Abstract
Background
The importance of nutritional status and chronic inflammation has been emphasized in cancer. We investigated the impact of Onodera's prognostic nutritional index (OPNI) on clinical outcomes in small cell lung cancer (SCLC) patients.
Methods
Data from 220 SCLC patients treated with first‐line platinum‐based chemotherapy from 2006 to 2017 were retrospectively reviewed. The OPNI was calculated as 10 × serum albumin level (g/dL) + 0.005 × absolute lymphocyte count (/mm3). Patients with an OPNI of > 45, 40–45, or < 40 were categorized in high, intermediate, or low OPNI groups, respectively.
Results
The proportion of non‐responders to first‐line therapy increased as the OPNI decreased (high, intermediate, low OPNI groups: 6.7%, 18.0%, and 30.8%, respectively; P < 0.001). Early discontinuation of first‐line therapy because of treatment toxicity occurred more frequently in the lower OPNI groups (high, intermediate, low OPNI groups: 5.8%, 21.3%, and 25.6%, respectively; P < 0.001). The one‐year progression‐free and overall survival rates in the high, intermediate, and low OPNI groups were 29%, 19%, and 3%, and 61%, 46%, and 23%, respectively. In multivariate analyses, the low OPNI group was independently associated with poor progression‐free (hazard ratio 1.592; 95% confidence interval 1.009–2.511; P = 0.046) and overall (hazard ratio 1.911; 95% confidence interval 1.208–3.024; P = 0.006) survival compared to the high OPNI group.
Conclusion
SCLC patients with an OPNI < 40 showed a low tolerance to chemotherapy and a poor prognosis. Further evaluation is needed to validate these findings.
Keywords: Cachexia, lymphocyte, nutrition assessment, serum albumin, small cell lung carcinoma
Introduction
Small cell lung cancer (SCLC), characterized by rapid growth, early widespread metastasis, and frequent paraneoplastic syndromes,1 accounts for approximately 13% of all lung cancers.2 Over the last 30 years, platinum‐based doublet chemotherapy with and without concurrent thoracic radiotherapy has been the standard first‐line therapy for limited‐stage (LD) and extensive‐stage (ED) SCLC, respectively.3, 4 Traditionally, a poor performance status (PS), ED, old age, and an elevated serum lactate dehydrogenase (LDH) level are associated with a poor prognosis in SCLC patients.5, 6 However, novel pre‐treatment prognostic factors are still required to identify those patients who will benefit from first‐line therapy and those who require adjustment of their chemotherapy dose.
The importance of nutritional status has been emphasized in cancer. A number of studies have indicated that malnutrition is associated with intolerance to chemotherapy, decreased quality of life, greater psychological distress, and poor survival in patients with cancer.7, 8, 9 Among several markers of nutritional status, Onodera's prognostic nutritional index (OPNI) has recently been considered useful because of its simplicity and clinical implications. The OPNI was initially designed to assess the risk of postoperative complications in gastrointestinal surgical patients using the serum albumin level and absolute lymphocyte count.10 Researchers have since reported relationships between the OPNI and clinical outcomes of patients with various types of cancer.11, 12, 13, 14 One study reported that the risk of death in SCLC patients with a low OPNI was increased by 40% compared to those with a high OPNI.15 However, the authors only reported associations of the OPNI with baseline clinicopathologic factors and overall survival (OS).
In the present study, we investigated the prognostic value of the OPNI in patients with SCLC treated with first‐line platinum‐based chemotherapy. To understand how the OPNI affects the prognosis of these patients, we assessed their response to first‐line therapy, treatment‐related toxicities, and treatment compliance.
Methods
Patients
After the institutional review board approved this study, we retrospectively reviewed the data of all consecutive SCLC patients recorded in the electronic medical record system between July 2006 and February 2017 at the Gyeongsang National University Hospital. Among them, 238 patients treated with platinum‐based chemotherapy as first‐line regimen without active infection at the time of chemotherapy initiation were screened. Eighteen patients were excluded from the study for the following reasons: chemotherapy commenced at another hospital (n = 11), inconsistent histology results (n = 3), a lack of clinical information (n = 2), and either no treatment or treatment with a non‐platinum‐based regimen (n = 2). Finally, a total of 220 patients were included in the study.
Assessments
Baseline assessments included demographics, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), tumor stage, treatment history, complete blood count (CBC), and serum chemistry including albumin and LDH levels. The baseline laboratory values measured closest to the date of chemotherapy initiation were recorded. The mean interval from the measurement of the CBC and serum albumin level to the initiation of chemotherapy was 1.7 ± 1.9 days (median 1 day; range: 0–12 days). Serum LDH was not measured in 63 patients within two weeks before chemotherapy initiation, and thus their LDH baseline values were considered missing. Response Evaluation Criteria in Solid Tumors version 1.1 via computed tomography was used to assess tumor response to the first‐line regimen. Grade ≥ 3 adverse events resulting from the first‐line chemotherapy were evaluated by National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Treatment‐related mortality (TRM) was defined as death as a probable or possible result of chemotherapy‐related toxicity during the treatment period or within 30 days of the last dose of chemotherapy. Premature cessation of the scheduled treatment because of chemotherapy‐related toxicity was referred to as early discontinuation of treatment. Additional dose reductions because of chemotherapy‐related toxicity were also reviewed. The OPNI was calculated as follows: OPNI = 10 × serum albumin level (g/dL) + 0.005 × absolute lymphocyte count (/mm3). As originally designed, patients with an OPNI of > 45, 40–45, or < 40 were categorized in the high, intermediate, or low OPNI groups, respectively.10
Statistical analysis
The chi‐squared test for trend and Spearman's rank correlation were used to assess the relationships between the OPNI group and categorical variables and between the OPNI group and continuous variables, respectively. The reverse Kaplan–Meier method was used to estimate the median follow‐up duration. Progression‐free survival (PFS) was defined as the interval from chemotherapy initiation to either the first progression, death from any cause, or the final follow‐up. OS was defined as the interval from chemotherapy initiation to either death from any cause or the final follow‐up. Survival curves were plotted using the Kaplan–Meier method and compared using the log‐rank test. All variables with a P value < 0.20 in univariate analyses were included in multivariate analysis, using the Cox regression model and the enter selection method. Variables with a missing value were excluded from multivariate analyses. A P value < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using Stata version 14.1 (Stata Corp., College Station, TX, USA).
Results
Study population
Of the 220 total patients, 120 were assigned to the high OPNI group, 61 to the intermediate, and 39 to the low. The OPNI ranged from 28.0 to 65.2. The patient characteristics according to the OPNI are described in Table 1. The overall median age was 68 years (range: 43–86). The majority of patients were male and smokers, without significant differences among the three OPNI groups. A higher OPNI level was significantly associated with a poor ECOG PS, advanced stage, and high LDH level (all P < 0.001). The most commonly used regimen was etoposide/cisplatin. The low OPNI group more frequently received alternative regimens, such as etoposide/carboplatin and irinotecan/cisplatin, compared to the high OPNI group (P = 0.005). The treatment response to the first‐line regimen was worse as the OPNI decreased, regardless of tumor stage (P < 0.001, P = 0.004, and P = 0.022 in total, LD, and ED, respectively). None of the patients in the low OPNI group showed a complete response and 30.8% (12/39) did not achieve an objective response. After excluding the patients prematurely withdrawn from treatment because of treatment toxicity (n = 30) and those who declined further treatment (n = 13), the proportion of patients who did not respond to the first‐line regimen was highest in the low OPNI group (high, intermediate, and low OPNI groups: 5.6%, 13.6%, and 26.9%, respectively; P < 0.001). Given the advanced stage and worse treatment response, the low thoracic radiotherapy and prophylactic cranial irradiation rates seen in the low OPNI group might be expected.
Table 1.
Patient characteristics and treatment response
| Factor | OPNI > 45 (n = 120) | OPNI 40–45 (n = 61) | OPNI < 40 (n = 39) | P |
|---|---|---|---|---|
| OPNI | ||||
| Median | 49.0 | 43.2 | 37.2 | |
| Mean (± std dev) | 50.5 (± 4.4) | 42.9 (± 1.5) | 37.1 (± 2.4) | < 0.001 |
| Range | 45.2–65.2 | 40.0–45.0 | 28.0–39.9 | |
| Age, years | ||||
| Median | 68 | 70 | 66 | |
| Mean (± SD) | 66.4 (± 8.4) | 68.9 (± 7.7) | 66.6 (±7.3) | 0.475 |
| Range | 43–85 | 48–86 | 49–82 | |
| Sex | 0.292 | |||
| Male | 109 (90.8) | 50 (82.0) | 34 (87.2) | |
| Female | 11 (9.2) | 11 (18.0) | 5 (12.8) | |
| Smoking | 0.142 | |||
| Non‐smoker | 2 (1.7) | 5 (8.2) | 2 (5.1) | |
| Smoker | 118 (98.3) | 56 (91.8) | 37 (94.9) | |
| ECOG PS | < 0.001 | |||
| 0–1 | 99 (82.5) | 35 (57.4) | 19 (48.7) | |
| 2–3 | 21 (17.5) | 26 (42.6) | 20 (51.3) | |
| Stage | < 0.001 | |||
| LD | 62 (51.7) | 22 (36.1) | 6 (15.4) | |
| ED | 58 (48.3) | 39 (63.9) | 33 (84.6) | |
| LDH, U/L (n = 157) | ||||
| Median | 227 | 269 | 309 | |
| Mean ± std dev | 289 ±255 | 298 ±136 | 478 ±443 | < 0.001 |
| Elevated | 45 (51.7) | 24 (63.2) | 24 (75.0) | 0.019 |
| First‐line regimen | 0.005 | |||
| EP | 93 (77.5) | 39 (63.9) | 22 (56.4) | |
| EC | 21 (17.5) | 18 (29.5) | 12 (30.8) | |
| IP | 6 (5.0) | 4 (6.6) | 5 (12.8) | |
| Thoracic RT | < 0.001 | |||
| Yes | 65 (54.2) | 20 (32.8) | 5 (12.8) | |
| No | 55 (45.8) | 41 (67.2) | 34 (87.2) | |
| Prophylactic cranial irradiation | < 0.001 | |||
| Yes | 57 (47.5) | 23 (37.7) | 4 (10.3) | |
| No | 63 (52.5) | 38 (62.3) | 35 (89.7) | |
| Response to first‐line regimen | ||||
| (Total / in LD / in ED) | < 0.001/0.004/0.022 | |||
| CR | 20 (16.7) / 19 (30.7) / 1 (1.7) | 3 (4.9) / 3 (13.6) / 0 (0.0) | 0 (0.0) / 0 (0.0) / 0 (0.0) | |
| PR | 92 (76.7) / 42 (67.7) / 50 (86.2) | 47 (77.1) / 18 (81.8) / 29 (74.4) | 27 (69.2) / 4 (66.7) / 23 (69.7) | |
| SD, PD, and NA | 8 (6.7) / 1 (1.6) / 7 (12.1) | 11 (18.0) / 1 (4.6) / 10 (25.6) | 12 (30.8) / 2 (33.3) / 10 (30.3) | |
Bold font indicates significant P values. Categorical variables are presented as number of patients (%).
CR, complete response; EC, etoposide/carboplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; ED, extensive‐stage; EP, etoposide/cisplatin; IP, irinotecan/cisplatin; LD, limited‐stage; LDH, lactate dehydrogenase; NA, not available; OPNI, Onodera's Prognostic Nutritional Index; PD, progressive disease; PR, partial response; RT, radiotherapy; SD, stable disease; std dev, standard deviation.
Treatment‐related toxicity and compliance
Table 2 presents the details of the treatment‐related toxicities and compliance with the first‐line regimen according to the OPNI. As the OPNI decreased, the incidences of grade 3/4 and grade 4 thrombocytopenia increased (high, intermediate, and low OPNI groups: 19.2% and 2.5%, 27.9% and 11.5%, and 35.9% and 18.0%, respectively; P = 0.026 and P = 0.001). Otherwise, no differences in anemia, neutropenia, febrile neutropenia, or non‐hematologic toxicities were observed among the three OPNI groups. TRM occurred in 5.9% (13/220) of the total patients, without a significant difference among the three groups.
Table 2.
Toxicities and treatment compliance
| Factor | Grade | OPNI > 45 (n = 120) | OPNI 40–45 (n = 61) | OPNI < 40 (n = 39) | P |
|---|---|---|---|---|---|
| Any hematologic toxicity | G3/4 | 109 (90.8) | 57 (93.4) | 32 (82.1) | 0.230 |
| G4 | 81 (67.5) | 37 (60.7) | 23 (59.0) | 0.265 | |
| Anemia | G3 | 21 (17.5) | 12 (19.7) | 9 (23.1) | 0.439 |
| Thrombocytopenia | G3/4 | 23 (19.2) | 17 (27.9) | 14 (35.9) | 0.026 |
| G4 | 3 (2.5) | 7 (11.5) | 7 (18.0) | 0.001 | |
| Neutropenia | G3/4 | 108 (90.0) | 56 (91.8) | 31 (79.5) | 0.150 |
| G4 | 80 (66.7) | 37 (60.7) | 22 (56.4) | 0.214 | |
| Febrile neutropenia | G3/4/5 | 18 (15.0) | 15 (24.6) | 9 (23.1) | 0.149 |
| G4/5 | 7 (5.8) | 7 (11.5) | 3 (7.7) | 0.458 | |
| Any non‐hematologic toxicity | G3/4/5 | 38 (31.7) | 23 (37.7) | 17 (43.6) | 0.157 |
| G4/5 | 14 (11.7) | 10 (16.4) | 8 (20.5) | 0.150 | |
| Treatment‐related mortality | 6 (5.0) | 4 (6.6) | 3 (7.7) | 0.506 | |
| Early discontinuation of treatment | 7 (5.8) | 13 (21.3) | 10 (25.6) | < 0.001 | |
| Additional dose reduction | 41 (34.2) | 24 (39.3) | 9 (23.1) | 0.377 | |
Bold font indicates significant P values. Categorical variables are presented as number of patients (%).
OPNI, Onodera's Prognostic Nutritional Index.
In terms of treatment compliance, the lower OPNI groups were associated more frequently with early discontinuation of treatment. While only 5.8% (7/120) of the high OPNI group discontinued treatment prematurely because of treatment toxicity, 21.3% (13/61) and 25.6% (10/39) discontinued in the intermediate and low OPNI groups, respectively (P < 0.001). There was no difference in the incidence of an additional dose adjustment among the three groups.
Survival
The median follow‐up duration was 4.1 years in all patients, and 178 deaths had occurred at the time of analysis. In the entire cohort, the median PFS and OS rates were 6.5 (95% confidence interval [CI] 6.2–6.9) and 11.7 (95% CI 9.8–13.6) months, respectively. The median PFS rates of the high, intermediate, and low OPNI groups were 6.9 (95% CI 6.3–8.1), 6.6 (95% CI 6.2–8.4), and 4.5 (95% CI 2.9–5.6) months, respectively (Fig 1a). The one‐year PFS rates of the high, intermediate, and low OPNI groups were 29% (95% CI 21–37%), 19% (95% CI 10–30%), and 3% (95% CI 0–13%), respectively. There were significant differences in PFS between the low and high OPNI groups (P < 0.001) and between the low and intermediate OPNI groups (P < 0.001), but not between the intermediate and high OPNI groups (P = 0.268). In patients with LD, the low OPNI group had poorer PFS (median 2.9 months, 95% CI 0.1–not reached) than the intermediate (median 9.3 months, 95% CI 1.6–6.7; P < 0.001) and high (median 11.1 months, 95% CI 6.8–15.9; P < 0.001) OPNI groups (Fig 1c). In patients with ED, there was a trend toward worse PFS in the low OPNI group (median 4.5 months, 95% CI 2.9–5.6) compared to the high OPNI group (median 6.2 months, 95% CI 5.4–6.9; P = 0.057) (Fig 1e).
Figure 1.
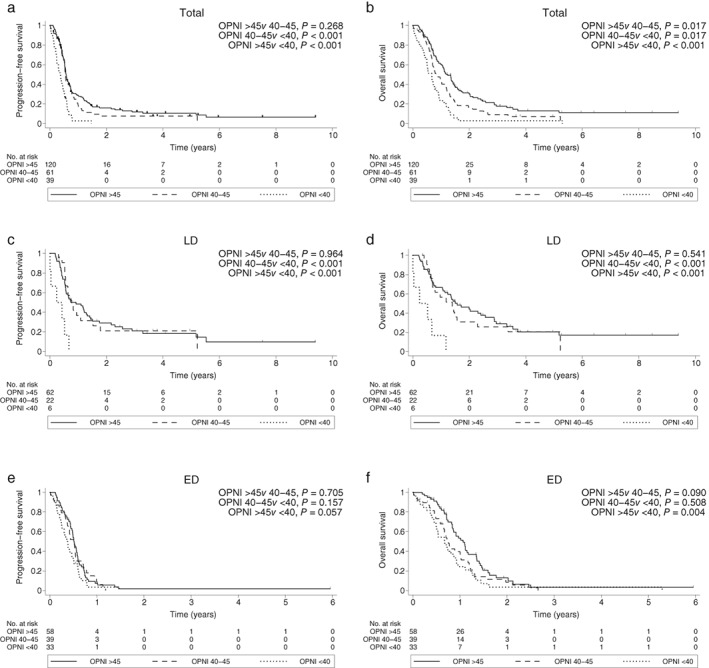
Progression‐free survival in (a) all patients, (c) patients with limited‐stage (LD), and (e) patients with extensive‐stage (ED). Overall survival in (b) all patients, (d) patients with LD, and (f) patients with ED. OPNI, Onodera's Prognostic Nutritional Index.
Similar results were observed for OS. The median OS rates of the high, intermediate, and low OPNI groups were 14.9 (95% CI 12.2–17.2), 9.8 (95% CI 8.3–14.2), and 8.0 (95% CI 5.3–9.3) months, respectively (Fig 1b). The one‐year OS rates of the high, intermediate, and low OPNI groups were 61% (95% CI 51–69%), 46% (95% CI 32–58%), and 23% (95% CI 11–38%), respectively. There were significant differences in OS among all three groups (Fig 1b). In patients with LD, the low OPNI group had poorer OS (median 2.9 months, 95% CI 0.1–not reached) than the intermediate (median 16.8 months, 95% CI 8.3–27.5; P < 0.001) and high (median 18.9 months, 95% CI 12.8–30.9; P < 0.001) OPNI groups (Fig 1d). In patients with ED, OS was significantly worse in the low OPNI group (median 8.1 months, 95% CI 5.9–10.9) than in the high OPNI group (median 12.8 months, 95% CI 10.1–16.2; P = 0.004) (Fig 1f).
After adjusting for potential confounding variables such as age, ECOG PS, stage, and chemotherapy regimen, the low OPNI group was found to be independently associated with a poor PFS relative to the high OPNI group (hazard ratio 1.592, 95% CI 1.009–2.511; P = 0.046) (Table 3). The low OPNI group was also associated with poor OS compared to the high OPNI group in multivariate analysis (hazard ratio 1.911, 95% CI 1.208–3.024; P = 0.006). There were no significant differences in PFS (P = 0.403) or OS (P = 0.909) between the high and intermediate OPNI groups in multivariate analyses.
Table 3.
Cox regression for PFS and OS
| Factor | PFS | OS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | ||||||||||||
| ≤ 65 | Ref. | Ref. | Ref. | Ref. | ||||||||
| > 65 | 1.271 | 0.949–1.704 | 0.108 | 1.340 | 0.964–1.862 | 0.081 | 1.434 | 1.057–1.946 | 0.021 | 1.575 | 1.113–2.229 | 0.010 |
| Gender | ||||||||||||
| Male | Ref. | Ref. | ||||||||||
| Female | 0.815 | 0.522–1.272 | 0.368 | 0.877 | 0.550–1.397 | 0.580 | ||||||
| ECOG PS | ||||||||||||
| 0–1 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 2 | 1.965 | 1.444–2.673 | < 0.001 | 1.634 | 1.134–2.355 | 0.008 | 2.582 | 1.871–3.563 | < 0.001 | 2.045 | 1.419–2.948 | < 0.001 |
| Stage | ||||||||||||
| LD | Ref. | Ref. | Ref. | Ref. | ||||||||
| ED | 2.701 | 1.971–3.699 | < 0.001 | 2.536 | 1.815–3.545 | < 0.001 | 2.114 | 1.534–2.913 | < 0.001 | 2.043 | 1.431–2.917 | < 0.001 |
| Regimen | ||||||||||||
| EP | Ref. | Ref. | Ref. | Ref. | ||||||||
| EC | 1.412 | 1.013–1.968 | 0.041 | 1.255 | 0.883–1.786 | 0.206 | 1.541 | 1.092–2.176 | 0.014 | 1.135 | 0.791–1.628 | 0.492 |
| IP | 1.517 | 0.887–2.594 | 0.128 | 1.024 | 0.571–1.835 | 0.937 | 1.247 | 0.716–2.171 | 0.436 | 0.827 | 0.451–1.518 | 0.540 |
| OPNI | ||||||||||||
| > 45 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 40–45 | 1.202 | 0.861–1.678 | 0.279 | 0.854 | 0.590–1.236 | 0.403 | 1.516 | 1.077–2.134 | 0.017 | 1.022 | 0.701–1.491 | 0.909 |
| < 40 | 2.680 | 1.825–3.937 | < 0.001 | 1.592 | 1.009–2.511 | 0.046 | 2.697 | 1.823–3.992 | < 0.001 | 1.911 | 1.208–3.024 | 0.006 |
Bold font indicates significant P values. CI, confidence interval; EC, etoposide/carboplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; ED, extensive‐stage; EP, etoposide/cisplatin; HR, hazard ratio; IP, irinotecan/cisplatin; LD, limited‐stage; OPNI, Onodera's Prognostic Nutritional Index; OS, overall survival; PFS, progression‐free survival.
Discussion
We demonstrated the prognostic value of the OPNI in SCLC patients receiving first‐line chemotherapy. The lower OPNI group exhibited a higher tumor burden (stage and serum LDH) and poorer PS. In addition, the highest proportions of patients refractory (30.8%) or intolerant to (25.6%) first‐line chemotherapy were found in the low OPNI group. The low OPNI group was associated with a poor prognosis, with median PFS and OS of only 4.5 and 8 months, respectively.
Cancer cachexia is defined as a multifactorial syndrome with ongoing loss of muscle mass with or without loss of fat mass that cannot be fully reversed by conventional nutritional support and is associated with reduced survival.16 Chronic inflammation has been demonstrated as one of the main contributors involved in cancer cachexia.17, 18 Pro‐inflammatory cytokines derived from tumor or immune cells activate the altered metabolic pathways associated with skeletal muscle wasting.18 For example, IL‐6 regulates altered mitochondrial biogenesis, which precedes the decrease in muscle mitochondrial content.19 Tumor necrosis factor‐alpha is involved in proteolysis, increased gluconeogenesis, and loss of adipose tissue.20 IL‐1 increases the plasma levels of tryptophan and serotonin, which lead to anorexia.21 However, the relationship between cancer cachexia and inflammation may not be explained solely by the cytokine alone because cancer cachexia develops from a complex interaction of factors.21 Rather, the acute‐phase proteins (APP), which are increased in up to 50% of cancer patients, may better explain the general inflammatory condition in cachectic cancer patients.20, 22 Among the APPs, albumin is a good indicator of the extent of cachexia, because it is also associated with body cell mass, as well as systemic inflammation.23 In SCLC, the C‐reactive protein/albumin ratio and albumin/globulin ratio were reported to be associated with patient prognosis.24, 25 Another contributor involved in cancer cachexia is impaired immune function. It was reported that immune suppression occurs as early as the pre‐cachectic state26 and may induce cancer cachexia via the STAT3 signaling pathway.27 Lymphocytes have been largely studied with respect to the relationship between the immune system and cancer.28, 29 In addition, the absolute lymphocyte count is reported to decrease as the severity of malnutrition increases.30 Given that albumin and lymphocytes reflect chronic inflammation and immune function, which are closely related to cancer cachexia, as well as nutritional status, the OPNI may be an excellent biomarker of cancer cachexia. Consequently, our study confirmed the negative impact of cancer cachexia on the prognosis of cancer patients.
Another negative impact of cancer cachexia is intolerance to anticancer therapy.16 Sarcopenia, one component of the diagnostic criteria for cancer cachexia, is associated with frequent dose‐limiting toxicities, TRM, and early discontinuation of treatment in cancer patients treated with chemotherapy.31, 32, 33 In previous studies of gastrointestinal cancer and non‐small cell lung cancer (NSCLC), patients with self‐reported weight loss experienced severe stomatitis and plantar‐palmar syndrome34 and failed to complete the scheduled chemotherapy,35 respectively, more frequently compared to patients that did not experience weight loss. Malnutrition and hypoalbuminemia are associated with major chemotherapy‐induced toxicities in NSCLC.36 In our study, the SCLC patients with a lower OPNI experienced early discontinuation of treatment and grade 3/4 thrombocytopenia more frequently than those with a higher OPNI. This finding is the first observation to show the relationship between the OPNI and chemotherapy‐related toxicity in SCLC patients and supports the adverse effects of cancer cachexia on the tolerance to anticancer therapy. Given the retrospective nature of this study, specific adverse events caused by chemotherapy (non‐hematologic toxicity in particular) may have been underreported in this study. Therefore, a prospective study is needed to evaluate the association between the OPNI and treatment‐related toxicity in SCLC.
The cutoff values of the OPNI varied among previous studies. The following two OPNI cutoff values are commonly used for cancer patients: 40 (applied in our study), originally defined by Onodera et al.;10, 37, 38, 39 and a range of 45–51, determined by receiver operating characteristic curve analyses.11, 13, 40, 41, 42 A pre‐defined OPNI cutoff value of 40 is the most conservative approach used to dichotomize or categorize continuous variables. A possible disadvantage of using this value is that it can result in unequally sized study groups and therefore reduce the statistical power of the test.43 The 45–51 value range, determined from an outcome‐oriented approach, has also been widely used to dichotomize continuous variables in clinical studies. However, applying this range to survival data is problematic because of the presence of censored observations and potential competing risks.44 The only previous study that applied the OPNI to SCLC defined a cutoff value of 52.48 using the minimal P value approach,15 which can inflate the type I error rate.45 We suggest using a predefined OPNI of 40 as the cutoff to perform reasonable comparisons among studies and to reduce the bias caused by the data‐driven technique.
The main limitation of this study is its retrospective nature. This resulted in the potential underestimation of some clinical data, as well as selection bias. As described above, non‐treatment‐related hematologic toxicities could not be investigated thoroughly. In addition, there were missing values for the serum LDH level and self‐reported weight loss, which are important in the study of SCLC and cancer cachexia.
In conclusion, this study showed that a low OPNI was associated with intolerance to first‐line platinum‐based chemotherapy and poor prognosis in SCLC patients. We suggest that intensive supportive care may be required to improve treatment response and prognosis in those with a low OPNI. Additionally, we hope that the OPNI will be useful as a surrogate marker of cancer cachexia in future studies.
Disclosure
No authors report any conflict of interest.
Acknowledgment
No funding was received for the present study.
References
- 1. Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: Have we made any progress over the last 25 years? Oncologist 2007; 12: 1096–104. [DOI] [PubMed] [Google Scholar]
- 2. Govindan R, Page N, Morgensztern D et al Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006; 24: 4539–44. [DOI] [PubMed] [Google Scholar]
- 3. Evans WK, Shepherd FA, Feld R, Osoba D, Dang P, Deboer G. VP‐16 and cisplatin as first‐line therapy for small‐cell lung cancer. J Clin Oncol 1985; 3: 1471–7. [DOI] [PubMed] [Google Scholar]
- 4. Kubota K, Hida T, Ishikura S et al Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited‐stage small‐cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): A randomised phase 3 study. Lancet Oncol 2014; 15: 106–13. [DOI] [PubMed] [Google Scholar]
- 5. Paesmans M, Sculier JP, Lecomte J et al Prognostic factors for patients with small cell lung carcinoma: Analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow‐up of 5 years. Cancer 2000; 89: 523–33. [DOI] [PubMed] [Google Scholar]
- 6. Cao S, Jin S, Shen J et al Selected patients can benefit more from the management of etoposide and platinum‐based chemotherapy and thoracic irradiation‐a retrospective analysis of 707 small cell lung cancer patients. Oncotarget 2017; 8: 8657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sachlova M, Majek O, Tucek S. Prognostic value of scores based on malnutrition or systemic inflammatory response in patients with metastatic or recurrent gastric cancer. Nutr Cancer 2014; 66: 1362–70. [DOI] [PubMed] [Google Scholar]
- 8. Santarpia L, Contaldo F, Pasanisi F. Nutritional screening and early treatment of malnutrition in cancer patients. J Cachexia Sarcopenia Muscle 2011; 2: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capra S, Ferguson M, Ried K. Cancer: Impact of nutrition intervention outcome‐‐nutrition issues for patients. Nutrition 2001; 17: 769–72. [DOI] [PubMed] [Google Scholar]
- 10. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi 1984; 85: 1001–5. [PubMed] [Google Scholar]
- 11. Jian‐Hui C, Iskandar EA, Cai Sh I et al Significance of Onodera's prognostic nutritional index in patients with colorectal cancer: A large cohort study in a single Chinese institution. Tumour Biol 2016; 37: 3277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe M, Iwatsuki M, Iwagami S, Ishimoto T, Baba Y, Baba H. Prognostic nutritional index predicts outcomes of gastrectomy in the elderly. World J Surg 2012; 36: 1632–9. [DOI] [PubMed] [Google Scholar]
- 13. Shoji F, Morodomi Y, Akamine T et al Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non‐small cell lung cancer. Lung Cancer 2016; 98: 15–21. [DOI] [PubMed] [Google Scholar]
- 14. Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: A systematic review and meta‐analysis. J Cancer Res Clin Oncol 2014; 140: 1537–49. [DOI] [PubMed] [Google Scholar]
- 15. Hong S, Zhou T, Fang W et al The prognostic nutritional index (PNI) predicts overall survival of small‐cell lung cancer patients. Tumour Biol 2015; 36: 3389–97. [DOI] [PubMed] [Google Scholar]
- 16. Fearon K, Strasser F, Anker SD et al Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011; 12: 489–95. [DOI] [PubMed] [Google Scholar]
- 17. Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cachexia and sarcopenia: Mechanisms and potential targets for intervention. Curr Opin Pharmacol 2015; 22: 100–6. [DOI] [PubMed] [Google Scholar]
- 18. Argiles JM, Busquets S, Stemmler B, Lopez‐Soriano FJ. Cancer cachexia: Understanding the molecular basis. Nat Rev Cancer 2014; 14: 754–62. [DOI] [PubMed] [Google Scholar]
- 19. White JP, Puppa MJ, Sato S et al IL‐6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle 2012; 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel HJ, Patel BM. TNF‐alpha and cancer cachexia: Molecular insights and clinical implications. Life Sci 2017; 170: 56–63. [DOI] [PubMed] [Google Scholar]
- 21. Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol 2015; 7: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deans DA, Tan BH, Wigmore SJ et al The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro‐oesophageal cancer. Br J Cancer 2009; 100: 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 2001; 39: 210–3. [DOI] [PubMed] [Google Scholar]
- 24. Zhou T, Zhan J, Hong S et al Ratio of C‐reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small‐cell lung cancer. Sci Rep 2015; 5: 10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou T, He X, Fang W et al Pretreatment albumin/globulin ratio predicts the prognosis for small‐cell lung cancer. Medicine (Baltimore) 2016; 95: e3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faber J, Vos AP, Kegler D et al Impaired immune function: An early marker for cancer cachexia. Oncol Rep 2009; 22: 1403–6. [DOI] [PubMed] [Google Scholar]
- 27. Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol 2016; 54: 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol 2002; 3: 991–8. [DOI] [PubMed] [Google Scholar]
- 29. Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural‐killer‐cell surveillance and therapy of cancer. Nat Rev Cancer 2002; 2: 850–61. [DOI] [PubMed] [Google Scholar]
- 30. Seiler WO. Clinical pictures of malnutrition in ill elderly subjects. Nutrition 2001; 17: 496–8. [DOI] [PubMed] [Google Scholar]
- 31. Go SI, Park MJ, Song HN et al Prognostic impact of sarcopenia in patients with diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle 2016; 7: 567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prado CM, Baracos VE, McCargar LJ et al Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009; 15: 2920–6. [DOI] [PubMed] [Google Scholar]
- 33. Go SI, Park MJ, Song HN et al Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer 2016; 24: 2075–84. [DOI] [PubMed] [Google Scholar]
- 34. Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998; 34: 503–9. [DOI] [PubMed] [Google Scholar]
- 35. Ross PJ, Ashley S, Norton A et al Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 2004; 90: 1905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arrieta O, Michel Ortega RM, Villanueva‐Rodriguez G et al Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non‐small cell lung cancer treated with paclitaxel‐cisplatin chemotherapy: A prospective study. BMC Cancer 2010; 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nozoe T, Kohno M, Iguchi T et al The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today 2012; 42: 532–5. [DOI] [PubMed] [Google Scholar]
- 38. Maeda K, Shibutani M, Otani H et al Low nutritional prognostic index correlates with poor survival in patients with stage IV colorectal cancer following palliative resection of the primary tumor. World J Surg 2014; 38: 1217–22. [DOI] [PubMed] [Google Scholar]
- 39. Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 2011; 98: 268–74. [DOI] [PubMed] [Google Scholar]
- 40. Kanda M, Mizuno A, Tanaka C et al Nutritional predictors for postoperative short‐term and long‐term outcomes of patients with gastric cancer. Medicine (Baltimore) 2016; 95: e3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wei GB, Lu YY, Liao RW, Chen QS, Zhang KQ. Prognostic nutritional index predicts prognosis in patients with metastatic nasopharyngeal carcinoma. Onco Targets Ther 2016; 9: 5955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Broggi MS, Patil D, Baum Y et al Onodera's prognostic nutritional index as an independent prognostic factor in clear cell renal cell carcinoma. Urology 2016; 96: 99–105. [DOI] [PubMed] [Google Scholar]
- 43. Katz MH. Multivariable Analysis: A Practical Guide for Clinicians and Public Health Researchers. Cambridge University Press, New York: 2011. [Google Scholar]
- 44. Steyerberg EW, Vickers AJ, Cook NR et al Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010; 21: 128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using "optimal" cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994; 86: 829–35. [DOI] [PubMed] [Google Scholar]


