Abstract
Key points
In most patients with obstructive sleep apnoea (OSA), there is a spontaneous resolution of the breathing disorders during slow wave sleep (SWS) for yet unknown reasons related to non‐anatomical factors.
Some recently identified forms of neural memory specific of upper airway muscles may play a role in this phenomenon.
In the present study, we show for the first time that a form of memory of the genioglossus (tongue) muscle is greatly enhanced during SWS compared to non‐rapid eye movement stage 2 sleep.
The present study represents a step forward in understanding the mechanisms responsible for the spontaneous development of stable breathing during SWS in OSA patients and may help the discovery of novel therapeutic strategies for this disease.
Abstract
Several studies have shown that obstructive sleep apnoea (OSA) improves during slow wave sleep (SWS) for reasons that remain unclear. Recent studies have identified forms of neural memory such as short‐term potentiation or after‐discharge that can occur in response to upper airway obstruction. Neural memory may play a role in the development of stable breathing during SWS by increasing upper airway muscles activity in this sleep stage. We hypothesize that the after‐discharge of the genioglossus muscle following upper airway obstruction is enhanced during SWS compared to non‐rapid eye movement stage 2 (N2). During sleep, we performed five‐breath drops in continuous positive airway pressure (CPAP‐drop) to simulate obstructive events and reflexively activate the genioglossus. Immediately afterwards, CPAP was returned to an optimal level. Once the post‐drop ventilation returned to eupnoea, the genioglossus after‐discharge was measured as the time it took for genioglossus activity to return to baseline levels. In total, 171 CPAP‐drops were analysed from a group of 16 healthy subjects and 19 OSA patients. A mixed‐model analysis showed that after‐discharge duration during SWS was 208% (95% confidence interval = 112% to 387%, P = 0.022) greater than during N2 after adjusting for covariates (ventilatory drive, CPAP levels). There was also a non‐significant trend for a –35% reduction in after‐discharge duration following an arousal vs. no‐arousal from sleep (95% confidence interval = –59.5% to 5%, P = 0.08). Genioglossus after‐discharge is two‐fold greater in SWS vs. N2, which could partly explain the breathing stabilization described in OSA patients during this sleep stage.
Keywords: genioglossus, obstructive sleep apnea, slow wave sleep
Key points
In most patients with obstructive sleep apnoea (OSA), there is a spontaneous resolution of the breathing disorders during slow wave sleep (SWS) for yet unknown reasons related to non‐anatomical factors.
Some recently identified forms of neural memory specific of upper airway muscles may play a role in this phenomenon.
In the present study, we show for the first time that a form of memory of the genioglossus (tongue) muscle is greatly enhanced during SWS compared to non‐rapid eye movement stage 2 sleep.
The present study represents a step forward in understanding the mechanisms responsible for the spontaneous development of stable breathing during SWS in OSA patients and may help the discovery of novel therapeutic strategies for this disease.
Introduction
Previous studies have shown that obstructive sleep apnoea (OSA) improves during slow wave sleep (SWS) (Ratnavadivel et al. 2009), although the reason for this improvement is not fully understood. Several non‐anatomical factors such as a reduced arousability (Ratnavadivel et al. 2010), a lower loop gain of the respiratory control system (i.e. greater breathing stability) (Landry et al. 2018) and an increased activation of upper airway dilator muscles (McSharry et al. 2013; Carberry et al. 2016; Hicks et al. 2017) compared to other sleep stages may contribute to this improvement in breathing. Understanding the mechanisms responsible for the spontaneous development of stable breathing during SWS may be important with respect to discovering novel therapeutic strategies for OSA.
Previous human research demonstrated that the genioglossus muscle activity is increased during SWS compared to lighter stages of sleep (Basner et al. 1991; McSharry et al. 2013; Carberry et al. 2016). However it is not clear whether this increase in activity is secondary to a sustained increase in ventilatory drive that reflexively activates the upper airway muscles (Ratnavadivel et al. 2010; de Melo et al. 2017; Hicks et al. 2017) or, instead, a primary, intrinsic mechanism involving a change in neural control of the genioglossus during this sleep stage.
It has been repeatedly shown that the respiratory system reflexively responds to feedback from both chemical and mechanical stimuli (Malhotra et al. 2000, 2002). Central and local reflexes help regulate activation of the diaphragm and other respiratory muscles, including the upper airway dilator muscles such as the genioglossus (tongue), via phrenic and hypoglossal nerves, respectively. In addition to fast‐acting reflexes, the respiratory control system also has a longer‐acting memory component (i.e. it remembers the effect of previous stimuli). Recent animal and human studies identified some forms of vagally‐mediated neural memory (i.e. short‐term potentiation, after discharge, long‐term facilitation of hypoglossal motor nucleus) as potential compensatory mechanisms (Mateika & Syed, 2013; Younes et al. 2014; Song & Poon, 2017).
One important form of neural memory related to the respiratory control system is long‐term facilitation (LTF), comprising an increase in the activity of respiratory muscles in response to repetitive stimulation with intermittent hypoxia or intermittent obstruction of the upper airway. LTF typically lasts for minutes or hours after termination of the stimulation (Fuller et al. 2000). Human and animal studies have shown that stimulation with intermittent hypoxia elicits LTF in both phrenic and hypoglossal motoneurons throughout the activation of serotonin receptors (Fuller et al. 2001). Along with chemical stimuli, mechanical feedback from the respiratory system also contributes to LTF via vagal inputs. This form of LTF is enhanced by adrenergic receptors and preferentially affects the upper airway dilator muscles over the diaphragm (Tadjalli et al. 2010). The benefit of LTF is that it tends to stabilize breathing during sleep and, in particular, it should improve pharyngeal patency via its effect on hypoglossal motoneurons.
Respiratory short‐term potentiation (STP) is another form of neural memory that has been demonstrated in humans, and it can affect both the diaphragm and the genioglossus muscle (Georgopoulos et al. 1990; Jordan et al. 2002; Younes et al. 2014). During respiratory STP, following an increase in muscle activity as a result of stimulation, the level of activity continues to increase thereafter despite the stimulus levels remaining constant. The gradual decay in muscle activity after the cessation of the primary stimulus is called after‐discharge (AD) and this can last for some seconds or minutes (Eldridge & Gill‐Kumar, 1978). In awake humans, a brief (<1 min) hypoxic exposure, terminated by oxygen breathing, is followed by a gradual return of ventilation to baseline without undershoot, thereby indicating the presence of short‐term memory in the diaphragmatic activation (Georgopoulos et al. 1990, 1995; Mateika & Syed, 2013). Similar to STP, another form of short‐term neural memory that occurs after repeated stimulation is post‐tetanic potentiation. This is characterized by an AD of shorter duration (30 s to 4 min) compared to STP and, in contrast to LTF and STP, it is probably mediated by simple pre‐synaptic mechanisms according to in vitro studies (Schulz & Fitzgibbons, 1997).
Three studies have investigated AD in the genioglossus (Jordan et al. 2002; Younes et al. 2014; Cori et al. 2017). Jordan et al. (2002) found that phasic genioglossus activity during wakefulness declined gradually in healthy humans following a brief hypoxic exposure and that the decline followed the same time course as the diaphragm. For this reason, it appeared that the diaphragm and the genioglossus muscle were closely coupled during respiratory AD. Indeed, the gradual decline in genioglossus activity tracked the negative pharyngeal pressure produced during the gradual fall in diaphragmatic activity. This suggests that genioglossus AD may be related to the negative pressure reflex produced by persistent diaphragm activity (i.e. it is a phenomenon secondary to the diaphragmatic AD).
In another study conducted in asleep OSA patients, Younes et al. (2014) demonstrated that tonic genioglossus activity exhibits a short‐term form of memory that is specific to the genioglossus muscle and independent of diaphragmatic ventilatory drive. This memory probably contributes to recruitment of the genioglossus during obstructive episodes in sleep and results in sustained tonic activity beyond the obstructive phase and even when ventilation returns to baseline levels. This form of AD could potentially prevent the recurrence of obstruction.
The contribution of arousals from sleep on the AD of the genioglossus muscle still remains a matter of debate. Younes et al. (2014) describe an inhibitory effect of arousal on AD, whereas Cori et al. (2017) hypothesize a role for arousals in AD enhancement.
Neural memory can be mediated by several mechanisms (Zucker & Regehr, 2002). For example, it can be produced by ‘synaptic plasticity’, which adjusts the synaptic strength between respiratory neurons based on their previous activity. Other causes are related to circuit mechanisms involving feedback loops and changes in the balance of inhibitory and excitatory inputs (Zucker & Regehr, 2002; Tadjalli et al. 2010; Puentes‐Mestril & Aton, 2017). These various mechanisms may play a role in the development of stable breathing during SWS by increasing genioglossus activity. In the present study, we performed a retrospective analysis measuring genioglossus AD during non‐rapid eye movement stage 2 (N2) and SWS in a group of healthy controls and OSA patients. We hypothesized that AD is enhanced during SWS compared to N2. We also determined the contribution of arousal to AD in the same subjects.
Methods
Ethical approval
The present study conformed to the standards set by the latest revision of the Declaration of Helsinki and was approved by Partners Human Research Committee, Brigham and Women Hospital's Institutional Review Board (protocol number: 2014P001033), except for registration in a database. All subjects provided their written informed consent prior to study enrolment.
Subjects
Patients originally enrolled into four clinical trials performed between 2015 and 2017 were included in this analysis. Subjects aged between 21 and 70 years were considered as healthy controls if they had no history of OSA and an apnoea hypopnea index (AHI) < 5 events h–1 during the study night. OSA patients had a clinical diagnosis of OSA with a polysomnogram showing an AHI > 10 events h–1 during the study night. Individuals were excluded if they were taking medications known to influence breathing or sleep–wake physiology. Subjects with significant comorbidities (including renal or heart failure, stroke, epilepsy) were also excluded.
Equipment
Subjects were instrumented with standard polysomnography equipment for sleep staging: electroencephalography, chin electromyography (EMG) and electrooculography were recorded for sleep staging. Piezo‐electric bands around the chest and abdomen monitored respiratory movements. Electrocardiography body position and arterial oxygen saturation were also measured.
In addition to the standard clinical montage, subjects breathed through a sealed nasal mask attached to a pneumotachometer (Hans‐Rudolph, Kansas City, MO, USA) and a pressure transducer (Validyne, Northridge, CA, USA). An exhaust port was placed between pneumotachometer and ventilator tubing. Mask pressure was monitored with a pressure transducer (Validyne) referenced to atmosphere. Epiglottic pressure was determined with a small, flexible pressure‐tipped catheter (Millar Instruments, Houston, TX, USA) as described previously (Taranto‐Montemurro et al. 2016b)
EMG activity from the genioglossus muscle (EMGGG) muscle was recorded using unipolar intramuscular electrodes as described previously (Jordan et al. 2009, 2010; Eckert et al. 2013). All data were acquired using Spike 2 software with a 1401 interface (Cambridge Electronic Design, Cambridge, UK).
Upper airway physiology using continuous positive airway pressure (CPAP) manipulation
Subjects were placed supine and connected to a positive/negative pressure source (Philips‐Respironics, Murrysville, PA, USA) to enable rapid switching between pressure levels. When stable sleep was reached, the pressure in the mask was increased to the required level to abolish flow limitation (optimum CPAP), as determined by the airflow waveform and epiglottic pressure signals. Following a baseline recording period of 5 min, the CPAP level was reduced acutely to subtherapeutic levels that generated flow limitation and genioglossus muscle activation. After five breaths at a subtherapeutic level, mask pressure was returned to the optimum level that abolished flow‐limitation (Fig. 1).
Figure 1. EMGGG remains elevated after a CPAP drop beyond the return of ventilation to baseline.
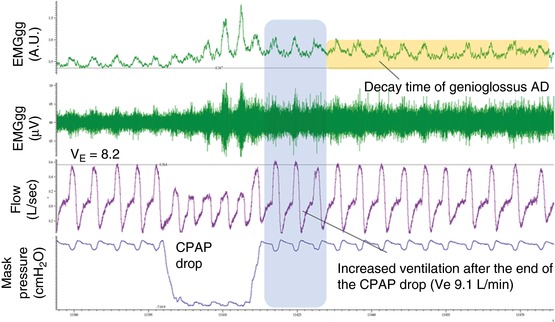
Example of a CPAP drop and calculation of the genioglossus AD duration. After a CPAP drop lasting five breaths, the CPAP is increased back to the optimum pressure. At this pressure, the ventilation matches the ventilatory drive because the airways are completely open. The increased ventilatory drive in the first breaths after the CPAP dial‐up is considered as the response to transient hypoxia/hypercapnia (with a possible contribution from diaphragmatic AD) (Jordan et al. 2002). In this example, the ventilation returned to baseline (pre‐drop) values after four breaths (right of shaded region). After this time, the genioglossus activity remained elevated above baseline (pre‐drop) levels for multiple breaths (orange horizontal box); note that the tonic (i.e. minimum value in expiration) in particular is increased, suggesting that a neural memory specific to the genioglossus (independent of the diaphragm and phasic/respiratory‐related activity) is at play at this time. Genioglossus AD was measured from the time in which ventilatory drive (ventilation) was back to eupnoea after the CPAP drop to the time in which the genioglossus was back to baseline (pre‐drop) levels. From top to bottom: EMGGG moving time average (arbitrary units, A.U.), EMGGG raw data (μV), ventilatory flow (L s–1) and continuous positive airway pressure level (mask pressure, cmH2O). [Color figure can be viewed at http://wileyonlinelibrary.com]
Data analysis
The raw EMGGG was band‐pass filtered (between 30 Hz and 1 kHz), rectified and integrated using a 100 ms window. The EMGGG was quantified in arbitrary units derived by signal processing of the raw signal and as a percentage of baseline, where baseline was determined as the average peak and tonic values of 10 breaths on optimum CPAP before each CPAP drop. EMGGG analysis was performed on a breath‐by‐breath basis. The maximum and minimum values during inspiration and expiration were taken as the EMGGG peak and EMGGG tonic, respectively. The difference between peak and tonic values was used to estimate the respiratory‐related phasic activity.
AD was quantified as the decay time of genioglossus activity to baseline levels following a CPAP drop (Fig. 1). We measured the decay time starting from when the ventilatory drive was back to eupnoea (pre‐CPAP drop level) and ending when the genioglossus activity was back to baseline (pre‐CPAP drop level) (Fig. 1). Because the upper airway was completely opened by the CPAP when on optimum pressure, the ventilatory drive is considered to match the actual ventilation under this condition. AD was also quantified as decay time constant (reduction by 63.2% starting from when the ventilatory drive was back to eupnoea) as an alternative measure that was considered to be more robust in the event of a failure of activity to return completely to the pre‐drop baseline.
CPAP drops were analysed regardless of the presence of an arousal at the end of the drop. However, the following drops were excluded:
all CPAP drops that did not result in inspiratory flow limitation or genioglossusmuscle activation, as the stimulus may be insufficient to trigger any form of neural memory in these cases;
if there was a change in sleep stage during or after the CPAP drop because the genioglossus AD could not clearly be assigned to N2 or SWS in these cases;
all CPAP drops in which the arousal happened during the drop (i.e. before the fifth breath) or more than 10 s after the end of the CPAP drop because, in those cases, the contribution of the arousal to genioglossus AD could not be clearly determined;
if the arousal at the end of the drop was longer than 16 s, thus leading to an epoch being scored as wakefulness. These drops were excluded because the elevation of genioglossus activity during wakefulness could be related to behavioural inputs such as voluntary tongue movements rather than AD.
Statistical analysis
A linear mixed‐effect model was used to estimate the effect of various covariates on the decay time of genioglossus activity after CPAP drops. This method of analysis takes into account the correlated nature of repeated measures for the same subject, a possible between‐subjects random effect on the genioglossus AD, and allows subjects with missing data to be included in the analysis (i.e. whenever CPAP drops were performed only in N2 or in SWS). In the mixed model analysis, the absolute differences between N2 and SWS are determined based on the subset of participants with both N2 and SWS data. The remaining participants (with data in N2 only or SWS only) do not contribute to the estimation of the N2‐SWS difference and its P value. Rather, they contribute to the accurate estimation of the confounding effects of ventilatory drive, arousals and the intercept (i.e. average effect in N2 without arousals). The genioglossus AD was treated as a dependent variable (after log10 + 1 transformation as a result of the skewed distribution of data) and was back‐transformed for presentation of results. Sleep stage and presence of arousal after CPAP drop were primary and secondary independent variables (fixed effects). We also included an interaction between OSA and sleep stage (OSA × stage) to test whether the presence of OSA modified the effect of stage on genioglossus AD (fixed effect). In a separate exploratory analysis, age, body mass index (BMI), sex, ventilation (ventilatory drive) following the CPAP drop (breath 1) and CPAP optimum pressure were included as additional covariates (fixed effects). Subjects were included as random effects to handle within‐subject correlations. Ventilation after each CPAP drop was included in the model to capture differences within and between subjects in the magnitude of the ventilatory drive (diaphragm output) stimulus for genioglossus activation as a result of varying CPAP drop levels; such variation was considered to potentially alter the duration of genioglossus AD (i.e. the stronger the stimulus, the longer the AD). CPAP optimum pressure was also included as a covariate because it has been shown that CPAP levels are inversely related to tonic and phasic genioglossus activity (Deegan et al. 1996). CPAP optimum pressure levels are also higher in OSA subjects vs. controls. The same mixed models analyses were also performed using AD duration starting from the end of the obstructive event (at the end of CPAP drop) as the dependent variable.
Repeated data were compared using Student's paired t test. P < 0.05 was considered statistically significant. Data are expressed as the mean ± SD unless otherwise specified. Statistical analysis was performed using the Matlab R2016b (Mathworks, Natick, MA, USA) and Prism, version 6.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Sixteen healthy controls and nineteen OSA patients were included in the analysis. Subject characteristics are provided in Table 1. The CPAP drops performed were on average 10.3 ± 6.0 per participant of which 4.4 ± 3.6 matched the inclusion criteria and were used for this analysis. The CPAP‐drops analysed were always five breaths long. The time length was similar between stages (21.7 ± 4.7 s in N2 vs. 23.7 ± 9.8 in SWS, P = 0.20). In total, 171 CPAP drops were analysed: 55 during SWS and 116 during N2. Only four drops performed during SWS ended with an arousal, whereas 49 drops analysed in N2 were followed by an arousal. The lower number of drops with arousal during SWS is consistent with the previously described increase in the respiratory arousal threshold during this sleep stage (Ratnavadivel et al. 2010). No CPAP drops were performed in REM sleep.
Table 1.
Characteristics of the subjects
| All | Healthy controls | OSA | |
|---|---|---|---|
| N | 35 | 16 | 19 |
| Age (years) | 43.9 ± 14.9 | 34.4 ± 11.5 | 52 ± 12.7 |
| BMI (kg m–2) | 29.2 ± 5.8 | 26.2 ± 5.1 | 31.6 ± 5.4 |
| Females, n (%) | 16 (47) | 9 (56) | 7 (37) |
| AHI, events h–1 | 20.1 ± 25.2 | 1.2 ± 1.6 | 35.9 ± 24.8 |
Data are expressed as the mean ± SD.
The mixed model analysis including sleep stage, presence of arousal and OSA × sleep stage as independent variables and the genioglossus tonic AD as the dependent variable showed that the only significant factor affecting the genioglossus AD was the sleep stage, with AD during SWS being 208% (95% confidence interval = 112% to 387%) of AD during N2 (P = 0.022) (for individual data examples, see Figs 2 and 3; for group data, see Fig. 4 A). There was also a trend for a –35% reduction (95% confidence interval = –59.5% to 5%, P = 0.08) in AD duration when the drop was followed by an arousal from sleep. Figure 4 B shows the AD duration in N2, SWS and arousal conditions starting from the end of the CPAP drop. No relationship between OSA × stage and AD duration was present (P = 0.28), indicating that the presence of OSA had no effect on stage dependency of AD. The same relationship between sleep state and genioglossus AD remained after including other possible confounding variables in the model, such as ventilatory drive post CPAP drop, CPAP optimum pressure, age, sex and BMI.
Figure 2. Example of CPAP drops in deep sleep (SWS) and N2.
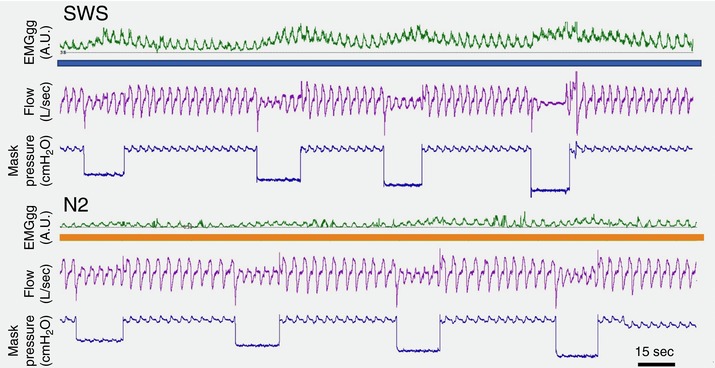
CPAP drops generated wide ‘waves’ of EMGGG during SWS (upper, blue line) and only small variations of EMGGG in N2 sleep (lower, orange line), suggesting an increased short term (or tetanic) potentiation during the obstructed period followed by a longer AD during SWS compared to N2. Data from a control subject (without sleep apnoea) are shown. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3. Example of ventilatory drive, tonic and phasic genioglossus activity after a CPAP‐drop in SWS and N2.
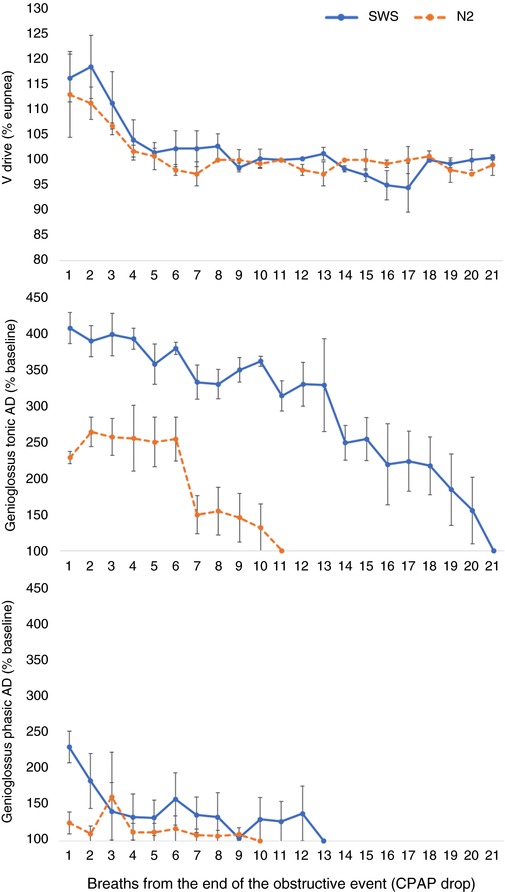
Ventilatory drive (Vdrive) (upper), tonic (middle) and phasic (lower) EMGGG are represented for several breaths after CPAP returned to optimal level in stage N2 (orange dashed line) and SWS (blue line) in an example control subject. Here, the decay time of tonic EMGGG is 10 breaths longer during SWS compared to N2. Phasic EMGGG returned to pre‐drop levels more quickly in both sleep stages, soon after ventilatory drive was back to eupnoea (100%). Genioglossus activity (middle and lower) is presented as the increase above baseline (units of % baseline) and calculated for each breath after the CPAP dial up. 100 on the y‐axis represents the return to baseline (pre‐drop) values. Data represent the mean of four drops performed during SWS and four performed during N2. Error bars indicate the SE. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4. Effect of sleep stage and arousal on genioglossus AD duration following five‐breath CPAP drops during sleep.
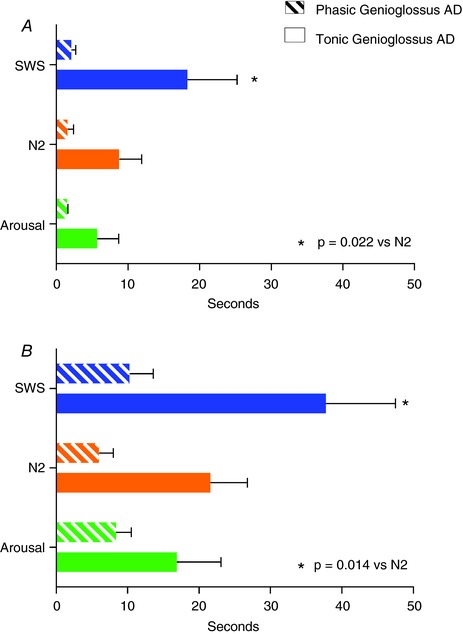
A, AD calculated from when the ventilatory drive was back to eupnoeic ventilation (pre‐CPAP drop levels). B, AD calculated starting from the end of the obstructive events. Tonic AD duration during SWS was twice the value compared to AD during N2 sleep (*). There was a trend for a shorter tonic AD duration when an arousal was present compared to without arousal (P = 0.08, values shown for NREM2). Phasic AD lasted a few seconds under all conditions, supporting the view that the respiratory activity of genioglossus muscle mimics the diaphragm activity and its duration is minimal after the ventilatory drive is back to the eupnoeic value. Data are based on mixed model analysis and represents group mean ± SEM; to promote normality, durations in seconds were transformed using Y = log10(time + 1) for analysis and then were back‐transformed for presentation. [Color figure can be viewed at http://wileyonlinelibrary.com]
No significant relationship was found when phasic genioglossus AD was used as the dependent variable in the same mixed model analysis (P = 0.37 vs. stage, P = 0.75 vs. presence of arousal) (Figs 2, 3 and 4).
A secondary analysis for tonic genioglossus activity was performed including only those subjects in whom the CPAP drops were analysed in both N2 and SWS and the results did not change compared to the mixed model analysis. Epiglottic pressure, CO2 levels and AD time constant for this subset of patients are shown in Table 2.
Table 2.
Comparison of AD duration and time constant, CPAP drop, CO2 and epiglottic pressure (Pepi) in the subgroup of patients (N = 16) with CPAP drops performed and analysed in both N2 and SWS
| N2 | SWS | Arousal | |
|---|---|---|---|
| AD duration (s) | 18.4 ± 16.5 | 33.0 ± 20.4* | 13.3 ± 7.9 |
| AD time constant (s) | 13.6 ± 12.0 | 27.5 ± 20.9† | 9.8 ± 5.9 |
| CPAP drop (cmH2O) | 5.6 ± 5.5 | 6.4 ± 6.5 | 6.5 ± 7.0 |
| End tidal CO2 (mmHg) | 40.1 ± 3.0 | 40.2 ± 2.6 | 40.1 ± 3.5 |
| Pepi (cmH2O) | 4.3 ± 3.9 | 4.6 ± 5.7 | 4.1 ± 4.4 |
*0.015 vs. N2, †0.009 vs. N2, Student's t test. Data are expressed as the mean ± SD. CO2 and epiglottic pressure data are taken as the median during the period of assessment of AD.
Discussion
The main finding of the present study was that, in a mixed group of 19 OSA and 16 healthy controls, tonic genioglossus AD measured after inducing a transient obstruction to the upper airway was more than two‐fold longer during SWS compared to stage N2.
Previous data from Younes et al. (2014) revealed the presence of an AD limited to the tonic genioglossus muscle that lasted (with high variability) between 6 and 30 s in OSA patients. In their study, three breaths of an obstruction were induced with a CPAP drop to 1 cmH2O from the optimum pressure in room air or preceded by hypoxia and/or hypercapnia when measuring genioglossus activity at different levels of ventilatory stimulation (Younes et al. 2014). These data did not discriminate between drops performed in different sleep stages. As in the study by Younes et al. (2014), we only took into account the AD after the ventilatory drive was back to the pre‐CPAP drop level on optimum CPAP, thus eliminating the possibility that elevation in genioglossus muscle activity was secondary to intrathoracic or upper airway pressure changes (Jordan et al. 2002) or to different levels of CO2 accumulated during the CPAP drops (Wellman et al. 2013).
Similar to Younes et al. (2014), we found a trend for a shorter tonic AD when the CPAP drop was followed by an arousal from sleep. The lack of a significant P value could be because arousals were not discriminated based on their intensity (Amatoury et al. 2016), although the arousals lasting longer than 16 s (probably the most intense), resulting in wakefulness epochs, were excluded from the analysis. The results reported by Younes et al. (2014) and our own results are partially in contrast with those of Cori et al. (2017) who showed that arousals induced by an auditory stimulus in ventilated healthy subjects could enhance the AD of the genioglossus following the return to sleep. One important difference between the results of Cori et al. (2017) and those of other studies is that the auditory stimulus of 0.5 s applied to the sleeping subjects generated arousals not associated with upper airway opening, and they were probably less intense compared to the arousals associated with the upper airway manipulation with CPAP. Further studies are needed to fully understand the role of arousals and their intensity on genioglossus AD during sleep.
In accordance with Jordan et al. (2002), we found that the phasic component of genioglossus AD was almost absent on average after the normalization of the ventilatory drive, suggesting that the phasic AD is secondary to the diaphragm AD (i.e. as a response to the negative pressure reflex in the upper airway). Nevertheless, given that the phrenic and hypoglossal motor pools receive a common respiratory drive signal, we cannot rule out the possibility that the common drive signal itself undergoes potentiation. Notably, previous data obtained in five healthy subjects and reported by Basner et al. (1991) showed that phasic genioglossus activity increased from N2 to SWS despite a lack of differences in minute ventilation or end‐tidal PCO2. This suggests that there are potentiating mechanisms active during non‐REM sleep that enhance phasic and tonic genioglossus activity independent of changes in chemical drive (PCO2).
Our data support the idea that genioglossus activity exhibits a form of neural memory that is specific to this muscle (and possibly to other upper airway muscles) and is independent of diaphragmatic ventilatory drive (Tadjalli et al. 2010). This memory contributes to the recruitment of the genioglossus during obstructive episodes in sleep and results in sustained tonic activity beyond the obstructive phase, even when ventilation returns to baseline levels and thus could potentially prevent the recurrence of obstruction. The fact that this memory is enhanced during SWS also corroborates this hypothesis because, during SWS, most patients with OSA can spontaneously recover from recurrent obstructions.
Although the mechanism of STP and AD in the tonic activation of the genioglossus is still uncertain, recent human and animal research has shown that tonic upper airway muscle activity is strongly influenced by adrenergic inputs, and the administration of adrenergic drugs to sleeping humans increases tonic more than phasic genioglossus activity (Chan et al. 2006; Taranto‐Montemurro et al. 2016a). Moreover, recent animal data have shown that the administration of the α2‐antagonist yohimbine greatly increased the genioglossus activity after the induction of repeated upper airway obstruction during sleep, suggesting that it may be possible to pharmacologically enhance genioglossus‐specific forms of neural memory (Song & Poon, 2017).
The mechanisms responsible for the link between increased genioglossus memory and SWS are still largely unknown. Most of the previous literature related to the effects of SWS on synaptic plasticity investigated forebrain mechanisms, which probably cannot be translated to the brainstem given the deeply different networks in which these circuits are embedded (Huber et al. 2004; Chauvette et al. 2012; Puentes‐Mestril & Aton, 2017).
Given the short time course (15–60 s) and decrementing nature of the observed AD, the underlying mechanism probably involves some form of short‐term neural plasticity, as opposed to mechanistically distinct long‐term forms of plasticity, which persist over much longer timescales (Schulz & Fitzgibbons, 1997). Specifically, the time course of the AD is most consistent with the occurrence of augmentation and/or post‐tetanic potentiation types of short‐term plasticity. These forms of plasticity are generally considered to stem from brief bursts of repetitive activity that result in transient accumulations of presynaptic calcium (Citri & Malenka, 2008). Moreover, post‐tetanic potentiation is associated with an elevated probability of spontaneous neurotransmitter release, which can result in increased tonic firing in postsynaptic neurons (Zucker & Regehr, 2002). An increased probability of spontaneous neurotransmitter release could account for our observation of increased tonic genioglussus muscle activity. One possible explanation for the enhanced post‐tetanic potentiation/augmentaion in SWS is that, during this sleep stage, input to the hypoglossal motor pool is more purely respiratory. In other words, in deep sleep, behavioural drives to the hypoglossal motor nucleus are withdrawn given the low arousability, leaving uninterrupted, repetitive respiratory input that may better enable potentiation mechanisms.
Limitations
The present study has several limitations. First, because this was a retrospective observational study, the study population was very heterogeneous in terms of age, BMI, OSA presence and severity. However, given that our statistical model showed an association between sleep stage and tonic genioglossus AD duration regardless of the other possible confounding factors, the variety of the population studied may confirm the general validity of this finding.
Second, the number of CPAP drops analysed was relatively low and one‐half of the subjects had only CPAP drops in N2 or SWS but not in both stages. For this reason, we used a mixed model analysis that has been used and validated previously in the case of repeated measures with missing values at the same time as taking into account within‐subjects correlations (Harrell et al. 1996). Nevertheless, even when we analysed only those subjects with CPAP drops performed in both stages, the findings related to genioglossus memory did not change. It needs to be recognized that these type of studies involve invasive equipment that often has the effect of worsening sleep quality and, for this reason, it is extremely difficult to collect complete data for all sleep stages in all subjects, even after studying several subjects.
Third, despite the finding of an association between SWS and genioglossus memory, we cannot conclude with certainty that this mechanism is in play at atmospheric pressure (e.g. during spontaneous cyclic respiratory events and stable breathing in OSA patients) because the genioglossus activity was measured only on optimum CPAP, which was as high as 14 cmH2O in the OSA patients. Therefore, more studies are needed to address the possibility that an improvement in genioglossus memory during spontaneous breathing has the potential to reduce OSA severity and plays a clinically meaningful role during SWS.
In conclusion, in the present study, we show that tonic genioglossus AD is enhanced during SWS and also that the increased duration of genioglossus activation following a mechanical stimulation could partly explain the previously demonstrated improvement of OSA during SWS. Additional studies are needed that investigate the neural mechanisms responsible for this phenomenon, as well as its relevance for the pathogenesis of sleep apnoea and the extent to which it may be a target for new OSA treatments.
Additional information
Competing interests
Dr L. Taranto‐Montemurro has received personal fees as a consultant for Novion Pharmaceuticals and Cambridge Sound Management outside of the submitted work. Dr D. A. Wellman works as a consultant for Varnum, Cambridge Sound Management, Nox, Bayer, Philips and Galvani, and has received grants from Pilips and Varnum. Dr L. Taranto‐Montemurro and Dr D. A. Wellman also have a financial interest in Apnimed Corp., a company developing pharmacological therapies for sleep apnoea. Their interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. Dr D. P. White receives personal fees as a consultant from Night Balance outside the submitted work, and he is the Chief Scientific Officer for Philips Respironics. Dr S. A. Sands receives personal fees as a consultant for Cambridge Sound Management, Merck and Nox Medical outside the submitted work. Dr A. Azarbarzin receives personal fees as a consultant for Somnifix. K. P. Grace, L. Messineo and R. Salant have no conflicts of interest to disclose.
Author contributions
LT‐M contributed to the study design. LT‐M and SAS contributed to the data collection. LT‐M, SAS, KPG, AA, LM, RS, DPW and DAW contributed to the data analysis and interpretation. LT‐M contributed to the drafting of the manuscript. LT‐M, SAS, KPG, AA, LM, RS, DPW and DAW contributed to the review of the manuscript. All authors qualify for authorship, and all those who qualify for authorship are include as authors. All authors approved the final version of the manuscript submitted for publication and agree to be accountable for all aspects of the work.
Funding
This research project received funding from Fan Hongbing, President of OMPA Corporation, Kaifeng, China, and the National Institutes of Health grants R01HL102321, P01HL095491 and UL1RR025758‐01. Dr L. Taranto‐Montemurro is supported by the American Heart Association (17POST33410436). Dr S. A. Sands is supported by the American Heart Association (15SDG25890059). Dr L. Messineo is supported by University of Brescia scholarship in respiratory medicine.
Biography
Luigi Taranto Montemurro is an Italian physician‐scientist who received his MD degree at Brescia University (Italy) in 2006. After medical school, he obtained specialty training in Respiratory and Sleep Medicine. In 2010 and 2011, he worked as a researcher at Toronto University focusing on the cardiovascular consequences of obstructive sleep apnoea. From January 2015 onward, he became part of Dr Andrew Wellman's research laboratory at Brigham and Women's Hospital and Harvard Medical School in Boston. His work at Harvard is focused on upper airway muscle activity during sleep and on the research for a pharmacological treatment for OSA.

Edited by: Scott Powers & Frank Powell
Linked articles This article is highlighted in a Translational Perspectives article by Younes. To read this article, visit http://doi.org/10.1113/JP276848.
References
- Amatoury J, Azarbarzin A, Younes M, Jordan AS, Wellman A & Eckert DJ (2016). Arousal intensity is a distinct pathophysiological trait in obstructive sleep apnea. Sleep 39, 2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner RC, Ringler J, Schwartzstein RM, Weinberger SE & Weiss JW (1991). Phasic electromyographic activity of the genioglossus increases in normals during slow‐wave sleep. Respir Physiol 83, 189–200. [DOI] [PubMed] [Google Scholar]
- Carberry JC, Jordan AS, White DP, Wellman A & Eckert DJ (2016). Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependent. Sleep 39, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E, Steenland HW, Liu H & Horner RL (2006). Endogenous excitatory drive modulating respiratory muscle activity across sleep‐wake states. Am J Respir Crit Care Med 174, 1264–1273. [DOI] [PubMed] [Google Scholar]
- Chauvette S, Seigneur J & Timofeev I. (2012). Sleep oscillations in the thalamocortical system induce long‐term neuronal plasticity. Neuron 75, 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A & Malenka RC (2008). Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33, 18–41. [DOI] [PubMed] [Google Scholar]
- Cori JM, Rochford PD, O'Donoghue FJ, Trinder J & Jordan AS (2017). The influence of CO2 on genioglossus muscle after‐discharge following arousal from sleep. Sleep 40, 10.1093/sleep/zsx160. [DOI] [PubMed] [Google Scholar]
- de Melo CM, Taranto‐Montemurro L, Butler JP, White DP, Loring SH, Azarbarzin A, Marques M, Berger PJ, Wellman A & Sands SA (2017). Stable breathing in patients with obstructive sleep apnea is associated with increased effort but not lowered metabolic rate. Sleep 40, 10.1093/sleep/zsx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deegan PC, Nolan P, Carey M & McNicholas WT (1996). Effects of positive airway pressure on upper airway dilator muscle activity and ventilatory timing. J Appl Physiol (1985) 81, 470–479. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, White DP, Jordan AS, Malhotra A & Wellman A (2013). Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets.Am J Respir Crit Care Med 188, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL & Gill‐Kumar P (1978). Lack of effect of vagal afferent input on central neural respiratory afterdischarge. J Appl Physiol Respir Environ Exerc Physiol 45, 339–344. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R & Mitchell GS (2000). Long term facilitation of phrenic motor output. Respir Physiol 121, 135–146. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL & Mitchell GS (2001). Phrenic long‐term facilitation requires 5‐HT receptor activation during but not following episodic hypoxia. J Appl Physiol (1985) 90, 2001–2006; discussion 2000. [DOI] [PubMed] [Google Scholar]
- Georgopoulos D, Bshouty Z, Younes M & Anthonisen NR (1990). Hypoxic exposure and activation of the afterdischarge mechanism in conscious humans. J Appl Physiol (1985) 69, 1159–1164. [DOI] [PubMed] [Google Scholar]
- Georgopoulos D, Mitrouska I, Argyropoulou P, Patakas D & Anthonisen NR (1995). Effect of hypoxic sensitivity on decay of respiratory short‐term potentiation.Chest 107, 150–155. [DOI] [PubMed] [Google Scholar]
- Harrell FE Jr, Lee KL & Mark DB (1996). Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15, 361–387. [DOI] [PubMed] [Google Scholar]
- Hicks A, Cori JM, Jordan AS, Nicholas CL, Kubin L, Semmler JG, Malhotra A, McSharry DGP & Trinder JA (2017). Mechanisms of the deep, slow‐wave, sleep‐related increase of upper airway muscle tone in healthy humans. J Appl Physiol (1985) 122, 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M & Tononi G (2004). Local sleep and learning. Nature 430, 78–81. [DOI] [PubMed] [Google Scholar]
- Jordan AS, Catcheside PG, O'Donoghue FJ, Saunders NA & McEvoy RD (2002). Genioglossus muscle activity at rest and in response to brief hypoxia in healthy men and women. J Appl Physiol (1985) 92, 410–417. [DOI] [PubMed] [Google Scholar]
- Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim‐Yeh S, Eikermann M, Smith SA, Stevenson KE & Malhotra A (2009). Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 32, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AS, White DP, Owens RL, Eckert DJ, Rahangdale S, Yim‐Yeh S & Malhotra A (2010). The effect of increased genioglossus activity and end‐expiratory lung volume on pharyngeal collapse. J Appl Physiol (1985) 109, 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry SA, Andara C, Terrill PI, Joosten SA, Leong P, Mann DL, Sands SA, Hamilton GS & Edwards BA (2018). Ventilatory control sensitivity in patients with obstructive sleep apnea is sleep stage dependent. Sleep 41, 10.1093/sleep/zsy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Fogel RB, Edwards JK, Shea SA & White DP (2000). Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med 161, 1746–1749. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, Hess D & White DP (2002). Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med 165, 71–77. [DOI] [PubMed] [Google Scholar]
- Mateika JH & Syed Z (2013) Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations. Respir Physiol Neurobiol 188, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry DG, Saboisky JP, Deyoung P, Matteis P, Jordan AS, Trinder J, Smales E, Hess L, Guo M & Malhotra A (2013). A mechanism for upper airway stability during slow wave sleep. Sleep 36, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puentes‐Mestril C & Aton SJ (2017). Linking network activity to synaptic plasticity during sleep: hypotheses and recent data. Front Neural Circuits 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD & Catcheside PG (2009). Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med 5, 519–524. [PMC free article] [PubMed] [Google Scholar]
- Ratnavadivel R, Stadler D, Windler S, Bradley J, Paul D, McEvoy RD & Catcheside PG (2010). Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax 65, 107–112. [DOI] [PubMed] [Google Scholar]
- Schulz PE & Fitzgibbons JC (1997). Differing mechanisms of expression for short‐ and long‐term potentiation. J Neurophysiol 78, 321–334. [DOI] [PubMed] [Google Scholar]
- Song G & Poon CS (2017). alpha2‐Adrenergic blockade rescues hypoglossal motor defense against obstructive sleep apnea. JCI Insight 2, e91456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjalli A, Duffin J & Peever J (2010). Identification of a novel form of noradrenergic‐dependent respiratory motor plasticity triggered by vagal feedback. J Neurosci 30, 16886–16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranto‐Montemurroy L, Edwards BA, Sands SA, Marques M, Eckert DJ, White DP & Wellman A (2016a). Desipramineincreases genioglossus activity and reduces upper airway collapsibility during non‐REM sleep in healthy subjects. Am J Respir Crit Care Med 194, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranto‐Montemurro L, Sands SA, Edwards BA, Azarbarzin A, Marques M, de Melo C, Eckert DJ, White DP & Wellman A (2016b). Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur Respir J 48, 1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, Passaglia CL, Jackson AC, Malhotra A & White DP (2013). A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) 114, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M, Loewen A, Ostrowski M & Hanly P (2014). Short‐term potentiation in the control of pharyngeal muscles in obstructive apnea patients. Sleep 37, 1833–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS & Regehr WG (2002). Short‐term synaptic plasticity. Annu Rev Physiol 64, 355–405. [DOI] [PubMed] [Google Scholar]


