Abstract
Background
The standard of care for fit locally advanced non‐small cell lung cancer (NSCLC) patients is concurrent chemoradiotherapy (CCRT). However, in a subset of patients with lung adenocarcinoma with mutant EGFR (LA‐mEGFR), the role of EGFR‐tyrosine kinase inhibitors (TKIs) is not clear. We compared CCRT versus TKIs for the treatment of stage IIIb LA‐mEGFR in a Taiwanese population.
Methods
We identified patients from the Taiwan Cancer Registry with good performance status at clinical stage IIIb LA‐mEGFR, diagnosed from June 2011 to December 2015 and treated with either TKIs or CCRT. Clinical covariables and survival status were also collected. The Cox regression method was used in the primary analyses and several propensity score methods and alternative study cohort definitions were used in additional analyses.
Results
We compared the data of 177 TKI and 22 CCRT patients and found no statistically significant difference in overall (adjusted hazard ratio of death 0.71, 95% confidence interval 0.34–1.47) or lung cancer‐specific survival (hazard ratio 0.65, 95% confidence interval 0.31–1.35). The results of most additional analyses were insignificant.
Conclusion
In this population‐based study from Taiwan with limited case numbers, no statistical difference in the survival outcomes of patients with clinical stage IIIb LA‐mEGFR treated with either EGFR‐TKIs or CCRT was determined. Further prospective studies are needed to clarify our findings.
Keywords: Concurrent chemoradiotherapy, lung adenocarcinoma, mutant epidermal growth factor receptor, tyrosine kinase inhibitor
Introduction
Lung cancer is the leading cause of cancer death worldwide.1 Non‐small cell lung cancer (NSCLC) accounts for the majority of lung cancer cases.2 For most clinical stage IIIb (American Joint Committee on Cancer, seventh edition [AJCC 7E]) NSCLC patients with good performance status, concurrent chemoradiotherapy (CCRT) is the standard of care,3, 4 and the addition of immunotherapy might improve progression‐free survival.3, 5
However, for a specific NSCLC subpopulation with mutant EGFR (mEGFR, usually adenocarcinoma), the optimal treatment is less clear. For stage IV NSCLC with sensitizing mEGFR, upfront EGFR‐tyrosine kinase inhibitors (TKIs, such as gefitinib, erlotinib, afatinib, or osimertinib) are the current standard of care.6 However, a consensus paper in 2016 stated that there was “no data supporting the use of EGFR TKIs in patients with stage I–III disease,”7 although a recent randomized study reported improved disease‐free survival with adjuvant gefitinib after resection8 while another randomized phase II trial reported improved progression‐free survival (PFS) for TKI plus radiotherapy for unresected stage III disease compared to CCRT.9 In addition, AJCC 7E clinical stage IIIb NSCLC patients with mEGFR are eligible for TKI therapy in some modern trials.10, 11
National Health Insurance (NHI) is a single‐payer, compulsory social insurance program that provides insurance coverage to almost all citizens in Taiwan.12 The benefit package is comprehensive (inpatient, outpatient, dental services, and even traditional Chinese medicine) and all medically necessary services are covered. Gefitinib has been reimbursed since June 2011 as first‐line treatment for stage IIIb–IV lung adenocarcinoma with mEGFR. Erlotinib and afatinib have also been reimbursed for the same indication since November 2013 and May 2014, respectively. Therefore, there is a unique opportunity to compare the effectiveness of TKIs in this population. The aim of our study was to compare TKIs versus CCRT for the treatment of AJCC 7E clinical stage IIIb lung adenocarcinoma with mEGFR (LA‐mEGFR) using population‐based data in Taiwan.
Methods
Data sources
The Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC) database provided complete information from the Taiwan Cancer Registry (TCR, until 2015), death registry (until 31 December 2016), and National Health Insurance (NHI) reimbursement data (until 31 December 2016) for the whole Taiwan population, provided by the Bureau of NHI.13 The quality of the TCR was verified in 2015.14 NHI data has been used in many population‐based studies. All data are compiled by the HWDC and de‐identified. The institutional review board approved this study (CMUH107‐REC3‐006).
Study design, setting, study population, and variables
Unresected AJCC 7E clinical stage IIIb lung adenocarcinoma patients diagnosed between 2011/6 and 2015 with mEGFR and good Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1, aged ≥ 18 were included in the study.15 Patients who underwent surgery as part of their treatment before disease progression, received palliative treatment, or had other cancer(s) within three years were excluded. Two treatment strategies were included for comparison: TKIs (gefitinib, erlotinib, or afatinib) and CCRT with platinum‐based chemotherapy and at least a 50 Gy radiotherapy dose (see Appendix A in supplementary data for the working definition). The threshold of 50 Gy was chosen based on a systemic review.16
The study outcomes were overall survival (OS) and lung cancer‐specific survival (LCSS), as identified by the death registry. We also identified patient demographics (age, gender, and residency region), disease factors (subtypes of stage IIIb), patient characteristics (comorbidity and smoking history), and diagnostic approach (use of positron emission tomography [PET]) as potential confounders. These confounders were selected and modified as per our experience in clinical practice and TCR/NHI related studies, and were defined as follows.17, 18, 19, 20 Patient residency was classified as northern Taiwan or elsewhere. Stage was classified as T3–4/N3 versus others according to AJCC 8th edition (stage IIIc vs. IIIb). Smoking history was classified as yes or no. Comorbidity was defined as with or without a modified Carlson comorbidity score ≥ 1. Use of PET (time window: 2 months before to 4 months after diagnosis) was classified as yes or no.21
Statistical methods and additional analyses
We used a log‐rank test and the Kaplan–Meier method for primary survival analyses. We then used the Cox regression model to include the potential confounders to reduce any bias. We calculated the E‐value in sensitivity analysis, as suggested by the literature, to evaluate the impact of potentially unmeasured confounder(s).22 We performed several additional analyses (AA). In the first and second AA, we used the propensity score (PS) method, as advocated in the literature, to balance the measured potential confounders to examine the robustness of our findings.23, 24 We used the covariables in the PS model via various methods (traditional logistic regression models, as well as machine learning, such as neural network [NN] or random forest [RF]) to estimate the possible PS value.25, 26, 27, 28 We then evaluated the covariate balance via standardized difference, as suggested by several review papers.25, 29, 30, 31 Finally, we used two methods: inverse probability weighting (IPW in the first AA) and PS matching (PSM, 1:1 paired matching in the second AA) to evaluate the effectiveness of TKI versus CCRT.24 In the 3rd–5th, AA we evaluated the impact of alternative study cohort definitions. In the third AA, we evaluated the impact of modifying patient age (< 75), as patients at older age are suggested as being ineligible for CCRT.32 In the fourth AA, we evaluated the impact of modifying the working definition if a radiotherapy dose of at least 60 Gy was required.3 In the fifth AA, we evaluated the impact if TKIs were limited to gefitinib only. In addition to the five AA conducted, we also evaluated subsequent treatments in the groups. We used SAS 9.4 (SAS Institute Inc., Cary, NC, USA), STATA/IC 11 (Stata Corp, College Station, TX, USA), and R 3.5.0 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria) to implement all analyses.
Results
Study population and descriptive data
Our study flowchart, as suggested by the STROBE guideline, is depicted in Figure 1.33 In brief, 199 (TKI 177, CCRT 22) unresected clinical stage IIIb LA‐mEGFR patients with good ECOG PS were included in our study (Table 1). The TKI group were older, more likely to be female, living in south Taiwan, and at T3–4N3 stage and less likely to be smokers than the CCRT group, although the results were only statistically significant for age, residency region, and smoking.
Figure 1.
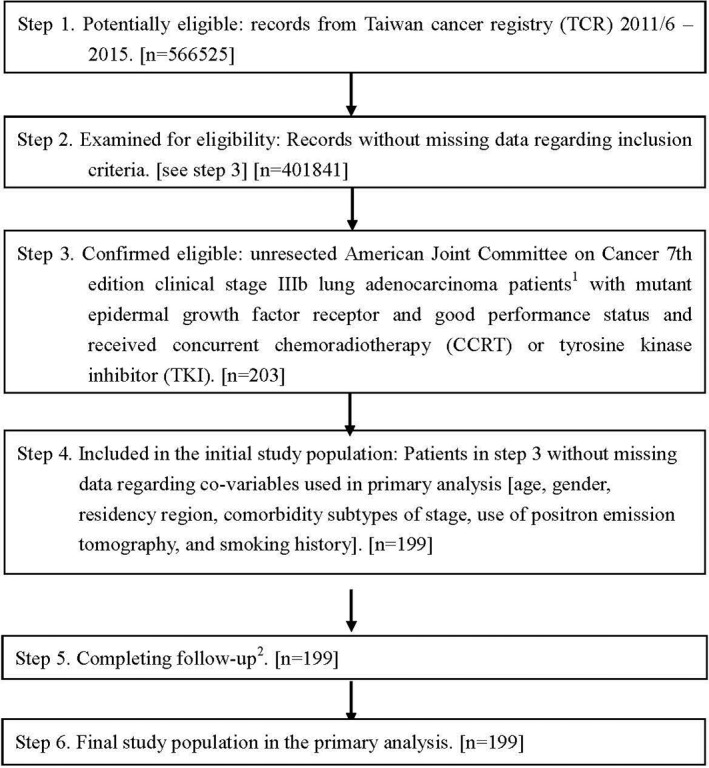
STROBE study flowchart and numbers of individuals at each stage of study. 1We only included those treated (class 1–2) at any single institution to ensure data consistency. 2Although patients with incomplete information were excluded from Step 4, 199 patients remained for Step 5.
Table 1.
Characteristics of the study population in primary analysis
| CCRT | TKI | ||
|---|---|---|---|
| Characteristic | Number (%)‡ or mean (SD) | Number (%)‡ or mean (SD) | P ‡ |
| Age | 60.14 (7.78) | 70.16 (10.90) | < 0.001 |
| Gender | |||
| Female | 12 (55) | 110 (62) | 0.49 |
| Male | 10 (45) | 67 (38) | |
| Residency region | |||
| Non‐north | 3 (14) | 107 (60) | < 0.001 |
| North | 19 (86) | 70 (40) | |
| Comorbidity | |||
| Without | 10 (45) | 75 (42) | 0.78 |
| With† | 12 (55) | 102 (58) | |
| Smoking history | |||
| Without | 11 (50) | 131 (74) | 0.019 |
| With | 11 (50) | 46 (26) | |
| Sub‐types of stage IIIb | |||
| T3–4N3 | 6 (27) | 68 (38) | 0.31 |
| Others | 16 (73) | 109 (62) | |
| Use of PET | |||
| Without | 11 (50) | 89 (50) | 0.98 |
| With | 11 (50) | 88 (50) |
Modified Carlson comorbidity score ≥ 1.
Rounded.
CCRT, concurrent chemoradiotherapy; PET, positron emission tomography; SD, standard deviation; TKI, tyrosine kinase inhibitor.
Outcome and results in primary analysis
After a median follow‐up of 23 months (range: 1–64), 90 patients had died (80 in the TKI and 10 in the CCRT group). The Kaplan–Meier OS curve is shown in Figure 2. There were no statistically significant differences between the groups (log rank test P = 0.51]. The three‐year OS rates for the TKI and CCRT groups were 55% and 60%, respectively. There was also no statistically significant difference after adjusting for potential confounders. The adjusted hazard ratio (HR) of death with a 95% confidence interval (CI) was 0.71 (0.34–1.47) for TKI versus CCRT (Table 2). The observed HR of 0.71 could be explained by an unmeasured confounder that was associated with both selections of treatment and life/death by a risk ratio of 1.85‐fold each (E‐value), but weaker confounding could not do so. The results for LCSS (HR 0.65, 95% CI 0.31–1.35) were not significant.
Figure 2.
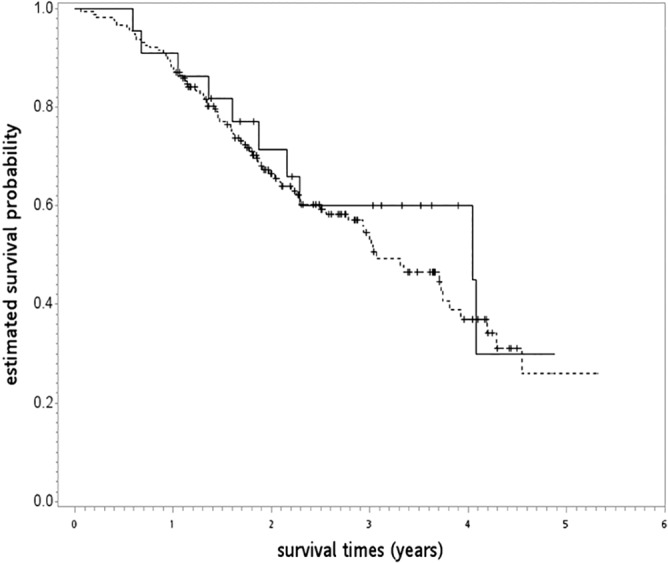
Kaplan–Meier overall survival curve (in years) in primary analysis. ( ) CCRT, concurrent chemoradiotherapy, (
) CCRT, concurrent chemoradiotherapy, ( ) TKI, tyrosine kinase inhibitors.
) TKI, tyrosine kinase inhibitors.
Table 2.
Survival analysis via Cox regression
| Characteristic | HR (95% CI)‡ | P ‡ |
|---|---|---|
| Age | 1.03 (1.01–1.06) | 0.009 |
| Gender | ||
| Female (reference) | ||
| Male | 1.12 (0.65–1.94) | 0.68 |
| Residency region | ||
| Non‐north (reference) | ||
| North | 0.73 (0.46–1.16) | 0.18 |
| Comorbidity | ||
| Without (reference) | ||
| With† | 1.01 (0.64–1.60) | 0.96 |
| Smoking history | ||
| Without (reference) | ||
| With | 0.73 (0.39–1.37) | 0.33 |
| Treatment | ||
| CCRT (reference) | ||
| TKI | 0.71 (0.34–1.47) | 0.36 |
| Subtypes of stage IIIb | ||
| T3–4N3 (reference) | ||
| Others | 0.51 (0.33–0.80) | 0.003 |
| Use of PET | ||
| Without (reference) | ||
| With | 0.73 (0.48–1.11) | 0.14 |
Modified Carlson comorbidity score ≥ 1.
Rounded.
CCRT, concurrent chemoradiotherapy; CI, confidence interval; HR, hazard ratio; PET, positron emission tomography; TKI, tyrosine kinase inhibitor.
Additional analyses
In the first AA, two of the seven covariables were well balanced (i.e. standardized difference [SDif] ≤ 0.1) before IPW (Table 3).29, 30 The IPW via RF‐based PS could achieve a better (but still not optimal) covariable balance in that three covariables could be well‐balanced and five could be moderately balanced (i.e. SDif < 0.25),26 whereas only one and five of the seven covariables could be well and moderately balanced, respectively, by LR‐based PS. Because RF but not NN achieved a better covariable balance, we only used RF to represent the results using machine learning in the following analyses. There was no statistically significant difference between TKI and CCRT, except when using RF approaches. The adjusted HR of death was 0.49 (95% CI 0.34–0.70; P < 0.0001) for IPW via RF and 1.57 (95% CI 0.74–3.31; P = 0.24) for IPW via LR. In the second AA, the covariable balance was better (but still not optimal) in the LR‐based (n = 30) than the RF‐based PSM (n = 14) in that two and six of the seven covariables could be well and moderately balanced, respectively, by LR, whereas only three and four of the seven covariables could be well and moderately balanced by RF (Table 4). There was still no statistically significant difference between TKI and CCRT. The adjusted HR of death was 1.23 (95% CI 0.32–4.67; P= 0.77) for LR‐based and 0.27 (95% CI 0.03–2.45; P = 0.25) for RF‐based PSM.
Table 3.
Covariable balance diagnostics: Before and after IPW
| Standardized difference† | ||||
|---|---|---|---|---|
| Characteristic | Pre‐IPW | IPW via LR | IPW via NN | IPW via RF |
| Age | 1.059 | 0.276 | 1.060 | 0.167 |
| Gender | 0.155 | 0.198 | 0.155 | 0.055 |
| Residency region | 1.108 | 0.106 | 1.109 | 0.381 |
| Comorbidity | 0.062 | 0.231 | 0.063 | 0.463 |
| Smoking history | 0.511 | 0.031 | 0.511 | 0.169 |
| Subtypes of stage IIIb | 0.239 | 0.240 | 0.238 | 0.089 |
| Use of PET | 0.006 | 0.317 | 0.007 | 0.031 |
Rounded.
IPW, inverse probability weighting; LR, logistic regression; NN, neural network; PET, positron emission tomography; RF, random forest.
Table 4.
Characteristics of the matched study population in the second additional analyses
| LR‐based PSM (n = 30) | RF‐based PSM (n = 14) | ||||||
|---|---|---|---|---|---|---|---|
| CCRT | TKI | Standardized difference‡ | CCRT | TKI | Standardized difference‡ | ||
| Characteristic | Number (%)‡ or mean (SD) | Number (%)‡ or mean (SD) | Number (%)‡ or mean (SD) | Number (%)‡ or mean (SD) | |||
| Age | 62.93 (6.33) | 60.47 (13.31) | 0.237 | 54.43 (7.81) | 55.86 (7.49) | 0.187 | |
| Gender | |||||||
| Female | 9 (60) | 8 (53) | 0.135 | § | § | 0.302 | |
| Male | 6 (40) | 7 (47) | § | § | |||
| Residency region | |||||||
| Non‐north | 3 (20) | 4 (27) | 0.158 | § | § | ||
| North | 12 (80) | 11 (73) | § | § | |||
| Comorbidity | |||||||
| Without | 8 (53) | 6 (40) | 0.270 | 3 (43) | 3 (43) | 0 | |
| With† | 7 (47) | 9 (60) | 4 (57) | 4 (57) | |||
| Smoking history | |||||||
| Without | 9 (60) | 9 (60) | 0 | § | § | 0.354 | |
| With | 6 (40) | 6 (40) | § | § | |||
| Subtypes of stage IIIb | |||||||
| T3–4N3 | 6 (40) | 6 (40) | 0 | § | § | 0 | |
| others | 9 (60) | 9 (60) | § | § | |||
| Use of PET | |||||||
| Without | 7 (47) | 8 (53) | 0.134 | 4 (57) | 4 (57) | 0 | |
| With | 8 (53) | 7 (47) | 3 (43) | 3 (43) | |||
Modified Carlson comorbidity score ≥ 1.
Rounded.
Exact numbers are not reported because the Health and Welfare Data Science Center database center policy is to avoid numbers in single cells ≤ 2.
CCRT, concurrent chemoradiotherapy; LR, logistic regression; PET, positron emission tomography; PSM, propensity‐score matching; RF, random forest; SD, standard deviation; TKI, tyrosine kinase inhibitor.
In the third AA, the result was robust to the modified age criteria (age < 75) as the adjusted HR of death was not statistically different (1.03, 95% CI 0.47–2.29) for 111 TKI versus 21 CCRT patients. In the fourth AA, the result was also robust to the treatment criteria modification (at least 60 Gy instead of 50 Gy for CCRT) as the adjusted HR of death was not statistically different (HR 0.77, 95% CI 0.36–1.63) for 177 TKI versus 21 CCRT patients. In the fifth AA, our results were similar to the treatment criteria modification (including only gefitinib) as the adjusted HR of death was 0.83 (95% CI 0.39–1.76) for 112 TKI versus 22 CCRT patients.
Regarding subsequent treatment, 83 of 177 TKI patients received platinum‐based chemotherapy or radiotherapy, whereas most (as per HWDC policy, numbers ≤ 2 cannot be reported) of the 22 CCRT patients received TKIs. For the 10 patients diagnosed in 2011, almost all (as per HWDC policy, the exact number cannot be reported) patients in the TKI group received subsequent platinum‐based chemotherapy or radiotherapy, whereas all patients in the CCRT group received subsequent TKIs.
Discussion
In this population‐based study from Taiwan, the survival outcome of AJCC 7E clinical stage IIIb LA‐mEGFR patients was not statistically different between those treated with EGFR‐TKIs or CCRT. To our knowledge, this is the first study to compare the outcomes of these two treatments in LA‐mEGFR patients.
The most relevant study we located was a 2013 Japanese study in which the treatment strategies of 49 NSCLC patients with mEGFR and inoperable stage III/IV disease were compared. EGFR‐TKIs (n = 32) achieved tumor shrinkage earlier than radiotherapy (n = 17), although no statistically significant difference in PFS was found (295 for TKI vs. 273 days for RT).34 However, all TKI patients had stage IV disease, while 94% patients in the radiotherapy group (88% combined with chemotherapy) had stage III disease.
The results of our study are comparable with those published in the literature. In the CCRT group, our five‐year OS result was approximately 30% (at 4.88/year) (Fig 2). A phase III trial reported five‐year OS rates in stage III NSCLC patients treated with CCRT of 23% to 32% (for 74 Gy and 60 Gy, respectively).35, 36 Furthermore, longer survival was reported in a 2017 study of locally advanced NSCLC patients with mEGFR (vs. wild EGFR) treated with CCRT,37 although the prognostic significance of mEGFR on recurrence was debated in a review paper published in 2016.38 In the TKI group, our five‐year OS result was 26%, close to the 25% estimated in a recent systematic review (see Appendix B).39
Obviously there were many limitations to our study. First, as a non‐randomized study, treatment decisions (CCRT or TKI) were made by the physician in charge and were not randomized; therefore, the treatment groups might be unbalanced in potential confounder(s), although we used conventional regression as well as the PS method in our analyses. However, possible unmeasured confounder(s), such as weight loss, tumor burden, or EGFR mutation subtypes were not available in our study. Second, our study sample was quite small, particularly in the CCRT group, and thus was not powered to investigate the difference in survival in TKI versus CCRT groups, especially with major confounders and imbalance in the numbers in both arms. Third, AJCC 8th edition cancer staging has been used since 2018, which is slightly different to AJCC 7E. However, most of the scenarios of AJCC 8E stage IIIb–IIIc are compatible with AJCC 7E stage IIIb (see Appendix C). Therefore, our results could be largely applied to AJCC 8E clinical stage IIIb–IIIc. Finally, other important endpoints, such as PFS or quality of life, were not investigated because of data limitations.
Given these limitations, our findings are not conclusive but we have provided a rationale to consider upfront TKI alone as a treatment alternative for locally advanced NSCLC with mEGFR, until the results of prospective clinical trials are available. However, when we searched http://www.clinicaltrials.gov/ using the keywords “condition/disease: lung cancer stage III & intervention/treatment: gefitinib (or erlotinib, afatinib, osimertinib)” on 24 July 2018 (66 trials: gefitinib [n = 19], erlotinib [n = 38], afatinib [n = 4], osimertinib [n = 5]), we did not find any current randomized studies comparing TKI versus CCRT for locally advanced NSCLC with mEGFR, although we did find the abovementioned randomized phase II trial comparing TKI plus radiotherapy versus CCRT, which reported improved PFS with TKI plus radiotherapy.9
In this limited sample population‐based study from Taiwan, there was no statistically significant difference in survival outcomes of AJCC 7E clinical stage IIIb LA‐mEGFR patients treated with either EGFR‐TKIs or CCRT. Further prospective studies are required to clarify our findings.
Disclosure
No authors report any conflict of interest.
Supporting information
Appendix A. Working definitions of tyrosine kinase inhibitors (TKI) or concurrent chemoradiotherapy (CCRT).
Appendix B. Estimation of five‐year survival of stage IIIb cases.
Appendix C. Scenarios of AJCC 8th versus AJCC 7th (including AJCC 8th IIIb–IIIc or AJCC 7th IIIb).
Acknowledgments
This work was supported by the Ministry of Science and Technology (MOST 107‐2314‐B‐039‐026‐). The Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan provided the data analyzed in this study. The corresponding author would like to thank Dr. Ya Chen Tina Shih as a mentor in health services research and the suggestions made for this study.
References
- 1. International Agency for Research on Cancer, World Health Organization . The Global Cancer Observatory. [Cited 15 Mar 2018.] Available from URL: https://gco.iarc.fr
- 2. Johnson DH, Schiller JH, Bunn PA Jr. Recent clinical advances in lung cancer management. J Clin Oncol 2014; 32: 973–82. (Published erratum appears in J Clin Oncol 2014; 32: 1520). [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network (NCCN) Guidelines for non‐small cell lung cancer. Version 9. 2017. [Cited 16 Nov 2017.] Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 4. Bayman N, Blackhall F, McCloskey P, Taylor P, Faivre‐Finn C. How can we optimise concurrent chemoradiotherapy for inoperable stage III non‐small cell lung cancer? Lung Cancer 2014; 83: 117–25. [DOI] [PubMed] [Google Scholar]
- 5. Antonia SJ, Villegas A, Daniel D et al Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med 2017; 377: 1919–29. [DOI] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network (NCCN) Guidelines for non‐small cell lung cancer, Version 1. 2018. [Cited 22 Nov 2017] Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 7. Tan DS, Yom SS, Tsao MS et al The International Association for the Study of Lung Cancer consensus statement on optimizing management of EGFR mutation‐positive non‐small cell lung cancer: Status in 2016. J Thorac Oncol 2016; 11: 946–63. [DOI] [PubMed] [Google Scholar]
- 8. Zhong WZ, Wang Q, Mao WM et al Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR‐mutant NSCLC (ADJUVANT/CTONG1104): A randomised, open‐label, phase 3 study. Lancet Oncol 2018; 19: 139–48. [DOI] [PubMed] [Google Scholar]
- 9. Xing L, Wu G, Wang L et al A multicenter, randomized, open‐label, phase II trial of erlotinib versus etoposide plus cisplatin with concurrent radiotherapy in unresectable stage III non‐small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) activating mutation. J Clin Oncol 2017; 35 (15 Suppl): 8531. [Google Scholar]
- 10. Wu YL, Zhou C, Liam CK et al First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: Analyses from the phase III, randomized, open‐label, ENSURE study. Ann Oncol 2015; 26: 1883–9. [DOI] [PubMed] [Google Scholar]
- 11. Shi YK, Wang L, Han BH et al First‐line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation‐positive lung adenocarcinoma (CONVINCE): A phase 3, open‐label, randomized study. Ann Oncol 2017; 28: 2443–50. [DOI] [PubMed] [Google Scholar]
- 12. National Health Insurance and Administration, Ministry of Health and Welfare . Universal Health Coverage in Taiwan [Cited 10 Mar 2018.] Available from URL: https://www.nhi.gov.tw/Resource/webdata/21717_1_UnversalHealthCoverage-2.pdf
- 13. Ministry of Health and Welfare . [The Health and Welfare Data Science Center Database] (In Chinese.) [Cited 10 Mar 2018.] Available from URL: http://dep.mohw.gov.tw/DOS/np-2497-113.html
- 14. Chiang CJ, You SL, Chen CJ, Yang YW, Lo WC, Lai MS. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn J Clin Oncol 2015; 45: 291–6. [DOI] [PubMed] [Google Scholar]
- 15. Reeves BC, Wells GA, Waddington H. Quasi‐experimental study designs series‐paper 5: A checklist for classifying studies evaluating the effects on health interventions‐a taxonomy without labels. J Clin Epidemiol 2017; 89: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown T, Pilkington G, Boland A et al Clinical effectiveness of first‐line chemoradiation for adult patients with locally advanced non‐small cell lung cancer: A systematic review. Health Technol Assess 2013; 17: 1–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tu CY, Hsia TC, Fang HY et al A population‐based study of the effectiveness of stereotactic ablative radiotherapy versus conventional fractionated radiotherapy for clinical stage I non‐small cell lung cancer patients. Radiol Oncol 2017; 52: 181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsia TC, Tu CY, Fang HY, Liang JA, Li CC, Chien CR. Cost and effectiveness of image‐guided radiotherapy for non‐operated localized lung cancer: A population‐based propensity score‐matched analysis. J Thorac Dis 2015; 7: 1643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chien CR, Hsia TC, Chen CY. Cost‐effectiveness of chemotherapy combined with thoracic radiotherapy versus chemotherapy alone for limited stage small cell lung cancer: A population‐based propensity‐score matched analysis. Thorac Cancer 2014; 5: 530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin CY, Fang HY, Feng CL, Li CC, Chien CR. Cost‐effectiveness of neoadjuvant concurrent chemoradiotherapy versus esophagectomy for locally advanced esophageal squamous cell carcinoma: A population‐based matched case‐control study. Thorac Cancer 2016; 7: 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dinan MA, Curtis LH, Carpenter WR et al Redistribution of health care costs after the adoption of positron emission tomography among medicare beneficiaries with non‐small‐cell lung cancer, 1998–2005. J Thorac Oncol 2014; 9: 512–8. [DOI] [PubMed] [Google Scholar]
- 22. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E‐value. Ann Intern Med 2017; 167: 268–74. [DOI] [PubMed] [Google Scholar]
- 23. Jagsi R, Bekelman JE, Chen A et al Considerations for observational research using large data sets in radiation oncology. Int J Radiat Oncol Biol Phys 2014; 90: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33: 1242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ali MS, Groenwold RH, Belitser SV et al Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: A systematic review. J Clin Epidemiol 2015; 68: 112–21. [DOI] [PubMed] [Google Scholar]
- 26. Matloff N. Statistical Regression and Classification: From Linear Models to Machine Learning. Chapman & Hall/CRC, New York: 2017. [Google Scholar]
- 27. Jackson JW, Schmid I, Stuart EA. Propensity scores in Pharmacoepidemiology: Beyond the horizon. Curr Epidemiol Rep 2017; 4: 271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deist TM, Dankers FJWM, Valdes G et al Machine learning algorithms for outcome prediction in (chemo)radiotherapy: An empirical comparison of classifiers. Med Phys 2018; 45: 3449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garrido MM, Kelley AS, Paris J et al Methods for constructing and assessing propensity scores. Health Serv Res 2014; 49: 1701–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yao XI, Wang X, Speicher PJ et al Reporting and guidelines in propensity score analysis: A systematic review of cancer and cancer surgical studies. J Natl Cancer Inst 2017; 109 (8): djw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34: 3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Ruysscher D, Botterweck A, Dirx M et al Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: A prospective, population‐based study. Ann Oncol 2009; 20: 98–102. [DOI] [PubMed] [Google Scholar]
- 33. von Elm E, Altman DG, Egger M et al The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–9. [DOI] [PubMed] [Google Scholar]
- 34. Imai H, Shukuya T, Takahashi T et al Comparison of the time‐to‐response between radiotherapy and epidermal growth factor receptor‐‐tyrosine kinase inhibitors for advanced non‐small cell lung cancer with EGFR mutation. Anticancer Res 2013; 33: 3279–84. [PubMed] [Google Scholar]
- 35. Senan S, Rusthoven CG, Slotman BJ, Siva S. Progress in radiotherapy for regional and oligometastatic disease in 2017. J Thorac Oncol 2018; 13: 488–96. [DOI] [PubMed] [Google Scholar]
- 36. Bradley JD, Hu C, Komaki RU et al Long‐term results of RTOG 0617: A randomized phase 3 comparison of standard dose versus high dose conformal chemoradiation therapy ± cetuximab for stage III NSCLC. Int J Radiat Oncol Biol Phys 2017; 99 (Suppl 2): S105. [Google Scholar]
- 37. Ishihara M, Igawa S, Sasaki J et al Evaluation of concurrent chemoradiotherapy for locally advanced NSCLC according to EGFR mutation status. Oncol Lett 2017; 14: 885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ochiai S, Nomoto Y, Watanabe Y et al The impact of epidermal growth factor receptor mutations on patterns of disease recurrence after chemoradiotherapy for locally advanced non‐small cell lung cancer: A literature review and pooled analysis. J Radiat Res 2016; 57: 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee CK, Davies L, Wu YL et al Gefitinib or erlotinib vs chemotherapy for EGFR mutation‐positive lung cancer: Individual patient data meta‐analysis of overall survival. J Natl Cancer Inst 2017; 109 (6): djw279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Working definitions of tyrosine kinase inhibitors (TKI) or concurrent chemoradiotherapy (CCRT).
Appendix B. Estimation of five‐year survival of stage IIIb cases.
Appendix C. Scenarios of AJCC 8th versus AJCC 7th (including AJCC 8th IIIb–IIIc or AJCC 7th IIIb).


