Abstract
Key points
The vascular endothelial growth factor (VEGF) responses to acute submaximal exercise and training effects in patients with heart failure with reduced ejection fraction (HFrEF) were investigated.
Six patients and six healthy matched controls performed knee‐extensor exercise (KE) at 50% of maximum work rate before and after (only patients) KE training. Muscle biopsies were taken to assess skeletal muscle structure and the angiogenic response.
Before training, during this submaximal KE exercise, patients with HFrEF exhibited higher leg vascular resistance and greater noradrenaline spillover. Skeletal muscle structure and VEGF response were generally not different between groups. Following training, resistance was no longer elevated and noradrenaline spillover was curtailed in the patients. Although, in the trained state, VEGF did not respond to acute exercise, capillarity was augmented. Muscle fibre cross‐sectional area and percentage area of type I fibres increased and mitochondrial volume density exceeded that of controls.
Structural/functional plasticity and appropriate angiogenic signalling were observed in skeletal muscle of patients with HFrEF.
Abstract
This study examined the response to acute submaximal exercise and the effect of training in patients with heart failure with reduced ejection fraction (HFrEF). The acute angiogenic response to submaximal exercise in HFrEF after small muscle mass training is debated. The direct Fick method, with vascular pressures, was performed across the leg during knee‐extensor exercise (KE) at 50% of maximum work rate (WRmax) in patients (n = 6) and controls (n = 6) and then after KE training in patients. Muscle biopsies facilitated the assessment of skeletal muscle structure and vascular endothelial growth factor (VEGF) mRNA levels. Prior to training, HFrEF exhibited significantly higher leg vascular resistance (LVR) (≈15%) and significantly greater noradrenaline spillover (≈385%). Apart from mitochondrial volume density, which was significantly lower (≈22%) in HFrEF, initial skeletal muscle structure, including capillarity, was not different between groups. Resting VEGF mRNA levels, and the increase with exercise, was not different between patients and controls. Following training, LVR was no longer elevated and noradrenaline spillover was curtailed. Skeletal muscle capillarity increased with training, as assessed by capillary‐to‐fibre ratio (≈13%) and number of capillaries around a fibre (N CAF) (≈19%). VEGF mRNA was now not significantly increased by acute exercise. Muscle fibre cross‐sectional area and percentage area of type I fibres both increased significantly with training (≈18% and ≈21%, respectively), while the percentage area of type II fibres fell significantly (≈11%), and mitochondrial volume density now exceeded that of controls. These data reveal structural and functional plasticity and appropriate angiogenic signalling in skeletal muscle of HFrEF patients.
Keywords: heart failure, skeletal muscle, VEGF
Key points
The vascular endothelial growth factor (VEGF) responses to acute submaximal exercise and training effects in patients with heart failure with reduced ejection fraction (HFrEF) were investigated.
Six patients and six healthy matched controls performed knee‐extensor exercise (KE) at 50% of maximum work rate before and after (only patients) KE training. Muscle biopsies were taken to assess skeletal muscle structure and the angiogenic response.
Before training, during this submaximal KE exercise, patients with HFrEF exhibited higher leg vascular resistance and greater noradrenaline spillover. Skeletal muscle structure and VEGF response were generally not different between groups. Following training, resistance was no longer elevated and noradrenaline spillover was curtailed in the patients. Although, in the trained state, VEGF did not respond to acute exercise, capillarity was augmented. Muscle fibre cross‐sectional area and percentage area of type I fibres increased and mitochondrial volume density exceeded that of controls.
Structural/functional plasticity and appropriate angiogenic signalling were observed in skeletal muscle of patients with HFrEF.
Introduction
Although heart failure with reduced ejection fraction (HFrEF) is fundamentally a disease that impacts central haemodynamics, a variety of skeletal muscle alterations, including muscle atrophy, a shift in fibre type, impaired oxidative capacity, reduced mitochondrial enzymes and decreased mitochondrial volume density, are typically associated with this pathology (Mancini et al. 1992; Harrington et al. 1997; Esposito et al. 2010 b, 2015; Kaur et al. 2018; Poole et al. 2018). In terms of the peripheral vasculature, HFrEF has also been linked to autonomic and cardiovascular dysregulation that leads to greater sympathetic vasoconstrictor tone (Magnusson et al. 1997; Kaur et al. 2018), decreased capillary‐to‐fibre ratio, reduced capillary density and smaller capillary diameter (Duscha et al. 1999; Piepoli et al. 2010 a, b ; Esposito et al. 2010 b; Poole et al. 2012). These findings imply that in addition to the inability of the heart to appropriately raise pumping capacity during exercise, both skeletal muscle and the peripheral vasculature also contribute to the exercise intolerance associated with HFrEF, a hallmark of this pathology.
To restore exercise tolerance, traditional cardiac rehabilitation has employed whole body (cycle) exercise which recruits a large muscle mass and therefore taxes the central circulation. Although this approach has consistently yielded significant improvements in exercise capacity in patients with HFrEF (Hambrecht et al. 1998; Piepoli et al. 2004; Hollriegel et al. 2016), because whole body exercise induces a complex interaction between central and peripheral haemodynamics, such observations leave doubt as to the role of central and peripheral adaptations in response to exercise training. In an attempt to address this uncertainty, a growing number of studies have employed small muscle mass training, unlikely to stimulate central haemodynamic adaptations (Magnusson et al. 1996; Tyni‐Lenne et al. 2001). Although this innovative approach has revealed that isolated muscle training can, indeed, improve whole body exercise capacity in patients with HFrEF (Magnusson et al. 1996; Tyni‐Lenne et al. 2001; Barrett‐O'Keefe et al. 2014; Poole et al. 2018), the sometimes indirect physiological assessments used in these studies has yet to yield a clear understanding of the skeletal muscle structural and functional response to such exercise training in this population.
Angiogenesis in skeletal muscle is an essential adaptive response to repeated exercise (i.e. training) resulting in an increase in skeletal muscle vascularity that, in turn, enhances O2 transport (Esposito et al. 2010). As vascular endothelial growth factor (VEGF) has a high specificity for vascular endothelial cells (Leung et al. 1989), these findings are in line with VEGF being a key angiogenic factor involved in structural and functional adaptations in skeletal muscle associated with exercise (Brodal et al. 1977; Zumstein et al. 1983; Hang et al. 1995; Olfert et al. 2009; Huey et al. 2016). Indeed, previously, both in healthy humans and in patients with HFrEF, a significant increase in skeletal muscle VEGF mRNA abundance has been documented in response to an acute small muscle mass exercise stimulus (Gustafsson et al. 1999; Richardson et al. 1999; Esposito et al. 2010 a). Furthermore, in healthy humans we have documented that the previously large VEGF mRNA response to acute exercise is significantly attenuated as a consequence of exercise training, adding credence to the concept that VEGF is important in the initial phase of exercise‐induced adaptation, but when significant angiogenesis has occurred the need and importance of this mechanism becomes greatly reduced. However, whether this holds true in patients with HFrEF, which is associated with a variety of maladaptations in the periphery, has yet to be determined.
Consequently, this study sought to examine skeletal muscle structure and function and the VEGF response to acute submaximal exercise in patients with HFrEF before and after exercise training. With the expectation that a negative feedback mechanism should exist to reduce the level of VEGF gene expression as exercise adaptation occurs, the purpose of this study was to (a) document the adaptations associated with exercise training in the skeletal muscle bed of patients with HFrEF and (b) test the hypothesis that in the face of such exercise training‐induced adaptations, the VEGF mRNA increase in patients with HFrEF, in response to acute exercise, would be significantly attenuated.
Methods
Ethical approval
After full explanation of the study design and experimental procedures, six male patients with HFrEF and six healthy control subjects gave written informed consent to participate in this study, which was approved by the University of California, San Diego, Human Subjects Protection Program (#990152). They were all free to withdraw at any time without jeopardy. The study conformed to the standards set out by the 1975 Declaration of Helsinki, except for registration in a database.
Subjects
All patients with HFrEF were clinically stable with symptoms compatible with New York Heart Association (NYHA) functional class II–III. Mean left ventricular ejection fraction in the HFrEF patients was 25 ± 3%. Patient medications were not altered because of the study, with the exception of beta‐blockers that were withheld for 48 h prior to each experimental day. Particular care was taken to match patients with HFrEF and controls in terms of age, sex and, in particular, physical activity by questionnaire and interview (Table 1). Several components of this study have been previously published with a focus on maximal exercise and the impact of exercise training in HFrEF (Esposito et al. 2011) and the VEGF response to acute submaximal exercise in HFrEF (Esposito et al. 2010 a). Although some overlap is acknowledged, the current work is novel in that it combines both the acute and the chronic responses to submaximal exercise in patients with HFrEF to better understand skeletal muscle plasticity in this population.
Table 1.
Characteristics of healthy controls and patients with heart failure with reduced ejection fraction (HFrEF) before (pre) and after (post) 8 weeks of knee‐extensor exercise training
| Controls | HFrEF pre | HFrEF post | |
|---|---|---|---|
| Age (years) | 51 ± 8 | 54 ± 14 | 54 ± 14 |
| Height (cm) | 179 ± 7 | 182 ± 6 | 182 ± 6 |
| Body mass (kg) | 90 ± 11 | 100 ± 4 | 101 ± 4 |
| Quadriceps muscle mass (kg) | 2.4 ± 0.4 | 2.6 ± 0.1 | 3.0 ± 0.1*# |
| NYHA class | − | II–III | II–III |
| KE maximum cardiac output (l/min) | 10.9 ± 1.2 | 9.0 ± 1.2 | 9.4 ± 1.1 |
| Peak pulmonary cycle (l/min) | 2.14 ± 0.10 | 1.63 ± 0.09# | 2.02 ± 0.15* |
| Peak pulmonary cycle (ml/kg/min) | 24.1 ± 1.1 | 15.3 ± 1.8# | 18.6 ± 1.6*# |
| Maximum cycle work rate (W) | 148 ± 8 | 115 ± 13# | 141 ± 16* |
| Cycle maximum cardiac output (l/min) | 16.8 ± 0.8 | 13.6 ± 1.2# | 14.3 ± 1.4# |
| Medications (fraction of users) | |||
| Digoxin | − | 5/5 | 5/5 |
| Diuretics | − | 5/5 | 5/5 |
| Long‐acting nitrates | − | 3/5 | 3/5 |
| Statins | − | 4/5 | 4/5 |
| Aspirin | − | 3/5 | 3/5 |
| β‐Blockers | − | 4/5 | 4/5 |
| Warfarin | − | 2/5 | 4/5 |
| ACE inhibitors | − | 2/5 | 4/5 |
| Ca2+ channel blockers | − | 2/5 | 4/5 |
NYHA, New York Heart Association; KE, knee‐extensor; , oxygen uptake. Data are expressed as mean ± SE. * P<0.05 post vs. pre; # P<0.05 HFrEF vs. controls.
Experimental design
An initial graded exercise test was performed on a cycle ergometer to document cycle maximum work rate (WRmax) and peak VO2 (). To determine knee‐extensor exercise (KE) WRmax subjects performed an 8–12 min graded KE test to exhaustion. Several days later, both patients with HFrEF and controls reported again to the laboratory and after a 5 min warm‐up performed 30 min of KE at 50% of WRmax. Both the resting and the exercised legs were biopsied within 1 h of the exercise stimulus. During KE studies, skeletal muscle blood flow and muscle were measured. To achieve this, two catheters (radial artery and left femoral vein) and a thermocouple (left femoral vein) were placed using a sterile technique to facilitate blood gas and blood flow measurements and O2 transport calculations, as previously reported (Richardson et al. 1993). These catheter and biopsy studies were then repeated in the HFrEF patients following 8 weeks of supervised KE training (three times/week, varied intensity – with overall intensity progressively increased based upon biweekly assessments, 50 min per session per leg), as previously described (Richardson et al. 2000; Lawrenson et al. 2003, 2004). Exercise training compliance was evaluated as a percentage of training sessions attended. Control subjects did not undergo training and, thus, were studied only once with the catheter and biopsy procedures.
Exercise apparatus
The knee‐extensor ergometer was used to produce the acute exercise stimulus, as previously described (Richardson et al. 1995). During exercise, the contraction rate was maintained at 60 repetitions per minute.
Vascular and metabolic measurements
The continuous infusion thermodilution approach was used to measure blood flow during exercise, as previously described (Richardson et al. 1993). Femoral arterial and venous blood pressures were continuously monitored at heart level by pressure transducers (model PX‐MK099, Baxter, Deerfield, IL, USA). Mean arterial pressure (MAP) and mean venous blood pressure (MVP) were computed by the integration of each pressure curve. Leg vascular resistance (LVR) was calculated as (MAP – MVP)/muscle blood flow. Before each blood flow measurement, 3–4 ml samples of arterial and femoral venous blood were withdrawn from the catheters anaerobically to measure , , pH, O2 saturation and haemoglobin concentration ([Hb]). All measurements were made on an IL 1306 blood gas analyser and IL 482 CO‐oximeter (Instrumentation Laboratories, Lexington, MA, USA). Arterial and venous O2 concentrations were calculated as [1.39 ml O2 × [Hb] g/100 ml × O2 saturation (%)] + [0.003 ml O2/100 ml of blood × (mmHg)]. Arterial–venous O2 concentration difference was calculated from the difference in radial artery and femoral venous O2 concentration measurements. Muscle was calculated as the product of blood flow and arterial–venous O2 difference, while O2 delivery () was calculated as the product of blood flow and arterial O2 concentration.
Norepinephrine spillover
Adrenaline (epinephrine) and noradrenaline (norepinephrine) were extracted from plasma using a cis‐diol‐specific affinity gel, acylated, derivatized enzymatically and then assayed by competitive ELISA using the microtitre plate format, as previously described (Wadley et al. 2006). The rate of noradrenaline spillover was determined, as described previously (Savard et al. 1989), using the following equation:
where Cv and Ca are plasma noradrenaline concentrations in the common femoral vein and radial artery, respectively. Ee is the fractional extraction of adrenaline, measured across the muscle bed, and LPF is the leg plasma flow, determined from leg blood flow and haematocrit.
KE training
Following the initial catheter‐based studies, subjects returned to the laboratory three times each week for 8 weeks to complete supervised KE training of varying intensity for 100 min exercise periods (50 min per leg), as previously described (Richardson et al. 2000).
Quadriceps muscle mass
Utilizing thigh length, circumferences and skin‐fold measurements, thigh volume was calculated to allow an estimate of quadriceps muscle mass, as utilized previously (Jones & Pearson, 1969; Andersen et al. 1985).
Muscle biopsy
All biopsies were taken from the vastus lateralis muscle at an approximate depth of 3–5 cm, 15 cm proximal to the knee and slightly distal to the ventral midline of the muscle using a Bergstrom needle, as described previously (Bergstrom, 1975). The muscle samples from each biopsy were either immediately frozen in liquid nitrogen and stored at −80°C for subsequent histochemical analysis or immersion‐fixed in glutaraldehyde fixative and later processed for electron microscopy and morphometry.
Histochemistry
Transverse sections 8 μm thick were cut at −24°C on a cryostat (Jung‐Reichert Cryocut 1800) and stained for fibre types I and II, and capillaries (Rosenblatt et al. 1987).
Electron microscopy
The glutaraldehyde‐fixed samples were processed for electron microscopy, as described previously (Mathieu‐Costello, 1987).
Morphometry
The relative cross‐sectional area and number of type I and type II fibres were estimated by point‐counting on a light microscope (250×) using an eyepiece square grid test A100 (Weibel, 1979). Capillary density (i.e. capillary number per fibre cross‐sectional area), capillary‐to‐fibre ratio (i.e. capillary number per fibre number), number of capillaries around a fibre (N CAF) and fibre cross‐sectional area were measured by point‐counting with a light microscope (400×). The volume density of mitochondria per volume of muscle fibre was estimated by point‐counting (490×).
VEGF mRNA levels
The relative VEGF mRNA levels in skeletal muscle from healthy controls and patients with HFrEF were measured by competitive reverse transcriptase polymerase chain reaction (RT‐PCR) analysis according to the method of Zachar et al. (1993). The gel was quantified by computer densitometry (GelPro Analyzer, Media Cybernetics, Silver Springs, MD, USA). VEGF mRNA signals were then expressed as the ratio of VEGF target DNA/internal standard in arbitrary units (AU). Note that VEGF protein expression was not assessed as it is well accepted that VEGF undergoes pretranslational regulation (Gustafsson & Kraus, 2001) and also because of the differing time course of VEGF mRNA and VEGF protein expression (1 h vs. 4 h, respectively; Gustafsson & Kraus, 2001); that would have required an additional biopsy, which was not performed for ethical reasons.
Statistical analysis
Subject characteristics for patients with HFrEF and healthy controls were compared using unpaired Student t‐tests. Variables assessed in the rested state and following acute exercise were assessed by ANOVA. Differences between groups and conditions were then identified using a Newman–Keuls post hoc analysis. Despite the relatively small sample size, a priori power calculations, based upon our previously published assessments of the acute VEGF mRNA response to exercise and muscle morphometry (Richardson et al. 1999, 2000; Esposito et al. 2010 a), revealed adequate power (>0.8) in the major variables of interest. Statistical significance was accepted at P < 0.05. All data are reported as mean ± SEM.
Results
Subject characteristics
Prior to exercise training, the only statistically significant different anthropometric characteristic between the patients and controls was body mass, which was higher in the patients with HFrEF. After training, body mass was unchanged while quadriceps muscle mass was 15% greater (Table 1).
Exercise training compliance
The individualized approach to the exercise training with supervision in the laboratory resulted in a 98% compliance to the prescribed exercise regimen. Due to sickness, a single patient with HFrEF did not complete the post‐training assessments and so was excluded from all analyses.
Maximal bike and KE before and after KE training
Cycle exercise WRmax and pulmonary were significantly lower in the patients with HFrEF compared to the controls before training, but, after training, both WRmax and pulmonary increased to equal that of the (untrained) controls (Table 1). Knee‐extensor maximum was also attenuated in the patients with HFrEF, but, as a consequence of the KE training, KE WRmax increased to equal that of the control subjects. Therefore, the initial 50% of KE WRmax, employed as the standard exercise stimulus before training, was, in absolute terms, lower than that for controls, but after training this relative exercise intensity was equal to the controls in both absolute and relative terms (Table 2).
Table 2.
Vascular and metabolic response to knee‐extensor exercise at 50% of maximum work rate (WRmax) in healthy controls and in patients with heart failure with reduced ejection fraction (HFrEF) before (pre) and after (post) 8 weeks of knee‐extensor exercise training
| Controls | HFrEF pre | HFrEF post | |
|---|---|---|---|
| 50% WRmax (W) | 20 ± 3 | 15 ± 2# | 21 ± 3 |
| HR (beats/min) | 94 ± 3 | 92 ± 6 | 90 ± 3 |
| leg (ml/min) | 313 ± 28 | 275 ± 23.5# | 324 ± 17* |
| Q leg (ml/min) | 2450 ± 223 | 2048 ± 159# | 2529 ± 238* |
| leg (ml/min) | 460 ± 41 | 401 ± 28# | 476 ± 48* |
| Hb (g/dl) | 13.9 ± 0.3 | 12.7 ± 0.6 | 13.1 ± 0.5 |
| (%) | 96 ± 1 | 97.1 ± 0.6 | 97.7 ± 0.4 |
| (ml/100 ml) | 18.8 ± 0.3 | 17.3 ± 0.9 | 18.1 ± 0.8 |
| (ml/100 ml) | 5.9 ± 0.5 | 4.4 ± 0.6 | 5.2 ± 0.9 |
| (mmHg) | 99.9 ± 3 | 95.3 ± 6.2 | 93.6 ± 3.5 |
| (mmHg) | 23.0 ± 1.1 | 19.6 ± 0.9 | 20.3 ± 1.2 |
| LVR (mmHg/l/min) | 41 ± 6 | 47 ± 7# | 39 ± 6*# |
| MAP (mmHg) | 121 ± 11 | 110 ± 6 | 112 ± 7 |
| MAP − MVP (mmHg) | 100 ± 12 | 95 ± 7 | 95 ± 6 |
| Noradrenaline spillover (nm/min) | 0.68 ± 0.2 | 3.30 ± 0.3# | 1.43 ± 0.5*# |
WRmax, maximum work rate; leg, leg oxygen uptake; HR, heart rate; Q leg, leg blood flow; leg, leg oxygen delivery; , arterial O2 saturation; , arterial O2 concentration; , venous O2 concentration; , arterial O2 partial pressure; , venous O2 partial pressure; LVR, leg vascular resistance; MAP, mean arterial pressure; MVP, mean venous pressure. Data are expressed as mean ± SE; * P < 0.05 (post vs. pre); # P < 0.05 (HFrEF vs. controls).
Muscle metabolic and vascular response to acute submaximal exercise
As the 50% of WRmax exercise stimulus was, before exercise training, in absolute terms, lower for the patients with HFrEF, muscle blood flow and muscle were lower than that exhibited by controls. Interestingly, prior to the exercise training, patients with HFrEF exhibited a ≈15% higher LVR than the controls which, following exercise training, was reduced to a level that was not different from the controls (Fig. 1). Furthermore, a reciprocal change in noradrenaline spillover was documented as a consequence of the exercise training (Fig. 1). Additional O2 transport and metabolic data are presented in Table 2.
Figure 1. Leg vascular resistance and noradrenaline spillover across the leg of untrained healthy controls and patients with chronic heart failure with reduced ejection fraction (HFrEF) before (pre) and after (post) knee‐extensor exercise (KE) training during KE at 50% of maximal KE capacity.
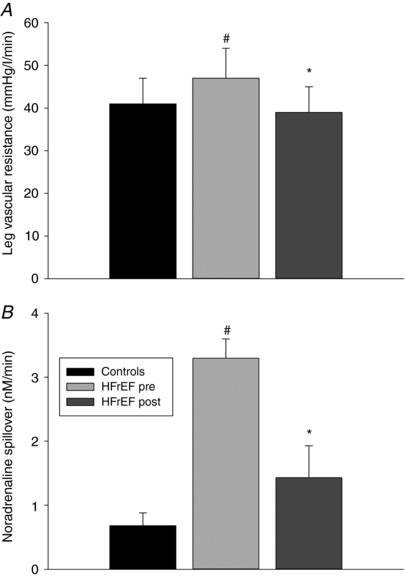
Data are presented as mean ± SE. * P < 0.05 post vs. pre; #P < 0.05 HFrEF vs. controls.
Muscle capillarity and VEGF mRNA
Prior to exercise training there was no significant difference in capillary‐to‐fibre ratio or number of capillaries around a fibre between patients and controls (Fig. 2). As a consequence of training, the patients exhibited a significant increase in capillary‐to‐fibre ratio and the number of capillaries around a fibre (Fig. 2). Representative VEGF mRNA gels for patients with HFrEF and controls are presented with the quantification of these signals in Fig. 2. In the rested leg, VEGF mRNA levels were not different in the controls and the patients with HFrEF both before and after exercise training (0.33 ± 0.04, 0.39 ± 0.03 and 0.38 ± 0.04 AU, respectively; P > 0.05) (Fig. 2). One hour following acute knee‐extensor exercise, both the controls and the patients with HFrEF before exercise training exhibited a significant increase in VEGF mRNA levels in the vastus lateralis muscle (0.78 ± 0.07 and 0.71 ± 0.07 AU in controls and HFrEF pre, respectively; Fig. 2). This increase in VEGF mRNA abundance, as a consequence of acute exercise, was not different in either the patients with HFrEF or controls. Consequently, the VEGF mRNA exercise‐to‐rest ratio was not different between controls and patients with HFrEF before training (2.5 ± 0.5 and 1.8 ± 0.4 AU, respectively; Fig. 2). In contrast, in the trained state, the acute exercise‐induced change in VEGF mRNA abundance in the patients with HFrEF (0.49 ± 0.04 AU) was not significantly different from rest (Fig. 2) and the VEGF mRNA exercise‐to‐rest ratio was attenuated compared to the controls and patients before training (1.3 ± 0.4 AU; Fig. 2).
Figure 2. Skeletal muscle capillary‐to‐fibre ratio, number of capillaries around a fibre, and the percentage increase in VEGF mRNA expression following knee‐extensor exercise (KE) at 50% of maximal KE work rate in untrained healthy controls and patients with chronic heart failure with reduced ejection fraction (HFrEF) before (pre) and after (post) KE training.
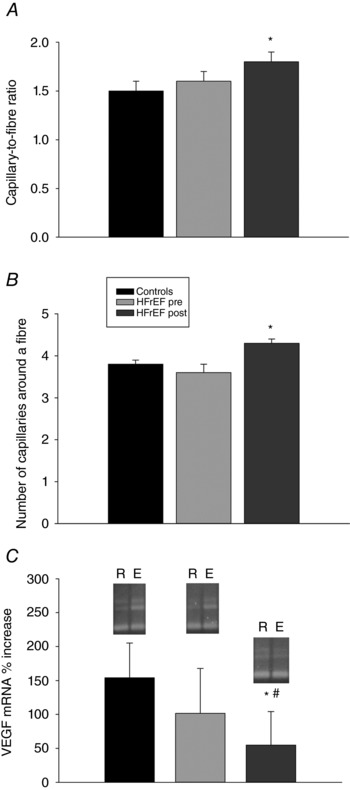
Data are presented as mean ± SE. * P < 0.05 post vs. pre; # P < 0.05 HFrEF vs. controls. R, rested leg; E, excercised leg.
Capillary density, fibre type and fibre area
Prior to exercise training, muscle structural characteristics for both patients with HFrEF and controls were very similar, with no significant difference in capillary density, percentage area of type I and II fibres, or fibre cross‐sectional area (Fig. 3). Interestingly, capillarity, in this case assessed as capillary density, was unchanged by the exercise training performed by the patients with HFrEF, probably because of concomitant muscle fibre changes. Specifically, the percentage area of type I fibres was significantly increased, while the percentage area of type II fibres was reduced. Furthermore, the exercise training induced an increase in fibre cross‐sectional area.
Figure 3. Skeletal muscle capillary density, fibre cross‐section area, percentage area type I fibres, and percentage area type II fibres in untrained healthy controls and patients with chronic heart failure with reduced ejection fraction (HFrEF) before (pre) and after (post) knee‐extensor exercise (KE) training.
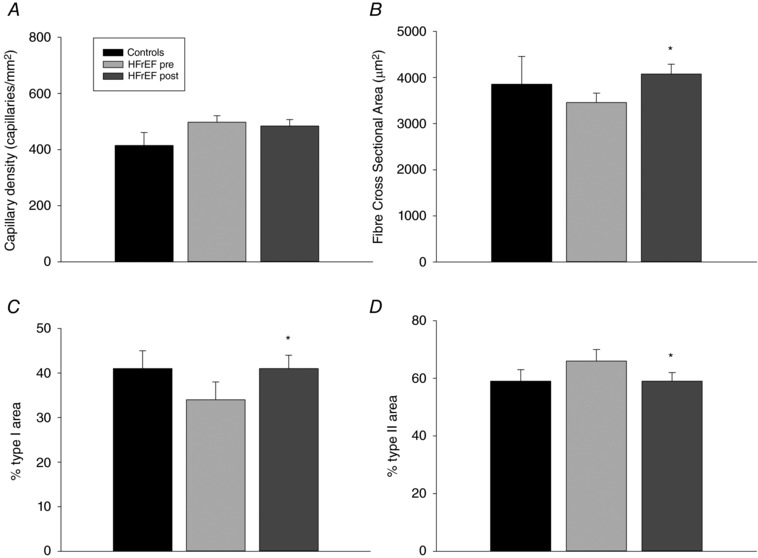
Data are presented as mean ± SE. * P < 0.05 post vs. pre; # P < 0.05 HFrEF vs. controls.
Mitochondrial volume density and capillarity
Prior to exercise training, mitochondrial volume density was significantly lower (22%, P < 0.05) in the patients with HFrEF compared to the controls (Fig. 4). As a result of the exercise training, the patients exhibited a significant increase in mitochondrial volume density. This increase in mitochondrial volume density was such that, after training, there was no longer a difference between patients and controls (Fig. 4). Interestingly, as illustrated in the inset to Fig. 4, there was a significant positive relationship between capillarity, assessed as N CAF, and mitochondrial volume density when these variables were considered before and after exercise training in the patients with HFrEF.
Figure 4. Skeletal muscle mitochondrial volume density in untrained healthy controls and patients with chronic heart failure with reduced ejection fraction (HFrEF) before (pre) and after (post) knee‐extensor exercise (KE) training.
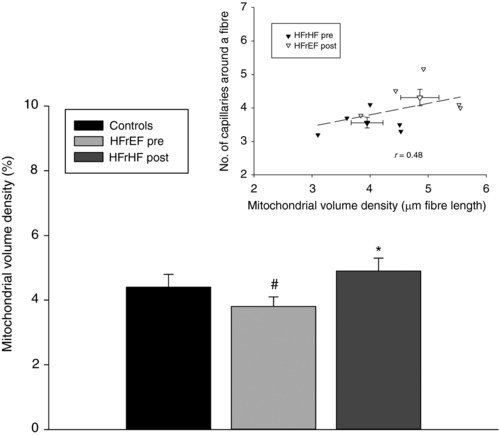
Inset, the relationship between capillarity and mitochondrial volume density content before and after KE training in the patients with HFrEF. Data are presented as mean ± SE. * P < 0.05 post vs. pre; # P < 0.05 HFrEF vs. controls.
Discussion
To better understand exercise‐induced skeletal muscle structural and functional plasticity in patients with HFrEF, this study sought to assess the response to acute submaximal exercise in such patients both before and after KE training and compare them with well‐matched controls. This approach yielded several interesting findings. In terms of function, prior to exercise training, assessed at 50% of WRmax, the patients with HFrEF exhibited higher LVR and much greater noradrenaline spillover. Skeletal muscle structure, with the exception of mitochondrial volume density (which was significantly lower in the patients with HFrEF compared to the controls), including measures of capillarity and fibre type, was not different between the two groups. Resting and acute exercise‐induced VEGF mRNA levels were not different between patients and controls. Following exercise training, the patients no longer exhibited elevated LVR and noradrenaline spillover was reduced. Skeletal muscle capillarity increased after exercise training, as assessed by capillary‐to‐fibre ratio and N CAF, and VEGF mRNA was no longer increased by the acute exercise stimulus. Muscle fibre cross‐sectional area was increased by the training and there was an augmented percentage area of type I fibres and fall in the area of the type II fibres. Finally, exercise training restored mitochondrial volume density, exceeding that of the controls. Collectively, these data provide evidence of structural and functional plasticity and appropriate angiogenic signalling in response to acute exercise in the skeletal muscle of patients with HFrEF.
Muscle metabolic and vascular response to acute exercise
A unique component of this study was that the physiological response to the acute KE stimulus was well documented in terms of metabolism and haemodynamics (Table 2). An initial important observation was that, before training, maximal WR and leg were significantly lower in the patients with HFrEF than in the well‐matched controls. Indeed, guided by the concept that training‐induced adaptations are predominantly stimulated by relative and not absolute WR, the pre‐training selection of 50% of WRmax in both groups resulted in this lower metabolic and vascular challenge for the patients than for the controls. Specifically, pre‐training leg muscle blood flow, and leg at 50% of WRmax were 16%, 13% and 12% lower, respectively, in the patients with HFrEF. However, following training, due to the improvement in maximal exercise capacity of the patients with HFrEF, there was no longer a difference in the absolute WR that equated to 50% of WRmax and, therefore, the metabolic and vascular stress imposed by the acute submaximal exercise was no longer lower than that of the controls and the response of the patients could now be, legitimately, compared to that of the controls.
Neurohumoral activation, including increased sympathetic nervous system activity (SNA), is a hallmark characteristic of advanced HFrEF (Esposito et al. 2010 b; Kaur et al. 2018) and, typically, those patients with the greatest SNA have the poorest chance of survival (Cohn et al. 1984). So strong is this link that almost every pharmacological intervention proven to increase survival in HFrEF interrupts this increase in SNA (Packer et al. 2001). Exercise training, on the other hand, has clear beneficial effects, but data regarding the effect of exercise training on SNA in HFrEF have been equivocal. For example, direct SNA measurements with microneurography revealed a reduction in resting SNA in patients with HFrEF following exercise training (Roveda et al. 2003). In contrast, a study, more similar to the current work (Gordon et al. 1997), evaluated patients with HFrEF before and after 8 weeks of two‐legged KE training, and although documenting many benefits of chronic exercise in this population, training did not alter resting noradrenaline levels. The current study supports the latter pre‐training findings, with evidence of elevated noradrenaline spillover and greater LVR in the patients with HFrEF. Of note, the elevated LVR was reversed by KE training and accompanied by a fall in noradrenaline spillover (Fig. 1). Thus, in contrast to our previous study focused on maximal exercise (Esposito et al. 2011), the current data support a link between the benefits of exercise training and a reduction in SNA at a submaximal exercise intensity in patients with HFrEF. It is, of course, plausible that other exercise training‐induced vascular adaptations, such as improved vascular endothelial function, contributed to the reduction in vascular resistance in the patients with HFrEF, but this is beyond the scope of the current study.
Skeletal muscle vasculature and VEGF
Prior to training, the muscle vascular characteristics of both patients with HFrEF and controls were very similar, with no significant differences in capillary‐to‐fibre ratio, N CAF or capillary density. As a consequence of training, the patients exhibited a significant increase in both capillary‐to‐fibre ratio and N CAF, but, ultimately, there was still no difference between groups in terms of these indices of capillarity (Fig. 2). In the current study and in our previous work (Esposito et al. 2010 a), the VEGF mRNA response to acute exercise in the untrained patients with HFrEF was not different from the healthy controls (Fig. 2). As VEGF functions as a direct angiogenic factor with a high specificity for vascular endothelial cells (Neufeld et al. 1999; Wahl et al. 2014) these findings are in line with the well‐accepted critical role of VEGF in the formation of new blood vessels in human skeletal muscle, including patients with HFrEF, in response to exercise (Gustafsson et al. 2001; Esposito et al. 2010 a).
Angiogenesis is an essential adaptive response in skeletal muscle to chronic exercise (i.e. training) resulting in an increase in the number of capillaries per muscle fibre that enhances O2 transport conductance between the microcirculation and mitochondria (Gustafsson et al. 2001; Esposito et al. 2010 a). The present data extend the link between VEGF and capillary proliferation to include the observation that following significant adaptations to exercise training, including angiogenesis, the previously large VEGF mRNA response to acute exercise in patients with HFrEF is significantly attenuated (Fig. 2). This finding adds credence to the concept that VEGF is important in the initial phase of exercise adaptation in patients with HFrEF, but when significant angiogenesis has occurred, due to training, the need and importance of this mechanism becomes significantly reduced. Note that although VEGF protein levels were not assessed in the current study, previous findings have documented proportional changes in VEGF mRNA and protein expression in this population (Gustafsson et al. 2001). Collectively, these data document the exciting and clinically relevant observation that the capacity of acute exercise to initiate the process of new capillary growth and the maintenance of existing vessels (i.e. increasing VEGF mRNA levels) is still intact in the skeletal muscle of patients with HFrEF.
Skeletal muscle mass and fibre type plasticity
Although HFrEF is often associated with muscle atrophy, this was not the case in the current patients in comparison to controls, despite the patients being predominantly defined as NYHA Class III. Indeed, probably due to the greater body mass in the patients, muscle mass actually tended to be greater in the patient group prior to KE training (Table 1). Following the 8 weeks of KE training, this initial tendency was translated into a significantly greater muscle mass in the patients compared to the, untrained, controls (Table 1). HFrEF is often associated with not only muscle atrophy and skeletal muscle dysfunction, but also with a shift in fibre types from ‘slow’, aerobic, fatigue‐resistant (myosin heavy chain I, MHC1) to the ‘fast’ more fatigable fibres (MHC2a and MHC2x) (Mancini et al. 1992; Narumi et al. 2015). Although not statistically different, in this regard, from controls, the current patients with HFrEF were no exception to this theme demonstrating a strong trend to a reduced percentage area of type I and increased percentage area of type II fibres in comparison with the controls (Fig. 4). Of note, considerable effort was made to find control subjects not only of similar age and with anthropometric characteristics, but also having a similar level of daily physical activity as the patients with HFrEF (i.e. relatively inactive) as this, in and of itself, may influence muscle structure. In agreement with this concept, KE training significantly increased the percentage of type I and decreased the percentage of type II fibres, with the patients, following training, now exhibiting fibre type proportions that were not different to the controls (Fig. 4).
Skeletal muscle mitochondrial volume density
Interestingly, the only morphometric difference prior to training between patients with HFrEF and controls was at the subcellular level with mitochondrial volume density in HFrEF patients being 22% lower than in controls (Fig. 4). Of further note, and germane to the current focus on vasculature, when training‐induced mitochondrial biogenesis restored mitochondrial volume density to levels in the patients with HFrEF to that of the controls, pre‐ and post‐training mitochondrial volume densities were significantly correlated with pre‐ and post‐training N CAF values (Fig. 4, inset). This finding reinforces the probable teleological link between the skeletal muscle metabolic machinery and the vasculature, and implies that this important relationship is still intact in patients with HFrEF (Poole & Mathieu‐Costello, 1996).
Perspective
The exercise‐induced skeletal muscle structural and functional improvements in patients with HFrEF, documented herein, provide evidence of significant peripheral vascular and metabolic plasticity in this population that can be developed with small muscle mass training and then harnessed to benefit whole body exercise capacity. Additionally, these findings highlight the intact nature of skeletal muscle‐specific processes such as angiogenesis and mitochondrial biogenesis in patients with HFrEF, allowing the contributions of these peripheral factors to be targeted, perhaps guiding clinical interventions in the future.
Additional information
Conflict of interests
None declared.
Author contributions
FE: conception and design of the study, acquisition, analysis and interpretation of data for the study, and drafting the article; OMC: conception and design of the study, analysis and interpretation of data, and critical revision for important intellectual content; PDW: conception and design of the study, analysis and interpretation of data, and critical revision for important intellectual content; RSR: conception and design of the study, acquisition, analysis and interpretation of data for the study, and critical revision for important intellectual content. All authors approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by a National Institutes of Health grant from the National Heart, Lung, and Blood Institute (HL‐091830), Veterans Affairs Rehabilitation Research and Development Merit Awards (E6910‐R, E1697‐R and E2323‐I), Veterans Affairs SPiRe Award (E1433‐P), and a Veterans Affairs Senior Research Career Scientist Award (E9275‐L). We declare no relationship with industry.
Translational perspectives.
This study has documented skeletal muscle structural and functional plasticity and appropriate angiogenic signalling in response to acute submaximal exercise in patients with HFrEF. In practical terms, small muscle mass exercise training appears to hold significant promise in terms of promoting peripheral adaptation in the apparently still responsive skeletal muscle of patients with HFrEF. These observations may contribute to a more focused, evidence‐based non‐pharmacological component of HFrEF treatment by physicians. The same strategic approach could also be proposed for other pathological scenarios, such as chronic obstructive pulmonary disease, where the central component of oxygen delivery is compromised but their disuse‐induced peripheral maladaptation at the skeletal muscle level could be reversed by small muscle mass physical training.
Acknowledgments
The authors thank all participants for their committed involvement in this study.
Biography
Fabio Esposito received his medical degree in 1990, after which he worked as Assistant Professor at the University of Brescia. He then joined the faculty at University of Milan in 2003, where he is currently Full Professor of Exercise and Sport Sciences at the Department of Biomedical Sciences for Health, head of the Exercise Physiology laboratory and Dean of the School of Exercise Sciences. His research field is Human Physiology, with particular interests in exercise physiology, muscle electro‐mechanical behaviour, pulmonary gas exchange and exercise‐induced cardioprotection.

Edited by: Scott Powers & Bruno Grassi
References
- Andersen P, Adams RP, Sjogaard G, Thorboe A & Saltin B (1985). Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 59, 1647–1653. [DOI] [PubMed] [Google Scholar]
- Barrett‐O'Keefe Z, Lee JF, Berbert A, Witman MA, Nativi‐Nicolau J, Stehlik J, Richardson RS & Wray DW (2014). Hemodynamic responses to small muscle mass exercise in heart failure patients with reduced ejection fraction. Am J Physiol Heart Circ Physiol 307, H1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J (1975). Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35, 609–616. [PubMed] [Google Scholar]
- Brodal P, Ingjer F & Hermansen L (1977). Capillary supply of skeletal muscle fibers in untrained and endurance‐trained men. Am J Physiol 232, H705–712. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB & Rector T (1984). Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. New England J Med 311, 819–823. [DOI] [PubMed] [Google Scholar]
- Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM & Annex BH (1999). Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II–III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol 33, 1956–1963. [DOI] [PubMed] [Google Scholar]
- Esposito F, Mathieu‐Costello O, Entin PL, Wagner PD & Richardson RS (2010. a). The skeletal muscle VEGF mRNA response to acute exercise in patients with chronic heart failure. Growth Factors 28, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Mathieu‐Costello O, Shabetai R, Wagner PD & Richardson RS (2010. b). Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol 55, 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Reese V, Shabetai R, Wagner PD & Richardson RS (2011). Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol 58, 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Wagner PD & Richardson RS (2015). Incremental large and small muscle mass exercise in patients with heart failure: evidence of preserved peripheral haemodynamics and metabolism. Acta Physiol (Oxf) 213, 688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A, Tyni‐Lenne R, Jansson E, Kaijser L, Theodorsson‐Norheim E & Sylven C (1997). Improved ventilation and decreased sympathetic stress in chronic heart failure patients following local endurance training with leg muscles. J Cardiac Fail 3, 3–12. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Bodin K, Sylven C, Gordon A, Tyni‐Lenne R & Jansson E (2001). Increased expression of VEGF following exercise training in patients with heart failure. Eur J Clin Invest 31, 362–366. [DOI] [PubMed] [Google Scholar]
- Gustafsson T & Kraus WE (2001). Exercise‐induced angiogenesis‐related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front Biosci 6, D75‐89. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Puntschart A, Kaijser L, Jansson E & Sundberg CJ (1999). Exercise‐induced expression of angiogenesis‐related transcription and growth factors in human skeletal muscle. Am J Physiol 276, H679–685. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J & Schuler G (1998). Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure [see comment]. Circulation 98, 2709–2715. [DOI] [PubMed] [Google Scholar]
- Hang J, Kong L, Gu JW & Adair TH (1995). VEGF gene expression is upregulated in electrically stimulated rat skeletal muscle. Am J Physiol Heart Circ Physiol 269, H1827–1831. [DOI] [PubMed] [Google Scholar]
- Harrington D, Anker SD, Chua TP, Webb‐Peploe KM, Ponikowski PP, Poole‐Wilson PA & Coats AJ (1997). Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol 30, 1758–1764. [DOI] [PubMed] [Google Scholar]
- Hollriegel R, Winzer EB, Linke A, Adams V, Mangner N, Sandri M, Bowen TS, Hambrecht R, Schuler G & Erbs S (2016). Long‐term exercise training in patients with advanced chronic heart failure: sustained benefits on left ventricular performance and exercise capacity. J Cardiopulm Rehabil Prev 36, 117–124. [DOI] [PubMed] [Google Scholar]
- Huey KA, Smith SA, Sulaeman A & Breen EC (2016). Skeletal myofiber VEGF is necessary for myogenic and contractile adaptations to functional overload of the plantaris in adult mice. J Appl Physiol (1985) 120, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PR & Pearson J (1969). Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204, 63P–66P. [PubMed] [Google Scholar]
- Kaur J, Senador D, Krishnan AC, Hanna HW, Alvarez A, Machado TM & O'Leary DS (2018). Muscle metaboreflex‐induced vasoconstriction in the ischemic active muscle is exaggerated in heart failure. Am J Physiol Heart Circ Physiol 314, H11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson L, Hoff J & Richardson RS (2004). Aging attenuates vascular and metabolic plasticity but does not limit improvement in muscle VO2 max. Am J Physiol Heart Circ Physiol 286, H1565–1572. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P & Richardson RS (2003). Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285, H1023–1031. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV & Ferrara N (1989). Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Gordon A, Kaijser L, Sylvén C, Isberg B, Karpakka J & Saltin B (1996). High intensity knee extensor training, in patients with chronic heart failure. Major skeletal muscle improvement. Eur Heart J 17, 1048–1055. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Sylvén C, Karlberg KE, Isberg B & Saltin B (1997). Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovasc Res 33, 297–306. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL & Wilson JR (1992). Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 85, 1364–1373. [DOI] [PubMed] [Google Scholar]
- Mathieu‐Costello O (1987). Capillary tortuosity and degree of contraction or extension of skeletal muscles. Microvasc Res 33, 98–117. [DOI] [PubMed] [Google Scholar]
- Narumi T, Watanabe T, Kadowaki S, Takahashi T, Yokoyama M, Kinoshita D, Honda Y, Funayama A, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyamoto T & Kubota I (2015). Sarcopenia evaluated by fat‐free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med 26, 118–122. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S & Poltorak Z (1999). Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13, 9–22. [PubMed] [Google Scholar]
- Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD & Breen EC (2009). Muscle‐specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587, 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK & DeMets DL (2001). Effect of carvedilol on survival in severe chronic heart failure. New England J Med 344, 1651–1658. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Davos C, Francis DP, Coats AJ & ExTra MC (2004). Exercise training meta‐analysis of trials in patients with chronic heart failure (ExTraMATCH) [see comment]. BMJ 328, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corra U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani G, Agostoni P, Working Group 'Exercise Physiology SC & Cardiac Rehabilitation ISoC (2010. a). Exercise intolerance in chronic heart failure: mechanisms and therapies. Part II. Eur J Cardiovasc Prev Rehabil 17, 643–648. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corra U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani GQ, Agostoni P, Working Group 'Exercise Physiology SC & Cardiac Rehabilitation ISoC (2010. b). Exercise intolerance in chronic heart failure: mechanisms and therapies. Part I. Eur J Cardiovasc Prev Rehabil 17, 637–642. [DOI] [PubMed] [Google Scholar]
- Poole DC, Hirai DM, Copp SW & Musch TI (2012). Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302, H1050–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC & Mathieu‐Costello O (1996). Relationship between fiber capillarization and mitochondrial volume density in control and trained rat soleus and plantaris muscles. Microcirculation 3, 175–186. [DOI] [PubMed] [Google Scholar]
- Poole DC, Richardson RS, Haykowsky MJ, Hirai DM & Musch TI (2018). Exercise limitations in heart failure with reduced and preserved ejection fraction. J Appl Physiol (1985) 124, 208–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B & Wagner PD (1995). Determinants of maximal exercise VO2 during single leg knee‐extensor exercise in humans. Am J Physiol 268, H1453–1461. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK & Wagner PD (1993). High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Henry R, Noyszewski EA & Wagner PD (1999). Human VEGF gene expression in skeletal muscle: effect of acute normoxic and hypoxic exercise. Am J Physiol Heart Circ Physiol 277, H2247–2252. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R & Wagner PD (2000). Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol 279, H772–778. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Kuzon WM, Jr , Plyley MJ, Pynn BR & McKee NH (1987). A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol 62, 85–92. [DOI] [PubMed] [Google Scholar]
- Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM & Negrao CE (2003). The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42, 854–860. [DOI] [PubMed] [Google Scholar]
- Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ & Saltin B (1989). Norepinephrine spillover from skeletal muscle during exercise: role of muscle mass. Am J Physiol 257, H1812–H1818. [DOI] [PubMed] [Google Scholar]
- Tyni‐Lenne R, Dencker K, Gordon A, Jansson E & Sylven C (2001). Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail 3, 47–52. [DOI] [PubMed] [Google Scholar]
- Wadley GD, Lee‐Young RS, Canny BJ, Wasuntarawat C, Chen ZP, Hargreaves M, Kemp BE & McConell GK (2006). Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab 290, E694–702. [DOI] [PubMed] [Google Scholar]
- Wahl P, Jansen F, Achtzehn S, Schmitz T, Bloch W, Mester J & Werner N (2014). Effects of high intensity training and high volume training on endothelial microparticles and angiogenic growth factors. PLoS One 9, e96024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E (1979). Practical Methods for Biological Morphometry. Academic Press, London. [Google Scholar]
- Zachar V, Thomas RA & Goustin AS (1993). Absolute quantification of target DNA: a simple competitive PCR for efficient analysis of multiple samples. Nucleic Acids Res 21, 2017–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumstein A, Mathieu O, Howald H & Hoppeler H (1983). Morphometric analysis of the capillary supply in skeletal muscles of trained and untrained subjects–its limitations in muscle biopsies. Pflugers Arch 397, 277–283. [DOI] [PubMed] [Google Scholar]


