Abstract
Key points
The mechanisms responsible for the high inter‐individual variability in blood pressure responses to exercise remain unclear.
Common genetic variants of genes related to the vascular transduction of sympathetic outflow have been investigated, but variants influencing skeletal muscle afferent feedback during exercise have not been explored.
Single nucleotide polymorphisms in TRPV1 rs222747 and BDKRB2 rs1799722 receptors present in skeletal muscle were associated with differences in the magnitude of the blood pressure response to static handgrip exercise but not mental stress.
The combined effects of TRPV1 rs222747 and BDKRB2 rs1799722 on blood pressure and heart rate responses during exercise were additive, and primarily found in men.
Genetic differences in skeletal muscle metaboreceptors may be a risk factor for exaggerated blood pressure responses to exercise.
Abstract
Exercise blood pressure (BP) responses demonstrate high inter‐individual variability, which could relate to differences in metabolically sensitive afferent feedback from the exercising muscle. We hypothesized that single‐nucleotide polymorphisms (SNPs) in genes encoding metaboreceptors present in group III/IV skeletal muscle afferents can influence the exercise pressor response. Two hundred men and women underwent measurements of continuous BP and heart rate at baseline and during 2 min of static handgrip exercise (30% maximal volitional contraction), post‐exercise circulatory occlusion and mental stress (serial subtraction; internal control). Participants were genotyped for SNPs in TRPV1 (rs222747; G/C), ASIC3 (rs2288645; G/A), BDKRB2 (rs1799722; C/T), PTGER2 (rs17197; A/G) and P2RX4 (rs25644; A/G). Exercise systolic BP (19 ± 10 vs. 22 ± 10 mmHg, P = 0.03) was lower in GG versus GC/CC minor allele carriers for TRPV1 rs222747, while exercise diastolic BP (14 ± 7 vs. 17 ± 7 mmHg, P = 0.007) and heart rate (12 ± 8 vs. 15 ± 9 beats min−1, P = 0.03) were lower in CC versus CT/TT minor allele carriers for BDKRB2 rs1799722. Individuals carrying both minor alleles for TRPV1 rs222747 and BDKRB2 rs1799722 had greater systolic (22 ± 11 vs. 17 ± 10 mmHg, P = 0.04) and diastolic (18 ± 7 vs. 14 ± 7 mmHg, P = 0.01) BP responses than those with no minor alleles; these differences were larger in men. No differences in BP or heart rate responses were detected during static handgrip with ASIC3 rs2288645, PTGER2 rs17197 or P2RX4 rs25644. None of the selected SNPs were associated with differences during mental stress. These findings demonstrate that variants in TRPV1 and BDKRB2 receptors can contribute to BP differences during static exercise in an additive manner.
Keywords: Single Nucleotide Polymorphisms, Muscle metaboreflex, Blood Pressure, Sex‐specific
Key points
The mechanisms responsible for the high inter‐individual variability in blood pressure responses to exercise remain unclear.
Common genetic variants of genes related to the vascular transduction of sympathetic outflow have been investigated, but variants influencing skeletal muscle afferent feedback during exercise have not been explored.
Single nucleotide polymorphisms in TRPV1 rs222747 and BDKRB2 rs1799722 receptors present in skeletal muscle were associated with differences in the magnitude of the blood pressure response to static handgrip exercise but not mental stress.
The combined effects of TRPV1 rs222747 and BDKRB2 rs1799722 on blood pressure and heart rate responses during exercise were additive, and primarily found in men.
Genetic differences in skeletal muscle metaboreceptors may be a risk factor for exaggerated blood pressure responses to exercise.
Introduction
Blood pressure (BP) responses to exercise demonstrate high inter‐individual variability (Ingelsson et al. 2007; Nunes et al. 2014; Notay et al. 2018), with exaggerated responses linked to an increased risk of future hypertension and cardiovascular mortality (Chaney & Eyman, 1988; Miyai et al. 2000, 2002; Weiss et al. 2010). The mechanisms responsible for the variability in exercise pressor responses are unclear. Activation of the sympathetic nervous system is critical for the maintenance of exercising BP and matching of cardiac output to skeletal muscle demand (Joyner & Casey, 2015), with accentuated sympathetic vasoconstriction considered a mechanism for a hypertensive response to exercise (Schultz & Sharman, 2014). During exercise, increases in sympathetic outflow occur primarily from feedforward signals from higher brain regions related to perception of effort (i.e. central command) and feedback signals from group III/IV skeletal muscle afferents sensitive to stretch and chemical stimuli, termed the muscle mechanoreflex and metaboreflex, respectively (Fisher et al. 2015). Stimulation of the muscle metaboreflex is known to potently activate the sympathetic nervous system (Mark et al. 1985), and its over‐activation is implicated in mediating larger BP responses during exercise in patients with hypertension (Delaney et al. 2010; Greaney et al. 2015).
Evidence suggests that genetic influences can contribute to the variability in BP responses to exercise; however, to date, most research has focused on single‐nucleotide polymorphisms (SNPs) in genes encoding adrenergic receptors (i.e. related to the vascular transduction of efferent sympathetic outflow), endothelial nitric oxide synthase, or components of the renin–angiotensin–aldosterone system (Eisenach et al. 2005; Ueno et al. 2005; Nieminen et al. 2006; Ingelsson et al. 2007; Dias et al. 2009; Nunes et al. 2014, 2016). SNPs are single‐base‐pair alterations in DNA that are prevalent throughout the human genome, and can influence, for example, gene expression or receptor function (Shastry, 2009). Whether genetic variations in metabolically sensitive receptors found in skeletal muscle group III/IV afferents contribute to the variability in BP responses to exercise is unknown. Functional differences in the chemical sensitivity of muscle metaboreceptors could modulate afferent feedback to brainstem regions controlling efferent sympathetic and parasympathetic outflow, and ultimately BP (Amann et al. 2015). Prior work has shown that group III/IV skeletal muscle afferents respond to a wide array of stimuli, and can contain acid sensing ion channel (ASIC), transient receptor potential vanilloid 1 (TRPV1), prostaglandin E2 (PTGER2), purinergic P2X (P2RX) and bradykinin B2 (BDKRB2) receptors (Greaney et al. 2015), though the specific contributions of individual receptor classes, particularly TRPV1 receptors, on the exercise pressor reflex remain controversial. Importantly, each of these genes possesses SNPs with common genetic variants (i.e. minor allele frequencies >10%).
The primary purpose of this study was to determine the effects of genetic variants of genes encoding metabolically sensitive receptors, present in group III/IV skeletal muscle afferents, on heart rate and BP responses during static handgrip exercise. This exercise mode was selected based on the large body of evidence that BP responses to static handgrip exercise are tightly linked to activation of the muscle metaboreflex (Mark et al. 1985; Delaney et al. 2010). To further isolate the actions of the muscle metaboreflex, BP responses were also assessed during post‐exercise circulatory occlusion (PECO). In addition, as many of the metaboreceptors found in group III/IV afferents are not selective to skeletal muscle, heart rate and BP responses were measured during a mental stress task (serial subtraction) to serve as an internal control (i.e. a stimulus that increases BP without feedback from group III/IV afferents). We hypothesized that genetic variants in metabolically sensitive receptors found in skeletal muscle would contribute to the high inter‐individual variability observed in BP responses to static handgrip exercise but not mental stress.
Methods
Ethics approval
The University of Guelph Research Ethics Board approved all procedures (REB no. 16‐12‐688/17‐05‐009) and the study conformed to the standards set by the Declaration of Helsinki, except for registration in a database. All participants provided informed, written consent prior to engaging in the study protocol.
Participants
Two hundred young healthy recreationally active men (n = 91) and women (n = 109) participated in the study between January 2017 and February 2018. All female participants were studied during the early follicular phase (between days 1 and 5) of the menstrual cycle and self‐reported that they had a regular 28‐day menstrual cycle; those on oral contraception (n = 47) were studied within the first 5 days of their placebo pill phase. All participants were free of known cardiovascular or metabolic disease, and did not consume any chronic medications other than oral contraception. The present cohort consisted almost exclusively of non‐Hispanic Caucasians with only five Hispanic and three black participants. A portion of the static handgrip blood pressure data from this study has been used previously to address an unrelated hypothesis (Notay et al. 2018).
Experimental protocol
The study consisted of a randomized crossover design examining BP responses to static handgrip exercise and mental stress. All participants underwent a familiarization visit to describe all aspects of the study protocol and practice performing maximal voluntary contractions on a handgrip dynamometer (Lafayette Instrument, Lafayette, LA, USA), as well as a single testing visit. Prior to the testing visit, participants were asked to refrain from caffeine, alcohol and vigorous exercise for a minimum of 24 h, and food or fluids for 1 h. Participants were studied in a light and temperature controlled laboratory. Following voiding and collection of anthropometric measurements, participants were positioned upright on a comfortable chair with their feet supported on an ottoman. Participants were asked to execute two maximal handgrip contractions in their left hand to determine maximal voluntary contraction (MVC); 16 participants were left‐handed (7 men; 9 women). Each contraction lasted ∼3 s and they were separated by at least 30 s of rest. If the MVC between the two contractions was >3 kg, a third MVC was required to be completed; however, this did not occur in any of the participants. The highest value was taken as MVC.
Next, participants were given 10 min of rest, after which continuous measures of heart rate and BP, as well as discrete minute‐to‐minute brachial BP, were collected simultaneously over a 5 min resting baseline period. Following this baseline period, participants were randomized to begin collection during either the static handgrip or the mental stress protocol; a minimum of 10 min rest was provided between the two stressors to ensure that all cardiovascular measures returned to baseline values. Continuous measurements of heart rate and BP were collected throughout both stressors. During the static handgrip protocol, participants completed a 2 min resting baseline, 2 min static handgrip contraction at 30% MVC, and 2 min of PECO. To complete PECO, a manual sphygmomanometer (DS400 Aneroid Sphygmomanometer; D.E. Hokanson Inc., Bellevue, WA, USA) was inflated to 220 mmHg (i.e. suprasystolic) in the upper left arm immediately prior to the completion of the static handgrip contraction. During the mental stress task, participants were randomly assigned to subtract 11 or 13 from a three‐ or four‐digit number, with a new number appearing on a personal computer every 5 s for a total of 2 min. The same 24 numbers were used for all participants. Answers were monitored to ensure that effort was given during the test; however, no verbal feedback was given to participants regarding correctness. Encouragement was also given to ensure participants continued to answer questions.
Measurements
Electrocardiography (Lead II) was used to continuously obtain beat‐to‐beat heart rate (ADInstruments Inc., Colorado Springs, CO, USA). Respiratory movements were monitored to ensure spontaneous breathing using a piezoelectric transducer positioned around the abdomen (Pneumotrace II, UFA, Morro Bay, CA, USA). To attain accurate recordings of blood pressure at rest, discrete left brachial blood pressure was logged on a minute‐to‐minute basis using an automated sphygmomanometer (BPTru Medical Devices, Coquitlam, Canada). A total of six discrete readings were taken, with the average of the last five recordings used for analysis. Both medium (cuff dimensions: 12 × 23 cm; n = 132) and large (cuff dimensions: 15 × 33 cm; n = 68) sized BP cuffs were used depending on the participant's arm circumference. Continuous beat‐to‐beat BP was recorded from the right middle finger using photoelectric plethysmography (Finometer MIDI, Finapres Inc., Enschede, Netherlands) to monitor haemodynamic responses during exercise and mental stress. Small (cuff fit: 45–55 mm; n = 27), medium (cuff fit: 55–65 mm; n = 139) and large (cuff fit: 65–75 mm; n = 34) sized finger cuffs were used depending on the participant's middle finger circumference. All continuous data were digitized and stored with LabChart (PowerLab, ADInstruments, Colorado Springs, CO, USA). Heart rate, respiration and blood pressure were recorded at a sampling frequency of 1000 Hz.
Selection of genetic variants and genotyping
The five candidate SNPs were chosen based on prior literature demonstrating associations with differences in BP at rest (Ko et al. 2008), investigated for differences in pulse pressure (Stokes et al. 2011), higher prevalence in hypertensive populations (Cui et al. 2005; Sato et al. 2007), or alterations in receptor structure or sensitivity (Wang et al. 2016). All SNPs had a minor allele frequency (MAF) >10% in a European population according to dbSNP (https://www.ncbi.nlm.nih.gov/projects/SNP/). DNA was extracted from either venous blood (n = 90) or saliva (n = 110), as previously described (Klingel et al. 2017). Briefly, 2 mL of saliva was collected in an Oragene DNA collection kit and DNA was extracted according to the manufacturer's instructions (DNA Genotek, Ottawa, ON, Canada). For venous blood, DNA was extracted using the Qiagen Paxgene Blood DNA kit, according to the manufacturer's instructions (Qiagen, Toronto, ON, Canada). Participants were genotyped for SNPs in TRPV1 (rs222747; G/C; MAF: 0.32), ASIC3 (rs2288645; G/A; MAF: 0.20), BDKRB2 (rs1799722; C/T; MAF: 0.43), P2RX4 (rs25644; A/G; MAF: 0.16) and PTGER2 (rs17197; A/G; MAF: 0.22) using the MassARRAY® Analyser 4 System (Agena Biosciences, San Diego, CA, USA) using iPlex gold chemistry and analysed using Typer 4.0 software at SickKid's Centre for Applied Genomics (Toronto, ON, Canada). Briefly, each locus was amplified by polymerase chain reaction and a third primer that flanks the polymorphism site was extended by one base. The extension reaction products were analysed using matrix‐assisted laser desorption/ionization time of flight (MALDI‐TOF) mass spectrometry to identify the amount and type of molecules present in the sample (Storm et al. 2003). Five DNA samples were randomly selected for replication and 100% concordance was achieved. Three quality control samples were also run and had 100% agreement.
Data and statistical analysis
Heart rate and BP measurements were averaged over a 2 min resting baseline and during the second minute of static handgrip exercise, PECO and mental stress. The change (∆) from baseline was calculated for statistical analysis. The coefficients of variation were calculated to measure the absolute variability of BP and heart rate responses during static handgrip exercise. All haemodynamic data were collected and analysed prior to obtaining genotyping results (i.e. blind to group allocation).
Statistical analyses were performed using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA) and Prism (GraphPad Software Inc., La Jolla, CA, USA). Deviation from Hardy–Weinberg equilibrium was tested for each SNP using the χ2 test. Due to the low number of homozygotic minor alleles for most SNPs, we combined heterozygous and minor homozygous subjects into a single group called ‘minor allele carriers’ for each SNP (Klingel et al. 2017). Thus, associations between genes and study endpoints were investigated using a dominant genetic model (i.e. MM vs. Mm+mm). BP and heart rate responses between genotype groups were compared using a one‐way analysis of covariance (ANCOVA). Age and sex were used as covariates for the comparison of resting baseline values, PECO and mental stress responses, while age, sex and MVC (Notay et al. 2018) were used as covariates during static handgrip exercise. Relative proportions of allele groups between men and women at baseline were tested using two‐tailed Fisher's exact tests. Subgroup analysis was conducted to determine the influence of sex on heart rate and BP responses to static handgrip exercise, PECO and mental stress using two‐way ANCOVAs. Age was used as a covariate for PECO and mental stress responses, while age and MVC were used during static handgrip exercise. Significant allele × sex interactions were probed using Bonferroni post hoc tests.
To assess the additive effects of significant SNPs, we computed a genetic risk score which labelled individuals as 0, 1 or 2, according to the number of minor alleles they were carrying for the TRPV1 rs222747 and BDKRB2 rs1799722 SNPs. Group 0 comprised individuals carrying no minor alleles, group 1 comprised individuals carrying one minor allele (irrespective of the gene) and group 2 comprised individuals carrying both minor alleles. BP and heart rate responses between genetic risk score groups were compared with an ANCOVA, using age, sex and MVC as covariates. Significant main effects between genetic risk score groups were probed using Bonferroni post hoc tests. Significance was considered P < 0.05. Data are presented as mean ± SD, unless otherwise stated.
Results
Complete BP, heart rate and genotype data were obtained in 200 participants with no adverse effects. Resting baseline characteristics of the cohort are presented in Table 1. All of the investigated SNPs were in Hardy–Weinberg equilibrium (P > 0.05).
Table 1.
Participant baseline characteristics
| Characteristic | Mean ± SD | Range |
|---|---|---|
| Sex (male/female) | 91/109 | — |
| Age (years) | 22 ± 3 | 18–30 |
| Height (cm) | 171 ± 9 | 147–196 |
| Weight (kg) | 69 ± 13 | 41–124 |
| Body mass index (kg m−2) | 23 ± 3 | 17–35 |
| Heart rate (beats min−1) | 67 ± 11 | 44–101 |
| Systolic blood pressure (mmHg) | 104 ± 8 | 83–133 |
| Diastolic blood pressure (mmHg) | 66 ± 7 | 50–87 |
| Maximal volitional contraction (kg) | 36 ± 13 | 13–79 |
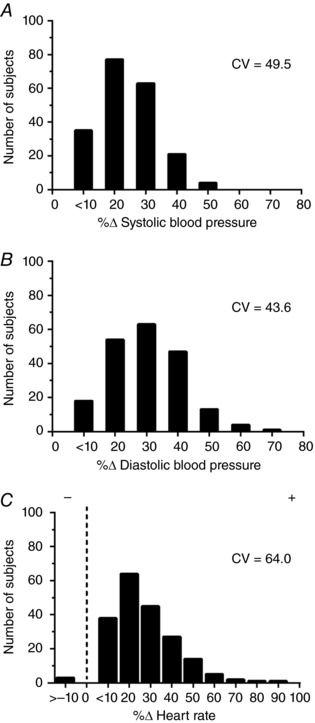
As expected, all participants experienced increases in both systolic and diastolic BP during static handgrip exercise. The majority of participants also had an increase in heart rate with the exception of three individuals who had a slight decrease (removal of these participants did not impact the results). There was no effect of testing order on any variable (P > 0.05). Figure 1 displays the distributions and coefficients of variation for changes in BP and heart rate with static exercise. Overall, the mean increase in systolic and diastolic BP during static handgrip exercise was 20 ± 10 and 16 ± 7 mmHg, respectively. These pressor responses were largely maintained during PECO, which had mean increases in systolic and diastolic BP of 18 ± 10 and 12 ± 6 mmHg, respectively. The mean increases in heart rate during static handgrip exercise and PECO were 14 ± 9 and 0 ± 6 beats min−1, respectively.
Figure 1. Distributions and coefficients of variation (CV) for changes in systolic BP (A), diastolic BP (B) and heart rate (C) during static handgrip exercise.
TRPV1 rs222747
Baseline characteristics were not significantly different between major (GG) and minor (CC + CG) allele carriers (Table 2). Systolic BP responses were smaller in GG versus CC + CG individuals during static handgrip exercise (19 ± 10 vs. 22 ± 10 mmHg, P = 0.03), but not PECO (17 ± 10 vs. 19 ± 11 mmHg, P = 0.29) or mental stress (14 ± 9 vs. 14 ± 12 mmHg, P = 0.94) (Fig. 2). Diastolic BP responses were not significantly different between GG versus CC + CG individuals during static handgrip exercise (16 ± 7 vs. 17 ± 7 mmHg, P = 0.15), PECO (12 ± 6 vs. 12 ± 6 mmHg, P = 0.65) or mental stress (10 ± 6 vs. 9 ± 5 mmHg, P = 0.14). A trend for smaller heart rate responses to static handgrip exercise was found in GG compared to CC + CG (13 ± 8 vs. 15 ± 9 beats min−1, P = 0.09) but not during PECO (0 ± 5 vs. 0 ± 7 beats min−1, P = 0.80) or mental stress (9 ± 9 vs. 9 ± 9 beats min−1, P = 0.95). Subgroup analysis did not detect any allele × sex interactions for static handgrip exercise, PECO or mental stress (all P > 0.05; data not shown).
Table 2.
Baseline characteristics for TRPV1 rs222747
| Characteristic | GG | CG + CC | P |
|---|---|---|---|
| Age (years) | 22 ± 2 | 22 ± 3 | 0.80 |
| Sex (male/female) | 43/53 | 48/56 | 0.89 |
| Height (cm) | 172 ± 9 | 170 ± 9 | 0.10 |
| Weight (kg) | 71 ± 13 | 67 ± 13 | 0.05 |
| Body mass index (kg m−2) | 24 ± 3 | 23 ± 3 | 0.14 |
| Heart rate (beats min−1) | 66 ± 11 | 68 ± 11 | 0.15 |
| Systolic blood pressure (mmHg) | 105 ± 8 | 104 ± 9 | 0.75 |
| Diastolic blood pressure (mmHg) | 66 ± 7 | 66 ± 7 | 0.97 |
| Mean arterial pressure (mmHg) | 79 ± 7 | 79 ± 7 | 0.92 |
| Maximal volitional contraction (kg) | 36 ± 12 | 36 ± 13 | 0.92 |
Data are shown as mean ± SD.
Figure 2. Mean changes (Δ) in systolic BP (A) and diastolic BP (B) during the second minute of static handgrip exercise (left), PECO (middle) and mental stress (right) in TRPV1 rs222747 between GG (n = 96) and CC + CG (n = 104) allele carriers.
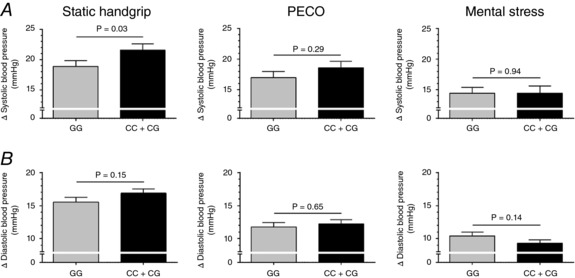
Data are mean ± SEM. P values adjusted for age, sex and maximal volitional contraction (static handgrip exercise only)
BDKRB2 rs1799722
Baseline diastolic BP was higher in major (CC) allele carriers than minor (CT + TT) (67 ± 8 vs. 65 ± 6 mmHg, P = 0.02); however, all other characteristics were not significantly different between groups (all P > 0.05) (Table 3). Adjusting for this small baseline difference in diastolic BP did not alter the results for static handgrip exercise, PECO or mental stress data. Systolic BP responses were not different between CC and CT + TT individuals during static handgrip exercise (19 ± 9 vs. 21 ± 10 mmHg, P = 0.16), PECO (16 ± 9 vs. 18 ± 11 mmHg, P = 0.26) or mental stress (15 ± 11 vs. 14 ± 11 mmHg, P = 0.46). In contrast, CC had smaller diastolic BP responses during static handgrip exercise (14 ± 7 vs. 17 ± 7 mmHg, P = 0.007) and PECO (11 ± 6 vs. 13 ± 7 mmHg, P = 0.05), but not mental stress (10 ± 6 vs. 10 ± 5 mmHg, P = 0.98). Heart rate exhibited similar attenuated responses in CC during static handgrip exercise (12 ± 8 vs. 15 ± 9 beats min−1, P = 0.03) but not PECO (0 ± 5 vs. 1 ± 7 beats min−1, P = 0.23) or mental stress (9 ± 10 vs. 8 ± 8 beats min−1, P = 0.61) (Fig. 3). Subgroup analysis demonstrated significant allele × sex interactions with attenuated systolic and diastolic BP responses during static handgrip exercise in CC vs. CC + CT men (21 ± 10 vs. 26 ± 9 mmHg, P = 0.01; and 15 ± 7 vs. 19 ± 7 mmHg, P = 0.001, respectively) but no differences in women (all P > 0.05) (Fig. 4). There were no sex differences in heart rate responses during static handgrip exercise, or heart rate and BP responses during PECO or mental stress (all P > 0.05).
Table 3.
Baseline characteristics for BDKRB2 rs1799722
| Characteristic | CC | CT + TT | P |
|---|---|---|---|
| Age (years) | 21 ± 2 | 22 ± 3 | 0.08 |
| Sex (male/female) | 25/40 | 66/69 | 0.18 |
| Height (cm) | 170 ± 8 | 172 ± 10 | 0.37 |
| Weight (kg) | 69 ± 12 | 69 ± 13 | 0.99 |
| Body mass index (kg m−2) | 24 ± 3 | 23 ± 3 | 0.45 |
| Heart rate (beats min−1) | 68 ± 11 | 67 ± 11 | 0.75 |
| Systolic blood pressure (mmHg) | 105 ± 9 | 104 ± 8 | 0.08 |
| Diastolic blood pressure (mmHg) | 67 ± 8 | 65 ± 6 | 0.02 |
| Mean arterial pressure (mmHg) | 80 ± 7 | 78 ± 6 | 0.03 |
| Maximal volitional contraction (kg) | 34 ± 12 | 37 ± 13 | 0.16 |
Data are shown as mean ± SD.
Figure 3. Mean changes (Δ) in systolic BP (A) and diastolic BP (B) during the second minute of static handgrip exercise (left), PECO (middle) and mental stress (right) in BDKRB2 rs222747 between CC (n = 65) and CT + TT (n = 135) allele carriers.
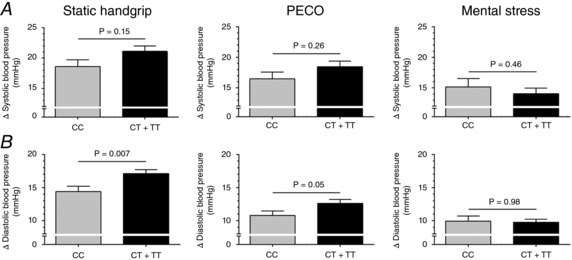
Data are mean ± SEM. P values adjusted for age, sex and maximal volitional contraction (static handgrip exercise only).
Figure 4. Mean changes (Δ) in systolic BP (A) and diastolic BP (B) during the second minute of static handgrip exercise in BDKRB2 rs222747 within male (left) CC (n = 25) and CT + TT (n = 66) allele carriers and within female (right) CC (n = 40) and CT + TT (n = 69) allele carriers.
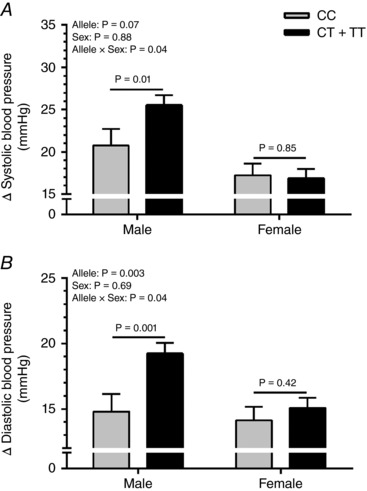
Data are mean ± SEM. P values adjusted for age and maximal volitional contraction.
ASIC3 rs2288645
All baseline characteristics were similar between major (GG) and minor (AA + AG) allele carrier groups, as were all BP and heart rate responses to static handgrip exercise, PECO, and mental stress (all P > 0.05; Table 4). Subgroup analysis did not yield any allele × sex interactions during static handgrip exercise or mental stress (all P > 0.05); however, GG men exhibited a larger systolic BP response during PECO (22 ± 11 vs. 17 ± 9 mmHg, P = 0.03).
Table 4.
PTGER2 rs17197 polymorphism on baseline participant characteristics and BP and heart rate responses to static handgrip exercise, PECO and mental stress
| AA | AG + GG | P | |
|---|---|---|---|
| Baseline characteristics | |||
| Number (n) | 125 | 75 | |
| Age (years) | 21 ± 2 | 22 ± 3 | 0.09 |
| Sex (male/female) | 58/67 | 33/42 | 0.77 |
| Height (cm) | 172 ± 9 | 170 ± 10 | 0.28 |
| Weight (kg) | 69 ± 12 | 68 ± 15 | 0.49 |
| Body mass index (kg m−2) | 23 ± 3 | 23 ± 4 | 0.80 |
| Heart rate (beats min−1) | 66 ± 11 | 69 ± 11 | 0.01 |
| Systolic blood pressure (mmHg) | 104 ± 8 | 104 ± 9 | 0.89 |
| Diastolic blood pressure (mmHg) | 65 ± 7 | 66 ± 7 | 0.75 |
| Mean arterial pressure (mmHg) | 78 ± 7 | 79 ± 7 | 0.87 |
| Maximal volitional contraction (kg) | 37 ± 13 | 34 ± 12 | 0.06 |
| Static handgrip exercise | |||
| ∆ Systolic blood pressure (mmHg) | 21 ± 10 | 19 ± 9 | 0.66 |
| ∆ Diastolic blood pressure (mmHg) | 17 ± 7 | 15 ± 7 | 0.78 |
| ∆ Heart rate (beats min−1) | 15 ± 9 | 13 ± 8 | 0.80 |
| PECO | |||
| ∆ Systolic blood pressure (mmHg) | 19 ± 11 | 16 ± 9 | 0.14 |
| ∆ Diastolic blood pressure (mmHg) | 13 ± 7 | 11 ± 6 | 0.09 |
| ∆ Heart rate (beats min−1) | 0 ± 6 | 0 ± 6 | 0.99 |
| Mental stress | |||
| ∆ Systolic blood pressure (mmHg) | 15 ± 11 | 13 ± 10 | 0.56 |
| ∆ Diastolic blood pressure (mmHg) | 10 ± 6 | 9 ± 5 | 0.70 |
| ∆ Heart rate (beats min−1) | 10 ± 9 | 7 ± 9 | 0.10 |
Data are shown as mean ± SD
PTGER2 rs17197
Baseline heart rate was lower in major (AA) allele carriers compared to minor (AG + GG) (66 ± 11 vs. 69 ± 11 beats min−1, P = 0.01), though no other characteristics were different between groups (all P > 0.05), as were all BP and heart rate responses during static handgrip exercise, PECO and mental stress (Table 5). Subgroup analysis did not yield any allele × sex interactions during static handgrip exercise or mental stress (all P > 0.05); however, AA men demonstrated larger systolic BP responses during PECO (23 ± 12 vs. 17 ± 7 mmHg, P = 0.01). A similar trend was observed for diastolic BP during PECO (interaction term, P = 0.06).
Table 5.
ASIC3 rs2288645 polymorphism on baseline participant characteristics and BP and heart rate responses to static handgrip exercise, PECO and mental stress
| GG | AA + AG | P | |
|---|---|---|---|
| Baseline characteristics | |||
| Number (n) | 132 | 68 | |
| Age (years) | 21 ± 2 | 22 ± 3 | 0.29 |
| Sex (male/female) | 56/76 | 35/33 | 0.23 |
| Height (cm) | 171 ± 9 | 172 ± 9 | 0.66 |
| Weight (kg) | 69 ± 13 | 68 ± 13 | 0.64 |
| Body mass index (kg m−2) | 24 ± 3 | 23 ± 3 | 0.30 |
| Heart rate (beats min−1) | 68 ± 11 | 66 ± 10 | 0.75 |
| Systolic blood pressure (mmHg) | 104 ± 9 | 105 ± 8 | 0.78 |
| Diastolic blood pressure (mmHg) | 65 ± 7 | 66 ± 7 | 0.24 |
| Mean arterial pressure (mmHg) | 78 ± 7 | 79 ± 7 | 0.35 |
| Maximal volitional contraction (kg) | 35 ± 13 | 36 ± 13 | 0.70 |
| Static handgrip exercise | |||
| ∆ Systolic blood pressure (mmHg) | 20 ± 10 | 21 ± 11 | 0.69 |
| ∆ Diastolic blood pressure (mmHg) | 16 ± 7 | 17 ± 7 | 0.34 |
| ∆ Heart rate (beats min−1) | 14 ± 9 | 14 ± 8 | 0.76 |
| PECO | |||
| ∆ Systolic blood pressure (mmHg) | 18 ± 11 | 17 ± 10 | 0.45 |
| ∆ Diastolic blood pressure (mmHg) | 12 ± 7 | 12 ± 6 | 0.51 |
| ∆ Heart rate (beats min−1) | 1 ± 7 | 0 ± 6 | 0.50 |
| Mental stress | |||
| ∆ Systolic blood pressure (mmHg) | 14 ± 10 | 16 ± 12 | 0.20 |
| ∆ Diastolic blood pressure (mmHg) | 10 ± 6 | 10 ± 6 | 0.37 |
| ∆ Heart rate (beats min−1) | 9 ± 9 | 8 ± 9 | 0.50 |
Data are shown as mean ± SD
P2RX4 rs25644
All baseline characteristics were similar between major (AA) and minor (AG + GG) allele carrier groups, as were all BP and heart rate responses to static handgrip exercise, PECO and mental stress (all P > 0.05; Table 6). Subgroup analysis did not yield any significant allele × sex interactions (all P > 0.05) (data not shown).
Table 6.
P2RX4 rs25644 polymorphism on baseline participant characteristics and BP and heart rate responses to static handgrip exercise, PECO and mental stress
| AA | AG + GG | P | |
|---|---|---|---|
| Baseline characteristics | |||
| Number (n) | 142 | 58 | |
| Age (years) | 21 ± 2 | 22 ± 3 | 0.001 |
| Sex (male/female) | 61/81 | 30/28 | 0.28 |
| Height (cm) | 171 ± 9 | 172 ± 9 | 0.34 |
| Weight (kg) | 68 ± 14 | 71 ± 12 | 0.24 |
| Body mass index (kg m−2) | 23 ± 3 | 24 ± 3 | 0.36 |
| Heart rate (beats min−1) | 68 ± 11 | 65 ± 10 | 0.13 |
| Systolic blood pressure (mmHg) | 104 ± 9 | 106 ± 8 | 0.47 |
| Diastolic blood pressure (mmHg) | 66 ± 7 | 66 ± 7 | 0.64 |
| Mean arterial pressure (mmHg) | 78 ± 7 | 79 ± 6 | 0.92 |
| Maximal volitional contraction (kg) | 35 ± 13 | 37 ± 11 | 0.32 |
| Static handgrip exercise | |||
| ∆ Systolic blood pressure (mmHg) | 20 ± 10 | 20 ± 10 | 0.77 |
| ∆ Diastolic blood pressure (mmHg) | 16 ± 7 | 16 ± 7 | 0.76 |
| ∆ Heart rate (beats min−1) | 14 ± 9 | 13 ± 8 | 0.71 |
| PECO | |||
| ∆ Systolic blood pressure (mmHg) | 18 ± 10 | 18 ± 11 | 0.82 |
| ∆ Diastolic blood pressure (mmHg) | 12 ± 6 | 12 ± 7 | 0.97 |
| ∆ Heart rate (beats min−1) | 0 ± 6 | 0 ± 6 | 0.74 |
| Mental stress | |||
| ∆ Systolic blood pressure (mmHg) | 14 ± 10 | 15 ± 12 | 0.25 |
| ∆ Diastolic blood pressure (mmHg) | 10 ± 6 | 10 ± 6 | 0.10 |
| ∆ Heart rate (beats min−1) | 9 ± 8 | 8 ± 10 | 0.40 |
Data are shown as mean ± SD
TRPV1–BDKRB2 genotype risk score
As shown in Fig. 5, individuals carrying both minor alleles (group 2) had larger systolic BP responses during static handgrip exercise than individuals carrying no minor alleles (group 0) (22 ± 11 vs. 17 ± 10 mmHg, P = 0.04). Differences between group 2 and those carrying one minor allele (group 1) (22 ± 11 vs. 20 ± 9 mmHg, P = 0.45) or between group 1 and group 0 (20 ± 9 vs. 17 ± 10 mmHg, P = 0.48) did not reach statistical significance. Diastolic BP responses during static handgrip exercise were larger in group 2 vs. group 0 (18 ± 7 vs. 14 ± 7 mmHg, P = 0.01) and tended to be larger between group 1 vs. group 0 (16 ± 6 vs. 14 ± 7 mmHg, P = 0.08). No differences were found between group 2 vs. group 1 (18 ± 7 vs. 16 ± 6 mmHg, P = 0.97). Heart rate responses during static handgrip exercise were larger in group 2 vs. group 1 (16 ± 10 vs. 12 ± 8 beats min−1, P = 0.04) and group 0 (16 ± 10 vs. 13 ± 9 beats min−1, P = 0.04). No differences were observed between group 1 vs. group 0 (13 ± 9 vs. 12 ± 8 beats min−1, P = 0.99). Subgroup analysis demonstrated that within men those in group 2 had larger systolic BP responses during static handgrip exercise than group 0 (27 ± 11 vs. 20 ± 9 mmHg, P = 0.01). Similarly, diastolic BP responses during static handgrip exercise were larger in group 2 vs. group 0 (19 ± 7 vs. 13 ± 7 mmHg, P = 0.001) and between group 1 vs. group 0 (18 ± 6 vs. 13 ± 7 mmHg, P = 0.004). Heart rate responses were larger in group 2 vs. group 0 (17 ± 11 vs. 11 ± 8 beats min−1, P = 0.02). In contrast, all BP and heart rate responses during static handgrip exercise were not significantly different in women (all P > 0.05).
Figure 5. Genetic risk score investigating the additive effects of TRPV1 rs222747 and BDKRB2 rs222747 on the mean changes (Δ) in systolic BP (A) and diastolic BP (B) during the second minute of static handgrip exercise across all participants (left), men (middle) and women (right).
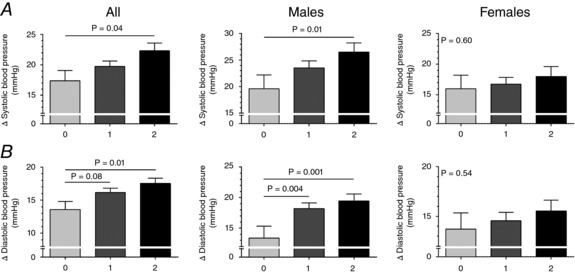
Group 0 comprised individuals carrying no minor alleles, group 1 comprised individuals carrying one minor allele (irrespective of the gene) and group 2 comprised individuals carrying both minor alleles. All participants, group 0 (n = 34), group 1 (n = 91), group 2 (n = 75); in men, group 0 (n = 13), group 1 (n = 40) and group 2 (n = 38); in women, group 0 (n = 21), group 1 (n = 51) and group 2 (n = 37). Data are mean ± SEM. P values adjusted for age, sex and maximal volitional contraction.
Discussion
The feedback of group III/IV skeletal muscle afferents is critical to maintaining the appropriate haemodynamic response during exercise (Amann et al. 2010). The present study sought to investigate whether genetic variants in metabolically sensitive receptors found in skeletal muscle afferents can influence BP responses to exercise. In support of our hypothesis, we observed that minor allele carriers of both TRPV1 rs222747 and BDKRB2 rs1799722 were associated with larger BP and/or heart rate responses during static handgrip exercise but not our internal control mental stress task. Further, the diastolic BP differences observed with BDKRB2 rs1799722 during static handgrip exercise were also present during isolation of the muscle metaboreflex using PECO. Although independently these relative changes were small (∆ 2–3 mmHg), the combination of these polymorphisms resulted in a ∼22–23% greater difference in systolic and diastolic BP responses (∆ 4–5 mmHg) between individuals carrying both minor alleles than individuals carrying no minor alleles. Interestingly, subgroup analysis determined that the haemodynamic effects of these two genetic variants were primarily in men, with even larger differences (∆ 6–7 mmHg) in exercise BP responses between groups. No differences in haemodynamic responses during exercise or mental stress were detected for PTGER2 rs17197, ASIC3 rs2288645 or P2RX4 rs25644. These results demonstrate that genetic variants in skeletal muscle metaboreceptors can independently and synergistically influence the magnitude of the BP response to exercise.
Prior investigations focusing on the contribution of genetic variants to the variability in exercise BP responses have focused primarily on polymorphisms in genes related to adrenergic and renin–angiotensin–aldosterone system receptors (Eisenach et al. 2005; Ueno et al. 2005; Nieminen et al. 2006; Ingelsson et al. 2007; Dias et al. 2009; Nunes et al. 2014, 2016). However, while these studies have demonstrated that such SNPs can influence pressor responses to exercise, they may be confounded by not measuring or controlling for potential differences in central sympathetic outflow to the vasculature. Afferent signals arising from skeletal muscle provide important feedback to the brainstem to maintain necessary BP and heart rate responses during exercise (Amann et al. 2010). The exercise pressor reflex is known to consist of mechanically and metabolically sensitive afferents, which relate primarily to group III and IV afferents, respectively (Fisher et al. 2015). The contribution of this reflex is well studied and activation of the muscle metaboreflex is known to be a potent stimulator of sympathetic vasoconstrictor outflow and BP responses during static handgrip exercise (Mark et al. 1985). A large number of studies, primarily in animal models (Stebbins et al. 1986, 1990; Rotto & Kaufman, 1988; Rotto et al. 1989; Symons et al. 1991; Pan et al. 1993; Kindig et al. 2005; Hayes et al. 2008; Smith et al. 2010; Mizuno et al. 2011; Stone et al. 2015), have sought to identify the roles of specific metabolites responsible for engaging the exercise pressor reflex, focusing specifically on stimuli to TRPV1, BDKRB2, ASIC, P2RX4 and PTGER2 receptor pathways (Greaney et al. 2015). However, the physiological contributions or role of each receptor class remain controversial and difficult to study in humans. Examining common genetic variants, especially those with known functional consequences, may represent a novel model to uncover the involvement of specific receptors.
Direct data investigating the role of TRPV1 receptors on the exercise pressor reflex in humans are limited. Recently, topical forearm application of capsaicin (a selective TRPV1 agonist) was shown to attenuate muscle sympathetic nerve activity during static handgrip and BP and muscle sympathetic responses during PECO, hypothesized to be secondary to desensitization of TRPV1 receptors (Vianna et al. 2018). Similarly, animal data from rats demonstrate that infusion of a TRPV1 antagonist attenuates the mean BP and heart rate responses to a static contraction by 7 mmHg and 2 beats min−1, respectively, through a metabolic, not mechanical, pathway (Smith et al. 2010). Further, blockade of TRPV1 receptors attenuates the overactive exercise pressor response in hypertensive rats (Mizuno et al. 2011). The present results support a role for TRPV1 in mediating the pressor responses to exercise in humans and demonstrate that the TRPV1 rs222747 polymorphism can contribute to the BP response to static handgrip exercise, with larger systolic BP responses seen in CC + GC individuals (independent of sex). We did not investigate the functional consequences of TRPV1 rs222747; however, in line with our findings, prior ex vivo culture work in human embryonic kidney suggests that this variant can be associated with increased calcium permeation and gain‐of‐function in response to acid or capsaicin (Wang et al. 2016).
Skeletal muscle contraction is associated with the release of bradykinin (Rett et al. 1989), and despite its known role as a vasodilator and potential regulator of exercise‐induced hyperaemia (Langberg et al. 2002), bradykinin can also stimulate group III/IV afferents, particularly at high intensities (Stebbins et al. 1990; Pan et al. 1993). Although data are limited in humans, BDKRB2 antagonism in cats attenuates the BP and heart rate during a 30 s electrically stimulated static hindlimb contraction by ∼41–50% (Pan et al. 1993). The present results support a role of bradykinin in activating the exercise pressor reflex in humans and demonstrate that BDKRB2 rs1799722 CT + TT individuals have greater diastolic BP responses during static handgrip exercise and PECO in comparison to major CC carriers. Subgroup analysis demonstrated that the systolic and diastolic BP responses to static handgrip exercise were impacted by sex, with differences present between CC vs. CT + TT allele carriers in men but not women. These findings align with prior work demonstrating the influence of another BDKRB2 polymorphism, rs5810761, found to be associated with systolic BP responses during treadmill exercise in men but not in women (Nunes et al. 2014). Future work is required to understand the mechanisms responsible for sex‐based differences in the influence of BDKRB2 polymorphisms on BP responses to exercise.
ASIC receptors have been shown to contribute to the exercise pressor reflex, as blunting lactic acid production during 20% MVC static handgrip exercise to failure decreases sympathetic and BP responses (Ettinger et al. 1991). However, it should be noted that the attenuated BP responses were observed primarily in the fourth to fifth minute of exercise immediately prior to fatigue (Ettinger et al. 1991). Thus, our 2 min 30% MVC static handgrip contraction may not have been of sufficient duration (or degree of fatigue) to activate this pathway. Similarly, systemic blockade of prostaglandin synthesis can attenuate mean BP and heart rate during the third minute of a 30% MVC static handgrip contraction (Fontana et al. 1995), while local blockade can attenuate the muscle sympathetic response prior to 30% MVC static contraction failure (Cui et al. 2007). However, not all work is consistent as prostaglandin blockade did not impact BP during a 40% MVC static handgrip to exhaustion (Davy et al. 1993). Again, it should be noted that prostaglandin accumulation during static exercise is greatest during high‐intensity or ischaemic exercise (Symons et al. 1991) and thus may not have been activated by our moderate intensity exercise protocol. Finally, P2RX receptors have been shown to contribute to the exercise pressor reflex in humans as infusion of a non‐specific P2 receptor agonist (pyridoxine hydrochloride) attenuates BP responses to a 30% MVC static handgrip contraction to failure (Cui et al. 2011). Again, this observation was seen in a static handgrip exercise response to failure (i.e. ischaemic model). Future work is required to establish the role of ASIC3, P2RX4 and PTGER2 polymorphisms using different exercise paradigms of varying intensity and contraction duration.
In addition to examining the BP responses to static handgrip exercise, we employed PECO in an attempt to isolate the effects of the muscle metaboreflex. Our study aligns with prior work (e.g. Mark et al. 1985) showing that BP responses to static handgrip exercise are largely maintained during PECO and thus considered to be mediated primarily by stimulation of the muscle metaboreflex. We did observe that the effects of BDKRB2 rs1799722 on diastolic BP responses demonstrated parallel group differences during static handgrip exercise and PECO; however, the effects of TRPV1 rs222747 on systolic BP responses and BDKRB2 rs1799722 on heart rate responses during static handgrip exercise were not accompanied by similar observations during PECO. The latter observations are not surprising given that heart rate responses during static handgrip exercise quickly return to baseline during PECO (present study: ∆ 0 ± 6 beats min−1). While this could be taken as evidence against a role of the muscle metaboreflex in controlling heart rate, other work suggests that metaboreflex‐mediated cardiac sympathetic activation is largely masked by parallel cardiac parasympathetic reactivation at moderate intensities (Fisher et al. 2010). Interestingly, we observed that ASIC3 rs2288645 and PTGER2 rs17197 both impacted systolic BP during PECO, but not static handgrip, in men. This is consistent with the need for a larger ischaemic stimulus to activate these pathways, and with evidence that men have greater BP response to static handgrip and PECO (Notay et al. 2018; Parmar et al. 2018). It is also important to consider methodological limitations of using PECO to isolate the haemodynamic contributions of the muscle metaboreflex. Mainly, the PECO model assumes that neural reflexes (e.g. mechanoreflexes, metaboreflexes, central command) contribute additively and does not account for potential interactions. For example, it has been postulated that central command may be modified by perception of effort (Williamson, 2010), which itself can be influenced by feedback from group III/IV skeletal muscle afferents (Amann et al. 2008, 2015). Thus, during static handgrip exercise, alterations in muscle metaboreflex activation could be interacting with other neural mechanisms, such as central command, to further modify the BP response. Finally, the recent discovery of subtypes of group III/IV afferents which respond selectively to low or high (noxious) levels of intramuscular metabolites raise questions regarding the applicability of PECO, particularly as it relates to freely perfused exercise modes (Amann et al. 2015).
In addition to supporting a role for TRPV1 and BDKRB2 receptors in mediating the exercise pressor reflex in humans, the present study has a number of stimulating clinical implications. First, a substantial body of evidence demonstrates the prognostic value of exaggerated BP reactivity to exercise (Schultz & Sharman, 2014). That is, in otherwise healthy or diseased individuals, a larger BP response to dynamic (Miyai et al. 2000, 2002; Weiss et al. 2010) and static handgrip (Chaney & Eyman, 1988) exercise is associated with heightened risk for future hypertension or cardiovascular events. The elucidation of genetic risk factors for identifying such individuals would be beneficial for providing early monitoring or aggressive treatment to reduce these future risks. Second, over‐activation of the muscle metaboreflex is considered to be a feature of the pathophysiology in both hypertension (Delaney et al. 2010; Greaney et al. 2015) and heart failure (Notarius et al. 2001). Older hypertensives display a ∼30% larger systolic and diastolic peak BP response during a 40% MVC static handgrip exercise than age‐matched normotensives (Delaney et al. 2010). In a cohort more comparable to the present study, young unmedicated prehypertensive men demonstrate ∼30% higher peak mean arterial pressure responses during a 50% MVC static handgrip exercise than normotensive controls (Choi et al. 2013). In the present study, the combination of the TRPV1 rs222747 and BDKRB2 rs1799722 polymorphisms in men resulted in individuals carrying minor alleles for both genes having 30% and 38% higher systolic and diastolic BP responses, respectively, during static handgrip exercise than those carrying no minor alleles. Thus, the BP differences caused by the TRPV1 rs222747 and BDKRB2 rs1799722 polymorphisms in men exhibit a similar magnitude difference as reported in those with prehypertension or hypertension. The functional consequences of an overactive muscle metaboreflex are more readily apparent in heart failure, where the result is exaggerated muscle sympathetic responses during static and dynamic exercise (Notarius et al. 2001, 2015), and restraint of exercising limb blood flow (Amann et al. 2014), providing a neural mechanism for exercise intolerance in this population. Whether the prevalence of TRPV1 rs222747 and BDKRB2 rs1799722 risk alleles in clinical populations further modifies BP or sympathetic responses warrants future investigation.
We acknowledge several limitations. First, the metabolically sensitive receptors found in group III/IV afferents are not specific to skeletal muscle and most can be found in several locations both centrally and peripherally. For example, TRPV1 receptors are expressed on both neural and non‐neural cells, such as the brain, skin, mast cells, hair follicles, urinary bladder, lungs and inner ear, and are involved in a wide range of diverse physiological functions (Messeguer et al. 2006). Nevertheless, we selected each of these receptors on the basis of prior evidence that they are involved in mediating the exercise pressor response (Greaney et al. 2015) and employed mental stress to serve as an internal control to provide greater certainty that BP and heart rate differences during exercise were attributable to receptors present in skeletal muscle. Second, we examined the associations between polymorphisms and haemodynamic responses and did not determine how the structure or expression of these receptors was altered between major and minor allele carriers. In line with our findings, prior studies have demonstrated altered sensitivity of TRPV1 rs222747 variants due to a Met315Ile missense mutation occurring in the exon region (Wang et al. 2016) and altered receptor expression of bradykinin receptors with BDKRB2 rs1799722 variants due to the T allele (Braun et al. 1996). Third, we employed a 2 min static handgrip exercise completed in the seated posture and our results may not be generalizable to other postures, contraction durations or exercise modes. Fourth, our cohort consisted of young healthy individuals to avoid the confounding influences of background pharmacological therapy or co‐morbidities; however, future research is necessary in those with hypertension shown to exhibit accentuated BP responses to exercise and heightened muscle metaboreflex activation (Delaney et al. 2010; Greaney et al. 2015). Fifth, the coefficients of variation were larger during PECO than static handgrip, which likely impaired our ability to see parallel statistical changes in both tests; however, the magnitude and direction of differences between groups was similar. Finally, our sample size limited our capacity to investigate the interactions of all studied SNPs, and we consider these results to represent pilot findings to direct future investigations in larger cohorts.
Conclusion
Herein, we demonstrate that TRPV1 and BDKRB2 polymorphisms can influence the haemodynamic responses to exercise but not mental stress. Although the modest effects of individual genetic variants on BP and heart rate during exercise are not surprising given the likely redundancy of the muscle metaboreflex and the integrative complexity of BP regulation during exercise (Stone et al. 2015), we observed additive effects of TRPV1 rs222747 and BDKRB2 rs1799722 resulting in ∼22–23% differences in systolic and diastolic BP responses between individuals carrying both minor alleles compared to individuals carrying no minor alleles. Subgroup analysis determined that these differences were observed primarily in men, and corresponded similarly to reported differences between BP responses during static handgrip exercise in normotensive and hypertensive populations (Delaney et al. 2010; Choi et al. 2013). Future studies are required to test whether polymorphisms in both afferent and efferent pathways similarly produce additive responses and can explain a larger proportion of BP variance.
Additional information
Competing interests
The authors declare no conflicts of interest relevant to the content of this study.
Author contributions
K.N. and P.J.M. conceived and designed the research; K.N., S.L.K., J.B.L., C.J.D., J.D.S. and P.J.M. performed the experiments; K.N. and M.S. analysed the data; K.N., D.M.M. and P.J.M. interpreted the results; K.N. prepared the figures and drafted the manuscript; K.N., D.M.M. and P.J.M. edited and revised the manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This research was supported by a Natural Science and Engineering Research Council of Canada (NSERC) Discovery Grant (P.J.M., no. 06019), the Canada Foundation for Innovation (P.J.M., no. 34379), a University of Guelph‐Humber Research Fund Grant (P.J.M), the Ontario Ministry of Research, Innovation, and Science (P.J.M., no. 34379), and the Ontario Ministry of Agriculture, Food, and Rural Affairs (D.M.M., no. 2093). A NSERC Alexander Graham Bell Canada Graduate Scholarship supported K.N. An Ontario Graduate Scholarship supported J.B.L. A Queen Elizabeth II Graduate Scholarship in Science and Technology supported S.L.K.
Biography
Karambir Notay was an MSc student in the laboratory of P.J.M. in the Department of Human Health and Nutritional Sciences at the University of Guelph. His work focused on examining the mechanisms responsible for inter‐individual variations in blood pressure responses to exercise. He is now attending the University of Toronto's Doctor of Dental Surgery program to further continue his studies.

Edited by: Harold Schultz & Philip Ainslie
Linked articles This article is highlighted in a Perspectives article by Kaur et al. To read this article, visit http://doi.org/10.1113/JP276971.
References
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF & Dempsey JA (2008). Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol 105, 1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Sidhu SK, Weavil JC, Mangum TS & Venturelli M (2015). Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton Neurosci Basic Clin 188, 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MAH, Groot HJ, Walter Wray D, Stehlik J & Richardson RS (2014). Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Kammerer S, Maier E, Böhme E & Roscher AA (1996). Polymorphisms in the gene for the human B2‐bradykinin receptor. New tools in assessing a genetic risk for bradykinin‐associated diseases. Immunopharmacology 33, 32–35. [DOI] [PubMed] [Google Scholar]
- Chaney RH & Eyman RK (1988). Blood pressure at rest and during maximal dynamic and isometric exercise as predictors of systemic hypertension. Am J Cardiol 62, 1058–1061. [DOI] [PubMed] [Google Scholar]
- Choi H‐M, Stebbins CL, Lee O‐T, Nho H, Lee J‐H, Chun J‐M, Kim K‐A & Kim J‐K (2013). Augmentation of the exercise pressor reflex in prehypertension: roles of the muscle metaboreflex and mechanoreflex. Appl Physiol Nutr Metab 38, 209–215. [DOI] [PubMed] [Google Scholar]
- Cui J, Leuenberger UA, Blaha C, King NC & Sinoway LI (2011). Effect of P2 receptor blockade with pyridoxine on sympathetic response to exercise pressor reflex in humans. J Physiol 589, 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, McQuillan P, Momen A, Blaha C, Moradkhan R, Mascarenhas V, Hogeman C, Krishnan A & Sinoway LI (2007). The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol 293, H1861–H1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JA, Melista EA, Chazaro IA, Zhang YB, Zhou XB, Manolis AJE, Baldwin CTB, DeStefano ALC & Gavras HA (2005). Sequence variation of bradykinin receptors B1 and B2 and association with hypertension. J Hypertens 23, 55–62. [DOI] [PubMed] [Google Scholar]
- Davy KP, Herbert WG & Williams JH (1993). Effect of indomethacin on the pressor responses to sustained isometric contraction in humans. J Appl Physiol 75, 273–278. [DOI] [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ & Farquhar WB (2010). Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299, H1318–H1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias RG, Alves M‐JNN, Pereira AC, Rondon MUPB, dos Santos MR, Krieger JE, Krieger MH & Negrão CE (2009). Glu298Asp eNOS gene polymorphism causes attenuation in nonexercising muscle vasodilatation. Physiol Genomics 37, 99–107. [DOI] [PubMed] [Google Scholar]
- Eisenach JH, Barnes SA, Pike TL, Sokolnicki LA, Masuki S, Dietz NM, Rehfeldt KH, Turner ST & Joyner MJ (2005). Arg16/Gly β2‐adrenergic receptor polymorphism alters the cardiac output response to isometric exercise. J Appl Physiol 99, 1776–1781. [DOI] [PubMed] [Google Scholar]
- Ettinger S, Gray K, Whisler S & Sinoway L (1991). Dichloroacetate reduces sympathetic nerve responses to static exercise. Am J Physiol Heart Circ Physiol 261, H1653–H1658. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH & Fadel PJ (2010). Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol 588, 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Young CN & Fadel PJ (2015). Autonomic adjustments to exercise in humans. Compr Physiol 5, 475–512. [DOI] [PubMed] [Google Scholar]
- Fontana GA, Pantaleo T, Bongianni F, Cresci F, Lavorini F, Guerra CT & Panuccio P (1995). Prostaglandin synthesis blockade by ketoprofen attenuates respiratory and cardiovascular responses to static handgrip. J Appl Physiol 78, 449–457. [DOI] [PubMed] [Google Scholar]
- Greaney JL, Wenner MM & Farquhar WB (2015). Exaggerated increases in blood pressure during isometric muscle contraction in hypertension: role for purinergic receptors. Auton Neurosci Basic Clin 188, 51–57. [DOI] [PubMed] [Google Scholar]
- Hayes SG, McCord JL, Rainier J, Liu Z & Kaufman MP (2008). Role played by acid‐sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295, H1720–H1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson E, Larson MG, Vasan RS, O'Donnell CJ, Yin X, Hirschhorn JN, Newton‐Cheh C, Drake JA, Musone SL, Heard‐Costa NL, Benjamin EJ, Levy D, Atwood LD, Wang TJ & Kathiresan S (2007). Heritability, linkage, and genetic associations of exercise treadmill test responses. Circulation 115, 2917–2924. [DOI] [PubMed] [Google Scholar]
- Joyner MJ & Casey DP (2015). Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol Rev 95, 549–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindig AE, Heller TB & Kaufman MP (2005). VR‐1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol 288, H1867–H1873. [DOI] [PubMed] [Google Scholar]
- Klingel SL, Roke K, Hidalgo B, Aslibekyan S, Straka RJ, An P, Province MA, Hopkins PN, Arnett DK, Ordovas JM, Lai C‐Q & Mutch DM (2017). Sex differences in blood HDL‐c, the total cholesterol/HDL‐c ratio, and palmitoleic acid are not associated with variants in common candidate genes. Lipids 52, 969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y‐L, Hsu L‐A, Wu S, Teng M‐S, Chang H‐H, Chen C‐C & Cheng C‐F (2008). Genetic variation in the ASIC3 gene influences blood pressure levels in Taiwanese. J Hypertens 26, 2154–2160. [DOI] [PubMed] [Google Scholar]
- Langberg H, Bjørn C, Boushel R, Hellsten Y & Kjaer M (2002). Exercise‐induced increase in interstitial bradykinin and adenosine concentrations in skeletal muscle and peritendinous tissue in humans. J Physiol 542, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C & Wallin BG (1985). Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57, 461–469. [DOI] [PubMed] [Google Scholar]
- Messeguer A, Planells‐Cases R & Ferrer‐Montiel A (2006). Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol 4, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T & Nishio I (2002). Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension 39, 761–766. [DOI] [PubMed] [Google Scholar]
- Miyai N, Arita M, Morioka I, Miyashita K, Nishio I & Takeda S (2000). Exercise BP response in subjects with high‐normal BP Exaggerated blood pressure response to exercise and risk of future hypertension in subjects with high‐normal blood pressure. J Am Coll Cardiol 36, 1626–1631. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Murphy MN, Mitchell JH & Smith SA (2011). Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589, 6191–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen T, Lehtimäki T, Laiho J, Rontu R, Niemelä K, Kööbi T, Lehtinen R, Viik J, Turjanmaa V & Kähönen M (2006). Effects of polymorphisms in β1‐adrenoceptor and α‐subunit of G protein on heart rate and blood pressure during exercise test. The Finnish Cardiovascular Study. J Appl Physiol 100, 507–511. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Atchison DJ & Floras JS (2001). Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol 280, H969–H976. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Millar PJ, Murai H, Morris BL, Marzolini S, Oh P & Floras JS (2015). Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age‐matched healthy subjects. J Physiol 593, 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notay K, Lee JB, Incognito AV, Seed JD, Arthurs AA & Millar PJ (2018). Muscle strength influences pressor responses to static handgrip in men and women. Med Sci Sports Exerc 50, 778–784. [DOI] [PubMed] [Google Scholar]
- Nunes RAB, Barroso LP, Pereira AdaC, Krieger JE & Mansur AJ (2014). Gender‐related associations of genetic polymorphisms of α‐adrenergic receptors, endothelial nitric oxide synthase and bradykinin B2 receptor with treadmill exercise test responses. Open Heart 1, e000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes RAB, Pereira Barroso L, da Costa Pereira A, Pinto Brandão Rondon MU, Negrão CE, Krieger JE & Mansur AJ (2016). Alpha2A‐adrenergic receptor and eNOS genetic polymorphisms are associated with exercise muscle vasodilatation in apparently healthy individuals. Int J Cardiol Heart Vasc 13, 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HL, Stebbins CL & Longhurst JC (1993). Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol 75, 2061–2068. [DOI] [PubMed] [Google Scholar]
- Parmar HR, Sears J, Molgat‐Seon Y, McCulloch CL, McCracken LA, Brown CV, Sheel AW, Dominelli PB (2018). Oral contraceptives modulate the muscle metaboreflex in healthy young women. Appl Physiol Nutr Metab 43, 460–466. [DOI] [PubMed] [Google Scholar]
- Rett K, Wicklmayr M, Fink E, Maerker E, Dietze G & Mehnert H (1989). Local generation of kinins in working skeletal muscle tissue in man. Biol Chem Hoppe Seyler 370, 445–449. [DOI] [PubMed] [Google Scholar]
- Rotto DM & Kaufman MP (1988). Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64, 2306–2313. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Massey KD, Burton KP & Kaufman MP (1989). Static contraction increases arachidonic acid levels in gastrocnemius muscles of cats. J Appl Physiol 66, 2721–2724. [DOI] [PubMed] [Google Scholar]
- Sato M, Nakayama T, Soma M, Aoi N, Kosuge K, Haketa A, Izumi Y, Matsumoto K, Sato N & Kokubun S (2007). Association between prostaglandin E2 receptor gene and essential hypertension. Prostaglandins Leukot Essent Fatty Acids 77, 15–20. [DOI] [PubMed] [Google Scholar]
- Schultz MG & Sharman JE (2014). Exercise hypertension. Pulse 1, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry BS (2009). SNPs: impact on gene function and phenotype. Methods Mol Biol 578, 3–22. [DOI] [PubMed] [Google Scholar]
- Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH & Garry MG (2010). The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol 588, 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins CL, Carretero OA, Mindroiu T & Longhurst JC (1990). Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol 69, 1225–1230. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Maruoka Y & Longhurst JC (1986). Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res 59, 645–654. [DOI] [PubMed] [Google Scholar]
- Stokes L, Scurrah K, Ellis JA, Cromer BA, Skarratt KK, Gu BJ, Harrap SB & Wiley JS (2011). A loss‐of‐function polymorphism in the human P2X4 receptor is associated with increased pulse pressure. Hypertension 58, 1086–1092. [DOI] [PubMed] [Google Scholar]
- Stone AJ, Copp SW, Kim JS & Kaufman MP (2015). Combined, but not individual, blockade of ASIC3, P2X, and EP4 receptors attenuates the exercise pressor reflex in rats with freely perfused hindlimb muscles. J Appl Physiol 119, 1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm N, Darnhofer‐Patel B, van den Boom D & Rodi CP (2003). MALDI‐TOF mass spectrometry‐based SNP genotyping. Methods Mol Biol 212, 241–262. [DOI] [PubMed] [Google Scholar]
- Symons JD, Theodossy SJ, Longhurst JC & Stebbins CL (1991). Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol 71, 1837–1842. [DOI] [PubMed] [Google Scholar]
- Ueno LM, Frazzatto EST, Batalha LT, Trombetta IC, do Socorro Brasileiro M, Irigoyen C, Brum PC, Villares SMF & Negrão CE (2005). α2B‐Adrenergic receptor deletion polymorphism and cardiac autonomic nervous system responses to exercise in obese women. Int J Obes 30, 214–220. [DOI] [PubMed] [Google Scholar]
- Vianna LC, Fernandes IA, Barbosa TC, Teixeira AL & Claudio Lucas da Nóbrega A (2018). Capsaicin‐based analgesic balm attenuates the skeletal muscle metaboreflex in healthy humans. J Appl Physiol 125, 362–368. [DOI] [PubMed] [Google Scholar]
- Wang S, Joseph J, Diatchenko L, Ro JY & Chung M‐K (2016). Agonist‐dependence of functional properties for common nonsynonymous variants of human transient receptor potential vanilloid 1. Pain 157, 1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF & Mora S (2010). Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation 121, 2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JW (2010). The relevance of central command for the neural cardiovascular control of exercise. Exp Physiol 95, 1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]


