Abstract
Key points
Lifestyle modifications that include the regular performance of exercise are probably important for counteracting the negative consequences of obesity on postprandial myofibrillar protein synthetic responses to protein dense food ingestion.
We show that the interactive effect of resistance exercise and feeding on the stimulation of myofibrillar protein synthesis rates is diminished with obesity compared to normal weight adults.
The blunted myofibrillar protein synthetic response with resistance exercise in people with obesity may be underpinned by alterations in muscle anabolic signalling phosphorylation (p70S6K and 4E‐BP1).
The results obtained in the present study suggest that further exercise prescription manipulation may be necessary to optimize post‐exercise myofibrillar protein synthesis rates in adults with obesity.
Abstract
We aimed to determine whether obesity alters muscle anabolic and inflammatory signalling phosphorylation and also muscle protein synthesis within the myofibrillar (MYO) and sarcoplasmic (SARC) protein fractions after resistance exercise. Nine normal weight (NW) (21 ± 1 years, body mass index 22 ± 1 kg m−2) and nine obese (OB) (22 ± 1 years, body mass index 36 ± 2 kg m−2) adults received l‐[ring‐13C6]phenylalanine infusions with blood and muscle sampling at basal and fed‐state of the exercise (EX) and non‐exercise (CON) legs. Participants performed unilateral leg extensions and consumed pork (36 g of protein) immediately after exercise. Basal muscle Toll‐like receptor 4 (TLR4) protein was similar between OB and NW groups (P > 0.05) but increased at 300 min after pork ingestion only in the OB group (P = 0.03). Resistance exercise reduced TLR4 protein in the OB group at 300 min (EX vs. CON leg in OB: P = 0.04). Pork ingestion increased p70S6K phosphorylation at 300 min in CON and EX of the OB and NW groups (P > 0.05), although the response was lower in the EX leg of OB vs. NW at 300 min (P = 0.05). Basal MYO was similar between the NW and OB groups (P > 0.05) and was stimulated by pork ingestion in the EX and CON legs in both groups (Δ from basal NW: CON 0.04 ± 0.01% h−1; EX 0.10 ± 0.02% h−1; OB: CON 0.06 ± 0.01% h−1; EX 0.06 ± 0.01% h−1; P < 0.05). MYO was more strongly stimulated in the EX vs. CON legs in NW (P = 0.02) but not OB (P = 0.26). SARC was feeding sensitive but not further potentiated by resistance exercise in both groups. Our results suggest that obesity may attenuate the effectiveness of resistance exercise to augment fed‐state MYO.
Keywords: strength training, leucine, muscle mass, mTORC1, inflammation, TLR4
Key points
Lifestyle modifications that include the regular performance of exercise are probably important for counteracting the negative consequences of obesity on postprandial myofibrillar protein synthetic responses to protein dense food ingestion.
We show that the interactive effect of resistance exercise and feeding on the stimulation of myofibrillar protein synthesis rates is diminished with obesity compared to normal weight adults.
The blunted myofibrillar protein synthetic response with resistance exercise in people with obesity may be underpinned by alterations in muscle anabolic signalling phosphorylation (p70S6K and 4E‐BP1).
The results obtained in the present study suggest that further exercise prescription manipulation may be necessary to optimize post‐exercise myofibrillar protein synthesis rates in adults with obesity.
Introduction
Obesity is associated with metabolic alterations during the postprandial period (Golay et al. 1986), many of which stem from impaired skeletal muscle metabolism (e.g. insulin resistance) (Bajpeyi et al. 2011). Specifically, several studies have reported a reduced stimulation of the muscle protein synthetic response to amino acid provision in obese adults compared to normal weight controls (Guillet et al. 2009; Chevalier et al. 2015; Murton et al. 2015; Beals et al. 2016; Smeuninx et al. 2017). Interestingly, it appears that this anabolic resistance of postprandial muscle protein synthesis rates in response to exogenous amino acid administration may be confined to distinct muscle protein subfractions (Guillet et al. 2009; Chevalier et al. 2015; Murton et al. 2015; Beals et al. 2016, 2017). For example, we have recently demonstrated an impairment in the stimulation of postprandial myofibrillar protein synthesis rates (Beals et al. 2016) but not mitochondrial protein synthesis rates (Beals et al. 2017) after ingestion of protein‐dense food in sedentary obese vs. normal‐weight adults. The apparent lack of a stimulation of postprandial myofibrillar protein synthesis rates with obesity may be related to alterations in anabolic and/or inflammatory signalling in response to increased dietary amino acid availability in the circulation (Beals et al. 2016, 2017). This obesity‐related anabolic resistance of myofibrillar proteins to dietary protein is concerning because this protein pool is the most primary and abundant repository of amino acids within skeletal muscles. Hence, the maintenance of the myofibrillar protein fraction is especially relevant for maintaining overall muscle mass and quality throughout adult life.
Resistance exercise is widely recognized as a fundamental anabolic stimulus by increasing mammalian target of rapamycin complex 1 (mTORC1) phosphorylation and subsequently facilitating more effective use of circulating dietary amino acids for the stimulation of postprandial myofibrillar protein synthesis rates but not the sarcoplasmic protein fraction (Moore et al. 2009; Burd et al. 2011 b) compared to the resting‐state in healthy young adults (Moore et al. 2009; Pennings et al. 2011). Importantly, the performance of acute resistance exercise prior to eating a protein dense meal has been shown to maximize the postprandial myofibrillar protein synthetic response in other populations with anabolic resistance, such as the elderly (Yang et al. 2012). Despite this, the efficacy of resistance exercise to augment postprandial muscle protein subfractional synthetic rates has yet to be examined in adults with obesity.
Therefore, the present study aimed to gain an understanding into how human skeletal muscle tissue remodels within the myofibrillar and sarcoplasmic protein fractions in response to the main anabolic stimuli to skeletal muscle tissue, protein ingestion and exercise, in obese and normal weight adults. In addition, we aimed to assess the impact of obesity, exercise and their relationship to muscle inflammatory responses during post‐exercise recovery. We used a unilateral exercise model to allow us to simultaneously assess the effects of food ingestion in the exercise and non‐exercise states. We hypothesized that resistance exercise prior to protein dense food ingestion (36 g of protein and 4 g of fat) would potentiate the phosphorylation of mTORC1 signalling and myofibrillar protein synthesis rates but not the sarcoplasmic fraction compared to the non‐exercise leg in both obese and normal weight adults. Moreover, we hypothesized that muscle inflammatory pathways related to TLR4/MyD88 and nuclear factor kappa B (NF‐κB) signalling would be upregulated with obesity (Beals et al. 2017) and remain unresponsive, irrespective of fat mass, to resistance exercise prescribed to maximize myofibrillar protein synthesis (i.e. 4 sets × 10–12 repetitions performed to volitional fatigue) (Burd et al. 2010 a).
Methods
Participants and ethical approval
Nine normal‐weight (NW) (21 ± 1 years, body mass index 21.9 ± 0.5 kg m−2) and nine obese (OB) (22 ± 1 years, body mass index 35.7 ± 2.3 kg m−2) men and women were enrolled to participate in the present study. Participants were not involved in a regular exercise‐training programme and were considered as insufficiently active based on a Godin leisure‐time exercise questionnaire (GLTEQ < 14 units) (Godin & Shephard, 1997). Participant characteristics are presented in Table 1. All participants were considered healthy based on responses to a routine medical screening questionnaire and had no prior history of participating in stable isotope amino acid tracer experiments. Each participant was informed of the purpose of the study, the experimental procedures and all of the potential risks prior to providing their written consent to participate. The study was approved by the University of Illinois Institutional Review Board (IRB approval #16699) and conformed to standards for the use of human participants in research as outlined in the Declaration of Helsinki. This trial was registered at clinicaltrials.gov as NCT03411681.
Table 1.
Participant characteristics
| Variable | NW | OB |
|---|---|---|
| n (females) | 9 (5) | 9 (4) |
| Age (years) | 21 ± 1 | 22 ± 1 |
| Ht (m) | 1.70 ± 0.04 | 1.71 ± 0.02 |
| Wt (kg) | 63.9 ± 3.8 | 104.0 ± 5.9‡ |
| Body mass index (kg m−2) | 21.9 ± 0.5 | 35.7 ± 2.3 |
| % Body fat | 23.3 ± 2.7 | 36.8 ± 2.7‡ |
| Lean mass (kg) | 47.8 ± 4.3 | 63.2 ± 3.5‡ |
| Lean mass, EX leg (kg) | 8.2 ± 0.8 | 10.8 ± 0.8‡ |
| Waist‐to‐hip ratio | 0.78 ± 0.02 | 0.89 ± 0.02‡ |
| Physical activity (GLTEQ score) | 6.7 ± 2.3 | 11.2 ± 1.5 |
| Fasting blood glucose (mg dL−1) | 79.7 ± 2.7 | 84.4 ± 3.5 |
| Fasting insulin (μIU mL−1) | 8.5 ± 1.1 | 19.0 ± 3.5‡ |
| HOMA‐IR | 1.7 ± 0.3 | 3.9 ± 0.7‡ |
| 120 min blood glucose (mg dL−1) | 81.6 ± 3.9 | 110.3 ± 8.3‡ |
| C‐reactive protein (mg L−1) | 0.95 ± 0.31 | 2.31 ± 0.73 |
Data are the mean ± SEM.
‡ P < 0.05 vs. NW.
Baseline procedures
Prior to the infusion trial, participants reported to the laboratory on two separate occasions in the morning. On the first visit, body weight and height were measured, as well as body composition by dual‐energy X‐ray absorptiometry (QDR 4500A; Hologic, Marlborough, MA, USA). Participant waist‐to‐hip ratio was also measured using the minimum waist‐maximum hip method (Shetterly et al. 1993). Finally, participants were familiarized with unilateral leg extension and the 10‐repetition maximum (10RM) procedure. On a separate occasion, each subject arrived at the laboratory after an overnight fast for the determination of oral glucose tolerance. Blood glucose concentrations were determined before and after consumption of 75 g of glucose dissolved in 500 mL of water. At the end of this visit, participant's unilateral 10RM for leg extension was assessed and used to determine exercise loads for the infusion protocol. The leg selected for exercise was randomized and counterbalanced for leg dominance across groups and sex. Obtained 10RMs were then used to estimate the participants’ single repetition maximum (Brzycki, 1993; Nascimento et al. 2007). In addition, participants were instructed to refrain from vigorous physical activity and alcohol, and to maintain their normal dietary intakes. Food records were obtained during the 3 days prior to the infusion using the Automated Self‐Administered Recall System (ASA24 version 2016; National Cancer Institute, Rockville, MD, USA) (Subar et al. 2012). Dietary macronutrient intakes are reported in Table 2.
Table 2.
Macronutrient intake
| Variable | NW | OB |
|---|---|---|
| Energy intake | ||
| Total (kcal day−1) | 1969 ± 311 | 2289 ± 333 |
| Relative (kcal kg BW−1 day−1) | 30 ± 4 | 23 ± 2 |
| Protein intake | ||
| Total (g day−1) | 70.4 ± 14.3 | 114.0 ± 18.8‡ |
| Relative (g kg BW−1 day−1) | 1.1 ± 0.1 | 1.2 ± 0.2 |
| Energy from protein (% kcal day−1) | 15 ± 1 | 20 ± 3 |
| Carbohydrate (CHO) intake | ||
| Total (g day−1) | 246.4 ± 50.3 | 221.9 ± 62.3 |
| Relative (g kg BW−1 day−1) | 3.8 ± 0.7 | 2.2 ± 0.6 |
| Energy from CHO (%kcal day−1) | 48 ± 3 | 36 ± 6 |
| Fiber (g day−1) | 14.3 ± 1.6 | 14.7 ± 2.7 |
| Fat intake | ||
| Total (g day−1) | 79.9 ± 11.8 | 106.5 ± 8.4 |
| Energy from fat (% kcal day−1) | 37 ± 3 | 44 ± 4 |
| Saturated fat (g day−1) | 22.8 ± 2.9 | 37.8 ± 2.1‡ |
| Cholesterol (mg day−1) | 291.0 ± 68.5 | 443.5 ± 68.0 |
BW, body weight. Data are the mean ± SEM
‡ P < 0.05 vs. NW.
Infusion protocol
The protocol for the infusion trials is presented in Fig. 1. On the day of the infusion trial, participants reported to the laboratory at ∼07.00 h after a 10 h fast. An iv catheter was inserted into an antecubital vein for baseline blood sample collection, after which a primed [2 μmol kg lean body mass (LBM)−1] continuous infusion (0.05 μmol kg LBM−1·min−1) of l‐[ring‐13C6]phenylalanine was initiated (t = −180 min) and maintained until the end of the trial. A second iv catheter was placed in a contralateral dorsal hand vein and placed in a heated blanket for repeated arterialized blood sampling. In the post‐absorptive state, muscle biopsies of the vastus lateralis were collected at 0 min of infusion from the non‐exercised control leg (CON). Subsequently, participants performed unilateral leg extension exercise [4 × 10–12 at 65–70% one repetition maximum (1RM)] to volitional failure. Upon completion, participants consumed 170 g of lean ground pork (36 g protein, ∼3.3 g leucine, 4 g fat). Additional muscle biopsies were collected from both the exercised (EX) and CON legs at 120 and 300 min after pork ingestion. The biopsies were collected from the middle region of the vastus lateralis (∼15 cm above the patella) with a Bergström needle under local anaesthesia. All muscle biopsy samples were freed from any visible adipose, connective tissue and blood, immediately frozen in liquid nitrogen, and stored at −80˚C until subsequent analysis. Arterialized blood samples were drawn every 30 or 60 min during the post‐absorptive and postprandial‐states and placed in pre‐chilled EDTA tubes. Blood samples (8 mL) were collected in EDTA‐containing tubes and centrifuged at 3000 g at 4˚C for 10 min. Aliquots of plasma were frozen and stored at −80˚C until subsequent analysis.
Figure 1. Study timeline.
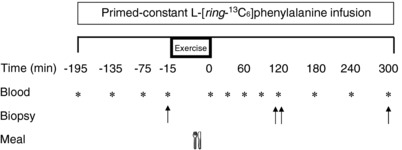
The top bar indicates intravenous infusion of labelled amino acids throughout the day. At t = 0, subjects received a pork meal. Blood samples are indicated with an asterisk (*) and muscle biopsy samples are indicated with an arrow (↑).
Meal composition
Lean centre‐cut pork loins were homogenized, ground and then individually packaged and stored at −20°C until each experimental trial. Prior to the infusion trial, the pork was thawed overnight at 4°C and grilled until the inner temperature reached 65°C. Proximate analysis of centre‐cut pork loin was performed as described previously (Novakofski et al. 1989; Beals et al. 2016). The ground pork patty (170 g) provided 36 g of protein and 4 g of fat. This amount of pork was selected because it represents a typical amount of protein consumed at dinner by US adults (USDA Agricultural Research Service, 2016).
Blood analysis
Glucose and lactate concentrations were analysed in whole blood using an automated biochemical analyser (YSI 2300 Stat Plus; YSI, Yellow Springs, OH, USA). Plasma insulin and C‐reactive protein (CRP) concentrations were determined using commercially available enzyme‐linked immunosorbent assays (ALPCO, Salem, NH, USA). Plasma amino acid enrichments and concentrations were determined by gas chromatography/mass spectrometry (GC/MS) analysis (Model 7890A GC/5975C; Agilent Technologies Inc., Santa Clara, CA, USA) as described previously (Beals et al. 2016). Briefly, plasma samples were deproteinized and converted into tert‐butyldimethylsilyl derivatives prior to GC/MS analysis. Plasma l‐[ring‐13C6]phenylalanine enrichments were determined using electron impact ionization by ion monitoring at m/z 336 (m+0) and 342 (m+6) for unlabelled and labelled phenylalanine, respectively. Amino acid concentrations were quantified using AMDIS, version 2.71 (NIST, Gaithersburg, MD, USA) and standards with known concentrations.
Muscle protein synthesis
Muscle intracellular (IC) amino acids were extracted from ∼15 mg of wet muscle in 0.6 м perchloric acid. Myofibrillar and sarcoplasmic protein‐enriched fractions were extracted from ∼50 mg of wet muscle as described previously (Burd et al. 2015). A portion (100 μL) of the resultant muscle homogenate was reserved for sarcoplasmic protein synthesis measurements. The remaining muscle homogenate was stored at −80°C for subsequent western blot analyses. Myofibrillar and sarcoplasmic protein fractions were hydrolysed overnight in 6 м HCl at 110°C. The resultant free amino acids (IC, myofibrillar, sarcoplasmic protein fractions) were purified using cation exchange chromatography (Dowex 50W‐X8‐200 resin; Acros Organics, Geel, Belgium) and dried under vacuum. Free amino acids were re‐suspended in 60% methanol and centrifuged before analysis using 5500 QTRAP (Sciex, Redwood City, CA, USA) liquid chromatography–tandem mass spectrometry in accordance with a previously reported methodology (Beals et al. 2016, 2017). The L‐[ring‐13C6]phenylalanine enrichments were determined by multiple reaction monitoring at m/z 166.0 → 103.0 and 172.0 → 109.0 for unlabelled and labelled l‐[ring‐13C6]phenylalanine, respectively. Analyst, version 1.6.2 (Sciex) was used for data acquisition and analysis.
Western blotting
A portion of muscle homogenates isolated during the myofibrillar protein extractions was used for western blotting analysis. Phosphorylation status and total protein content of mammalian target of rapamycin complex 1 at Ser2448 (mTORC1: 1° dilution 1:500, catalogue number 2971; Cell Signaling Technology, Beverly, MA USA), 70 kDa S6 protein kinase 1 at Thr389 (p70S6K: 1° dilution 1:500, catalogue number 9205; Cell Signaling Technology), eukaryotic initiation factor 4E binding protein 1 at Thr37/46 (4E‐BP1: 1° dilution 1:1000, catalogue number 9459; Cell Signaling Technology) and nuclear factor‐kappa B (NF‐κB) at Ser468 (NF‐κB: 1° dilution 1:1000, catalogue number 3039; Cell Signaling Technology) were determined using antibodies purchased from Cell Signaling Technology. Muscle protein content was also determined for Toll‐like receptor 4 (TLR4: 1° dilution 1:500, catalogue number 13556; Abcam, Cambridge, MA, USA) and myeloid differentiation factor 88 (MyD88: 1° dilution 1:1000, catalogue number 4283; Cell Signaling Technology). Protein content of the homogenate was determined by Bradford Assay (Bio‐Rad, Hercules, CA, USA) and then equal amounts of protein were separated by SDS‐PAGE before being transferred to either nitrocellulose (mTORC1 and TLR4) or polyvinyl difluoride membranes (p70S6K, 4E‐BP1, MyD88 and NF‐κB). After blocking with either 5% BSA (mTORC1, 4E‐BP1, MyD88, NF‐κB) or 5% milk (p70S6K, TLR4), membranes were incubated in primary antibodies overnight at 4°C. Membranes from the respective proteins were then incubated with appropriate secondary antibodies and protein content was detected using West Femto Maximum Sensitivity substrate (SuperSignal; Thermo Scientific, Waltham, MA, USA) and the ChemiDoc XRS+ Imaging System (Bio‐Rad) and by comparing molecular weights with pre‐stained protein standards (Kaleidoscope; Bio‐Rad). After detection of phosphorylated proteins, membranes were stripped with western blot stripping buffer (Restore; Thermo Scientific) and re‐incubated with antibodies against total protein. Western blot data were normalized to an internal control (α‐tubulin: 1° dilution 1:5000, catalogue number 4074; Abcam). Bands were quantified using ImageJ (NIH, Bethesda, MD, USA), normalized to a control sample run on each blot to account for inter‐blot variability.
Calculations
Homeostasis model assessment of insulin resistance (HOMA‐IR) was calculated using the fasting glucose and insulin values from the oral glucose tolerance test (OGTT) (Glucosefast × Insulinfast/22.5) (Matthews et al. 1985). The fractional synthetic rates (FSR) of myofibrillar and sarcoplasmic proteins were calculated using standard precursor‐product methods by dividing the increment in tracer enrichment in the protein fractions by the enrichment of IC free precursor surrogate pools over time. For the calculation of muscle protein FSR at 0–300 min, the change in phenylalanine labelling between the muscle biopsies collected at t = −15 and 300 min was used. For the calculation of FSR at 0–120 min, the increment in muscle tracer enrichment between t = −15 and 120 min was used. For the calculation of FSR at 120–300 min, the increment in muscle tracer enrichment from t = 120 and 300 min was used. The baseline muscle‐bound enrichment for the calculation of resting muscle protein synthesis rates was estimated using mixed proteins in plasma collected prior to initiation of the infusion as described in detail elsewhere (Burd et al. 2011 a, 2012).
Statistical analysis
Differences in myofibrillar and sarcoplasmic protein synthesis, plasma and intracellular enrichments, muscle signalling proteins, blood glucose and lactate, and plasma insulin were determined using factorial ANOVA with repeated measures on time. Bonferroni post hoc tests were used to compare means whenever significant interactions were observed. Body composition, demographics, exercise variables, dietary intake, HOMA‐IR and plasma CRP, as well as net area under the blood glucose, plasma insulin and AA curves (AUC), were analysed using unpaired Student's t tests. For all analyses, P < 0.05 was considered statistically significant. All calculations were performed using SPSS, version 20 (IBM Corp., Armonk, NY, USA). Data are expressed as the mean ± SEM.
Results
OGTT
Data obtained during the OGTT are presented in Table 1. Blood glucose was similar among groups at baseline (P = 0.33). However, at 120 min post‐beverage consumption, blood glucose was greater in the OB (110.3 ± 8.3 mg dL−1) compared to the NW (81.6 ± 3.9 mg dL−1; P = 0.008) group. Fasting insulin was greater (P = 0.01) in the OB (19.0 ± 3.2 μIU mL−1) compared to the NW (8.5 ± 1.1 μIU mL−1) group. Similarly, HOMA‐IR was greater (P = 0.01) in the OB (3.88 ± 0.70) compared to the NW (1.73 ± 0.25) group. Fasting CRP was not different between groups (P = 0.19).
Resistance exercise
Exercise performance variables are presented in Table 3. Total external work [repetitions × load (kg)] performed during resistance exercise was similar between groups (NW: 1610 ± 164 kg; OB: 1888 ± 154 kg, P = 0.23). However, total external work relative to the lean mass of the EX leg (leg LM) was greater in the NW (196.4 ± 3.1 kg kg leg LM−1) compared to the OB (176.0 ± 7.7 kg kg leg LM−1; P = 0.03) group. Resistance exercise increased blood lactate concentrations similarly between the NW and OB groups (absolute change from pre‐exercise NW: 3.80 ± 0.93 mM; OB: 3.40 ± 0.52 mM; Exercise effect: P < 0.001; Group effect: P = 0.30).
Table 3.
Exercise variables
| Variable | NW | OB |
|---|---|---|
| Unilateral leg extension 10 RM (kg) | 31.8 ± 3.0 | 37.8 ± 2.8 |
| Pre‐exercise blood lactate (mM) | 0.99 ± 0.09 | 1.22 ± 0.20 |
| Post‐exercise blood lactate (mM) | 4.79 ± 1.00 | 4.61 ± 0.66 |
| Total work (kg) | 1610 ± 164 | 1888 ± 154 |
| Relative work (kg kg 1 RM−1) | 38.1 ± 1.3 | 37.3 ± 0.9 |
| Work: leg LM ratio (kg kg leg LM−1) | 196.4 ± 3.1 | 176.0 ± 7.7‡ |
1RM, one repetition maximum; LM, lean mass. Data are the mean ± SEM.
‡ P < 0.05 vs. NW.
Blood variables
Blood glucose concentrations did not change after pork ingestion (P < 0.05), with no differences between the NW and OB groups (P < 0.05) (Fig. 2 A). Basal insulin concentrations were ∼3.2‐fold greater in the OB (19.0 ± 3.4 μIU mL−1) compared to the NW (8.2 ± 0.9 μIU mL−1) group (Fig. 2 B). Plasma insulin concentrations increased similarly above basal in both groups (Time effect: P = 0.02; Group × Time: P = 0.36). However, plasma insulin concentrations were greater in the OB group throughout the infusion trial compared to the NW group (Group effect: P = 0.002). Plasma essential amino acid (EAA) concentrations were not different at baseline (P = 0.89) and increased similarly after pork ingestion in the NW and OB groups (Time effect: P < 0.001) with peak values at 90 min of the postprandial period (Fig. 2 C). Moreover, the net area under the concentration × time curve (AUC) for EAA was similar between groups (P = 0.92). Similarly, plasma branched chain amino acid (BCAA) concentrations increased after pork ingestion, with no differences between groups (Time effect: P < 0.001) (Fig. 2 D).
Figure 2. Blood variables.
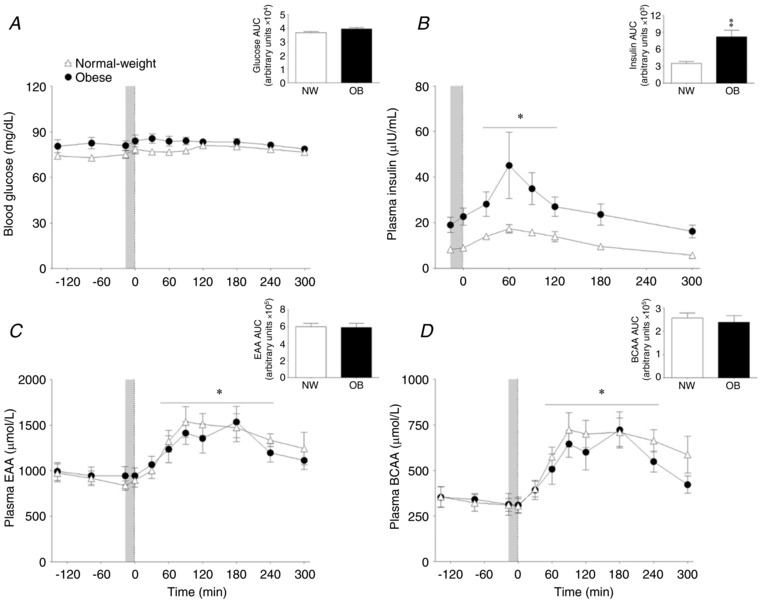
Blood glucose (A) and plasma insulin (B) EAA (C) and BCAA (D) concentrations in the basal state and after pork ingestion (n = 9 per group). Inset are the respective area under the time curves. Grey bar indicates a bout of unilateral leg extension exercise. Dashed vertical line refers to pork ingestion. Data are the mean ± SEM. Insulin concentrations – Group effect: P = 0.002. * P < 0.05 vs. basal. ‡P < 0.05 vs. NW.
Muscle inflammatory signalling
Basal muscle TLR4 protein content was similar among groups (P = 0.22) (Fig. 3 A). Pork ingestion did not affect muscle TLR4 protein concentrations in either the CON or EX legs of NW (all P > 0.05). By contrast, muscle TLR4 protein content in the muscle of the OB group was elevated above basal in both the CON and EX legs at 300 min (P < 0.05); however, muscle TLR4 protein content was reduced in the EX compared to the CON leg of the OB group (P = 0.04). Total MyD88 protein contents were stable, with no effect of pork ingestion or exercise in the CON or EX legs of the NW and OB groups (all P > 0.05) (Fig. 3 B).
Figure 3. Muscle inflammatory signaling.
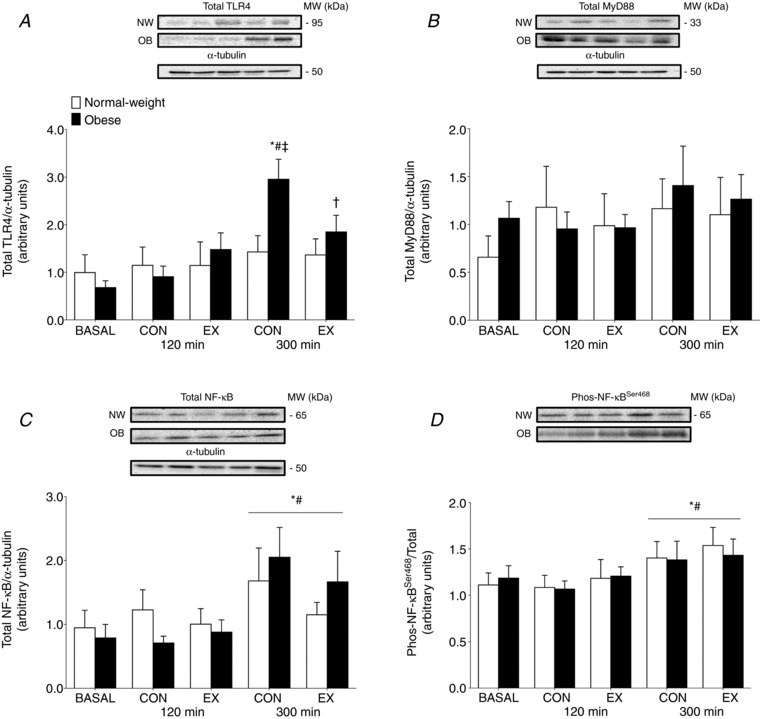
Muscle protein content for TLR4 (A), MyD88 (B), total protein (C) and phosphorylation of NF‐κB at Ser468 (D) at basal and after the ingestion of pork (n = 9 per group) in both the non‐exercised (CON) and exercised (EX) leg. Immediately prior to pork ingestion, participants performed a bout of unilateral leg extension exercise. Data are the mean ± SEM. * P < 0.05 vs. basal, #P < 0.05 vs. 120 min, †P < 0.05 vs. CON leg, ‡P < 0.05 vs. NW.
Muscle relative abundance of NF‐κB protein is shown in Fig. 3 C. Relative NF‐κB protein content was similar among groups throughout the trial (Group effect: P = 0.97). Pork ingestion increased NF‐κB protein content at 300 min in both the EX and CON legs of both groups (Time effect: P = 0.002), with no differences between the EX and CON legs (P > 0.05). The phosphorylation state of NF‐κB at Ser468 was also similar among groups during the infusion trials (P = 0.98) (Fig. 3 D). The phosphorylation state of NF‐κB increased in response to pork ingestion similarly in in both the EX and CON legs of each group (Time effect: P = 0.006). There were no differences in the phosphorylation state of NF‐κB between the EX and CON legs in either group (P = 0.85).
Muscle anabolic signalling
Relative total protein content for mTORC1 were stable after pork ingestion (P = 0.21) or exercise (P = 0.93) in both the NW and OB groups. However, total mTORC1 protein content was 2.3‐fold greater at basal in the OB group compared to the NW group (Group effect: P = 0.03) (Fig. 4 A). The phosphorylation state of mTORC1 at Ser2448 was also unaffected by pork ingestion (P = 0.84) or exercise (P = 0.66) in the NW and OB groups throughout the 0–300 min postprandial period. However, there was a trend for an increased mTORC1 phosphorylation state in NW compared to the OB group (Group effect: P = 0.08) (Fig. 4B).
Figure 4. Muscle anabolic signaling.
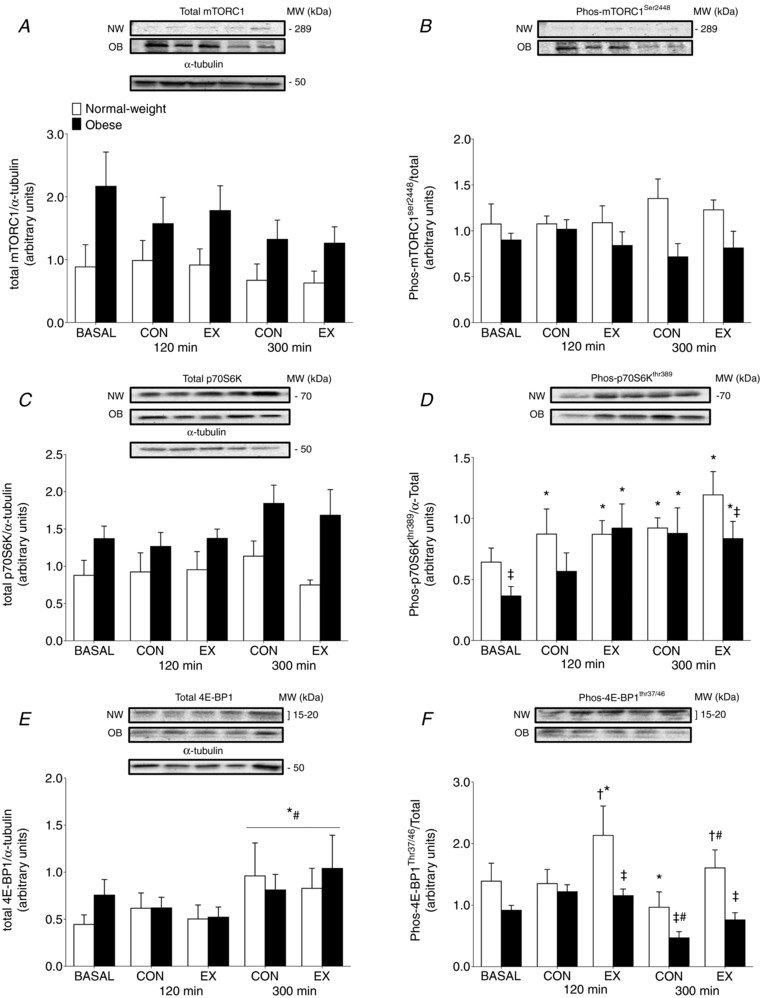
Muscle protein content and phosphorylation of the mTORC1 at Ser2448 (A and B), p70S6K at Thr389 (C and D) and 4E‐BP1 at Thr37/46 (E and F) at basal and after the ingestion of pork (n = 9 per group) in both the non‐exercised (CON) and exercised (EX) leg. Immediately prior to pork ingestion, participants performed a bout of unilateral leg extension exercise. Data are the mean ± SEM. Total mORC1 – Group effect: P = 0.03. Total p70S6K – Group effect: P = 0.02. * P < 0.05 vs. basal, #P < 0.05 vs. 120 min, †P < 0.05 vs. CON leg, ‡P < 0.05 vs. NW.
Total p70S6K protein content was 1.6‐fold greater at basal in the OB group compared to the NW group (Group effect: P = 0.02) (Fig. 4 C). However, protein ingestion (P = 0.27) or resistance exercise (P = 0.26) did not alter total p70S6K protein content in either group throughout the 0–300 min postprandial period. At basal, p70S6K phosphorylation at Thr389 was lower in OB group compared to the NW group (P = 0.04) (Fig. 4 D). The phosphorylation state of p70S6K was increased above basal at 120 and 300 min in the CON and EX legs of the NW group (both P < 0.05), with no differences between CON and EX (all P > 0.05). By contrast, at 120 min of the postprandial period, the p70S6K phosphorylation state was only increased above basal in the EX leg of OB group (P = 0.01). At 300 min, the p70S6K phosphorylation state was increased in both the CON and EX legs (both P > 0.05). However, in the EX leg, there was a lower p70S6K phosphorylation state in the OB group compared to the NW group (P = 0.05).
Relative total muscle 4E‐BP1 protein content is shown in Fig. 4 E. Muscle 4E‐BP1 protein content was similar among groups throughout the trial (Group effect: P = 0.57). 4E‐BP1 protein content increased similarly among groups at 300 min after pork ingestion in both the EX and CON legs of both groups (Time effect: P = 0.002), with no differences between the EX and CON legs (P>0.05). Phosphorylation of 4E‐BP1 at Thr37/46 was not different between groups at basal (P = 0.19). The phosphorylation state of 4E‐BP1 decreased at 300 min after pork ingestion in the CON leg of both groups (P < 0.05). Resistance exercise increased the phosphorylation state of 4E‐BP1 at 120 min after pork ingestion in the NW group (P = 0.03) but not the OB group (P = 0.62).
The phosphorylation state of 4E‐BP1 was lower in both the EX and CON legs of the OB group at 300 min after pork ingestion compared to the NW group (P = 0.01).
Plasma and intracellular precursor enrichments
Plasma and IC free l‐[ring‐13C6]phenylalanine enrichments are shown in Fig. 5. Plasma l‐[ring‐13C6]phenylalanine enrichments were steady during the post‐absorptive period, but decreased after pork ingestion at 90 and 120 min of the postprandial period (Time effect: P = 0.002), with no group differences (P = 0.29). IC l‐[ring‐13C6]phenylalanine enrichments also decreased at 120 min of the postprandial period (Time effect: P = 0.01), with no differences observed between groups (group effect: P = 0.22). In addition, IC enrichments were also greater in EX compared to CON legs (exercise effect: P = 0.03).
Figure 5. Plasma and intracellular precursor enrichments.
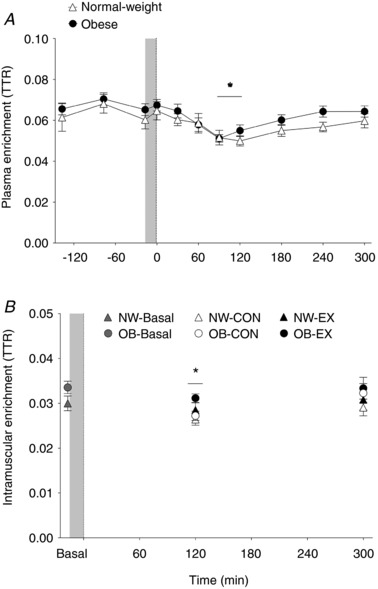
Plasma (A) and muscle‐free (B) L‐[ring‐13C6]phenylalanine enrichments [tracer‐to‐tracee ratio (TTR)] in the basal state and after pork ingestion (n = 9 per group). Dashed vertical line refers to pork ingestion. Grey bar indicates a bout of unilateral leg extension exercise. Data are the mean ± SEM. IC enrichments – EX effect: P = 0.03. * P < 0.05 vs. basal.
Muscle protein synthesis
Sarcoplasmic and myofibrillar protein synthesis rates calculated using the intracellular precursor pool are presented in Fig. 6. Basal sarcoplasmic protein synthesis rates did not differ between the NW and OB groups (P = 0.72) (Fig. 6 A). Postprandial sarcoplasmic protein synthesis rates increased similarly in CON leg after pork ingestion throughout the 0–300 min postprandial period in the NW and OB groups (P = 0.45). Moreover, there were no differences observed in the EX vs. CON legs throughout the 0–300 min postprandial period in the NW or OB groups (P = 0.52). The time course of stimulation of sarcoplasmic protein synthesis rates after pork ingestion is shown in Table 4. Sarcoplasmic protein synthesis rates increased in the CON leg at 0–120 min and 120–300 min (P < 0.05), with no group differences (P = 0.30). However, postprandial sarcoplasmic protein synthesis rates only increased in the EX leg of the OB group at 0–120 min and were greater than the response observed in the NW group (P = 0.03). Sarcoplasmic protein synthesis rates were similar at 120–300 min after pork ingestion in the EX leg in the NW and OB groups (P = 0.31).
Figure 6. Muscle protein synthesis.
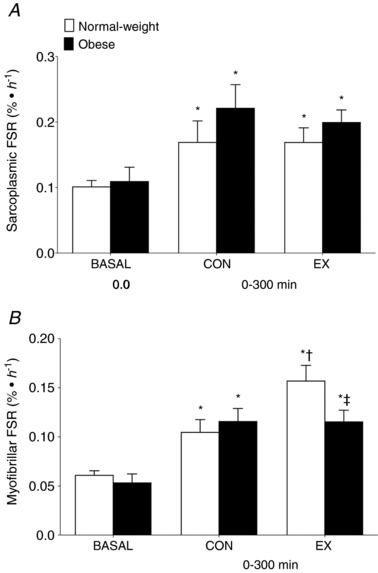
Sarcoplasmic (A) and myofibrillar (B) FSR calculated using the muscle‐free l‐[ring‐13C6]phenylalanine as a precursor at basal and after (0–300 min) the ingestion of pork (n = 9 per group) in both the non‐exercised (CON) and exercised (EX) leg. Immediately prior to pork ingestion, participants performed a bout of unilateral leg extension exercise. Data are the mean ± SEM. * P < 0.05 vs. basal, †P < 0.05 vs. CON leg, ‡P < 0.05 vs. NW group.
Table 4.
Temporal myofibrillar and sarcoplasmic protein synthetic responses in the exercised (EX) and non‐exercised control (CON) legs before and after pork ingestion (n = 9 per group)
| Time (min) | ||||||
|---|---|---|---|---|---|---|
| 0–120 | 120–300 | |||||
| MPS (% h−1) | Group | Basal | CON | EX | CON | EX |
| Sarcoplasmic | NW | 0.10 ± 0.01 | 0.18 ± 0.03* | 0.10 ± 0.04 | 0.20 ± 0.05* | 0.22 ± 0.04*, # |
| OB | 0.11 ± 0.02 | 0.18 ± 0.04* | 0.20 ± 0.02*, ‡ | 0.25 ± 0.05* | 0.20 ± 0.03* | |
| Myofibrillar | NW | 0.06 ± 0.01 | 0.12 ± 0.02* | 0.18 ± 0.02*, † | 0.09 ± 0.01* | 0.14 ± 0.03*, † |
| OB | 0.05 ± 0.01 | 0.12 ± 0.02* | 0.12 ± 0.01*, ‡ | 0.11 ± 0.01* | 0.11 ± 0.02* | |
Data are the mean ± SEM.
* P < 0.05 vs. basal
# P < 0.05 vs. 0‐120
† P < 0.05 vs. CON leg
‡ P < 0.05 vs. NW.
Basal myofibrillar protein synthesis rates were similar between NW and OB groups (P = 0.42) (Fig. 6 B). Cumulative (0–300 min) myofibrillar protein synthesis rates were stimulated in the EX and CON legs after pork ingestion in both the NW and OB groups. However, the postprandial myofibrillar protein synthetic response was stimulated to a greater extent in the EX vs. CON legs in the NW group (absolute difference from CON: 0.052 ± 0.016 % h−1; P = 0.03) but not in the OB group (absolute difference from CON: −0.001 ± 0.015 % h−1; P = 1.00). The time course of stimulation of the myofibrillar protein synthetic responses is shown in Table 4. Pork ingestion stimulated postprandial myofibrillar protein synthesis rates above basal values in the CON legs at 0–120 and 120–300 min of the postprandial period with no group differences (all P > 0.05). Postprandial myofibrillar protein synthesis rates were greater in EX than CON leg at 0–120 and 120–300 min after pork ingestion in the NW group (P < 0.05). However, postprandial myofibrillar protein synthesis rates in the EX‐state were not stimulated above those of CON (P > 0.05) and were lower in the OB compared to the NW group (P < 0.05).
Discussion
To our knowledge, this is the first assessment of muscle anabolic and inflammatory signalling, as well as changes in myofibrillar and sarcoplasmic protein synthesis rates, after protein dense food ingestion in the non‐exercise and exercise‐states in people with obesity. Here, we show that myofibrillar and sarcoplasmic protein synthesis rates are similarly enhanced with pork ingestion in the non‐exercise leg in both the NW and OB groups. However, resistance exercise potentiated the fed‐state synthetic response only within the myofibrillar protein fraction in the NW group, and not in the OB group. The stimulation of sarcoplasmic protein synthesis rates was feeding responsive, with no additive effect of prior resistance exercise on this protein fraction, irrespective of body mass index score. What is noteworthy is that this blunted post‐exercise myofibrillar protein synthetic response with obesity occurred despite a lack of clear differences in habitual physical activity levels or systemic inflammation between the groups (Table 1). As such, our results demonstrate that excess adiposity attenuated the effectiveness of resistance exercise to potentiate the fed‐state myofibrillar protein synthetic response in otherwise healthy young adults.
Previous reports have emphasized the importance of resistance exercise volume (Burd et al. 2010 a) and the achievement of bulk muscle fibre recruitment (Burd et al. 2010 b) as important anabolic factors for maximizing the postprandial myofibrillar protein synthetic response during recovery from resistance exercise in healthy young adults. Based on this, we provided (or what we considered to be) a potent resistance exercise prescription to maximize fed‐state myofibrillar protein synthesis rates. Specifically, the volunteers performed resistance exercise at four sets × 10–12 repetitions, with each set performed to volitional failure, which generally serves as a proxy indicator for a strong stimulus being provided to maximize muscle fibre recruitment patterns in both the NW and OB groups (Potvin & Fuglevand, 2017). Moreover, both the NW and OB groups performed similar amounts of external total work [repetitions × load (kg)] in both absolute terms and when expressed relative to maximal strength (Table 3), which resulted in similar blood lactate concentrations immediately after resistance exercise. The amount of external work performed during resistance exercise has been shown to directly influence both the amplitude and the duration of the stimulation of post‐exercise myofibrillar protein synthesis rates (Burd et al. 2010 b). Hence, it would appear that a sufficient and equivalent resistance exercise stimulus was provided to both the NW and OB groups. What is noteworthy, however, is that obesity is associated with greater total lean mass compared to normal weight individuals in this data set and others (Forbes & Welle, 1983; Beals et al. 2016). Therefore, we compared the amount of external work performed relative to the lean mass of the exercised leg. We observed that the NW group performed more total work per leg lean mass, despite both groups exercising at a high level of effort and until voluntary failure. Thus, it could be speculated that the ‘quality’ of the overall contractile stimulus on skeletal muscle tissue was reduced in the OB compared to the NW group. We did not assess electromyography during exercise and, as such, it is not possible to determine whether muscle fibre recruitment patterns were different between the OB and NW groups. Notwithstanding, studies in ageing populations, who also display anabolic resistance (Wall et al. 2015), have shown that more resistance exercise volume is required to elicit a robust post‐exercise myofibrillar protein synthetic response compared to their younger‐counterparts (Kumar et al. 2012). Therefore, it is possible that we under‐prescribed resistance exercise and subsequently missed an opportunity to maximize the anabolic potential of resistance exercise to counteract the negative consequences of obesity on myofibrillar protein remodelling, despite providing an exercise prescription exceeding current exercise recommendations for novice weight lifters (ACSM, 2009; Donnelly et al. 2009; CSEP, 2011).
Similar to previous efforts (Moore et al. 2009; Burd et al. 2011 b), we show that resistance exercise had no additional effect on the stimulation of postprandial sarcoplasmic protein synthesis rates in the OB or NW groups. Interestingly, the time course in the stimulation of sarcoplasmic protein synthesis rates was different at 2 h after protein dense food ingestion in the EX‐state between NW and OB groups. Specifically, the postprandial sarcoplasmic protein response in the EX‐state was greater at 0–2 h in the OB group compared to the NW group. Given that the synthesis rates of myofibrillar protein fraction were robustly stimulated in the NW group at 0–2 h compared to the OB group, it appears that perhaps with resistance exercise the dietary amino acids remained confined to the more rapidly turning over sarcoplasmic pool in obese muscles as opposed to being used for post‐exercise myofibrillar protein accretion.
The cellular mechanisms that regulate myofibrillar protein synthesis rates are assumed to be centered on anabolic pathways such as mTORC1 signalling (Abou Sawan et al. 2018). We previously showed dysregulated mTORC1 signalling in muscles of obese vs. normal weight adults (Beals et al. 2016). In particular, muscles of individuals with obesity demonstrated an increased relative content of total mTORC1 protein, and increased phosphorylation, compared to the NW adults. Here, we also observed elevated mTORC1 protein content and phosphorylation in the basal and fed‐states in the OB group compared to the NW group. Indeed, when assessing the ratio of phosphorylation to total mTORC1 protein, there were no observed differences between the OB and NW groups (Figure 4), although these normalized data mask the aberrant mTORC1 signalling that occurred in obese vs. healthy‐weight muscles. This notion is further emphasized by an obesity‐related decrease in p70S6K phosphorylation in the basal‐state and at 300 min of the exercise leg compared to the NW group (Figure 4). Previously, it has been shown that the amplitude of increase in post‐exercise p70S6K phosphorylation at Thr389 is sensitive to resistance exercise volume in normal weight adults (Terzis et al. 2010). Moreover, it has been demonstrated that doubling the resistance exercise volume (six sets > three sets at 75% of 1RM) in older adults induces a more robust increase in p70S6K phosphorylation during post‐exercise recovery (Kumar et al. 2012). We also observed a blunted phosphorylation of 4E‐BP1 at Thr37/46 in response to resistance exercise in people with obesity compared to their normal‐weight counterparts (Figure 4). Although not a universal finding (Dreyer et al. 2006), the phosphorylation state of 4E‐BP1 is typically quite sensitive to resistance exercise in young adults (Kumar et al. 2009; Burd et al. 2010 b; Vliet et al. 2017). However, 4E‐BP1 phosphorylation has also been shown to be blunted during recovery from resistance exercise (Kumar et al. 2009), which may partly explain the blunted myofibrillar protein synthetic responses to resistance exercise observed in these groups. Thus, we speculate that anabolic signalling mechanisms may be desensitized to exercise in obese muscles such that a higher threshold of volume may be required to restore the anabolic sensitivity of the muscle protein synthetic machinery.
The results from our laboratory (Beals et al. 2017) and other previous studies (Reyna et al. 2008; Timmerman et al. 2016) have shown that individuals with obesity have elevated muscle TLR4 protein content. However, we did not observe this in the present study. In agreement with our previous studies, we observed an obesity‐specific increase in muscle TLR4 protein content after protein‐dense food ingestion. By contrast to our previous work (Beals et al. 2017), we observed no differences between groups in muscle MyD88, an adaptor protein downstream of TLR4 signalling. We also observed that total NF‐κB protein content and phosphorylation state was similar among groups at basal and throughout the postprandial period, which was unaffected by acute resistance exercise. Our findings are in contrast to previous reports where older adults with altered post‐exercise anabolic signalling also displayed elevated NF‐κB phosphorylation at Ser468 (Rivas et al. 2012). Furthermore, resistance exercise has been shown to suppress NF‐κB phosphorylation in young untrained individuals (Rivas et al. 2012). In the present study, we observed a main effect of pork ingestion during the postprandial period in the EX and CON legs for increases in both total and phosphorylated NF‐κB in both groups. However, the activation of NF‐κB kinase did not interfere with anabolic signalling mechanisms or the stimulation of myofibrillar protein synthesis rates in the NW group. Thus, the role of activation of NF‐κB signalling on the post‐exercise skeletal muscle adaptive responses in young adults warrants further examination. Interestingly, we demonstrated that an acute bout of resistance exercise suppresses the feeding‐induced increase in muscle TLR4 protein content in people with obesity. This receptor has been implicated in the development of insulin resistance (Shi et al. 2006) and reductions in TLR4 protein may be a mechanism for resistance exercise‐induced increases in insulin sensitivity (Croymans et al. 2013). Relatively few studies have attempted to capture the acute or prolonged effect of exercise on muscle TLR4 protein (Lambert et al. 2008; Ghosh et al. 2015; Li et al. 2015). Twelve weeks of combined endurance and resistance exercise training has been shown to be more effective than diet‐induced weight loss at reducing total muscle TLR4 protein content in older adults with obesity (Lambert et al. 2008). In support, 16 weeks of aerobic exercise reduces muscle TLR4 protein in non‐obese older adults to amounts that are similar to younger adults (Ghosh et al. 2015). In the present study, we show that acute resistance exercise can modify and reduce total muscle TLR4 protein content in obese muscles. Certainly, more work is ultimately needed to determine how acute vs. chronic exercise modulates muscle inflammation in people with obesity and the associated phenotypic consequence.
There are conflicting results with regards to the effects of obesity on the stimulation of muscle protein synthesis rates in response to elevated plasma amino acid availability in the resting‐state compared to normal‐weight controls. By contrast to some prevous studies (Murton et al. 2015; Beals et al. 2016; Smeuninx et al. 2017), but not all (Chevalier et al. 2015; Hector et al. 2015), we showed that postprandial myofibrillar protein synthesis rates were stimulated in the CON leg of people with obesity. From a physiological perspective, several noteworthy differences exist between the obese group used in our present study compared to the OB group included in our previous investigations (Beals et al. 2016). Specifically, we observed no differences in markers of low‐grade systemic and muscle inflammation (fasting plasma CRP, as well as muscle TLR4 protein content and phosphorylation state of NF‐κB, respectively) or plasma branched‐chain amino acids concentrations between the NW and OB group in the present study. Elevated plasma BCAA concentrations have been shown to differentiate between metabolically healthy and unhealthy obese phenotypes (Bagheri et al. 2018). Altogether, these studies highlight the notion that metabolic health varies within obese populations (Samocha‐Bonet et al. 2014) and differences in volunteer characteristics may have contributed to the differential findings of the present study vs. our previous work (Beals et al. 2016). Moreover, it is challenging to directly compare results from a unilateral exercise model (as used in the present study) vs. a study that was conducted in the resting‐state (Beals et al. 2016). Furthermore, volunteers consumed a single food source containing 36 g of protein and 4 g of fat in the present study. It is far more common to consume mixed meals within a normal eating pattern and, thus, understanding the potential anabolic action of mix meal ingestion and/or the impact of several meal‐times throughout a more prolonged recovery on muscle protein synthesis rates with obesity is a logical progression for the field. Overall, our study design allowed us to clearly demonstrate that resistance exercise failed to augment postprandial myofibrillar protein synthesis rates within an acute meal setting in young adults with obesity. This anabolic defect manifested in participants with apparently normal fed‐state myofibrillar protein synthesis rates in the CON leg compared to NW group.
In conclusion, we show that excess fat mass diminishes the interactive nature of feeding and resistance exercise on the stimulation of myofibrillar protein synthesis in sedentary young adults. We observed no defect in the stimulation of sarcoplasmic protein synthesis rates between obese and normal weight adults. This finding highlights the value of studying muscle protein subfractional responses in people with obesity. In addition, we show that muscle anabolic signalling mechanisms are also reduced in obese vs. normal weight adults. The focus of future research should aim to determine the influence of various exercise prescription manipulations (exercise mode, type, intensity, etc.) with respect to re‐sensitizing the anabolic machinery and myofibrillar protein synthesis rates in people with obesity.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
JWB and NAB contributed to the conception and the design of the experiment. JWB, SKS, CFM, EGP, SAF, SvV, IGM, AVU, ZL, SAP and NAB contributed to the collection, analysis and interpretation of data. JWB and NAB contributed to drafting or revising intellectual content of the manuscript. JWB and NAB had primary responsibility for final content. JWB, SKS, CFM, EGP, SAF, SvV, IGM, AVU, ZL, SAP and NAB read, edited and approved the final version of the manuscript submitted for publication. The researchers were responsible for the study design, data collection and analysis, decision to publish, and preparation of the manuscript. The American College of Sports Medicine Foundation approved the study design.
Funding
This research was supported by an ACSM Foundation Doctoral Student Research Grant from the American College of Sports Medicine Foundation.
Acknowledgements
We are grateful to the participants who volunteered for this study. We also would like to thank Anna Dilger and the Meat Science Laboratory for the proximate analysis of the pork loin.
Biography
Joseph Beals received his Master's Degree from Colorado State University, where he studied Health and Exercise Science. After graduation, Joseph received his doctoral training in the Division of Nutritional Sciences at the University of Illinois under the supervision of Nicholas Burd. Joseph's dissertation work focused on the effect of human obesity on muscle protein synthesis. Joseph is continuing his research in people with obesity as a postdoctoral scholar at the Washington University School of Medicine at the Center for Human Nutrition.

Edited by: Scott Powers & Paul Greenhaff
This is an Editor's Choice article from the 1 November 2018 issue.
References
- Abou Sawan S, van Vliet S, Parel JT, Beals JW, Mazzulla M, West DWD, Philp A, Li Z, Paluska SA, Burd NA & Moore DR (2018). Translocation and protein complex co‐localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise. Physiol Rep 6, e13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACSM (2009). American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41, 687–708. [DOI] [PubMed] [Google Scholar]
- Bagheri M, Farzadfar F, Qi L, Yekaninejad MS, Chamari M, Zeleznik OA, Kalantar Z, Ebrahimi Z, Sheidaie A, Koletzko B, Uhl O & Djazayery A (2018). Obesity‐related metabolomic profiles and discrimination of metabolically unhealthy obesity. J Proteome Res 17, 1452–1462. [DOI] [PubMed] [Google Scholar]
- Bajpeyi S, Pasarica M, Moro C, Conley K, Jubrias S, Sereda O, Burk DH, Zhang Z, Gupta A, Kjems L & Smith SR (2011). Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J Clin Endocrinol Metab 96, 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals JW, Mackenzie RWA, van Vliet S, Skinner SK, Pagni BA, Niemiro GM, Ulanov AV, Li Z, Dilger AC, Paluska SA, De Lisio M & Burd NA (2017). Protein‐rich food ingestion stimulates mitochondrial protein synthesis in sedentary young adults of different BMIs. J Clin Endocrinol Metab 102, 3415–3424. [DOI] [PubMed] [Google Scholar]
- Beals JW, Sukiennik RA, Nallabelli J, Emmons RS, van Vliet S, Young JR, Ulanov AV, Li Z, Paluska SA, Lisio MD & Burd NA (2016). Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein‐dense food is reduced in overweight and obese young adults. Am J Clin Nutr 104, 1014–1022. [DOI] [PubMed] [Google Scholar]
- Brzycki M (1993). Strength testing – predicting a one‐rep max from reps‐to‐fatigue. J Phys Educ Recreat Dance 64, 88–90. [Google Scholar]
- Burd NA, Groen BBL, Beelen M, Senden JMG, Gijsen AP & van Loon LJC (2012). The reliability of using the single‐biopsy approach to assess basal muscle protein synthesis rates in vivo in humans. Metabolism 61, 931–936. [DOI] [PubMed] [Google Scholar]
- Burd NA, Holwerda AM, Selby KC, West DWD, Staples AW, Cain NE, Cashaback JGA, Potvin JR, Baker SK & Phillips SM (2010. a). Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol 588, 3119–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, Tardif N, Rooyackers O & van Loon LJC (2015). Optimizing the measurement of mitochondrial protein synthesis in human skeletal muscle. Appl Physiol Nutr Metab Physiol Appliquée Nutr Métabolisme 40, 1–9. [DOI] [PubMed] [Google Scholar]
- Burd NA, West DW, Rerecich T, Prior T, Baker SK & Phillips SM (2011. a). Validation of a single biopsy approach and bolus protein feeding to determine myofibrillar protein synthesis in stable isotope tracer studies in humans. Nutr Metab 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, West DWD, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK & Phillips SM (2011. b). Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr 141, 568–573. [DOI] [PubMed] [Google Scholar]
- Burd NA, West DWD, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK & Phillips SM (2010. b). Low‐load high volume resistance exercise stimulates muscle protein synthesis more than high‐load low volume resistance exercise in young men. PLoS ONE 5, e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier S, Burgos SA, Morais JA, Gougeon R, Bassil M, Lamarche M & Marliss EB (2015). Protein and glucose metabolic responses to hyperinsulinemia, hyperglycemia, and hyperaminoacidemia in obese men. Obesity 23, 351–358. [DOI] [PubMed] [Google Scholar]
- Croymans DM, Paparisto E, Lee MM, Brandt N, Le BK, Lohan D, Lee CC & Roberts CK (2013). Resistance training improves indices of muscle insulin sensitivity and β‐cell function in overweight/obese, sedentary young men. J Appl Physiol 115, 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSEP (2011). Canadian Society for Exercise Physiology – Home. Available at: http://csep.ca/home [Accessed 15 March 2018].
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK & American College of Sports Medicine (2009). American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 41, 459–471. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E & Rasmussen BB (2006). Resistance exercise increases AMPK activity and reduces 4E‐BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 576, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes GB & Welle SL (1983). Lean body mass in obesity. Int J Obes 7, 99–107. [PubMed] [Google Scholar]
- Ghosh S, Lertwattanarak R, Garduño J de J, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S & Musi N (2015). Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol A Biol Sci Med Sci 70, 232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G & Shephard RJ (1997). Godin lesiure‐time exercise questionnaire. Med Sci Sports Exerc 29 June Supplement, S36–S38. [Google Scholar]
- Golay A, Swislocki A l. m., Chen Y d. i., Jaspan J b. & Reaven G m. (1986). Effect of obesity on ambient plasma glucose, free fatty acid, insulin, growth hormone, and glucagon concentrations. J Clin Endocrinol Metab 63, 481–484. [DOI] [PubMed] [Google Scholar]
- Guillet C, Delcourt I, Rance M, Giraudet C, Walrand S, Bedu M, Duche P & Boirie Y (2009). Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab 94, 3044–3050. [DOI] [PubMed] [Google Scholar]
- Hector AJ, Marcotte GR, Churchward‐Venne TA, Murphy CH, Breen L, von Allmen M, Baker SK & Phillips SM (2015). Whey protein supplementation preserves postprandial myofibrillar protein synthesis during short‐term energy restriction in overweight and obese adults. J Nutr 145, 246–252. [DOI] [PubMed] [Google Scholar]
- Kumar V, Atherton PJ, Selby A, Rankin D, Williams J, Smith K, Hiscock N & Rennie MJ (2012). Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol Ser A 67, 1170–1177. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N & Rennie MJ (2009). Age‐related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert CP, Wright NR, Finck BN & Villareal DT (2008). Exercise but not diet‐induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol 105, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Liu J‐Y, Zhang H‐X, Li Q & Zhang S‐W (2015). Exercise training attenuates sympathetic activation and oxidative stress in diet‐induced obesity. Physiol Res 64, 355–367. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF & Turner RC (1985). Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA & Phillips SM (2009). Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murton AJ, Marimuthu K, Mallinson JE, Selby AL, Smith K, Rennie MJ & Greenhaff PL (2015). Obesity appears to be associated with altered muscle protein synthetic and breakdown responses to increased nutrient delivery in older men, but not reduced muscle mass or contractile function. Diabetes 64, 3160–3171. [DOI] [PubMed] [Google Scholar]
- do Nascimento MA, ES Cyrino, Nakamura FY, Romanzini M, Pianca HJC & Queiróga MR (2007). Validation of the Brzycki equation for the estimation of 1‐RM in the bench press. Rev Bras Med Esporte 13, 47–50. [Google Scholar]
- Novakofski J, Park S, Bechtel PJ & McKeith FK (1989). Composition of cooked pork chops: effect of removing subcutaneous fat before cooking. J Food Sci 54, 15–17. [Google Scholar]
- Pennings B, Koopman R, Beelen M, Senden JM, Saris WH & Loon LJ van (2011). Exercising before protein intake allows for greater use of dietary protein–derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 93, 322–331. [DOI] [PubMed] [Google Scholar]
- Potvin JR & Fuglevand AJ (2017). A motor unit‐based model of muscle fatigue. PLoS Comput Biol 13, e10005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna SM, Ghosh S, Tantiwong P, Meka CSR, Eagan P, Jenkinson CP, Cersosimo E, DeFronzo RA, Coletta DK, Sriwijitkamol A & Musi N (2008). Elevated Toll‐like receptor 4 expression and signaling in muscle from insulin‐resistant subjects. Diabetes 57, 2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas DA, Morris EP, Haran PH, Pasha EP, Morais M da S, Dolnikowski GG, Phillips EM & Fielding RA (2012). Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J Appl Physiol 113, 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samocha‐Bonet D, Dixit VD, Kahn CR, Leibel RL, Lin X, Nieuwdorp M, Pietiläinen KH, Rabasa‐Lhoret R, Roden M, Scherer PE, Klein S & Ravussin E (2014). Metabolically healthy and unhealthy obese – the 2013 Stock Conference report. Obes Rev Off J Int Assoc Study Obes 15, 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetterly SM, Marshall JA, Baxter J & Hamman RF (1993). Waist‐hip ratio measurement location influences associations with measures of glucose and lipid metabolism. The San Luis Valley Diabetes Study. Ann Epidemiol 3, 295–299. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H & Flier JS (2006). TLR4 links innate immunity and fatty acid‐induced insulin resistance. J Clin Invest 116, 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeuninx B, Mckendry J, Wilson D, Martin U & Breen L (2017). Age‐related anabolic resistance of myofibrillar protein synthesis is exacerbated in obese inactive individuals. J Clin Endocrinol Metab 102, 3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, Willis G, Islam NG, Baranowski T, McNutt S & Potischman N (2012). The Automated Self‐Administered 24‐Hour Dietary Recall (ASA24): a resource for researchers, clinicians and educators from the National Cancer Institute. J Acad Nutr Diet 112, 1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P & Blomstrand E (2010). The degree of p70S6k and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur J Appl Physiol 110, 835–843. [DOI] [PubMed] [Google Scholar]
- Timmerman KL, Connors ID, Deal MA & Mott RE (2016). Skeletal muscle TLR4 and TACE are associated with body fat percentage in older adults. Appl Physiol Nutr Metab 41, 446–451. [DOI] [PubMed] [Google Scholar]
- USDA Agricultural Research Service (2016). Energy intakes: percentages of energy from protein, carbohydrate, fat, and alcohol, by gender and age, what we eat in America, NHANES 2013–2014. Available at: https://doi.org/www.ars.usda.gov/ba/bhnrc/fsrg [Accessed 4 December 2017].
- van Vliet S, EL Shy, Sawan SA, Beals JW, West DW, Skinner SK, Ulanov AV, Li Z, Paluska SA, Parsons CM, Moore DR & Burd NA (2017). Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am J Clin Nutr 106, 1401–1412. [DOI] [PubMed] [Google Scholar]
- Wall BT, Gorissen SH, Pennings B, Koopman R, Groen BBL, Verdijk LB & van Loon LJC (2015). Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PloS ONE 10, e0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Breen L, Burd NA, Hector AJ, Churchward‐Venne TA, Josse AR, Tarnopolsky MA & Phillips SM (2012). Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 108, 1780–1788. [DOI] [PubMed] [Google Scholar]


