored with a telemetry system (DynaScope DS‐3140, Fukuda Denshi, Tokyo, Japan). Arterial blood pressure (AP) was non‐invasively and continuously measured with a Finometer® (Finapres Medical Systems BV, Arnhem, the Netherlands), whose cuff was attached to the left middle or index finger. The AP waveform was sampled at a frequency of 200 Hz. The beat‐to‐beat values of mean AP (MAP) and heart rate (HR) were obtained throughout the experiments. Simultaneously, the beat‐to‐beat values of cardiac output (CO), stroke volume (SV) and total peripheral resistance (TPR) were calculated from aortic pressure waveform by using Modelflow® software (BeatScope 1.1, Finapres Medical Systems).
EMG activities of the four muscles of the right arm (the triceps and biceps brachii muscles and flexor and extensor carpi radialis muscles; FCR and ECR), contributing to arm cranking, were measured using a pair of silver‐bar electrodes attached on the muscle belly (Bagnoli‐4 EMG System, Delsys, Boston, MA, USA). The EMG signals were amplified (×1000) and passed through a bandpass filter between 20 and 2000 Hz.
Experimental protocols
All subjects performed two types of one‐armed cranking: voluntary and passive mode. To minimise an effect of movement artefact on the NIRS signals, the subjects were asked to prevent head movement, contraction of facial and masseter muscles, and excess eye blink as much as possible. In a voluntary cranking task, the exercise intensity was set at 30% and 60% of the MVE. Before performing each voluntary cranking task, the subjects were informed about a given exercise intensity. Then, the subjects were given a verbal instruction of ‘please start exercise whenever you want after you have calmed down and rested sufficiently’, and they then started exercise arbitrarily without any cue. In a passive cranking task, one‐armed cranking movement was driven by a motor of the ergometer without any verbal cue. To minimise the chance of anticipating the start of passive movement, the subjects were not informed of when the passive movement would start. The rating of perceived exertion (RPE), which is regarded as the degree of motor effort, was requested after each bout of exercise, according to the Borg 6–20 unit scale (Borg, 1970). The order of the three tasks was randomised.
Probe locations identified by TMS and three‐dimensional digitiser
At the end of the experiments, TMS was utilised to verify whether a putative primary motor area, estimated by the NIRS channel that accompanied the greatest Oxy‐Hb response during voluntary one‐armed cranking, actually projected to the contralateral arm muscles. The location of the estimated NIRS channel and the surrounding sites were stimulated five times at the same intensity (1.2× the motor threshold) using a figure‐of‐eight‐shaped coil (90 mm mean diameter) connecting to a magnetic stimulator (Magstim 200, Magstim, Whitland, UK). Motor evoked potentials (MEPs) of the contralateral muscles were recorded according to TMS on the putative primary motor area as exemplified in Fig. 1. In addition, the three‐dimensional locations of the NIRS probes on the skull were determined with a magnetic space digitiser (Fastrak, Polhemus, Colchester, VT, USA). Then, NIRS‐SPM software was used to localise the coordinates of the NIRS probes and channels on the Montreal Neurological Institute standard brain template and to estimate probabilistically brain regions for the coordinates (Singh et al. 2005; Ye et al. 2009). The anatomical labelling for the coordinates was obtained based on the Brodmann area (BA) obtained by the MRIcro programme (Rorden and Brett, 2000).
In this study, we determined the anatomical locations of the NIRS channels based on the following criteria: an estimated BA of which probability was over 60% was adopted as the anatomical location of a certain NIRS channel. Exceptionally, the anatomical locations of channel 28 and 30, in which TMS evoked MEPs, were regarded as the arm area of the primary motor cortex, even though the probability of the other BA was over 60%. In addition, since MEPs were recorded on channel 23 and 26 in 10 subjects, the anatomical locations of channel 23 and 26 were regarded as the arm area of the primary motor cortex in the subjects. Following the criteria, the NIRS channels were classified into seven cortical areas: the frontopolar area (BA 10), the dorsal and lateral parts of the DLPFC (dorsal‐DLPFC (BA 9) and lateral‐DLPFC (BA 46), respectively), ventrolateral PFC (VLPFC; i.e. pars triangularis Broca's area, BA45), PM‐SMA (BA 6), primary motor cortex (BA 4), and somatosensory cortex (including BA 1–3, 5 and 7) (Fig. 1). The data of the following channels were excluded from the data analysis, because of lower probability of any BA (channel 1, 4, 29 and 38) or low signal quality (channel 10 and 13).
Data analysis
The data of the developed torque and crank displacement of the ergometer, AP, ECG and EMG signals were stored to computers at a sampling frequency of 1000 Hz using A/D converters and accessary software (MP150, Biopack Systems, Santa Barbara, CA, USA and PowerLab 16/35, ADInstruments, Castle Hill, Australia) for off‐line analysis. The Oxy‐ and Deoxy‐Hb data and the crank displacement data were stored using the NIRS system and software. All data were aligned at the onset of crank displacement, which was defined as ‘time = 0’. The data of two female subjects were rejected from data analysis because their three‐dimensional locations of the NIRS probes on the skull were different from others due to a small head. In addition, their NIRS data in the frontopolar area and DLPFC involved great movement artefacts in association with eye blink. Thus the following data analysis was performed in 20 subjects (11 males and 9 females).
We manually excluded the NIRS data including movement artefacts according to a previous study (Koseki et al. 2013) and confirmed that the baseline noise level (defined by taking the standard deviation over the baseline) was similar to the level after adopting the noise removal technique reported by Gagnon et al. (2014). The baseline values were defined as the averages during the pretask resting period before each cranking intervention. In a voluntary cranking task, the 10 s period prior to the onset of exercise was excluded from the baseline. The cardiovascular and NIRS signals from the baseline values were sequentially averaged every 1 s. Since the subjects performed one to four trials in each type of cranking (voluntary one‐armed cranking at 30% and 60% MVE and passive one‐armed cranking), the data of multiple trials were averaged for each cranking type in a given subject. Then the NIRS signals of each channel were pooled in every cortical area.
Statistical analysis
The baseline values of the cardiovascular variables were compared among the three cranking interventions by a one‐way repeated measures ANOVA and Holm–Sidak post hoc test. We have reported that the prefrontal Oxy‐Hb increased from 5 s before the onset of voluntary cycling exercise independently of the actual exercise intensity (Matsukawa et al. 2015; Asahara et al. 2016). Taking into account the finding and a time delay of 3–6 s from the cortical activation to the Oxy‐Hb response (Furubayashi et al. 2013), the NIRS responses from −5 to 5 s from the onset of voluntary cranking (defined as the start period) are expected to reflect the feedforward cortical activation before and at the exercise onset. Based on this rationale, the significant NIRS and cardiovascular responses from the baseline were analysed at the start period of voluntary cranking by one‐way repeated measures ANOVA and the Holm–Sidak post hoc test to examine the hypothesis that the DLPFC is one of the specific cortical area activated independently of the actual exercise intensity prior to arbitrary motor execution.
To examine whether the EMG activities in the contracting muscles were augmented during exercise irrespective of the constant torque output, the EMG activities and torque output at the early (11–20 s) and late periods (51–60 s) of voluntary cranking were compared by using Student's paired t‐test. Augmentation of the EMG activities with a constant‐load exercise suggests effort‐related increases in the overall number, firing rate and synchronisation of the active motor units. To examine whether the DLPFC oxygenation increased with such EMG augmentation, the NIRS and cardiovascular responses at the early and late periods of voluntary cranking were analysed by one‐way repeated measures ANOVA and the Holm–Sidak post hoc test. Similar analysis was adopted in the case of passive cranking to examine an influence of mechanical afferents by rhythmic arm movement on the cortical oxygenation. The late Oxy‐Hb response of the primary motor and somatosensory cortices was compared among cranking interventions using one‐way repeated measures ANOVA and the Holm–Sidak post hoc test, to examine whether the Oxy‐Hb increased in association with the exercise intensity. If either normality or equal variance test failed in the one‐way ANOVA, a Friedman repeated measures ANOVA on ranks with the appropriate post hoc test (Dunnett's or Tukey's method) was performed.
To examine the hypothesis that the Oxy‐Hb response of the DLPFC during voluntary cranking was correlated with motor effort, the relationship between the RPE and the late Oxy‐Hb responses of both cranking tasks at 30% and 60% MVE was analysed using Pearson's correlation with a sample size of 40 (20 subjects × 2 exercise intensities). However, there is a possibility that an increase in RPE during exercise would cause increases in MAP and CO, resulting in an increase in cortical Oxy‐Hb (Ide et al. 2000; Ogoh et al. 2005; Ogoh and Ainslie, 2009). To examine the degree of relationship between the RPE and the late Oxy‐Hb responses after excluding the effects of MAP and CO on the Oxy‐Hb response, we conducted partial correlation analysis (with a sample size of 40) that measures the degree of a linear relationship between two variables while controlling for the effect of one or more other variables as used previously (Bohnen et al. 2009; Sakakibara et al. 2016). The partial correlation between the RPE and the Oxy‐Hb response is the correlation between the residuals (R RPE and R Oxy‐Hb) resulting from the linear regression of the RPE with MAP and CO and of the Oxy‐Hb with MAP and CO, respectively. The MAP‐ and CO‐derived components involved in the Oxy‐Hb response are reduced by the linear regression. Thus partial correlation was likely to reveal the actual relationship between the RPE (i.e. motor effort) and the Oxy‐Hb response better than Pearson's correlation did. The Pearson's and partial correlation analyses were followed by a Holm–Sidak method for multiple comparisons. The α level was set at 0.05. Most statistical analyses were conducted using SigmaPlot version 12.5 (Systat Software, San Jose, CA, USA), while partial correlation analysis was conducted using free software (PAleontological STatistics version 3.18, Hammer et al. 2001). All variables are expressed as means ± SD, unless otherwise stated.
Results
Torque output and EMG activity during voluntary one‐armed cranking
The torque output and EMG activity of the contracting arm muscles during voluntary one‐armed cranking are summarised in Fig. 2. The torque output was constant from the early to late period of cranking at both intensities. All EMG activities became greater (P < 0.05) during the late period of cranking at 60% MVE than during the early period, while such augmentation of the EMG activity was not evident in the FCR muscle in the cranking at 30% MVE. The RPE was greater (P < 0.05) in the cranking at 60% MVE than in the cranking at 30% MVE (Table 1).
Figure 2. The developed torque and electromyogram (EMG) activity of the contracting right arm muscles during voluntary one‐armed cranking at 30% (black lines) and 60% MVE (white lines) in 20 subjects.

Grey area indicates the cranking period; yellow areas indicate the early (11–20 s) and late (51–60 s) period of voluntary cranking. Each variable was sequentially calculated every 1 s. Values are means ± SD. *Significant difference (P < 0.05) from the value at the early period of cranking at 60% MVE. †Significant difference (P < 0.05) from the value at the early period of cranking at 30% MVE. Biceps, biceps brachii muscle; ECR, extensor carpi radialis muscle; FCR, flexor carpi radialis muscle; Triceps, triceps brachii muscle; VOL30%, voluntary one‐armed cranking at 30% MVE; VOL60%, voluntary one‐armed cranking at 60% MVE.
Table 1.
Baseline values of the cardiovascular variables and the rating of perceived exertion
| VOL 60% | VOL 30% | Passive | |
|---|---|---|---|
| HR (beats min−1) | 77 ± 13 | 78 ± 11 | 75 ± 12 |
| SV (mL) | 70 ± 13 | 71 ± 12 | 72 ± 13 |
| CO (L min−1) | 5.4 ± 1.3 | 5.5 ± 1.2 | 5.4 ± 1.2 |
| MAP (mmHg) | 103 ± 10* | 103 ± 9* | 97 ± 8 |
| TPR (mmHg L−1 min−1) | 21 ± 6* | 20 ± 6 | 19 ± 5 |
| RPE (Borg scale) | 14.3 ± 1.1*† | 11.4 ± 1.7* | 7.4 ± 1.8 |
∗Significant difference (P < 0.05) from baseline values of passive one‐armed cranking. †Significant difference (P < 0.05) between voluntary one‐armed cranking at 30% and 60% MVE. Values are means ± SD for 20 subjects. CO, cardiac output; HR, heart rate; MAP, mean arterial blood pressure; MVE, maximal voluntary effort; Passive, passive one‐armed cranking; RPE, rating of perceived exertion; SV, stroke volume; TPR, total peripheral resistance; VOL 30%, voluntary one‐armed cranking at 30% MVE; VOL 60%, voluntary one‐armed cranking at 60% MVE.
Cardiovascular responses during voluntary one‐armed cranking
The baseline HR, SV and CO were similar among the cranking tasks (Table 1), although baseline MAP and TPR were slightly different (P < 0.05) between voluntary and passive cranking tasks. Figure 3 represents the time courses of the average cardiovascular responses to voluntary one‐armed cranking. During cranking at either intensity, HR, CO and MAP increased and TPR decreased (P < 0.05), whereas SV did not change significantly. In transition from the early to late period of cranking at either intensity, TPR increased toward the baseline and MAP increased further. The late HR response became greater than the early HR response in the case of cranking at 60% MVE.
Figure 3. Time courses of the cardiovascular responses to voluntary one‐armed cranking at 30% (black lines) and 60% (white lines) MVE in 20 subjects.

Grey area indicates the cranking period; yellow areas indicate the early and late period of voluntary cranking. Each variable was sequentially calculated every 1 s. Values are means ± SD. *Significant difference (P < 0.05) from the baseline. †Significant difference (P < 0.05) from the value at the early period of cranking. CO, cardiac output; HR, heart rate; MAP, mean arterial blood pressure; SV, stroke volume; TPR, total peripheral resistance.
Cortical oxygenation responses during voluntary one‐armed cranking
Figure 4 demonstrates the time courses of the average Oxy‐Hb responses in all NIRS channels during voluntary one‐armed cranking. Figure 5 summarises the Oxy‐Hb responses, pooled into the seven cortical areas, at the start period (from −5 to 5 s) of voluntary cranking. In the case of cranking at 60% MVE, a significant rise in the Oxy‐Hb was found (P < 0.05) in all cortical areas except the middle frontopolar area and dorsal‐DLPFC and the bilateral PM‐SMA. In the case of cranking at 30% MVE, such an Oxy‐Hb increase occurred in the right dorsal‐DLPFC and both sides of the lateral‐DLPFC, VLPFC, and primary motor and somatosensory cortices. In contrast, significant changes of Deoxy‐Hb did not occur in all cortical areas in both cranking tasks, except the right frontopolar area and the dorsal‐DLPFC in the cranking at 30% MVE and the left VLPFC and the somatosensory cortices in the cranking at 60% MVE (data not shown).
Figure 4. Time courses of the oxygenated haemoglobin (Oxy‐Hb) responses in the frontal (A) and frontoparietal areas (B) during voluntary one‐armed cranking at 30% (black lines) and 60% MVE (white lines) in 20 subjects.

Grey areas indicate the cranking period. Horizontal scatter lines indicate the baseline level. The Oxy‐Hb was sequentially calculated every 1 s and expressed as mean without SD.
Figure 5. Time courses of the Oxy‐Hb responses in the frontal (A) and frontoparietal areas (B) at the start period of voluntary one‐armed cranking at 30% (black circles) and 60% MVE (white circles) in 20 subjects.

Grey areas indicate the cranking period. Horizontal scatter lines indicate the baseline level. The Oxy‐Hb responses from the baseline were analysed at the start period (from −5 to 5 s) of voluntary cranking by a one‐way repeated measures ANOVA and Holm–Sidak post hoc test. The Oxy‐Hb was expressed as mean ± SD within the start period and mean without SD outside the start period. *Significant difference (P < 0.05) from the baseline of cranking at 60% MVE. †Significant difference (P < 0.05) from the baseline of cranking at 30% MVE.
Figure 6 summarises the pooled Oxy‐Hb response at the early and late periods of voluntary one‐armed cranking. At the early period of cranking at 60% MVE, a significant increase in Oxy‐Hb was found (P < 0.05) in both sides of the frontopolar area, lateral‐DLPFC, VLPFC, and somatosensory cortices and the left primary motor cortex. Thereafter, the brain areas with increased Oxy‐Hb expanded to other cortical areas. The late Oxy‐Hb responses were greater in all cortical areas (except the right VLPFC) than the early Oxy‐Hb responses. At the early period of cranking at 30% MVE, the Oxy‐Hb did not change (P > 0.05) from the baseline in all cortical areas. At the late period of the cranking, the Oxy‐Hb increased (P < 0.05) in the frontoparietal area and some of the frontal area (the left frontopolar area, the right lateral‐DLPFC, and the middle dorsal‐DLPFC). The late Oxy‐Hb responses were greater than the early Oxy‐Hb responses in all cortical areas, except the right frontopolar area, the bilateral lateral‐DLPFC and VLPFC, and the right dorsal‐DLPFC. The Deoxy‐Hb changed slightly (by −0.0093 to 0.0085 mM cm) but significantly (P < 0.05) in the following areas: the bilateral frontopolar areas and lateral‐DLPFC, the left dorsal‐DLPFC, and the right PM‐SMA in the cranking at 30% MVE and, in addition to these areas, the middle frontopolar area and the left VLPFC and somatosensory cortex in the cranking at 60% MVE (data not shown).
Figure 6. The Oxy‐Hb responses in the frontal (A) and frontoparietal areas (B) at the early and late period of voluntary one‐armed cranking at 30% (black circles) and 60% MVE (white circles) in 20 subjects.
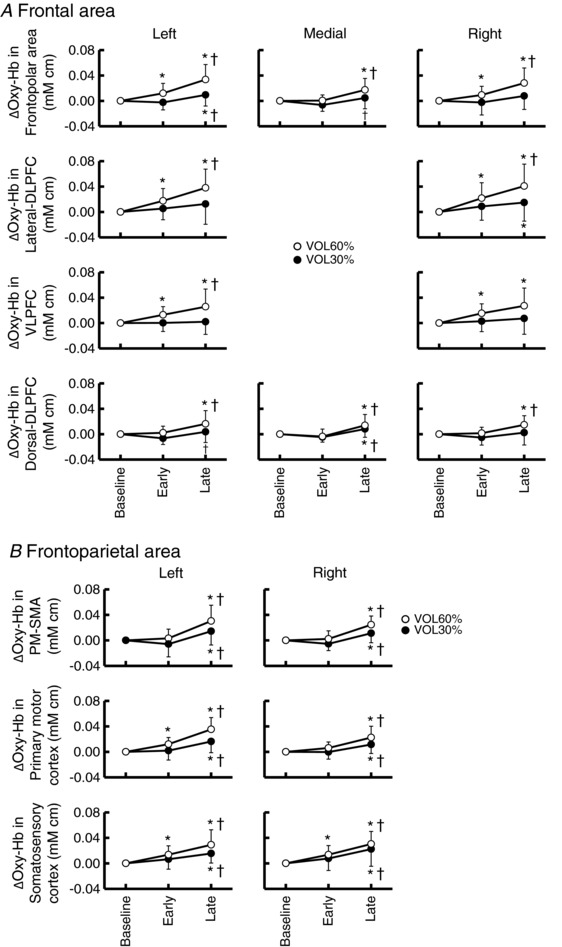
Values are means ± SD. *Significant difference (P < 0.05) from the baseline. †Significant difference (P < 0.05) from the value at the early period of cranking.
Relationship between motor effort and cortical oxygenation response
Table 2 summarises the relationships between the RPE and the Oxy‐Hb responses. Pearson's correlation analysis with a Holm–Sidak method revealed the significant relationship between them in all cortical areas. However, after statistically excluding the effects of MAP and CO on the Oxy‐Hb response by using the partial correlation analysis with a Holm–Sidak method, the significant relationship between the RPE and the Oxy‐Hb responses existed only in all the frontopolar areas (r = 0.47–0.49) and the lateral‐DLPFC (r = 0.46) and VLPFC (r = 0.48) on the right side (Table 2).
Table 2.
Relationships between the RPE and the Oxy‐Hb responses
| Cortical area | r Pearson's | P | r partial | P | |
|---|---|---|---|---|---|
| Frontopolar area | Left | 0.57 | <0.001* | 0.49 | 0.002* |
| Medial | 0.45 | 0.004* | 0.47 | 0.003* | |
| Right | 0.52 | <0.001* | 0.49 | 0.002* | |
| Lateral‐DLPFC | Left | 0.53 | <0.001* | 0.28 | 0.089 |
| Right | 0.6 | <0.001* | 0.46 | 0.003* | |
| VLPFC | Left | 0.56 | <0.001* | 0.43 | 0.006 |
| Right | 0.6 | <0.001* | 0.48 | 0.002* | |
| Dorsal‐DLPFC | Left | 0.47 | 0.002* | 0.33 | 0.044 |
| Medial | 0.36 | 0.024* | 0.2 | 0.211 | |
| Right | 0.45 | 0.003* | 0.33 | 0.044 | |
| PM‐SMA | Left | 0.52 | <0.001* | 0.23 | 0.161 |
| Right | 0.48 | 0.002* | 0.31 | 0.06 | |
| Primary motor cortex | Left | 0.54 | <0.001* | 0.26 | 0.118 |
| Right | 0.44 | 0.004* | 0.35 | 0.032 | |
| Somatosensory cortex | Left | 0.41 | 0.006* | 0.1 | 0.536 |
| Right | 0.38 | 0.017* | 0.22 | 0.175 |
r Pearson's: Pearson's correlation coefficient. r partial: partial correlation coefficient. Both correlation analyses were conducted with a sample size of 40 (20 subjects × 2 exercise intensities; 30% and 60% MVE). *Significant correlation after the correction with the Holm–Sidak method that adjusts the rejection criteria. DLPFC, dorsolateral prefrontal cortex; PM‐SMA, premotor and supplementary motor areas; VLPFC, ventrolateral prefrontal cortex.
Cortical oxygenation responses during passive one‐armed cranking
Motor‐driven passive one‐armed cranking slightly increased HR, CO and MAP (7 ± 4 beats min−1, 0.6 ± 0.6 L min−1 and 6 ± 4 mmHg, respectively), but not SV and TPR (data not shown). The RPE of the passive cranking was much lower (P < 0.05) than that of the voluntary cranking tasks (Table 1). The influences of passive one‐armed cranking on the Oxy‐Hb in all channels are summarised in Fig. 7. The Oxy‐Hb did not change (P > 0.05) at the start period and then decreased gradually in the frontal area (Fig. 8). On the other hand, regarding the frontoparietal area, the significant decrease in Oxy‐Hb was localised on the right side of the PM‐SMA and primary motor cortex. The Deoxy‐Hb in the frontal area did not change significantly (P > 0.05) during passive cranking, although a slight decrease in Deoxy‐Hb was detected in the bilateral PM‐SMA and primary motor cortices (data not shown).
Figure 7. Time courses of the Oxy‐Hb responses in the frontal (A) and frontoparietal areas (B) during passive one‐armed cranking in 20 subjects.

Grey areas indicate the cranking period. Horizontal scatter lines indicate the baseline level. The Oxy‐Hb was sequentially calculated every 1 s and expressed as mean without SD.
Figure 8. The Oxy‐Hb responses in the frontal (A) and frontoparietal areas (B) at the early and late period of passive one‐armed cranking in 20 subjects.

In the frontopolar area and the dorsal‐DLPFC, each part (right, middle and left) of the cortical areas is indicated in white, grey and black triangle, respectively. Values are means ± SD. *Significant difference (P < 0.05) from the baseline in the left cortical areas. †Significant difference (P < 0.05) from the baseline in the right cortical areas. #Significant difference (P < 0.05) from the baseline in the middle cortical areas.
Forehead skin blood flow responses during voluntary and passive one‐armed cranking
Figure 9 summarises the individual forehead skin blood flow responses during voluntary and passive one‐armed cranking in eight subjects. In both voluntary cranking tasks, the forehead skin blood flow on the right side did not change significantly (P > 0.05) prior to the exercise onset, and then increased during cranking (Fig. 9 A and B). The forehead skin blood flow responses to the voluntary cranking did not correlate with the Oxy‐Hb responses of the right lateral‐DLPFC and with the RPE (P > 0.05, analysed by Pearson's correlation) as shown in Fig. 9 C. During passive one‐armed cranking, the forehead skin blood flow did not change significantly (P > 0.05).
Figure 9. Forehead skin blood flow responses during voluntary and passive one‐armed cranking.

A, the individual forehead skin blood flow responses on the right side during voluntary and passive one‐armed cranking in 8 subjects. B, the average forehead skin blood flow response at the start period of voluntary one‐armed cranking at 30% (black circles) and 60% MVE (white circles) in 8 subjects. *Significant difference (P < 0.05) from the baseline of cranking at 60% MVE. †Significant difference (P < 0.05) from the baseline of cranking at 30% MVE. C, the relationship between the skin blood flow response and the Oxy‐Hb response of the lateral‐DLPFC on the right side or the RPE with a sample size of 16 (8 subjects × 2 exercise intensities (30% and 60% MVE)). N.S., not significant (P > 0.05).
Discussion
This study has examined using a multichannel NIRS system whether oxygenation of a specific cortical area does change independently of the actual exercise intensity prior to arbitrarily started exercise and in proportion to the motor effort during exercise. The cortical Oxy‐Hb response suggests changes in oxygen supply (i.e. changes in regional tissue blood flow) as reported previously (Hoshi et al. 2001; Suzuki et al. 2004; Furubayashi et al. 2013; Derosière et al. 2014; Matsukawa et al. 2015; Asahara et al. 2016; Ishii et al. 2017 b). The major findings of this study are that (1) at the start period of voluntary one‐armed cranking at both 30% and 60% MVE, oxygenation increased in the lateral‐DLPFC and VLPFC on both sides, the dorsal‐DLPFC on the right side, and the bilateral primary motor and somatosensory cortices; (2) irrespective of the constant torque output, the oxygenation increased gradually in all cortical areas during the cranking at 60% MVE, while the increase in oxygenation was confined to the frontoparietal area and some of the frontal area during the cranking at 30% MVE; (3) the RPE in response to the voluntary cranking was correlated with the oxygenation response of the lateral‐DLPFC and VLPFC on the right side and all the frontopolar areas; (4) passive one‐armed cranking decreased oxygenation in most cortical areas, except the contralateral PM‐SMA and primary motor cortex and the bilateral somatosensory cortices. Taken together, the above novel findings suggest that the lateral‐DLPFC and VLPFC on the right side are a specific cortical area responding in a feedforward‐ and motor effort‐dependent manner prior to and during voluntary exercise of the ipsilateral arm. The lateral‐DLPFC, which has projection to caudal autonomic nuclei (Arnsten, 1997; Uylings et al. 2003), may play a role in triggering off central command. On the other hand, afferent inputs from skeletal muscles may operate to decrease the cortical oxygenation widely.
Feedforward oxygenation response during voluntary one‐armed cranking
Cortical regions triggering off central command should respond independently of the actual exercise intensity in a feedforward manner, i.e. preceding the onset of exercise. Based on the previous findings (Furubayashi et al. 2013; Matsukawa et al. 2015; Asahara et al. 2016), the Oxy‐Hb response at the start period (from −5 to 5 s) of voluntary exercise was regarded as an index of the feedforward cortical activation before and at the exercise onset as described above. We found an increase in oxygenation of the lateral‐DLPFC and VLPFC on both sides, the dorsal‐DLPFC on the right side, and the bilateral primary motor and somatosensory cortices at the start period of voluntary one‐armed cranking at both low and moderate intensities (Fig. 5). The oxygenation response is unlikely to be evoked by influences of the changes in extracranial skin blood flow, HR, perfusion pressure and/or partial pressure of arterial carbon dioxide, for the following reasons. First, although the above factors should have systemic influences, the feedforward oxygenation response was localised in the specific cortical areas. Second, forehead skin blood flow (near the NIRS channel 9) did not increase prior to voluntary cranking (Fig. 9), suggesting that extracranial skin blood flow is unlikely to account for the feedforward oxygenation responses. Third, the pre‐exercise increase in Oxy‐Hb occurred in the lateral‐DLPFC and VLPFC before a significant HR change (which occurred at the exercise onset). In addition, spontaneous fluctuation of HR (approximately by 5 to 10 beats min−1) during a baseline condition did not affect the Oxy‐Hb. Finally, our previous studies (Matsukawa et al. 2015; Asahara et al. 2016, 2018) demonstrated that prior to voluntary exercise, the prefrontal oxygenation increased before forehead skin blood flow, HR, MAP, and/or end‐tidal carbon dioxide () exhibited some changes. Thus it is likely that the increased cortical oxygenation at the start period of voluntary cranking reflects a rise in regional CBF, following feedforward neural activation. The feedforward oxygenation response does not seem to be a startle and/or emotionally evoked response but is related to arbitrary motor intention, because such an increase in oxygenation did not occur at the start period of passive one‐armed cranking (Fig. 7). The pre‐exercise oxygenation response (∼4 s prior to the onset of exercise) in the lateral‐DLPFC and the VLPFC supports strongly the feedforward cortical activation hypothesis. The feedforward cortical activation is early enough to evoke the cardiovascular responses around the onset of exercise, taking into account a time delay involving neural conduction, synaptic transmission, and reaction time of cardiovascular effectors (Ishii et al. 2016). For instance, HR responds within 3 s to activation of the cardiac sympathetic nervous system (Mokrane and Nadeau, 1998) and 300 ms to activation of the cardiac parasympathetic nervous system (Williamson et al. 1995), whereas the vasomotor system responds much more slowly (5–6 s) to activation of the sympathetic nervous system (Cooper & Kerslake, 1955; Gandevia et al. 1993).
Effects of mechanosensitive afferent signals on the cortical oxygenation
Motor‐driven passive cranking of the right arm caused reduction of the oxygenation in most cortical areas (Figs. 7 and 8), whereas oxygenation of the contralateral (left) PM‐SMA and primary motor cortex and the somatosensory cortices did not decrease. The oxygenation reduction in the broad area is unlikely to be explained by changes in skin blood flow, HR, perfusion pressure and/or (Matsukawa et al. 2015; Asahara et al. 2016; Asahara and Matsukawa, 2018; Fig. 9 in the present study). Instead, the decreased cortical oxygenation during passive arm movement would be caused by a decrease in CBF, of which the mechanism is unknown. One possible mechanism responsible for the decreased oxygenation is that afferent signals evoked by dynamic limb movement may operate to cause decreases of CBF and cortical oxygenation due to cortical deactivation and/or cerebral vasoconstriction. On the other hand, the maintained oxygenation level in the contralateral frontoparietal areas may be because a broad CBF‐reducing effect, as mentioned above, may be counterbalanced by a regional CBF‐increasing effect caused by afferent neural inputs to the areas (Bernard et al. 2002; Francis et al. 2009) and by a minor motor control‐related cortical activation in association with passive movement. If so, the above CBF‐increasing effects would be augmented during voluntary cranking. Indeed, the cortical oxygenation during voluntary cranking was increased in comparison to that during the passive cranking (P < 0.05, Fig. 10 B).
Figure 10. Regional difference of cortical oxygenation responses during voluntary and passive one‐armed cranking.

A, time courses of the individual Oxy‐Hb responses of the lateral‐DLPFC on the right side and the left primary motor cortex during voluntary and passive one‐armed cranking in 20 subjects. B, time courses of the average Oxy‐Hb responses in the lateral‐DLPFC and the primary motor and somatosensory cortices during voluntary one‐armed cranking at 30% (white lines) and 60% MVE (red lines) and during passive one‐armed cranking (black lines) in 20 subjects. Grey areas indicate the cranking period. Horizontal scatter lines indicate the baseline level. The Oxy‐Hb responses was sequentially calculated every 1 s. Values are means ± SEM for data visibility.
Motor effort‐dependent oxygenation response during voluntary one‐armed cranking
Cortical regions triggering off central command may respond in proportion to motor effort during exercise. Williamson et al. (2001) examined the effort‐related cortical regions by changing effort sense to constant‐load cycling using hypnosis in which the subjects perceived uphill or downhill cycling. They found that an increase in RPE was accompanied by an increase in regional CBF of the insular cortex and thalamic region as well as the increases in HR and MAP. Furthermore, when comparing the regional CBF responses between imagery of uphill and downhill cycling, the uphill cycling imagery evoked greater increases in regional CBF of the DLPFC, PM‐SMA, sensorimotor areas, thalamus and cerebellum as well as greater increases in HR and ventilation (Thornton et al. 2001). We found that the oxygenation of most cortical areas, as well as HR and MAP, increased when EMG activities in the contracting muscles were augmented probably due to a rise in motor effort (Figs 2 and 6). Pearson's correlation analysis showed the positive relationship between the RPE and the late oxygenation response in all cortical areas (Table 2). However, these findings do not deny the possibility that the increase in RPE during exercise may cause increases in MAP and CO that may result in the increases in CBF and cortical Oxy‐Hb (Ide et al. 2000; Ogoh et al. 2005; Ogoh and Ainslie, 2009). Thus we attempted to examine the degree of the relationship between the RPE and the cortical oxygenation responses to voluntary cranking by statistically removing the effects of the systemic haemodynamic changes on the oxygenation response. As a result, the oxygenation response in the lateral‐DLPFC and VLPFC on the right side and the frontopolar areas correlated with the RPE (Table 2). The forehead skin blood flow response did not account for the relationship between the RPE and late oxygenation response, because there was no significant relationship between the late skin blood flow response and the RPE or the late oxygenation response (Fig. 9 C). It is likely that the oxygenation behaving in a feedforward‐ and motor effort‐dependent manner was observed only in the lateral‐DLPFC and VLPFC on the right side.
Limitations
This study involved several limitations. First, this study dropped the data of several NIRS channels and did not examine all cortical areas such as the temporal cortex, occipital cortex and deeper cortical areas (e.g. anterior cingulate and insular cortices). Thus, in addition to the lateral‐DLPFC and VLPFC, the possibility that other cortical areas have the feedforward‐ and motor effort‐dependent features cannot be neglected. Second, brain activity was non‐invasively estimated as changes in cortical oxygenation following metabolically evoked change in regional CBF. However, it remained unknown whether the increases in regional CBF and oxygenation involve excitatory or inhibitory neural activation or both, even though their changes may reflect mass neural activity in a local brain region. Third, the coordination for the NIRS channels was not defined based on the subjects’ brain images. Fourth, the degree of motor effort was evaluated based on the subjective RPE score. Finally, we did not use the specific techniques used by other investigators (Gagnon et al. 2014; Guerrero‐Mosquera et al. 2016) to remove the contamination of movement artefact or background noise in the NIRS data, although the data clearly including movement artefacts were excluded manually. The NIRS signal in this study, however, seemed to have a good quality as described above. Also, we conducted sequential averaging of the NIRS signals every 1 s, which reduced the influence of high frequency noise on the NIRS signals. Thus it is likely that movement artefacts and high frequency background noise had a minimal effect on the NIRS signals presented in our study. In the data process, an inverse oxygenation response did not seem to occur in both the lateral‐DLPFC and the primary motor cortex (as shown in Fig. 10 A), while the magnitude of oxygenation response in the lateral‐DLPFC differed greatly among the subjects. Interestingly, the oxygenation response in the lateral‐DLPFC on the right side significantly correlated with the RPE to voluntary cranking tasks (Table 2).
Conclusion
In conclusion, the oxygenation of the lateral‐DLPFC and VLPFC on the right side may increase in a feedforward‐ and motor effort‐dependent manner prior to and during voluntary cranking of the ipsilateral arm. On the other hand, afferent inputs from skeletal muscles in association with rhythmic arm movement may decrease the cortical oxygenation.
Additional information
Competing interests
No competing interests declared.
Author contributions
The study was conducted in the laboratory at Hiroshima University. K.I. contributed to the conception and design of the study, acquisition, analysis and interpretation of the data, and drafting the manuscript. K.M. and N.L. contributed to the conception and design of the study, acquisition and interpretation of the data, and revising the draft critically for important intellectual content. R.A. and M.T. contributed to acquisition and interpretation of the data, and revising the draft critically for important intellectual content. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the study. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by Grants‐in‐Aid for Scientific Research (B) (grant number 15H03061), for Exploratory Research (grant number 16K12935), and for Young Scientists (B) (grant number 26750187) from the Japan Society for the Promotion of Science.
Perspective.
The dorsolateral prefrontal cortex (DLPFC) in primates projects directly to caudal autonomic nuclei such as the locus coeruleus and raphe nuclei (Arnsten, 1997; Uylings et al. 2003), although the DLPFC is known to have multiple higher brain functions such as executive control of behaviour (Kübler et al. 2006), willed action (Frith et al. 1991) and working memory (Zhang et al. 2003). Thus we speculate that the lateral‐DLPFC activation in association with motor intention and effort may play a role in triggering off central ‘cardiovascular’ command by relaying a certain caudal neural circuit (Matsukawa, 2012). From this point of view, the oxygenation response of the lateral‐DLPFC on the right side may suggest that central ‘cardiovascular’ command operates not only prior to cranking at 60% but also throughout the exercise, regardless of gradual activation of the contralateral sensorimotor area (as represented in Fig. 10). The present finding may help us to understand impaired regulation of the cardiovascular system in association with lesion of the prefrontal cortex (Verberne et al. 1987; Shoemaker and Goswami, 2015). On the other hand, the ventrolateral prefrontal cortex (VLPFC) has connections to the premotor and supplementary motor areas (Ford et al. 2010) and can be activated during observation of grasping movement (Rizzolatti et al. 1996; Binkofski and Buccino, 2004) and during preparation, intention and execution of actual movement (Parsons et al. 1995; Thoenissen et al. 2002). Thus the right VLPFC may at least partly contribute to triggering off central ‘motor’ command rather than central ‘cardiovascular’ command. Further studies are required to examine the neural connectivity of the lateral‐DLPFC and the VLPFC in humans and the direct relationship between the activation of these cortical areas and cardiovascular or motor responses.
Biography
Kei Ishii completed a PhD at Hiroshima University in 2014, where he investigated the central regulation of muscle blood flow during exercise. For the research, he received the Hiroshi and Aya Irisawa Memorial Promotion Award for Young Physiologists in 2015. He is currently a research fellow in the National Institute of Advanced Industrial Science and Technology, investigating the central mechanism of cardiovascular regulation during exercise and how cerebral and muscle blood flow are regulated during cardiac arrhythmia.

Edited by: Harold Schultz & Diego Contreras
References
- Arnsten AF (1997). Catecholamine regulation of the prefrontal cortex. J Psychopharmacol 11, 151–162. [DOI] [PubMed] [Google Scholar]
- Asahara R, Endo K, Liang N & Matsukawa K (2018). An increase in prefrontal oxygenation at the start of voluntary cycling exercise was observed independently of exercise effort and muscle mass. Eur J Appl Physiol 118, 1689–1702. [DOI] [PubMed] [Google Scholar]
- Asahara R & Matsukawa K (2018). Decreased prefrontal oxygenation elicited by stimulation of limb mechanosensitive afferents during cycling exercise. Am J Physiol Regul Integr Comp Physiol 315, R230–R240. [DOI] [PubMed] [Google Scholar]
- Asahara R, Matsukawa K, Ishii K, Liang N & Endo K (2016). The prefrontal oxygenation and ventilatory responses at start of one‐legged cycling exercise have relation to central command. J Appl Physiol 121, 1115–1126. [DOI] [PubMed] [Google Scholar]
- Bernard RA, Goran DA, Sakai ST, Carr TH, McFarlane D, Nordell B, Cooper TG & Potchen EJ (2002). Cortical activation during rhythmic hand movements performed under three types of control: an fMRI study. Cogn Affect Behav Neurosci 2, 271–281. [DOI] [PubMed] [Google Scholar]
- Binkofski F & Buccino G (2004). Motor functions of the Broca's region. Brain Lang 89, 362–369. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Müller ML, Kuwabara H, Constantine GM & Studenski SA (2009). Age‐associated leukoaraiosis and cortical cholinergic deafferentation. Neurology 72, 1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G (1970). Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2, 92–98. [PubMed] [Google Scholar]
- Cooper KE & Kerslake DM (1955). Vasoconstriction in the hand during electrical stimulation of the lumber sympathetic chain in man. J Physiol 127, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai TH, Liu JZ, Sahgal V, Brown RW & Yue GH (2001). Relationship between muscle output and functional MRI‐measured brain activation. Exp Brain Res 140, 290–300. [DOI] [PubMed] [Google Scholar]
- Derosière G, Alexandre F, Bourdillon N, Mandrick K, Ward TE & Perrey S (2014). Similar scaling of contralateral and ipsilateral cortical responses during graded unimanual force generation. Neuroimage 85, 471–477. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE & Waldrop TG (1981). Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211, 844–846. [DOI] [PubMed] [Google Scholar]
- Ford A, McGregor KM, Case K, Crosson B & White KD (2010). Structural connectivity of Broca's area and medial frontal cortex. Neuroimage 52, 1230–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S, Lin X, Aboushoushah S, White TP, Phillips M, Bowtell R & Constantinescu CS (2009). fMRI analysis of active, passive and electrically stimulated ankle dorsiflexion. Neuroimage 44, 469–479. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF & Frackowiak RS (1991). Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci 244, 241–246. [DOI] [PubMed] [Google Scholar]
- Furubayashi T, Mochizuki H, Terao Y, Arai N, Hanajima R, Hamada M, Matsumoto H, Nakatani‐Enomoto S, Okabe S, Yugeta A, Inomata‐Terada S & Ugawa Y (2013). Cortical hemoglobin concentration changes underneath the coil after single‐pulse transcranial magnetic stimulation: a near‐infrared spectroscopy study. J Neurophysiol 109, 1626–1637. [DOI] [PubMed] [Google Scholar]
- Gagnon L, Yücel MA, Boas DA & Cooper RJ (2014). Further improvement in reducing superficial contamination in NIRS using double short separation measurements. Neuroimage 85, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Killian K, McKenzie DK, Crawford M, Allen GM, Gorman RB & Hales JP (1993). Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J Physiol 470, 85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL & Paterson DJ (2008). Identification of neurocircuitry controlling cardiovascular function in humans using functional neurosurgery: implications for exercise control. Exp Physiol 93, 1022–1028. [DOI] [PubMed] [Google Scholar]
- Guerrero‐Mosquera C, Borragán G & Peigneux P (2016). Automatic detection of noisy channels in fNIRS signal based on correlation analysis. J Neurosci Methods 271, 128–138. [DOI] [PubMed] [Google Scholar]
- Hammer Ø, Harper DAT & Ryan PD (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electronica 4, 1–9. [Google Scholar]
- Hoshi Y, Kobayashi N & Tamura M (2001). Interpretation of near‐infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol 90, 1657–1662. [DOI] [PubMed] [Google Scholar]
- Ide K, Boushel R, Sørensen HM, Fernandes A, Cai Y, Pott F & Secher NH (2000). Middle cerebral artery blood velocity during exercise with β‐1 adrenergic and unilateral stellate ganglion blockade in humans. Acta Physiol Scand 170, 33–38. [DOI] [PubMed] [Google Scholar]
- Ishii K, Matsukawa K, Asahara R, Liang N, Endo K, Idesako M, Michioka K, Sasaki Y, Hamada H, Yamashita K, Watanabe T, Kataoka T & Takahashi M (2017. a). Central command increases muscular oxygenation of the non‐exercising arm at the early period of voluntary one‐armed cranking. Physiol Rep 5, e13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Matsukawa K & Liang N (2017. b). Increased oxygenation of the dorsolateral prefrontal cortex prior to the onset of voluntary one‐armed cranking. J Physiol Sci 67 (Suppl 1), S156. [Google Scholar]
- Ishii K, Matsukawa K, Liang N, Endo K, Idesako M, Asahara R, Kadowaki A, Wakasugi R & Takahashi M (2016). Central command generated prior to arbitrary motor execution induces muscle vasodilatation at the beginning of dynamic exercise. J Appl Physiol 120, 1424–1433. [DOI] [PubMed] [Google Scholar]
- Kadowaki A, Matsukawa K, Wakasugi R, Nakamoto T & Liang N (2011). Central command does not decrease cardiac parasympathetic efferent nerve activity during spontaneous fictive motor activity in decerebrate cats. Am J Physiol Heart Circ Physiol 300, H1373–H1385. [DOI] [PubMed] [Google Scholar]
- King AB, Menon RS, Hachinski V & Cechetto DF (1999). Human forebrain activation by visceral stimuli. J Comp Neurol 413, 572–582. [PubMed] [Google Scholar]
- Koseki S, Noda T, Yokoyama S, Kunisato Y, Ito D, Suyama H, Matsuda T, Sugimura Y, Ishihara N, Shimizu Y, Nakazawa K, Yoshida S, Arima K & Suzuki S (2013). The relationship between positive and negative automatic thought and activity in the prefrontal and temporal cortices: a multi‐channel near‐infrared spectroscopy (NIRS) study. J Affect Disord 151, 352–359. [DOI] [PubMed] [Google Scholar]
- Krogh A & Lindhard J (1913). The regulation of respiration and circulation during the initial stages of muscular work. J Physiol 47, 112–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler A, Dixon V & Garavan H (2006). Automaticity and reestablishment of executive control—an fMRI study. J Cogn Neurosci 18, 1331–1342. [DOI] [PubMed] [Google Scholar]
- Matsukawa K (2012). Central command: control of cardiac sympathetic and vagal efferent nerve activity and the arterial baroreflex during spontaneous motor behaviour in animals. Exp Physiol 97, 20–28. [DOI] [PubMed] [Google Scholar]
- Matsukawa K, Ishii K, Liang N, Endo K, Ohtani R, Nakamoto T, Wakasugi R, Kadowaki A & Komine H (2015). Increased oxygenation of the cerebral prefrontal cortex prior to the onset of voluntary exercise in humans. J Appl Physiol 119, 452–462. [DOI] [PubMed] [Google Scholar]
- Matsukawa K, Murata J & Wada T (1998). Augmented renal sympathetic nerve activity by central command during overground locomotion in decerebrate cats. Am J Physiol Heart Circ Physiol 275, H1115–H1121. [DOI] [PubMed] [Google Scholar]
- Mizuguchi N, Nakata H & Kanosue K (2014). Activity of right premotor‐parietal regions dependent upon imagined force level: an fMRI study. Front Hum Neurosci 8, 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrane A & Nadeau R (1998). Dynamics of heart rate response to sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol 275, H995–H1001. [DOI] [PubMed] [Google Scholar]
- Nowak M, Holm S, Biering‐Sørensen F, Secher NH & Friberg L (2005). “Central command” and insular activation during attempted foot lifting in paraplegic humans. Hum Brain Mapp 25, 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S & Ainslie PN (2009). Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol 107, 1370–1380. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, O‐Yurvati A & Raven PB (2005). The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol 569, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP & Hachinski VC (1992). Cardiovascular effects of human insular cortex stimulation. Neurology 42, 1727–1732. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, Jerabek PA & Lancaster JL (1995). Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature 375, 54–58. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D & Fazio F (1996). Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res 111, 246–252. [DOI] [PubMed] [Google Scholar]
- Rorden C & Brett M (2000). Stereotaxic display of brain lesions. Behav Neurol 12, 191–200. [DOI] [PubMed] [Google Scholar]
- Sakakibara E, Homae F, Kawasaki S, Nishimura Y, Takizawa R, Koike S, Kinoshita A, Sakurada H, Yamagishi M, Nishimura F, Yoshikawa A, Inai A, Nishioka M, Eriguchi Y, Matsuoka J, Satomura Y, Okada N, Kakiuchi C, Araki T, Kan C, Umeda M, Shimazu A, Uga M, Dan I, Hashimoto H, Kawakami N & Kasai K (2016). Detection of resting state functional connectivity using partial correlation analysis: A study using multi‐distance and whole‐head probe near‐infrared spectroscopy. Neuroimage 142, 590–601. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK & Goswami R (2015). Forebrain neurocircuitry associated with human reflex cardiovascular control. Front Physiol 6, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Okamoto M, Dan H, Jurcak V & Dan I (2005). Spatial registration of multichannel multi‐subject fNIRS data to MNI space without MRI. Neuroimage 27, 842–851. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Miyai I, Ono T, Oda I, Konishi I, Kochiyama T & Kubota K (2004). Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. Neuroimage 23, 1020–1026. [DOI] [PubMed] [Google Scholar]
- Thoenissen D1, Zilles K & Toni I (2002). Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci 22, 9024–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JM, Guz A, Murphy K, Griffith AR, Pedersen DL, Kardos A, Leff A, Adams L, Casadei B & Paterson DJ (2001). Identification of higher brain centres that may encode the cardiorespiratory response to exercise in humans. J Physiol 533, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ & Kolb B (2003). Do rats have a prefrontal cortex? Behav Brain Res 146, 3–17. [DOI] [PubMed] [Google Scholar]
- Verberne AJ, Lewis SJ, Worland PJ, Beart PM, Jarrott B, Christie MJ & Louis WJ (1987). Medial prefrontal cortical lesions modulate baroreflex sensitivity in the rat. Brain Res 426, 243–249. [DOI] [PubMed] [Google Scholar]
- Williamson JW (2010). The relevance of central command for the neural cardiovascular control of exercise. Exp Physiol 95, 1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB & Morgan WP (2001). Hypnotic manipulation of effort sense during dynamic exercise: cardiovascular responses and brain activation. J Appl Physiol 90, 1392–1399. [DOI] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB & Morgan WP (2002). Brain activation by central command during actual and imagined handgrip under hypnosis. J Appl Physiol 92, 1317–1324. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Nóbrega AC, Winchester PK, Zim S & Mitchell JH (1995). Instantaneous heart rate increase with dynamic exercise: central command and muscle‐heart reflex contributions. J Appl Physiol 78, 1273–1279. [DOI] [PubMed] [Google Scholar]
- Wong SW, Massé N, Kimmerly DS, Menon RS & Shoemaker JK (2007). Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage 35, 698–708. [DOI] [PubMed] [Google Scholar]
- Yasui Y1, Breder CD, Saper CB & Cechetto DF (1991). Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303, 355–374. [DOI] [PubMed] [Google Scholar]
- Ye JC, Tak S, Jang KE, Jung J & Jang J (2009). NIRS‐SPM: statistical parametric mapping for near‐infrared spectroscopy. Neuroimage 44, 428–447. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Leung HC & Johnson MK (2003). Frontal activations associated with accessing and evaluating information in working memory: an fMRI study. Neuroimage 20, 1531–1539. [DOI] [PubMed] [Google Scholar]


