Abstract
Background
The role of sorafenib in patients with hepatocellular carcinoma (HCC) recurrence after liver transplantation (LT) has been rarely studied. The aim of this study was to evaluate the efficacy of sorafenib in post-LT era.
Methods
Consecutive patients with post-transplant HCC recurrence not eligible to resection or locoregional therapy were included. Patients receiving best supportive care (BSC) until 2007 were compared with those treated by sorafenib thereafter.
Results
Of a total of 65 patients, 20 patients received BSC and 45 received sorafenib. Clinical characteristics were similar between two groups except that sorafenib group received tacrolimus and mammalian target-of-rapamycin inhibitors more frequently than BSC group. Treatment with sorafenib conferred a survival advantage as compared with BSC for survival after recurrence (median, 14.2 vs. 6.8 months; P = 0.01). In multivariate analyses, high serum α-fetoprotein level, synchronous intrahepatic recurrence and distant metastasis at the time of recurrence, and BSC were independently associated with poorer survival after recurrence. Sorafenib treatment was associated with better survival after recurrence as compared with BSC (hazard ratio, 0.25; 95% confidence interval, 0.10–0.62; P = 0.002). In addition, sorafenib group showed tolerable toxicity in the post-transplant setting.
Conclusion
Sorafenib may be beneficial in patients with post-transplant HCC recurrence.
Keywords: Sorafenib, Liver Transplantation, Hepatocellular Carcinoma, Recurrence
Graphical Abstract
INTRODUCTION
Liver transplantation (LT) is the most effective therapy in carefully selected patients with hepatocellular carcinoma (HCC).1 Patients within the Milan criteria (MC) have shown 5-year recurrence-free survival and overall survival (OS) rates of 83% and 75%, respectively.2 However, post-transplant HCC recurrence is reported up to 8%–20% of cases in spite of stringent selection of transplant candidates.1,3,4,5,6,7,8 The majority of patients with HCC recurrence after LT have systemic tumor spreading not amenable to resection or locoregional therapies.3,4,9,10 Moreover, transplanted patients are on multiple drugs including immunosuppressive agents, most of them are known to promote tumor growth.11,12 Therefore, patients with systemic tumor recurrence generally show a dismal prognosis with a median survival of less than one year.5,13 Management of these patients is a challenging issue, however, there is no consensus treatment strategy regarding HCC recurrence not amenable to resection or locoregional therapies.
Sorafenib, a multi-tyrosine kinase inhibitor, is the first drug to demonstrate a significant improvement in the OS of patients with advanced HCC.14 It might be considered in selected cases of HCC recurrence after LT, when systemic treatment is warranted. However, only a few retrospective, small sized studies are available regarding the efficacy of sorafenib in these settings. A recent small case-control study showed that sorafenib seems to be associated with better survival compared to best supportive care (BSC) in this setting.15 In contrast, several studies reported that sorafenib seems to be poorly tolerated because of drug to drug interactions with immunosuppressive agents, and rarely effective.16,17 Currently, the efficacy and safety of sorafenib in this setting are controversial.
In this study, we aimed to evaluate whether sorafenib, compared to BSC, could increase survival in patients with HCC recurrence after LT not amenable to surgical resection or locoregional therapies.
METHODS
Patients
This retrospective cohort study included patients who were diagnosed with recurrent HCC following LT between October 2000 and May 2015 at Seoul National University Hospital (Seoul, Korea). Patients were divided into two groups according to treatment regimens after development of HCC not amenable to resection or locoregional treatment (untreatable progression [UP]).15 Patients treated by sorafenib after presenting with UP were classified as the sorafenib group, and those received only BSC including palliative radiotherapy to extrahepatic metastasis were classified as the control group.
Treatment of recurrence and outcomes
After LT, all patients were monitored with dynamic computed tomography (CT) scans or magnetic resonance imaging (MRI) every 2–4 months for the first 2 years and every 3–6 months thereafter. HCC recurrence was either confirmed by histology or diagnosed according to the non-invasive criteria of the American Association for the Study of Liver Disease.18
Treatment of HCC recurrence was discussed and decided at multidisciplinary team meeting. Treatment strategy was carefully aimed, whenever possible, at complete surgical removal of recurrence. Radiofrequency ablation (RFA) was performed in patients with up to three intrahepatic recurrences (≤ 3 cm in size) for which resection was not feasible. Transarterial chemoembolization was considered in patients with 1) multi-nodular HCCs (more than three, > 3 cm in size); or 2) risky or inaccessible lesions for RFA (i.e., hepatic dome or perivascular lesions). When tumor was deemed not anymore eligible to resection or locoregional therapies according to these criteria, patients received systemic chemotherapy or BSC until 2007, and received sorafenib based treatment or BSC thereafter. Sorafenib was initiated at a dosage of 400 mg twice daily. In case of adverse events (AEs), the dose was tapered to 400 mg/day and eventually to 400 mg every other day, according to severity and persistence of symptoms. Sorafenib was withdrawn in case of serious AEs or radiological tumor progression according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.
The primary study objective was evaluation of the efficacy of sorafenib in patients with no otherwise treatable HCC recurrence after LT compared to BSC, according to survival time. Survival time was analyzed in two ways: 1) as the interval between tumor recurrence and death (survival after recurrence); 2) as the interval between UP and death (survival after UP).
A toxicity profile was also evaluated in those patients according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical analyses
The Mann-Whitney U test and Kruskal-Wallis test were used to analyze differences between the groups. The χ2 test or Fisher's exact test was used for categorical data. The cumulative rate of survival was calculated using the Kaplan-Meier method and the log-rank test was performed to compare the differences between the groups. Cox proportional hazards models were used to assess the influence of the clinical variables outcome. Considering that the investigated patients were selected as having UP, we used a left-truncated Cox proportional hazard regression model for the analysis of survival after recurrence to account for the interval between posttransplant HCC recurrence and assessment of UP (median, 11 months). Differences at P < 0.05 were considered statistically significant. The statistical analyses were performed using SPSS for Windows, version 22.0 (SPSS Inc., Chicago, IL, USA) and R language ver. 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics statement
The present study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki, and was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (IRB No.: H-1608-019-781). Documentation of informed consent was waived by the IRB because of the anonymous evaluation of data.
RESULTS
Baseline characteristics
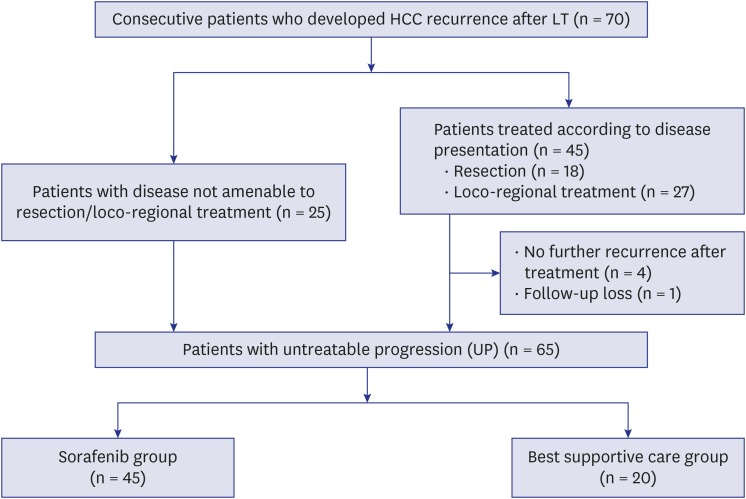
During the study period, 502 patients underwent LT for HCC, 70 of whom developed recurrent HCC. Twenty-five patients presented upfront with a disease not amenable to resection, ablation or locoregional treatments, while 45 received multiple treatments until development of UP. Of the 45 patients, four patients were cured by resection or ablation and did not need any further treatment, and one patient was lost to follow-up. Of the 65 patients presented with UP, 45 patients treated by sorafenib and 20 patients receiving BSC constituted our study population (Fig. 1).
Fig. 1. The diagram of patients flow.
HCC = hepatocellular carcinoma, LT = liver transplantation.
The baseline characteristics of patients are described in Table 1. The median age at HCC recurrence after LT was 55 years (interquartile range [IQR], 49–62 years) and 55 (84.6%) patients were males. Hepatitis B virus infection was the most common etiology of the underlying liver disease. Fifty-two patients (80.0%) underwent living-donor LT (LDLT). Forty-nine patients (75.4%) were beyond MC at pre-transplant staging, and micro-vascular invasion was present in 35 cases (53.8%) at explant pathology. The median time from LT to recurrence was 8.2 months (IQR, 3.2–12.3 months). Recurrence was limited to the liver in 5 patients (7.7%), was limited to extrahepatic lesion in 30 patients (46.2%), and was both intrahepatic and extrahepatic in 30 patients (46.2%) at the time of recurrence. Patients underwent resection or locoregional treatments (range, 0–8) until developing UP. The median time from LT to UP was 11.2 months (IQR, 4.4–20.0 months).
Table 1. Baseline characteristics of patients according to treatment group.
| Variables | Sorafenib (n = 45) | BSC (n = 20) | P value | |
|---|---|---|---|---|
| Age at recurrence, yr | 55 (49–61) | 53 (50–62) | 0.92 | |
| Sex, male | 41 (91.1) | 14 (70.0) | 0.06 | |
| Etiology of liver disease | 1.00 | |||
| HBV | 40 (88.9) | 18 (90.0) | ||
| HCV/other | 5 (11.1) | 2 (10.0) | ||
| No. of treatments before LT | 0.42 | |||
| < 2 | 22 (48.9) | 7 (35.0) | ||
| ≥ 2 | 23 (51.1) | 13 (65.0) | ||
| Tumor stage (pre–LT) | 1.00 | |||
| Within MC | 11 (24.4) | 5 (25.0) | ||
| Beyond MC | 34 (75.6) | 15 (75.0) | ||
| Type of LT | 0.09 | |||
| Living donor | 39 (86.7) | 13 (65.0) | ||
| Deceased donor | 6 (13.3) | 7 (35.0) | ||
| MVI (LT pathology) | 25 (55.6) | 10 (50.0) | 0.79 | |
| Main immunosuppression before recurrence | 0.03 | |||
| Cyclosporine | 0 | 3 (15.0) | ||
| Tacrolimus | 45 (100) | 17 (85.0) | ||
| mTOR inhibitor use | < 0.001 | |||
| Sirolimus | 36 (80.0) | 7 (35.0) | ||
| Everolimus | 6 (13.3) | 1 (5.0) | ||
| None | 3 (6.7) | 12 (60.0) | ||
| Episode of acute rejection | 8 (17.8) | 3 (15.0) | 0.92 | |
| Time-to-recurrence, mon | 6.5 (2.8–11.7) | 11.7 (6.1–14.6) | 0.18 | |
| Episode of acute rejection | 8 (17.8) | 3 (15.0) | 0.92 | |
| Time-to-recurrence, mon | 6.5 (2.8–11.7) | 11.7 (6.1–14.6) | 0.18 | |
| Initial patterns of recurrence | 0.77 | |||
| Extrahepatic | 20 (44.4) | 10 (50.0) | ||
| Intrahepatic | 3 (6.7) | 2 (10.0) | ||
| Both | 22 (48.9) | 8 (40.0) | ||
| AFP at recurrence | 31.1 (3.7–424.1) | 163.3 (7.8–3,376.5) | 0.27 | |
| PIVKA-II at recurrence | 110 (39–777) | 131 (40–1,776) | 0.78 | |
| MELD score at recurrence | 7.4 (6.4–10.0) | 7.9 (6.6–10.5) | 0.91 | |
| Initial treatment at recurrence | 0.65 | |||
| Resection | 11 (24.5) | 6 (30.0) | ||
| Locoregional treatment | 15 (33.3) | 8 (40.0) | ||
| Sorafenib/BSC | 19 (42.2) | 6 (30.0) | ||
| No. of treatments after recurrence until UP | 0.79 | |||
| < 2 | 30 (66.7) | 14 (70.0) | ||
| ≥ 2 | 15 (33.3) | 6 (30.0) | ||
| Time-to-UP, mon | 7.0 (3.2–12.3) | 3.7 (1.5–8.1) | 0.49 | |
Data are presented as median (interquartile range) or number (%), unless otherwise indicated.
BSC = best supportive care, HBV = hepatitis B virus, HCV = hepatitis C virus, LT = liver transplantation, MC = Milan criteria, MVI = microvascular invasion, mTOR = mammalian target-of-rapamycin, AFP = α-fetoprotein, PIVKA–II = prothrombin in vitamin K absence–II, MELD = model for end-stage liver disease, UP = untreatable progression.
Clinical characteristics were similar across the treatment groups except immunosuppressive strategy. All patients in the sorafenib group received tacrolimus as main immunosuppressant, while 3 in the BSC group received cyclosporine. Calcineurin inhibitors were maintained in 60.0% of patients in the BSC group, while 93.3% of the sorafenib group switched to mammalian target-of-rapamycin (mTOR) inhibitors after HCC recurrence (P < 0.001).
Survival analysis
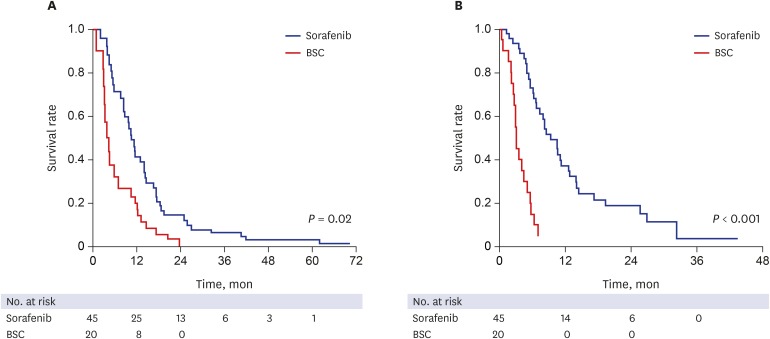
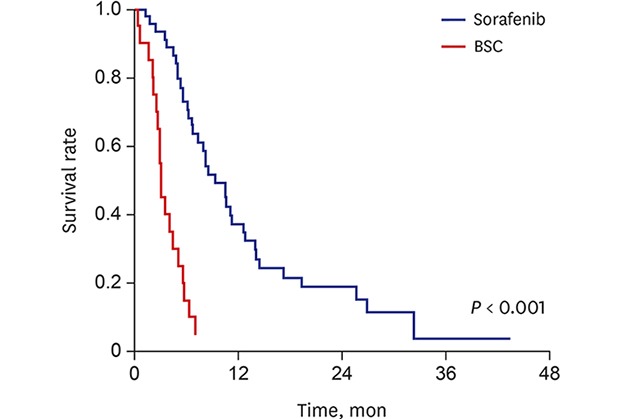
During the median follow-up period of 12.1 months (IQR, 5.7–20.9 months) after HCC recurrence, 57 out of 65 patients died. The median survival after recurrence and median survival after UP were 14.2 months (95% confidence interval [CI], 9.6–18.8) and 9.4 months (95% CI, 6.6–12.2) in the sorafenib group, while 6.8 (95% CI, 1.7–16.7) and 3.2 (95% CI, 2.8–3.6) months in the BSC group, respectively (Fig. 2). Treatment with sorafenib conferred a survival advantage as compared with BSC both for survival after recurrence (hazard ratio [HR], 0.59; 95% CI, 0.28–0.89; P = 0.02) and survival after UP (HR, 0.17; 95% CI, 0.09–0.34; P < 0.001), respectively. In multivariate Cox regression analyses, high serum AFP level, synchronous intrahepatic recurrence and distant metastasis at the time of recurrence, and receiving only BSC after UP were independently associated with poorer survival after recurrence (Table 2). Sorafenib treatment was independently associated with better survival after recurrence as compared with BSC (HR, 0.25; 95% CI, 0.10–0.62; P = 0.002). In addition, sorafenib was also independently associated with better survival after UP (HR, 0.13; 95% CI, 0.06–0.27; P < 0.001).
Fig. 2. Kaplan-Meier estimates of survival after recurrence and after UP. (A) Patients in the sorafenib group showed significantly longer survival after diagnosis of recurrence than those in the BSC group. (B) Patients in the sorafenib group showed significantly longer survival after UP than those in the BSC group.
UP = untreatable progression, BSC = best supportive care.
Table 2. Univariate and multivariate analyses of factors associated with survival after recurrence (corrected by time from recurrence to untreatable progression).
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Tumor stage (beyond MC vs. within MC) | 0.85 (0.45–1.60) | 0.62 | |||
| Vascular invasion (histology) | 0.97 (0.56–1.67) | 0.91 | |||
| Main immunosuppression (tacrolimus vs. cyclosporine) | 0.21 (0.06–0.73) | 0.01 | 0.53 (0.11–2.57) | 0.43 | |
| mTOR inhibitor use | 0.36 (0.19–0.69) | < 0.01 | 0.57 (0.25–1.28) | 0.17 | |
| AFP at recurrence (≥ 200 vs. < 200) | 2.49 (1.39–4.45) | < 0.01 | 1.99 (1.01–3.90) | 0.04 | |
| MELD at recurrence | 1.03 (0.99–1.08) | 0.14 | |||
| Time-to-recurrence (≥ 1 yr vs. < 1 yr) | 0.72 (0.37–1.40) | 0.33 | |||
| Initial patterns of recurrence | 0.02 | ||||
| Extrahepatic | 1.00 | 1.00 | |||
| Intrahepatic | 1.59 (0.59–4.29) | 1.00 (0.31–3.21) | 0.99 | ||
| Both | 2.40 (1.29–4.47) | 3.58 (1.79–7.18) | < 0.01 | ||
| Initial treatment at recurrence | 0.56 | ||||
| Resection | 1.00 | ||||
| Locoregional treatment | 1.25 (0.59–2.64) | ||||
| Sorafenib//BSC | 0.94 (0.44–2.01) | ||||
| No. of treatments after recurrence until UP (≥ 2 vs. < 2) | 0.61 (0.30–1.22) | 0.16 | |||
| Treatment after UP (sorafenib vs. BSC) | 0.59 (0.28–0.89) | 0.02 | 0.25 (0.10–0.62) | < 0.01 | |
HR = hazard ratio, CI = confidence interval, MC = Milan criteria, mTOR = mammalian target-of-rapamycin, AFP = α-fetoprotein, MELD = model for end-stage liver disease, BSC = best supportive care, UP = untreatable progression.
In the subgroup analysis, sorafenib enhanced survival after recurrence compared with BSC both in patients who presented with UP at recurrence and those who received multiple treatments until development of UP (P = 0.005 and 0.006, respectively, Supplementary Tables 1 and 2). In addition, sorafenib treatment was associated with better prognosis in patients with high α-fetoprotein (AFP) levels (≥ 200 ng/mL; P = 0.003), patients developing early recurrence after LT (< 1 year; P < 0.001), and patients who had recurrence limited to extrahepatic lesion at the time of recurrence (P = 0.04). Patients with low AFP levels (< 200 ng/mL), patients developing late recurrence after LT (≥ 1 year), and patients who had intrahepatic recurrence with/without distant metastasis also showed similar trends, although the differences were not significant because of the small number of patients.
AEs
The AEs during sorafenib were as following: hand-foot syndrome was observed in 4 patients (8.9%), diarrhea in 6 (13.3%), alopecia in one (2.2%), vomiting in one (2.2%) and abnormality of liver function test in 2 (4.4%), respectively. The AEs were well controlled by a dose reduction. Drug-related grade 4 or 5 toxicity did not occur. Sorafenib was withdrawn for progression of disease in 39 patients (86.7%), one patient for liver function test abnormality, one patient for vomiting, two patients for diarrhea and one patient for hand-foot syndrome.
DISCUSSION
In this largest single-center report of sorafenib for recurrent HCC following LT, sorafenib treatment was associated with better post-recurrence survival as compared with BSC. The associations were independent of other well-known prognostic factors including serum AFP level and patterns of tumor recurrence. In addition, treatment with sorafenib in the post-transplant setting showed tolerable toxicity.
According to the previous studies regarding post-transplant HCC recurrence, median survival after sorafenib treatment ranges between 17.8 and 38.5 months, suggesting an improvement of prognosis compared to BSC, albeit factors such as small sample size, the heterogeneity of the patients and treatment protocols may limit the conclusions.15,16,19 Similarly, the non-adjusted median survival after recurrence and UP more than doubled in the sorafenib group compared to BSC group in our study. Although sorafenib group were more often on mTOR inhibitors because they were treated in the most recent years, pre- and post-transplant characteristics associated with prognosis, such as MC in or out, microvascular invasion, or time-to recurrence were comparable between sorafenib and BSC group. In addition, treatment with mTOR inhibitors was not significantly associated with prognosis in the multivariate analysis. Furthermore, initial treatment at recurrence, number of treatment until UP, and time to UP did not differ between two groups, suggesting consistent treatment policy regardless of different eras. These findings indicate that the survival difference between two groups may be mainly due to sorafenib treatment.
Meanwhile, the post-recurrence survival of sorafenib group in our study was relatively shorter than that of previous studies performed in Western transplant centers.15,16,19,20 It may be resulted from different baseline characteristics of patients. First, more than half of the patients in our study had advanced HCC beyond the MC, and the median time to recurrence after LT was shorter compared with that of previous reports, suggesting more aggressive tumor biology of our cases.21 Second, most patients (83.3%) in our study received LDLT. In the LDLT setting, the acceptable outcome might be lower than that of deceased donor LT (DDLT) as grafts are not public resources.7,22 In our institute, we use expanded criteria based on AFP, PIVKAII and PET positivity, and consider LDLT even in patients with advanced HCC if there is no other effective therapy and the expected survival/risk of recurrence after LT is acceptable in both donor and recipient.23,24 As a result, patients with advanced HCC accounted more than half of the cases in our study. Furthermore, it has been suggested that LDLT is associated with higher post-transplant HCC recurrence rate, compared to DDLT because of release of growth factors that mediate rapid regeneration after implantation, shorter waiting time and fast track selection which might preclude the detection of aggressive tumor before LT.25,26,27,28,29 Therefore, the poorer outcome of sorafenib group in our study compared to previous studies may be attributed to different baseline characteristics and unique features associated with LDLT. Nevertheless, the beneficial effect of sorafenib on survival was observed consistently even in the predominance of LDLT for HCC beyond the MC, supporting the potential role of sorafenib in post-LT HCC recurrence.
Dose reduction or withdrawn of sorafenib caused by AEs occurred only in 5 patients, and the overall AEs were acceptable with a comparable toxicity profile to the previous reports.15,16,30 Our study shows that the tolerability of sorafenib for recurrent HCC after LT is comparable to the palliative setting of non-transplant HCC.
Combination of sorafenib and mTOR inhibitors has been an interesting issue because of the potential synergistic effects by targeting different major signaling pathways involved in hepatocarcinogenesis; b-Raf and mTOR/AKT.31 Preclinical reports suggest that the combination therapy has additive efficacy compared with sorafenib alone.32,33 However, recent randomized phase II trial revealed that combination of sorafenib and everolimus did not improve OS compared with sorafenib alone in the first-line treatment of advanced HCC, although objective response favored the combination group.34 Consistent with this, combination of sorafenib and mTOR inhibitors did not significantly improve prognosis compared to sorafenib alone in our patients with post-LT HCC recurrence, although the interpretation may be limited due to the small sample size. It would be better to test the combination strategy in target-enriched populations such as those with mTOR pathway activation.
Our study has several limitations. First, it is based on retrospective observational data. The different treatment eras related to the time of introducing sorafenib might act as a historical bias in comparing the sorafenib and BSC groups. However, both cohorts received similar treatment strategy over time as shown by similar time-to-recurrence, initial patterns and treatment at recurrence, number of treatments until UP, and time-to-UP. The different immunosuppression strategy between two groups might be a potential bias. However, previous reports suggesting a benefit of mTOR inhibitor are based on uncontrolled pilot studies, and recent multi-center randomized trial showed that sirolimus did not improve long-term progression-free survival and OS compared with mTOR inhibitor-free immunosuppression in patients undergoing LT for HCC.35 Therefore, the benefit of mTOR inhibitor in post-LT HCC is still not clear and more data are awaited. Second, although this study is the largest single-center report of sorafenib for recurrent HCC following LT to our knowledge, it is based on a relatively small sample size. Because of the low incidence of post-LT HCC recurrence, multi-center, prospective cohort studies are needed to investigate this issue more in depth.
Post-transplant HCC recurrence rates may be increased gradually due to the expansion of LT in HCC. Therefore, refinement of treatment strategy regarding HCC recurrence after LT is highly relevant in clinical practice. Our data suggest the intriguing possibility of sorafenib in patients with post-transplant HCC recurrence. Furthermore, sorafenib seems to be well tolerated in post-transplant setting. Further larger, prospective studies performed in real-life cohort are warranted to validate the present results.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Cho EJ. Data curation: Kang SH, Cho H, Cho EJ. Formal analysis: Kang SH, Cho H, Cho EJ. Writing - original draft: Kang SH, Cho H, Cho EJ. Writing - review & editing: Kang SH, Cho H, Cho EJ, Lee JH, Yu SJ, Kim YJ, Yi NJ, Lee KW, Suh KS, Yoon JH.
SUPPLEMENTARY MATERIALS
Factors associated with survival after recurrence in 25 patients who presented with UP at recurrence
Factors associated with survival after recurrence in 40 patients amenable to resection or locoregional therapy at recurrence
References
- 1.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 3.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13(1):e11–22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollebecque A, Decaens T, Boleslawski E, Mathurin P, Duvoux C, Pruvot FR, et al. Natural history and therapeutic management of recurrent hepatocellular carcinoma after liver transplantation. Gastroenterol Clin Biol. 2009;33(5):361–369. doi: 10.1016/j.gcb.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Davis E, Wiesner R, Valdecasas J, Kita Y, Rossi M, Schwartz M. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2011;17(Suppl 2):S162–S166. doi: 10.1002/lt.22361. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Lee HW, Suh KS. Liver transplantation for advanced hepatocellular carcinoma. Clin Mol Hepatol. 2016;22(3):309–318. doi: 10.3350/cmh.2016.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Li H, Shi B, Que W, Wang C, Fan J, et al. Prognostic factors in patients with recurrent hepatocellular carcinoma treated with salvage liver transplantation: a single-center study. Oncotarget. 2016;7(23):35071–35083. doi: 10.18632/oncotarget.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de'Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol. 2015;21(39):11185–11198. doi: 10.3748/wjg.v21.i39.11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park MS, Lee KW, Yi NJ, Choi YR, Kim H, Hong G, et al. Optimal tailored screening protocol after living donor liver transplantation for hepatocellular carcinoma. J Korean Med Sci. 2014;29(10):1360–1366. doi: 10.3346/jkms.2014.29.10.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L, Altaca G, Guarrera JV, et al. Long-term medical complications in patients surviving > or = 5 years after liver transplant. Transplantation. 2000;69(5):781–789. doi: 10.1097/00007890-200003150-00018. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Kim YH, Yi NJ, Kim HS, Lee HS, Lee BK, et al. Impact of immunosuppressant therapy on early recurrence of hepatocellular carcinoma after liver transplantation. Clin Mol Hepatol. 2014;20(2):192–203. doi: 10.3350/cmh.2014.20.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10(4):534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 15.Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, et al. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59(1):59–66. doi: 10.1016/j.jhep.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Weinmann A, Niederle IM, Koch S, Hoppe-Lotichius M, Heise M, Düber C, et al. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Dig Liver Dis. 2012;44(5):432–437. doi: 10.1016/j.dld.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, et al. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25(2):180–186. doi: 10.1097/MEG.0b013e328359e550. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18(1):45–52. doi: 10.1002/lt.22434. [DOI] [PubMed] [Google Scholar]
- 20.de'Angelis N, Landi F, Nencioni M, Palen A, Lahat E, Salloum C, et al. Role of sorafenib in patients with recurrent hepatocellular carcinoma after liver transplantation. Prog Transplant. 2016;26(4):348–355. doi: 10.1177/1526924816664083. [DOI] [PubMed] [Google Scholar]
- 21.Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morard I, et al. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci. 2013;20(3):342–347. doi: 10.1007/s00534-012-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC) 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver. 2015;9(3):267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HW, Suh KS. Expansion of the criteria for living donor liver transplantation for hepatocellular carcinoma. Curr Opin Organ Transplant. 2016;21(2):231–237. doi: 10.1097/MOT.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 24.Hong SK, Lee KW, Kim HS, Yoon KC, Yi NJ, Suh KS. Living donor liver transplantation for hepatocellular carcinoma in Seoul National University. Hepatobiliary Surg Nutr. 2016;5(6):453–460. doi: 10.21037/hbsn.2016.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park MS, Lee KW, Suh SW, You T, Choi Y, Kim H, et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation. 2014;97(1):71–77. doi: 10.1097/TP.0b013e3182a68953. [DOI] [PubMed] [Google Scholar]
- 26.Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant. 2013;27(1):140–147. doi: 10.1111/ctr.12031. [DOI] [PubMed] [Google Scholar]
- 27.Vakili K, Pomposelli JJ, Cheah YL, Akoad M, Lewis WD, Khettry U, et al. Living donor liver transplantation for hepatocellular carcinoma: increased recurrence but improved survival. Liver Transpl. 2009;15(12):1861–1866. doi: 10.1002/lt.21940. [DOI] [PubMed] [Google Scholar]
- 28.Man K, Fan ST, Lo CM, Liu CL, Fung PC, Liang TB, et al. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg. 2003;237(2):256–264. doi: 10.1097/01.SLA.0000048976.11824.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(5) Suppl 1:S277–S282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 30.Alsina AE, Makris A, Nenos V, Sucre E, Arrobas J, Franco E, et al. Can sorafenib increase survival for recurrent hepatocellular carcinoma after liver transplantation? A pilot study. Am Surg. 2014;80(7):680–684. doi: 10.1177/000313481408000723. [DOI] [PubMed] [Google Scholar]
- 31.Bhoori S, Toffanin S, Sposito C, Germini A, Pellegrinelli A, Lampis A, et al. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010;52(5):771–775. doi: 10.1016/j.jhep.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Zhou J, Fan J, Qiu SJ, Yu Y, Huang XW, et al. Effect of rapamycin alone and in combination with sorafenib in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2008;14(16):5124–5130. doi: 10.1158/1078-0432.CCR-07-4774. [DOI] [PubMed] [Google Scholar]
- 33.Piguet AC, Saar B, Hlushchuk R, St-Pierre MV, McSheehy PM, Radojevic V, et al. Everolimus augments the effects of sorafenib in a syngeneic orthotopic model of hepatocellular carcinoma. Mol Cancer Ther. 2011;10(6):1007–1017. doi: 10.1158/1535-7163.MCT-10-0666. [DOI] [PubMed] [Google Scholar]
- 34.Koeberle D, Dufour JF, Demeter G, Li Q, Ribi K, Samaras P, et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29) Ann Oncol. 2016;27(5):856–861. doi: 10.1093/annonc/mdw054. [DOI] [PubMed] [Google Scholar]
- 35.Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation. 2016;100(1):116–125. doi: 10.1097/TP.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Factors associated with survival after recurrence in 25 patients who presented with UP at recurrence
Factors associated with survival after recurrence in 40 patients amenable to resection or locoregional therapy at recurrence