Abstract
Key points
Threats to standing balance (postural threat) are known to facilitate soleus tendon‐tap reflexes, yet the mechanisms driving reflex changes are unknown.
Scaling of ramp‐and‐hold dorsiflexion stretch reflexes to stretch velocity and amplitude were examined as indirect measures of changes to muscle spindle dynamic and static function with height‐induced postural threat.
Overall, stretch reflexes were larger with threat. Furthermore, the slope (gain) of the stretch‐velocity vs. short‐latency reflex amplitude relationship was increased with threat.
These findings are interpreted as indirect evidence for increased muscle spindle dynamic sensitivity, independent of changes in background muscle activity levels, with a threat to standing balance.
We argue that context‐dependent scaling of stretch reflexes forms part of a multisensory tuning process where acquisition and/or processing of balance‐relevant sensory information is continuously primed to facilitate feedback control of standing balance in challenging balance scenarios.
Abstract
Postural threat increases soleus tendon‐tap (t‐) reflexes. However, it is not known whether t‐reflex changes are a result of central modulation, altered muscle spindle dynamic sensitivity or combined spindle static and dynamic sensitization. Ramp‐and‐hold dorsiflexion stretches of varying velocities and amplitudes were used to examine velocity‐ and amplitude‐dependent scaling of short‐ (SLR) and medium‐latency (MLR) stretch reflexes as an indirect indicator of spindle sensitivity. t‐reflexes were also performed to replicate previous work. In the present study, we examined the effects of postural threat on SLR, MLR and t‐reflex amplitude, as well as SLR‐stretch velocity scaling. Forty young‐healthy adults stood with one foot on a servo‐controlled tilting platform and the other on a stable surface. The platform was positioned on a hydraulic lift. Threat was manipulated by having participants stand in low (height 1.1 m; away from edge) then high (height 3.5 m; at the edge) threat conditions. Soleus stretch reflexes were recorded with surface electromyography and SLRs and MLRs were probed with fixed‐amplitude variable‐velocity stretches. t‐reflexes were evoked with Achilles tendon taps using a linear motor. SLR, MLR and t‐reflexes were 11%, 9.5% and 16.9% larger, respectively, in the high compared to low threat condition. In 22 out of 40 participants, SLR amplitude was correlated to stretch velocity at both threat levels. In these participants, the gain of the SLR–velocity relationship was increased by 36.1% with high postural threat. These findings provide new supportive evidence for increased muscle spindle dynamic sensitivity with postural threat and provide further support for the context‐dependent modulation of human somatosensory pathways.
Keywords: Stretch reflex, Muscle Spindle, Postural Threat
Key points
Threats to standing balance (postural threat) are known to facilitate soleus tendon‐tap reflexes, yet the mechanisms driving reflex changes are unknown.
Scaling of ramp‐and‐hold dorsiflexion stretch reflexes to stretch velocity and amplitude were examined as indirect measures of changes to muscle spindle dynamic and static function with height‐induced postural threat.
Overall, stretch reflexes were larger with threat. Furthermore, the slope (gain) of the stretch‐velocity vs. short‐latency reflex amplitude relationship was increased with threat.
These findings are interpreted as indirect evidence for increased muscle spindle dynamic sensitivity, independent of changes in background muscle activity levels, with a threat to standing balance.
We argue that context‐dependent scaling of stretch reflexes forms part of a multisensory tuning process where acquisition and/or processing of balance‐relevant sensory information is continuously primed to facilitate feedback control of standing balance in challenging balance scenarios.
Introduction
Sensorimotor and behavioural responses to threat are commonly observed in animals and probably serve as a self‐preservation mechanism in which movement is reduced to avoid predator detection (Balaban, 2002). When humans are exposed to a threat to standing balance, such as standing at the edge of a high surface, they change balance control behaviours (Carpenter et al., 1999, 2004), alter conscious perception of body sway (Cleworth and Carpenter, 2016) and modulate reflexes from balance‐relevant sensory inputs (Horslen et al., 2013, 2014, 2017; Naranjo et al., 2015, 2016). Functionally, the sensorimotor changes in humans probably serve to reduce movement to avoid a potentially injurious fall. However, the mechanisms via which balance behaviour and sensorimotor processing changes are achieved are not well understood.
A potential contributor to balance changes that occur with height‐induced postural threat could be modulations in muscle spindle dynamic sensitivity. Ackerley et al. (2017) reported that unpleasant emotional state may increase ankle dorsiflexor muscle spindle dynamic responses to sinusoidal stretch, suggesting that muscle spindle sensitivity can be tuned to emotional context. Further evidence from postural threat studies has shown altered monosynaptic stretch reflexes with threats to standing balance. Specifically, soleus tendon‐tap reflex (t‐reflex) amplitudes are increased (Davis et al., 2011; Horslen et al., 2013), and Hoffmann (H‐) reflexes are either unchanged (Horslen et al., 2013) or decreased (Llewllyn et al., 1990; Sibley et al., 2007), with a threat to standing balance. Although both H‐ and t‐reflexes can be modulated centrally (pre‐/post‐synapse or across motor neuron pool) in the spinal cord, only t‐reflexes are subject to changes in peripheral muscle spindle sensitivity (Pierrot‐Deseilligny and Burke, 2012). Therefore, the different effects of threat on H‐ and t‐reflexes within the same participants were interpreted as indirect evidence for an increase in muscle spindle sensitivity, possibly as a result of independent fusimotor drive (Horslen et al., 2013).
However, technical limitations may preclude direct comparison of H‐ and t‐reflexes for the purpose of evaluating fusimotor state (Pierrot‐Deseilligny and Burke, 2012) based on potential differences in: (i) shape and duration of the evoked afferent volley; (ii) sensitivity to central modulation; (iii) effects of the afferent volley on the motoneuron pool; and (iv) muscle spindle afferent firing properties represented in the reflex (t‐reflexes predominantly reflect dynamic responses from Ia afferents over Ia and/or II static firing profiles) (Burke et al., 1983). Therefore, central modulation of t‐reflexes cannot be ruled out as an explanation for the different H‐ and t‐reflex outcomes at this time. Furthermore, although postural threat might be expected to facilitate both spindle static and dynamic sensitivity (Prochazka et al., 1985), t‐reflexes are not affected by changes in spindle static (length‐dependent) sensitivity (Morgan et al., 1984) and thus relative scaling of static and dynamic sensitivity with threat remains unexplored. Although the gold‐standard for evaluating changes in human muscle spindle sensitivity would be direct microneurographic recording of Ia afferent activity in response to known stretch stimuli with participants standing at low and high surface heights, it would be extremely difficult to ensure stable recordings with the body orientation and muscle activity changes known to occur at height (Carpenter et al., 2001).
Changes in scaling of ramp‐and‐hold plantar flexor stretch reflexes to varying stretch velocities may serve as an indirect probe of changes in muscle spindle dynamic sensitivity. Ramp‐and‐hold stretches evoke short‐latency (SLR) and medium‐latency reflex (MLR) responses in the stretched muscle. In the lower limb, the SLR is considered to reflect the Ia myotatic stretch reflex (Nardone et al., 1996), which is velocity‐ but not amplitude‐dependent (Gottlieb and Agarwal, 1979; Allum and Mauritz, 1984; Grey et al., 2001). Thus, the SLR and t‐reflex are often taken as analogous reflexes. The mechanistic inferences that can be drawn from a change in mean stretch reflex amplitude (SLR or t‐reflex) with postural threat are inherently limited. Both central (e.g. motoneuron pool excitability, pre‐synaptic inhibition/excitation and primary afferent post‐activation depression) and peripheral factors (i.e. muscle spindle sensitivity and/or muscle thixotropy) can affect stretch reflex amplitudes to any given stretch (Pierrot‐Deseilligny and Burke, 2012). However, we propose that the slope (gain) of the SLR‐stretch velocity relationship would be sensitive to a threat‐induced increase in muscle spindle dynamic sensitivity. Fast stretches cause greater Ia firing rate during the dynamic (lengthening) portion of muscle stretch than slower stretches of the same amplitude (Cooper, 1961); more Ia firing translates into a larger stretch reflex. Increasing muscle spindle dynamic sensitivity (via gamma dynamic, γD, stimulation) further increases Ia dynamic sensitivity to stretch (Crowe and Matthews, 1964). Therefore, we propose that the relationship between SLR amplitude and stretch velocity for a given participant/testing session, in the absence of central reflex facilitation, indirectly reflects receptor sensitivity to stretch velocity.
An advantage to using ramp‐and‐hold stretch stimuli is that they also evoke MLR responses, which contain information beyond the SLR. By contrast to the dynamic SLR that originates from Ia afferent feedback, the lower limb MLR is considered to contain significant static (length‐dependent) contributions from muscle spindle type II, as well as Ia, afferents. Evidence for static contributions to the MLR comes from: (i) significant MLR suppression with tizanidine, an α2‐adrenergic agonist that suppresses afferent type II transmission to interneurons in the intermediate zone of the spinal cord, without impact on the SLR (Corna et al., 1995; Grey et al., 2001; Meskers et al., 2010; for contrast, see Kurtzer et al., 2018); (ii) the MLR being more delayed with nerve cooling than SLR responses (Schieppati and Nardone, 1997; Grey et al., 2001), suggesting the involvement of smaller‐diameter afferent fibres; and (iii) the MLR scaling to stretch amplitude (Gottlieb and Agarwal, 1979; Allum and Mauritz, 1984) or stretch duration (Lewis et al., 2005; Schuurmans et al., 2009), whereas the response to stretch velocity is mixed (Allum and Mauritz, 1984; Grey et al., 2001). Accordingly, the present study aimed to examine how the amplitude and length‐dependent scaling of MLRs change with postural threat, in conjunction with SLR and t‐reflex data. As a result of the mechanical limitations of our tilting platform (for details, see Methods), the amplitude‐dependent reflex scaling results have been excluded from this report.
The present study aimed to determine how height‐induced postural threat affects: (i) t‐reflex, SLR and MLR stretch reflex amplitudes within the same participants and (ii) velocity‐dependent scaling of the SLR. It was hypothesized that SLRs, MLRs and t‐reflexes would all be increased with postural threat. Furthermore, it was hypothesized that SLRs would scale linearly to stretch velocity, and that the slope of the SLR–velocity relationship would be steeper with threat, reflecting increased dynamic gain of the SLR.
Methods
Ethical approval
Forty‐three young healthy adults were recruited from the community. All participants provided their written informed consent prior to participation and the methods used were approved by the University of British Columbia Clinical Research Ethics Board (approval reference number H06‐70316). The study conformed with the 2013 version of the Declaration of Helsinki, except for registration in a database. Two participants withdrew from the study and one participant was excluded because of equipment malfunction. The remaining 40 participants were divided into two experimental groups, with 16 participants in Protocol 1 (nine female/seven male; mean ± SEM) age: 21.4 ± 0.47 years; height: 1.71 ± 0.02 m; weight: 68.5 ± 3.14 kg) and 24 participants in Protocol 2 (13 female/11 male; age: 20.9 ± 0.63 years; height: 1.72 ± 0.02 m; weight: 66.3 ± 1.92 kg). The two protocols were used in an attempt to overcome technical challenges associated with eliciting reliable length‐dependent scaling of the MLR. The data are presented combined across groups unless stated otherwise.
Protocols
Participants stood on a custom‐built servo‐controlled tilting platform surrounded by a non‐moving stage mounted at the edge of a hydraulic lift (M419‐207B01H01D; Pentalift, Guelph, Canada). As shown in Fig. 1 A, feet were positioned with the toes 20 cm from the edge of the lift and 25 cm apart, with the right foot on the tilting platform (5 cm from of the right edge of tilting platform) and the left foot on the stage (5 cm from a cut‐out for the left motor swingarm). Foot position was marked on the stage and platform, and a light‐weight plumb line was hung from the right medial tibial condyle for the entire experiment to allow visual monitoring by an experimenter to ensure that foot position and knee angle were maintained in all conditions. We utilized unilateral ankle stretches to minimize the potential confound of evoking balance perturbations or corrective responses (Corna et al., 1996), where stretch reflexes may be inhibited over repeated exposure because they are further destabilizing in support surface tilts (Keshner et al., 1987).
Figure 1. Experiment layout, threat and stimulus presentation.
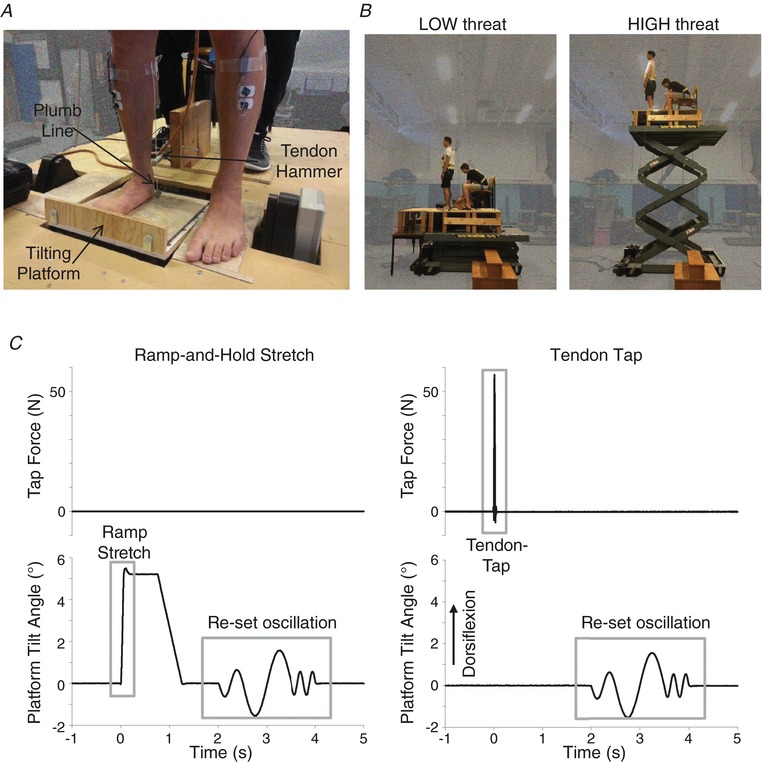
Participants stood with their right foot on a tilting plate and left foot on a stable even surround (A). A linear motor was positioned behind the right Achilles tendon to evoke t‐reflexes and a plumb line was hung from the tibial condyle to guide experimenter tracking of knee angle. The tilting plate was mounted at the edge of a hydraulic lift. The lift was lowered to its lowest level and a 60 cm wide support surface extension was fixed in front of the participant in the LOW threat condition (B); the extension was removed and the lift elevated for the HIGH threat condition (B). All stimuli began with the tilting plate locked in a horizontal position (0°); at time zero, either the plate tilted the foot through dorsiflexion (positive) or plantarflexion (negative) or a tendon‐tap was presented (C). Two seconds after stimulus presentation, the right foot was oscillated through a re‐set pattern (C) to ensure all stimuli ended with a comparable mechanical event. [Color figure can be viewed at http://wileyonlinelibrary.com]
In the low threat condition (LOW), the platform and stage surfaces were 1.1 m above the ground (minimum height of lift and stage). An additional support surface (width 60 cm) was positioned in front of the lift, creating an 80 cm continuous support surface (Fig. 1 B) to further minimize threat of falling off the stage. The additional support surface was removed and the platform was elevated to 3.5 m to increase postural threat in the high threat condition (HIGH). Absolute surface heights in both conditions were higher than levels previously used in our laboratory to accommodate the height of the tilting platform and stage, yet the 2.4 m change in height between conditions matches that of previous work (Horslen et al., 2013, 2014). The order of presentation of the threat conditions is known to influence threat‐related changes in balance control (Adkin et al., 2000); therefore, the LOW condition was always presented first to maximize the contrast between conditions. There were no safety rails bordering the lift; however, participants were harnessed into a safety line and an experimenter was within arm's reach at all times. No participants fell or required a step or external assistance to maintain balance during the experiment.
The output of four load cells mounted into the tilting platform (SSB‐250 with BSC4A‐C14; Interface Advanced Force Measurement, Scottsdale, AZ, USA) was used to monitor vertical load and anterior‐posterior (AP) and medial‐lateral (ML) torque on the plate under the right foot (sampled 1000 Hz; Power 1401 with Spike2 software; Cambridge Electronic Design Ltd, Cambridge, UK). Prior to any stimulation, participants stood quietly (at LOW height) for 2 min and baseline mean ± SD vertical load, AP and ML torques were calculated. Vertical load was monitored online by an experimenter to ensure that the weighting between the feet did not change across conditions. Visual feedback of real‐time AP and ML torque was projected (MS317; BenQ, Taipei, Taiwan) on the wall at eye level, ∼3.7 m in front of the participant (display: height 1.35 m, width 1.83 m). Participants were instructed to keep the real‐time torque position (projected as a green dot on white background) within a target box with dimensions equal to AP and ML mean ± SD from the baseline trial; the magnitude of the target with respect to the total visual display was scaled to each participant's preference, with total area not less than 2× SD about the baseline mean. The feedback disappeared 10 ms prior to each stimulus, and remained hidden for 4 s; participants were instructed not to attempt to control their lean when feedback was hidden. Participants were given a practice trial to familiarize themselves with the feedback and mitigate potential first‐trial effects; the practice trial included a single cued presentation of each stimulus used in the experiment as well as a five‐perturbation session where a random sample of stimuli were presented without cues, similar to the actual experimental trials.
The tilting platform was servo‐controlled with an analogue input voltage provided through a D/A system (Power 1401 with Spike2 software; Cambridge Electronic Design Ltd). Actual platform tilt angle was measured with a calibrated potentiometer and low‐pass filtered offline with a 50 Hz second‐order Butterworth filter passed in the forward and backward direction to remove phase shifts (sampled 5000 Hz; Power 1401 with Spike2; filter: Spike2) and differentiated offline to calculate platform velocity (Spike2). In both experiments, participants experienced five toes‐up dorsiflexion (DF) ramp‐and‐hold stretches for each of the five profiles (25 in total) which were presented in a random order. As shown in Fig 2 B and C, stretch profiles were grouped into three velocity profiles (Slow, Medium, Fast; used to examine SLR amplitude and SLR–velocity scaling) and three amplitude profiles (Small, Moderate, Large; for MLR amplitude and non‐examined MLR‐amplitude scaling). In Protocol 1, ramp profiles were designed to be similar to stimuli used in prior reactive balance studies (amplitude: 3–7.5°; velocity: 50–120° s−1) (Corna et al., 1995, 1996; Nardone et al., 1996; Schieppati & Nardone, 1997; Carpenter et al., 2004; Campbell et al., 2009). However, we found that the range of achieved tilt amplitudes within the MLR timeframe was too small (<1°) to assess scaling reliably (Table 1). Thus, Protocol 2 was designed to expand the range of actual angles by using platform angles and velocities that reached the maximum capability of our tilting platform under load (200° s−1, 7.5°) (Table 2). Each ramp stretch was followed by a 750 ms hold before returning to baseline position over 500 ms. Muscle stretch responses can be affected by previous history (e.g. stiction or thixotropic after‐effects) (Proske et al., 1993). Although a conditioning stretch or contraction prior to each event would be ideal for minimizing these effects (Proske et al., 2014), we did not wish to cue the participant to impending stimuli in case they attempted to voluntarily modify their response. Therefore, the tilting platform was oscillated through the same 2 s conditioning pattern of DF and plantarflexion single sine waves (one cycle at 2 Hz ± 0.6°, one cycle at 1 Hz ± 1.5° then two cycles at 4 Hz ± 0.5°) after each event to ensure that all stimuli ended with similar mechanical effects (Fig. 1 C). Participants were instructed not to unload the plate or resist platform movement during the oscillations. Participants then had to actively maintain the visual feedback within the target range for 1–10 s before the next stimulus was presented, ensuring an active contraction within a controlled target muscle length preceded each stimulus. Combined, the oscillations and pre‐stimulus contractions were expected to minimize any differences in muscle spindle stiction between threat conditions.
Figure 2. Ramp stretch reflex muscle activity and platform movement analyses.
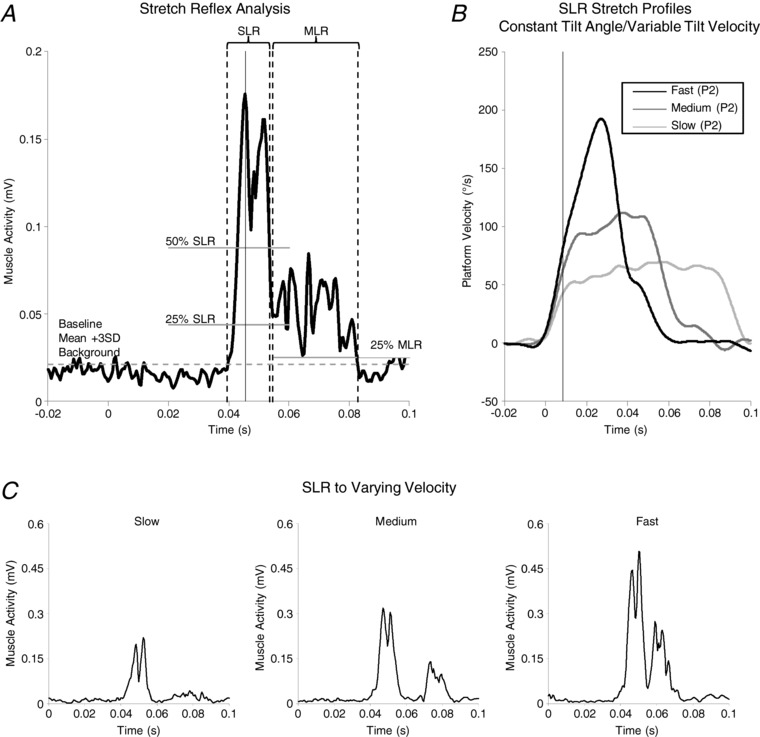
Soleus waveform averages (A) were used to establish analysis windows and significant points, such as SLR onset, peak and end, as well as MLR onset and end. A mean + 3 SD baseline threshold was established from a 100 ms period ending 10 ms before platform onset (dashed horizontal line) and was used to determine SLR onset (dashed vertical line). The peak of the SLR waveform (solid vertical line) was calculated from a fixed window (35–55 ms after platform onset). In some cases, the SLR did not return to baseline before MLR onset. In these cases, the lowest value of either the baseline mean + 3 SD, 25% SLR peak or 50% SLR peak reached in soleus rectified EMG before 65 ms post‐platform onset (continuous for 3 ms; 50% SLR peak in A) was used to define the end of the SLR (dashed vertical line). SLR onset and end were confirmed by visual inspection and SLR peak amplitude and latency were re‐calculated within the SLR window. MLR peak was calculated in a window spanning 5 ms after SLR end to 150 ms after platform onset. The MLR analysis window began (dashed vertical line) at the lowest point between SLR end and MLR peak and adjusted manually if necessary (adjusted here). MLR end (dashed vertical line) was defined as the point where the MLR dropped below the highest of either 25% MLR peak (solid horizontal line) or the baseline mean + 3 SD threshold used to determine SLR onset. SLR and MLR analysis windows were created for both LOW and HIGH trials and the earliest onsets and latest ends were used to set common analysis windows; analysis windows differed across participants. Peak platform movement parameters for SLR–specific velocity profiles (B) were referenced to the time of SLR peak amplitude (solid vertical line) by subtracting SLR onset latency from the latency of SLR peak. As shown with representative data, the SLR response increased with faster stretch speeds (C).
Table 1.
SLR–velocity scaling stimulus velocity and amplitude metrics
| Maximum achieved | |||||||
|---|---|---|---|---|---|---|---|
| Velocity (° s−1) by SLR peak | Protocol 1 | Protocol 2 | |||||
| Stimulus | Threat condition | Protocol 1 | Protocol 2 | Velocity (° s−1) | Amplitude (°) | Velocity (° s−1) | Amplitude (°) |
| Slow | LOW | 37.09 ± 2.04 | 37.02 ± 1.52 | 75.56 ± 0.35 | 5.46 ± 0.0023 | 78.37 ± 0.46 | 5.45 ± 0.0029 |
| HIGH | 39.68 ± 2.32 | 38.49 ± 1.71 | 75.41 ± 0.39 | 5.46 ± 0.0021 | 76.07 ± 0.39 | 5.44 ± 0.0022 | |
| Medium | LOW | 47.23 ± 2.23 | 55.65 ± 2.82 | 97.89 ± 0.65 | 5.51 ± 0.0034 | 117.46 ± 0.44 | 5.52 ± 0.0031 |
| HIGH | 50.26 ± 2.18 | 56.95 ± 2.80 | 96.55 ± 0.44 | 5.50 ± 0.0036 | 115.92 ± 0.34 | 5.51 ± 0.0028 | |
| Fast | LOW | 58.15 ± 2.77 | 71.95 ± 3.78 | 115.68 ± 0.39 | 5.54 ± 0.0041 | 193.83 ± 0.52 | 5.56 ± 0.0031 |
| HIGH | 57.89 ± 2.51 | 74.49 ± 3.80 | 114.11 ± 0.38 | 5.53 ± 0.0037 | 194.17 ± 0.35 | 5.56 ± 0.0028 | |
Values indicate the mean ± SEM.
Table 2.
MLR‐amplitude scaling stimulus velocity and amplitude metrics
| Maximum achieved | |||||||
|---|---|---|---|---|---|---|---|
| Amplitude (°) by MLR end | Protocol 1 | Protocol 2 | |||||
| Stimulus | Threat condition | Protocol 1 | Protocol 2 | Velocity (° s−1) | Amplitude (°) | Velocity (° s−1) | Amplitude (°) |
| Small | LOW | 3.06 ± 0.04 | 2.10 ± 0.02 | 91.44 ± 0.78 | 3.34 ± 0.0025 | 110.64 ± 0.66 | 2.24 ± 0.0020 |
| HIGH | 3.06 ± 0.03 | 2.10 ± 0.02 | 91.55 ± 0.42 | 3.33 ± 0.0019 | 110.65 ± 0.43 | 2.24 ± 0.0017 | |
| Moderate | LOW | 3.63 ± 0.22 | 4.60 ± 0.12 | 97.89 ± 0.65 | 5.51 ± 0.0034 | 193.83 ± 0.52 | 5.56 ± 0.0031 |
| HIGH | 3.66 ± 0.21 | 4.63 ± 0.12 | 96.55 ± 0.44 | 5.50 ± 0.0036 | 194.17 ± 0.35 | 5.56 ± 0.0028 | |
| Large | LOW | 3.85 ± 0.35 | 5.15 ± 0.21 | 97.44 ± 0.33 | 7.38 ± 0.0028 | 202.72 ± 0.55 | 7.48 ± 0.0024 |
| HIGH | 3.88 ± 0.35 | 5.20 ± 0.21 | 97.84 ± 0.46 | 7.37 ± 0.0019 | 202.42 ± 0.55 | 7.48 ± 0.0023 | |
Values indicate the mean ± SEM.
Within each height condition, the DF stimuli were randomly intermixed with five plantarflexion catch trials (5° at 80° s−1; not analysed) and 10 Achilles tendon‐taps to evoke t‐reflexes. Tendon‐taps were delivered using a magnetic linear motor (motor: LinMot PS01 –23 × 80; controller: LinMot E2000‐AT; software: LinMot, version 1.3.12; NTI Ltd, Elkhorn, WI, USA) stroking ∼1 cm in 10 ms (Davis et al., 2011; Horslen et al., 2013) and tendon‐tap impact force was monitored (Isotron Dynamic Force Sensor, Endevco‐Meggitt, Irvine, CA, USA; sampled at 5000 Hz; Power 1401 with Spike2).
Measures
Psychological and autonomic states were assessed using questionnaires and electrodermal activity (EDA), respectively. Prior to each trial, participants rated how confident they felt that they could maintain balance and avoid a fall (0–100%; higher values indicate greater confidence). After each trial, participants reported their experienced fear of falling (0–100%; higher values indicate greater fear), perceived stability (0–100%; higher scores indicate greater perceived stability) and state anxiety (16‐item questionnaire; 1–9 Likert scale for each question summed to a maximum of 144; higher scores indicate greater anxiety). These questionnaires have previously been shown to have moderate to high reliability in a height‐induced postural threat task for young healthy adults (Hauck, 2011). Galvanic skin conductance was measured (model 2501; Cambridge Electronic Design Ltd; sampling: 100 Hz) across the palm of the left hand with disposable electrodes (Kendall H59P Cloth Electrodes; Covidien, Dublin, Ireland) placed on the thenar and hypothenar eminences to quantify EDA, which indicates degree of sympathetic arousal (Boucsein et al., 2012). To avoid motor artefacts, EDA was clipped to 1 s bins immediately preceding each stimulus and averaged across trials within each threat condition (per Horslen et al., 2013).
Muscle activity was recorded with surface electromyography (EMG) from the right soleus (SOL) and tibialis anterior (TA) muscles with electrodes placed ∼2 cm apart over the muscle bellies (Kendall H59P). EMG data were bandpass filtered between 10 and 1500 Hz (Telemyo 2400 G2; Noraxon, Scottsdale, AZ, USA), sampled at 3000 Hz, and A/D converted at 2000 Hz (Power 1401 with Spike 2). EMG data were filtered offline (10–500 Hz second‐order Butterworth dual‐passed; Spike2) and baseline corrected.
Analyses
A custom algorithm (Spike2) was used to determine onset of platform displacement, reflex latencies and amplitudes, as well as to measure platform displacement parameters. Onset of platform movement for each stretch was determined from the load cell nearest to the participant's toes, and was set to the time where the load exceeded (min 6 ms duration) a mean ± 3 SD threshold from a 100 ms period preceding the event trigger; onset was confirmed by visual inspection of load, displacement and velocity data.
Similar to Horslen et al. (2013), SOL t‐reflexes were measured as peak‐to‐peak amplitudes of unrectified EMG and screened for changes in tap force. Individual stimuli were excluded post hoc if they were evoked with peak tap force outside of a mean ± 2 SD range of the least variable trial (defined as the trial with the smallest range of tap forces) (Davis et al., 2011; Horslen et al., 2013). t‐reflex amplitudes from stimuli that passed tap‐force screening were averaged within a threat condition. A minimum of five tendon‐taps per condition was required for the participant's t‐reflex data to be included in the final analysis. SOL and TA background muscle activity (preceding both t‐reflexes and ramp stretches) was calculated as the integrated area of the rectified EMG from a 100 ms period ending 10 ms before stimulus onset.
Ramp‐and‐hold stretch profiles were time‐locked to onset of platform movement to create separate waveform averages of rectified SOL EMG for SLR and MLR comparisons. Similar to Corna et al. (1995), participant‐specific SLR and MLR analysis windows were used to assess individual reflexes in each threat condition. SLRs were analysed from fixed‐amplitude, variable‐velocity stretches (for SLR–velocity scaling) and MLRs were analysed from variable‐amplitude, fixed‐velocity stretch profiles (for MLR‐length scaling). SLR onset, latency of peak rectified EMG and end time, as well as MLR onset and end, were determined with respect to background muscle activity for each threat condition (Fig. 2 A). Participant‐specific analysis window bounds were set by the earliest reflex onset and latest end across threat conditions for both SLR and MLR, and were confirmed by visual inspection. Latencies to SLR peak were averaged across conditions to obtain a value for use in platform velocity analyses. Across participants, bounds of the analysis windows were: SLR onset mean ± SEM (range) = 40.2 ± 0.5 ms (34–46 ms); SLR end = 57.9 ± 0.61 ms (49.5–64.5 ms); SLR peak = 48 ± 0.5 ms (41.3–54 ms); MLR onset = 60.4 ± 0.9 ms (50.5–73.5 ms); and MLR end = 85.1 ± 2.6 ms (67–145.5 ms).
SLR and MLR amplitudes were calculated for individual reflex events as the integrated area of rectified SOL EMG within defined analysis windows (Fig. 2 A) and averaged within each threat condition for main effects analyses. Similar to Corna et al. (1995), reflex area was measured even if no reflex was evoked and reflex areas were not referenced to background muscle activity. Individual reflexes were also related to corresponding platform velocity (described below) to examine scaling effects. Separate from establishing analysis window bounds, SLR and MLR onset latencies were measured for each event where a reflex was evoked. Onset latencies were determined as the point where rectified SOL EMG remained above a mean ± 3 SD threshold for a minimum of 3 ms and were confirmed by visual inspection; baseline activity was measured from a 100 ms period ending 10 ms before platform onset.
Platform velocity was measured in relation to latency of SLR peak activity to characterize SLR–velocity scaling. There is an inherent delay between mechanical stretch of a muscle and stretch reflex activity as a result of neural transmission and central processing. We operationalized SLR onset latency as the reflex delay and subtracted this delay from the latency to SLR peak to set the bounds for measurement of peak platform velocity (Fig. 2 A and B). We assumed that velocity‐dependent scaling of the SLR would be most prominent up to SLR peak, before differences in amplitude start to emerge. The SLR–velocity relationship was quantified for each participant and in each threat condition as the slope of the line of best fit between peak stretch velocity and SLR amplitude (custom Matlab script; Matlab R2017a; MathWorks, Natick, MA, USA). Changes in slope across threat conditions were used to assess threat effects on scaling. Because this method assumes the relationship between SLR amplitude and platform velocity is linear, a minimum regression value of r ≥ 0.25 in both threat conditions was required for a participant to be included in the analysis.
Compared to Protocol 1, Protocol 2 generated a larger difference in actual tilt angles between small and moderate tilts; however, a similar difference (<1°) was achieved between moderate and large tilts (Table 2). Small tilt amplitudes evoked MLRs in only 50% of participants, and only two of those participants had more than one MLR in both low and high threat conditions. It should be noted that a failure to evoke an MLR with small tilt angles (20 ms duration) is consistent with prior reports in the upper limb that reported minimum duration dependency of the MLR up to 40 ms (Lee and Tatton, 1982; Lewis et al., 2005; Schuurmans et al., 2009). As a result of the inability to generate sufficient change in actual tilt angles in Protocol 1 and 2, as well as the low number MLRs evoked for small tilts across threat conditions, no further analysis on MLR scaling was performed.
Statistical analysis
It was hypothesized that mean t‐reflex, SLR and MLR amplitudes would be larger in the HIGH compared to LOW postural threat condition. Furthermore, it was also hypothesized that the slope of the SLR–velocity relationship would be higher in the HIGH compared to LOW threat condition. We tested our hypotheses by evaluating differences in mean SLR and MLR amplitudes, t‐reflex amplitude and SLR–velocity slope between threat conditions with paired samples t tests (Matlab R2017a). We also evaluated changes in SOL/TA background muscle activity, EDA and psychological state questionnaires between threat conditions with paired samples t tests to assess the potency of our threat manipulation. In all cases, alpha was set to 0.05 and effect size was calculated as:
where t is the t test statistic and d.f. is the sample degrees of freedom. Post hoc correlations between changes in reflex amplitude and changes in background muscle activity were performed with SPSS (IBM Corp., Armonk, NY, USA). Normality of the distribution of change scores was assessed with Kolmogorov–Smirnov tests. Pearson correlations were used when normality assumptions were met. Spearman's rho correlations on rank ordered data were used for non‐normal data sets.
Results
Four out of 40 participants were excluded from the EDA analysis as a result of technical limitations and one participant failed to answer the fear of falling and perceived stability questions. Exposure to the HIGH threat condition evoked significant changes in participants’ psychological and autonomic states. Participants had significantly lower balance confidence and felt less stable in the HIGH compared to LOW threat condition (mean ± SEM balance confidence: LOW: 92.7 ± 1.8%; HIGH: 73.5 ± 3.2%; t 39 = 7.10, P < 0.001, r = 0.751; perceived stability: LOW: 90.1 ± 2.1%; HIGH: 66.6 ± 3.6%; t 38 = 7.62, P < 0.001, r = 0.778). Participants were also significantly more fearful of falling (LOW: 4.1 ± 1.2%; HIGH: 31.4 ± 4.4%; t 38 = −6.79, P < 0.001, r = 0.741) and anxious (LOW: 29.2 ± 1.9; HIGH: 46.9 ± 3.9; t 39 = −5.38, P < 0.001, r = 0.653) and had higher EDA (LOW: 20.61 ± 1.47 μS; HIGH: 27.70 ± 1.87 μS; t 35 = −6.27, P < 0.001, r = 0.727) in the HIGH compared to LOW condition.
Soleus tendon‐tap, short‐latency and medium‐latency reflex amplitudes were all increased with postural threat. Thirty‐one out of 40 participants met SOL t‐reflex tap‐force screening criteria; t‐reflex latencies were similar between height conditions (LOW: 39.3 ± 0.6 ms; HIGH: 39.6 ± 0.6 ms), yet amplitudes were 16.9% larger (representative subject in Fig. 3 A; group means in Fig. 3 C) in the HIGH compared to LOW condition (t 30 = −2.84, P = 0.008, r = 0.46). Platform DF ramp‐and‐hold stretches evoked SLR responses with onset latencies of 42.3± 0.5 ms and 42.3± 0.5 ms in the LOW and HIGH conditions, respectively, and MLR responses with latencies 63.9± 1.3 ms (LOW) and 64.5± 1.4 ms (HIGH). Mean SLR amplitudes across all velocity profiles were significantly larger in the HIGH compared to LOW condition (SLR: t 39 = −2.24, P = 0.031, r = 0.338), with an average increase of 11% (representative subject in Fig. 3 B; group means in Fig. 3 C). Similarly, mean SOL MLRs across all amplitude profiles were significantly larger in the HIGH compared to LOW condition (t 39 = −2.35, P = 0.024, r = 0.352), with an average increase of 9.5% (Fig. 3 B and C).
Figure 3. Height‐induced threat increased tendon‐tap and ramp stretch reflex amplitudes.
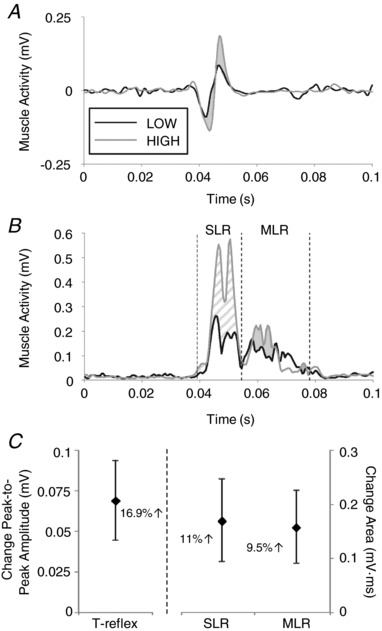
Waveform averages of unrectified t‐reflexes (A) and rectified SLR and MLR responses (B) from a single representative participant. LOW condition traces are drawn in black and HIGH in grey, with vertical dashed lines indicating the bounds of the region of interest for SLR and MLR traces and shading for effect to highlight where the HIGH response is greater than LOW (SLR patterned, MLR solid shading). Groupwide mean differences are shown in (C), with error bars indicating SE and the percentage change given for reference.
SOL and TA background muscle activity significantly increased with height‐induced threat. SOL background muscle activity increased from 1.36 ± 0.11 μV · s in the LOW to 1.45 ± 0.11 μV · s in the HIGH threat condition (6.4% change; t 39 = −3.18, P = 0.003, r = 0.454), and TA background activity increased from 0.26 ± 0.06 μV · s in the LOW to 0.49 ± 0.10 μV · s in the HIGH threat condition (84.8% change; t 39 = −2.35, P = 0.024, r = 0.352). Correlations between changes in background muscle activity and reflex amplitudes were examined post hoc to determine whether height‐induced changes in reflexes were related to changes in background muscle activity. Across participants, changes in SOL background activity with threat were not associated with height‐induced changes in SLR area (Spearman's rho = 0.225, P = 0.164) or t‐reflex amplitude (Pearson's r = 0.20, P = 0.280), although they were correlated with changes in MLR area (Pearson's r = 0.377, P = 0.017). Similarly, changes in TA background activity were not linked to changes in SLR (Spearman's rho = 0.203, P = 0.209) or MLR area (rho = 0.126, P = 0.439), nor to t‐reflexes across threat conditions (rho = 0.302, P = 0.099).
It was possible to investigate velocity scaling of SLR amplitude because the three velocity profiles achieved different tilt velocities by the latency of SLR peak in both Protocols 1 and 2 (Tables 1 and 2). SLR amplitude increased with increasing platform velocity (Figs. 2 C and 4 A) and the relationship scaled for 22 participants (Protocol 1, n = 7; mean r = 0.502, Protocol 2, n = 15; mean r = 0.54). The SLR–velocity slope was significantly steeper in the HIGH compared to LOW condition [LOW: 0.041 ± 0.005 (mV·ms)/(°/s); HIGH: 0.052 ± 0.007 (mV·ms)/(°/s); t 21 = −2.73, P = 0.013, r = 0.511] (Fig 4 B); on average, SLR–velocity slopes were 36.1% steeper in the HIGH compared to LOW condition.
Figure 4. Postural threat increased gain of SLR–tilt velocity relationship.
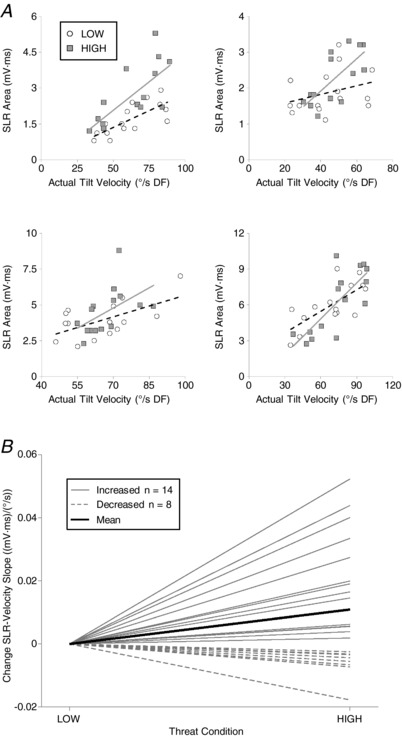
SLR amplitude was plotted against calculated platform velocity to calculate the slope (or gain) of the SLR–velocity relationship (A). As demonstrated in four representative participants (one from Protocol 1 and two from Protocol 2), the slope of the relationship was steeper in the HIGH (grey squares, solid line) compared to LOW (open circles, dashed line) threat condition. Change in SLR–velocity slope (HIGH–LOW) for all participants is shown in (B), with participants who increased gain indicated by solid grey lines, decreased gain indicated by dashed grey lines, and groupwide mean indicated in solid black.
Discussion
The present study aimed to determine how soleus SLR and MLR responses to ramp stretch, as well as tendon‐tap reflexes, are affected by height‐induced postural threat. We examined threat‐effects on SOL SLR and MLR responses to ramp‐and‐hold stretches of different amplitudes and velocities. The onset and pattern of SOL SLR and MLR responses observed in the low threat conditions were consistent with the reflexes reported previously using similar stretch stimuli in sitting (Gottlieb and Agarwal, 1979; Allum and Mauritz, 1984), standing (Corna et al., 1995, 1996; Allum et al., 1993; Carpenter et al., 2004) and walking (Grey et al., 2001). As hypothesized, postural threat increased SOL SLR and MLR amplitudes by 11% and 9.5%, respectively. Changes in reflex amplitudes with threat were either uncorrelated, or weakly correlated (maximum explained variance = 14%), with changes in background muscle activity in agonist or antagonist muscles. Furthermore, consistent with our hypothesis of an increased SLR dynamic gain, the slope of the SLR amplitude–stretch velocity relationship was increased by 36.1% with a height‐induced postural threat.
Our observation of increased mean SLR and MLR amplitudes differs from a previous observation made by our group where SLRs were not affected by height‐induced postural threat (Carpenter et al., 2004). Methodological differences between studies may explain the divergent findings. Carpenter et al. (2004) had a smaller sample size (n = 10) and used a slower tilt velocity (50° s−1) than that used in either protocol reported in the present study (∼75–200° s−1 DF). As shown in the present study, there is a greater contrast between SLR amplitudes across threat conditions at faster stretch velocities (Fig. 4 A). Furthermore, Carpenter et al. (2004) made participants stand with both feet on the tilting platform, whereas we only tilted the right foot. We used unilateral stimuli to reduce the confound of perturbing standing balance and minimize reactive postural responses in our experiment. Although unilateral stretches should evoke relatively smaller MLR responses (Corna et al., 1996), the bilateral perturbations used by Carpenter et al. (2004) would be more destabilizing and SLRs were probably suppressed to accommodate balance stabilization (Keshner et al., 1987). By contrast, we hypothesize that unilateral right‐side tilts and stretch reflexes are less destabilizing, and therefore less likely to be suppressed. As such, it is possible that stretch reflexes were relatively larger in the present study and, given the greater sample size and faster stretches used, our procedures may have been more sensitive to gain changes in SOL stretch reflexes than those used by Carpenter et al. (2004).
Consistent with previous human stretch reflex studies, we found that SLR amplitude scaled to stretch velocity. Previous studies using bilaterally‐applied support surface tilts (Allum et al., 1993) or translations (Diener et al., 1988) have shown that balance correcting responses and non‐balance correcting SLRs evoked during standing are larger with faster stretch velocities. Mean regression values between SOL SLR amplitude and platform velocity reported in the present study (r = 0.502 and 0.54 for Protocols 1 and 2, respectively) are comparable to the regressions between medial gastrocnemius SLR and translation velocity (r = 0.6319) (Diener et al., 1988) or later SOL responses (80–120 ms) and tilt velocity (r = 0.77) (Allum et al., 1993). The observed SLR–velocity scaling to unilateral stretch of SOL when standing is also consistent with observations of scaling from seated (Gottlieb and Agarwal, 1979; Allum and Mauritz, 1984) or walking participants (Grey et al., 2001), as well as from studies of upper‐limb reflexes (Lewis et al., 2005; Schuurmans et al., 2009; Meskers et al., 2010). Combined, these studies suggest that the SLR scales to stretch velocity, probably as a result of Ia afferents originating from spindle dynamic bag fibres.
Our novel contribution is the observation of threat‐dependent changes in scaling of SOL SLR responses to stretch velocity in standing humans. This observation is consistent with the theory that humans modulate sensorimotor gains to suit the context in which they stand. In this case, context changes in the form of a postural threat had a significant influence on the within‐subjects SLR–velocity relationship. On average, there was a 36.1% steeper SLR–velocity slope when participants were exposed to the HIGH postural threat condition (Fig. 4 B). This suggests the dynamic, velocity sensitive component of the stretch reflex is facilitated with postural threat.
Consistent with our previous work, we replicate an increase in t‐reflex amplitude with height‐induced threat (Davis et al., 2011; Horslen et al., 2013). The magnitude of threat‐related changes is relatively smaller in the present study than in our previous work: present study 0.07 mV or 16.9% increase compared to 0.1 mV change in Davis et al. (2011) and 0.558 mV or 35% in Horslen et al. (2013). We speculate that these differences may be a result of subtle task differences rather than different responses to threat because all studies used similar height paradigms and induced comparable changes in threat, anxiety and arousal. Our use of online visual feedback in the present study may have led to more conscious control of balance and/or altered attentional focus across threat conditions compared to previous studies in our laboratory. Postural threat is known to affect attention to movement (Huffman et al., 2009; Zaback et al., 2016) and changes in attention to movement have been shown to influence spindle discharge rates (Ribot‐Ciscar et al., 2007; Hospod et al., 2009). By guiding attention toward visual feedback, we may have muted attention changes that may have contributed to relatively larger t‐reflex changes in previous work (Davis et al., 2011; Horslen et al., 2013). Nonetheless, consistent observations of increased t‐reflexes across studies and lack of significant correlations between t‐reflex changes and background muscle activity suggests that threat‐related changes in stretch reflexes are not dependent on changes in background muscle activity or attention.
We hypothesize that a threat‐induced increase in muscle spindle sensitivity via elevated gamma dynamic (γD) fusimotor activity underlies the increase in t‐reflex, SLR and MLR amplitudes, and also the steeper SLR–velocity slopes in the present study, as well as previous studies (Davis et al., 2011; Horslen et al., 2013). Prochazka (1989) argued ‘the full power of fusimotion is reserved for novel and/or difficult tasks, where strong dynamic action causes very large increases in spindle primary responses to muscle displacement’. In the present study, participants were threatened with the risk of falling from a 3.5 m high platform, where a loss of balance would probably lead to injury. Increases in γD activity would lead to an increase in the gain of the stretch velocity–spindle afferent firing relationship. Greater afferent responses (more spikes and/or greater change in firing rate) with greater velocity would lead to larger reflex responses, and a steeper velocity–reflex amplitude relationship. The magnitude of stretch reflex increases in the current study (9.5–16.9%) is consistent with the 10% increase in spindle discharge rate to a soleus tendon tap with direct γD fusimotor stimulation in the anaesthetized cat (Morgan et al., 1984). There is now mounting evidence from human studies indicating that both muscle spindle static and dynamic sensitivity can be altered. Ackerley et al. (2017) recently used microneurographic recordings of human ankle dorsiflexor muscle spindle Ia afferent activity to demonstrate increases in spindle dynamic sensitivity with unpleasant emotional state (sad music). Similarly, microneurographic recordings have been used to demonstrate that both static and dynamic sensitivity can be increased when attending to changes in limb position or movement velocity (Hospod et al., 2007; Ribot‐Ciscar et al., 2009). Combined, the data from our present study and the microneurographic evidence suggest that humans are capable of modulating muscle spindle sensitivity, independent of background muscle activity, probably via changes to fusimotor activity. However, we acknowledge that both our reflex and the microneurography evidence to support this claim is indirect because it is not currently possible to either directly stimulate or record from fusimotor neurons in humans.
Other potential mechanisms that could explain our observation of larger reflexes in the HIGH threat condition include central reflex facilitation or peripheral changes in muscle spindle tension (independent of fusimotor drive). Recent transcranial magnetic stimulation evidence suggests that, although SOL corticospinal excitability is increased with task difficulty, height‐induced postural threat does not lead to larger SOL motor evoked potentials (Tokuno et al., 2018). This suggests that descending cortical facilitation is probably not mediating our change in stretch reflexes. However, serotonergic and noradrenergic projections from brainstem centres, including the reticular formation, can cause persistent inward currents (PICs) and subsequent plateau potentials in motor neurons (Johnson and Heckman, 2010), which can amplify and prolong the effects of Ia synaptic input to motor neurons (Lee and Heckman, 2000). Functionally, this may serve to decrease motor neuron firing thresholds and cause units to fire with relatively less synaptic input (Bawa and Murnaghan, 2009), meaning that a given stimulus may recruit more motor units and increase the magnitude of reflexes recorded with surface EMG. PICs might be driven by descending inputs from supraspinal regions, such as the reticular formation or vestibular nuclei, which are assumed to be excited by fear and/or anxiety networks activated by postural threat (Naranjo et al., 2016; Staab et al., 2013; Horslen et al., 2014). However, PICs could also manifest as a consequence of increased muscle spindle sensitivity. PICs build‐up with increasing Ia synaptic input (ElBasiouny et al., 2006) and triceps surae muscle spindles are considered to be sensitive to the small amplitude stretches that occur during postural sway (Peters et al., 2017). Therefore, sway‐related Ia afferent activity would be expected to increase with increased muscle spindle sensitivity in unconstrained standing. Accordingly, increased muscle spindle sensitivity may facilitate stretch reflex amplitudes both through larger afferent responses to the plate tilt, as well as by increasing motor neuron excitability as a consequence of increased Ia afferent traffic related to standing upright.
Alternatively, the changes in t‐reflex, SLR and MLR amplitudes might be explained by changes in muscle spindle sensitivity because of stiction, as opposed to independent fusimotor activity with increased postural threat. Stiction, or passive muscle spindle contractile fibre tension as a result of the formation of stable actin‐myosin cross‐bridges in intrafusal muscle fibres after contraction can lead to larger stretch reflexes independent of central modulation or fusimotor drive (Proske et al., 1993). Unfortunately, we could not make participants perform a conditioning contraction prior to each stretch to ensure that spindles were in a comparable state before each stimulus and between threat conditions (Proske et al., 2014). However, the platform was re‐set with the same oscillating pattern after each stimulus to attempt to break any after effects from the previous stimulus. Although this cannot account for changes in muscle spindle state when the participant stood quietly between stimuli, it does ensure that the effects cannot be explained by the presentation order of the stimuli.
Increasing the gain of sensory systems under threatening contexts may serve as a protective response to reduce movement and improve detection of imposed movements (Balaban, 2002). Although changes to spindle sensitivity have been proposed as a means to increase somatosensory gain to supraspinal centres under challenging/threatening conditions (Llewelyn et al., 1990), the lack of evidence for early increases in evoked cortical responses to tendon‐taps with height‐induced threat (Davis et al., 2011) suggests that increased somatosensory information is used by subcortical centres to facilitate balance and other protective reflexes. Increases in muscle spindle sensitivity (as suggested in the present study), in conjunction with increased vestibular gain (Horslen et al., 2014; Naranjo et al., 2015, 2016; Lim et al., 2017) and altered Ib reflexes (Horslen et al., 2017), might contribute to larger balance‐correcting responses to postural perturbations, as typically observed with increased postural threat (Brown and Frank, 1997; Carpenter et al., 2004; Cleworth et al. 2016), which are considered to involve supraspinal pathways (Horak and Macpherson, 2011). Similarly, increased muscle spindle sensitivity would enable people to maintain, or increase, the volume of muscle spindle afferent feedback despite reducing actual sway with threat. People tend to reduce amplitude and increase frequency of centre of pressure (Carpenter et al., 1999) and centre of mass displacements (Carpenter et al., 2001) when standing at the edge of an elevated platform; interestingly, despite actual reductions in amplitude, people perceive themselves to sway just as much in HIGH and LOW threat conditions (Cleworth and Carpenter, 2016). It has been argued that people gather balance‐relevant sensory information from normal postural sway (Carpenter et al., 2010; Murnaghan et al., 2011) and that increasing muscle spindle sensitivity (and other balance‐relevant senses; e.g. vestibular) could permit reductions of postural sway without compromising sensory feedback (Horslen et al., 2013, 2014); this may explain why changes in movement perception do not follow actual changes in movement.
In conclusion, the present study provides novel human evidence of threat‐related increases in SLR and MLR to ramp‐and‐hold stretches, as well as increased gain of dynamic, velocity‐dependent stretch reflexes in a posturally engaged muscle to increased postural threat. We interpret these data as indirect evidence in support of increased muscle spindle sensitivity, possibly via independent dynamic fusimotor drive. We argue that context‐dependent scaling of stretch reflexes forms part of a multisensory tuning process where acquisition and/or processing of balance‐relevant sensory information is continuously primed to facilitate feedback control of standing balance in challenging balance scenarios.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
All data were collected in the Neural Control of Posture and Movement Lab at the University of British Columbia. BCH, JTI, JSB and MGC contributed to the conception of the study. BCH, MZ and MGC contributed to the study design. BCH and MZ collected the data. BCH analysed the data and drafted the manuscript under the supervision of MGC. All authors contributed to interpretation of the data and critically reviewed the manuscript. All authors approved the manuscript submitted for publication and agree to be held accountable for all aspects of the work presented here.
Funding
The authors thank the Natural Sciences and Engineering Research Council of Canada (NSERC) for financial assistance. Specifically, we acknowledge NSERC 326910 to MGC, NSERC 356026 to JSB, NSERC 183666 to JTI, and doctoral awards to BCH and MZ.
Biography
Brian Horslen is a Banting postdoctoral fellow in the Wallace H. Coulter Department of Biomedical Engineering at Emory University and Georgia Tech in Atlanta, GA, USA. He has a PhD and MSc from the University of British Columbia and a BSc from the University of Waterloo. His current research focuses on how mechanical properties of muscle tissue shape muscle spindle sensory encoding, and how this information is used for control of upright standing balance. His goal is to develop a framework for understanding how sensory information is gathered and integrated to control standing balance.

Edited by: Janet Taylor & Richard Carson
References
- Ackerley R, Aimonetti JM & Ribot‐Ciscar E (2017). Emotions alter muscle proprioceptive coding of movements in humans. Sci Rep 7, 8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkin AL, Frank JS, Carpenter MG & Peysar GW (2000). Postural control is scaled to level of postural threat. Gait Posture 12, 87–93. [DOI] [PubMed] [Google Scholar]
- Allum JH, Honegger F & Schicks H (1993). Vestibular and proprioceptive modulation of postural synergies in normal subjects. J Vestib Res 3, 59–85. [PubMed] [Google Scholar]
- Allum JH & Mauritz KH (1984). Compensation for intrinsic muscle stiffness by short‐latency reflexes in human triceps surae muscles. J Neurophysiol 52, 797–818. [DOI] [PubMed] [Google Scholar]
- Balaban CD (2002). Neural substrates linking balance control and anxiety. Physiol Behav 77, 469–475. [DOI] [PubMed] [Google Scholar]
- Bawa P & Murnaghan CD (2009). Motor unit rotation in a variety of human muscles. J Neurophysiol 102, 2265–2272. [DOI] [PubMed] [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S, Ben‐Shakhar G, Roth WT, Dawson ME, Filion DL & Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures (2012). Publication recommendations for electrodermal measurements. Psychophysiology 49, 1017–1034. [DOI] [PubMed] [Google Scholar]
- Brown LA & Frank JS (1997). Postural compensations to the potential consequences of instability: kinematics. Gait Posture 6, 89–97. [Google Scholar]
- Burke D, Gandevia SC & McKeon B (1983). The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol 339, 535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AD, Dakin CJ & Carpenter MG (2009). Postural responses explored through classical conditioning. Neuroscience 164, 986–997. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Adkin AL, Paton A & Allum JHJ (2004). Influence of postural anxiety on postural reactions to multi‐directional surface rotations. J Neurophysiol 92, 3255–3265. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS & Silcher CP (1999). Surface height effects on postural control: a hypothesis for a stiffness strategy for stance. J Vestib Res 9, 277–286. [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP & Peysar GW (2001). The influence of postural threat on the control of upright stance. Exp Brain Res 138, 210–218. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Murnaghan CD & Inglis JT (2010). Shifting the balance: evidence of an exploratory role for postural sway. Neuroscience 171, 196–204. [DOI] [PubMed] [Google Scholar]
- Cleworth TW & Carpenter MG (2016). Postural threat influences conscious perception of postural sway. Neurosci Lett 620, 127–131. [DOI] [PubMed] [Google Scholar]
- Cleworth TW, Chua R, Inglis JT & Carpenter MG (2016). Influence of virtual height exposure on postural reactions to support surface translations. Gait Posture 47, 96–102. [DOI] [PubMed] [Google Scholar]
- Cooper S (1961). The responses of the primary and secondary endings of muscle spindles with intact motor innervation during applied stretch. Q J Exp Physiol Cogn Med Sci 46, 389–398. [DOI] [PubMed] [Google Scholar]
- Corna S, Galante M, Grasso M, Nardone A & Schieppati M (1996). Unilateral displacement of lower limb evokes bilateral EMG responses in leg and foot muscles in standing humans. Exp Brain Res 109, 83–91. [DOI] [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A & Schieppati M (1995). Selective depression of medium‐latency leg and foot muscle responses to stretch by an alpha 2‐agonist in humans. J Physiol 484, 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A & Matthews PBC (1964). The effects of stimulation of static and dynamic fusimotor fibres on the response to stretching of the primary endings of muscle spindles. J Physiol 174, 109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JR, Horslen BC, Nishikawa K, Fukushima K, Chua R, Inglis JT & Carpenter MG (2011). Human proprioceptive adaptations during states of height‐induced fear and anxiety. J Neurophysiol 106, 3082–3090. [DOI] [PubMed] [Google Scholar]
- Diener HC, Horak FB & Nashner LM (1988). Influence of stimulus parameters on human postural responses. J Neurophysiol 59, 1888–1905. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ & Mushahwar VK (2006). Simulation of Ca2+ persistent inward currents in spinal motoneurones: mode of activation and integration of synaptic inputs. J Physiol 570, 355–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb GL & Agarwal GC (1979). Response to sudden torques about ankle in man: myotatic reflex. J Neurophysiol 42, 91–106. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, Nielsen JB & Sinkjaer T (2001). Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol 534, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck LJ (2011). Understanding the influence of fear of falling on clinical balance control – efforts in fall prediction and prevention. PhD Dissertation. UWSpace, http://hdl.handle.net/10012/6009. [Google Scholar]
- Horak FB & Macpherson JM (2011). Postural orientation and equilibrium In Compr Physiol, Supplement 29: Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems, ed. Rowell LB. & Shepherd JT, pp. 255–292. Oxford, NY. [Google Scholar]
- Horslen BC, Dakin CJ, Inglis JT, Blouin JS & Carpenter MG (2014). Modulation of human vestibular reflexes with increased postural threat. J Physiol 592, 3671–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horslen BC, Inglis JT, Blouin JS & Carpenter MG (2017). Both standing and postural threat decrease Achilles tendon reflex inhibition from tendon electrical stimulation. J Physiol 595, 4493–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horslen BC, Murnaghan CD, Inglis JT, Chua R & Carpenter MG (2013). Effects of postural threat on spinal stretch reflexes: evidence for increased muscle spindle sensitivity? J Neurophysiol 110, 899–906. [DOI] [PubMed] [Google Scholar]
- Hospod V, Aimonetti J, Roll J & Ribot‐Ciscar E (2007). Changes in Human Muscle Spindle Sensitivity during a Proprioceptive Attention Task. J Neurosci 27, 5172–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JL, Horslen BC, Carpenter MG & Adkin AL (2009). Does increased postural threat lead to more conscious control of posture? Gait Posture 30, 528–532. [DOI] [PubMed] [Google Scholar]
- Johnson MD & Heckman CJ (2010). Interactions between focused synaptic inputs and diffuse neuromodulation in the spinal cord. Ann NY Acad Sci 1198, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshner EA, Allum JH & Pfaltz CR (1987). Postural coactivation and adaptation in the sway stabilizing responses of normals and patients with bilateral vestibular deficit. Exp Brain Res 69, 77–92. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Bouyer LJ, Bouffard J, Jin A, Christiansen L, Nielsen JB & Scott SH (2018). Variable impact of tizanidine on the medium latency reflex of upper and lower limbs. Exp Brain Res 236, 665–677. [DOI] [PubMed] [Google Scholar]
- Lee RH & Heckman CJ (2000). Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20, 6734–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RG & Tatton WG (1982). long latency reflexes to imposed displacements of the human wrist: dependence on duration of movement. Exp Brain Res 45, 207–216. [DOI] [PubMed] [Google Scholar]
- Lewis G, Perreault E & MacKinnon C (2005). The influence of perturbation duration and velocity on the long‐latency response to stretch in the biceps muscle. Exp Brain Res 163, 361–369. [DOI] [PubMed] [Google Scholar]
- Lim SB, Cleworth TW, Horslen BC, Blouin JS, Inglis JT & Carpenter MG (2017) Postural threat influences vestibular‐evoked muscular responses. J Neurophysiol 117, 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn M, Yang JF & Prochazka A (1990). Human H‐reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res 83, 22–28. [DOI] [PubMed] [Google Scholar]
- Meskers CM, Schouten A, Rich ML, Groot J, Schuurmans J & Arendzen JH (2010). Tizanidine does not affect the linear relation of stretch duration to the long latency M2 response of m. flexor carpi radialis. Exp Brain Res 201, 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Prochazka A & Proske U (1984). Can fusimotor activity potentiate the responses of muscle spindles to a tendon tap? Neurosci Lett 50, 209–215. [DOI] [PubMed] [Google Scholar]
- Murnaghan CD, Horslen BC, Inglis JT & Carpenter MG (2011). Exploratory behavior during stance persists with visual feedback. Neuroscience 195, 54–59. [DOI] [PubMed] [Google Scholar]
- Naranjo EN, Allum JH, Inglis JT & Carpenter MG (2015). Increased gain of vestibulospinal potentials evoked in neck and leg muscles when standing under height‐induced postural threat. Neuroscience 293, 45–54. [DOI] [PubMed] [Google Scholar]
- Naranjo EN, Cleworth TW, Allum JH, Inglis JT, Lea J, Westerberg BD & Carpenter MG (2016). Vestibulo‐spinal and vestibulo‐ocular reflexes are modulated when standing with increased postural threat. J Neurophysiol 115, 833–842. [DOI] [PubMed] [Google Scholar]
- Nardone A, Grasso M, Giordano A & Schieppati M (1996). Different effect of height on latency of leg and foot short‐ and medium‐ latency EMG responses to perturbation of stance in humans. Neurosci Lett 206, 89–92. [DOI] [PubMed] [Google Scholar]
- Peters RM, Dalton BH, Blouin JS & Inglis JT (2017). Precise coding of ankle angle and velocity by human calf muscle spindles. Neuroscience 349, 98–105. [DOI] [PubMed] [Google Scholar]
- Pierrot‐Desseilligny E & Burke D (2012). The Circuitry of the Human Spinal Cord Spinal and Corticospinal Mechanisms Of Movement. Cambridge University Press, Cambridge. [Google Scholar]
- Prochazka A (1989). Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33, 281–307. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Zangger P & Appenteng K (1985). ‘Fusimotor set’: new evidence for α‐independent control of γ‐motoneurones during movement in the awake cat. Brain Res 339, 136–140. [DOI] [PubMed] [Google Scholar]
- Proske U, Tsay A & Allen T (2014). Muscle thixotropy as a tool in the study of proprioception. Exp Brain Res 232, 3397–3412. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL & Gregory JE (1993). Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol 41, 705–721. [DOI] [PubMed] [Google Scholar]
- Ribot‐Ciscar E, Hospod V, Roll J & Aimonetti J (2009). Fusimotor drive may adjust muscle spindle feedback to task requirements in humans. J Neurophysiol 101, 633–640. [DOI] [PubMed] [Google Scholar]
- Schieppati M & Nardone A (1997). Medium‐latency stretch reflexes of foot and leg muscles analysed by cooling the lower limb in standing humans. J Physiol 503, 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans J, de Vlugt E, Schouten AC, Meskers CG, de Groot JH & van der Helm FC (2009). The monosynaptic Ia afferent pathway can largely explain the stretch duration effect of the long latency M2 response. Exp Brain Res 193, 491–500. [DOI] [PubMed] [Google Scholar]
- Sibley KM, Carpenter MG, Perry JC & Frank JS (2007). Effects of postural anxiety on the soleus H‐reflex. Hum Mov Sci 26, 103–112. [DOI] [PubMed] [Google Scholar]
- Staab JP, Balaban CD & Furman JM (2013). Threat assessment and locomotion: clinical applications of an integrated model of anxiety and postural control. Semin Neurol 33, 297–306. [DOI] [PubMed] [Google Scholar]
- Tokuno CD, Keller M, Carpenter MG, Márquez G & Taube W (2018). Alterations in the cortical control of standing posture during varying levels of postural threat and task difficulty. J Neurophysiol 120, 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaback M, Carpenter MG & Adkin AL (2016). Threat‐induced changes in attention during tests of static and anticipatory postural control. Gait Posture 45, 19–24. [DOI] [PubMed] [Google Scholar]


