Abstract
Background
Advanced thymic epithelial tumors (TETs) are indolent and poorly responsive to chemotherapy. PD‐1/PD‐L1 inhibitors have shown remarkable clinical benefit in several cancers; however, many immunomodulatory molecules have been identified that affect the immune response. This study examined the progonostic roles of PD‐L1, transforming growth factor‐β (TGF‐β), and CD8+ tumor‐infiltrating lymphocytes (CD8+ TILs) in patients with TETs.
Methods
Retrospective analysis was performed on the data of 20 patients with stage IV thymic carcinoma and 13 with stage III/IV invasive thymoma. Tissue biopsies were obtained before first‐line chemotherapy was administered. Protein levels were assessed by immunohistochemistry. Objective response rate, overall survival (OS), and progression‐free survival (PFS) were analyzed.
Results
Patients with advanced thymic carcinoma exhibited higher levels of PD‐L1 and TGF‐β than patients with advanced invasive thymic carcinoma (PD‐L1: 65.0% vs. 46.2%, P = 0.472; TGF‐β: 65.0% vs. 15.4%, P = 0.011). Five advanced thymic carcinoma patients with low levels of PD‐L1 and TGF‐β exhibited high levels of CD8 staining. The median OS was 29.5 months patients with high TGF‐β expression versus 62.9 in patients with low TGF‐β (P = 0.052). In patients with advanced thymic carcinoma, the median PFS in the high PD‐L1 expression group was 13.3 months versus 23.5 (P = 0.043) in the low PD‐L1, and the median OS was 50.7 months in the high CD8 expression versus 15.1 in the CD8 low group (P = 0.154).
Conclusions
Our results showed the prognostic roles of PD‐L1, TGF‐β, and CD8+ TILs in patients with advanced TETs, and the potential for development of anti‐PD‐1/PD‐L1 therapies.
Keywords: Advanced thymic epithelial tumor, CD8+ tumor‐infiltrating lymphocyte, PD‐L1, transforming growth factor‐β
Introduction
The thymus is a primary lymphoid organ, dedicated to T cell differentiation. CD8+ T lymphocytes, for example, are stimulated via antigen presentation to become CD8+ cytotoxic T effector cells, which eliminate tumors. The incidence of thymic epithelial tumors (TETs) is < 0.15 cases per 100 000 people per year.1, 2 TETs can be classified into two categories: thymoma and thymic carcinoma. Thymoma is a kind of organotypic cancer with unique morphology, performing organ‐like functions.3, 4 Thymomas generate immature T cells and large amounts of autobodies; 30–40% of patients experience complications, including autoimmune diseases. By contrast, thymic carcinoma is non‐organotypic, similar to tumors found in locations such as the lungs, head, and neck, and does not exert organ‐like functions to promote T cell maturation.3, 4
The age of onset of thymoma and thymic carcinoma is usually between 40 and 60 years. The five‐year survival rates are 64% and 45% in patients with stage III and IV thymoma, and 33% and 24% in patients with stage III and IV thymic carcinoma, respectively. Late‐stage thymic malignancy with metastasis is considered to be unresectable, and chemotherapy with/without radiotherapy is recommended. However, thymoma and thymic carcinoma are indolent and poorly responsive to chemotherapy. Because there is no standard therapy for second‐line treatment, therapeutic development is urgently needed.
In the immune response against cancer, CD8+ T cell infiltration has contributed to provide immune‐surveillance to eliminate cancer. After stimulation in the lymph nodes, CD8+ cytotoxic lymphocytes (CTLs) are thought to migrate to and infiltrate tumor sites. The antigen‐specific recognition of cancer cells by CD8+ CTLs triggers the release of cytokines such as interferon‐gamma (IFN‐γ) to promote immune response, and the release of cytolytic enzymes (Granzyme B and perforin) to attack cancer cells. The number of CD8+ tumor‐infiltrating lymphocytes (TILs) positively correlates with prolonged survival in various cancers.5, 6, 7, 8, 9, 10, 11, 12, 13 PD‐L1/PD‐1 signaling is an immunosuppressive checkpoint that is highly upregulated within the tumor microenvironment to inhibit infiltrating cytolytic T cells (i.e. promote T cell exhaustion and suppress T cell proliferation) in various cancers.14, 15, 16 Transforming growth factor‐β (TGF‐β) promotes tumor development, causing versatile effects on not only cancer cells, but also various immune cells, including CTLs.17
In order to develop a tumor where CTLs emerge, thymic cancer cells may conduct an immunosuppressive program to suppress immune surveillance. Therefore, targeting cancer‐mediated immunosuppressive machinery could provide an additional strategy to control and eradicate advanced TETs.
In this study, we investigated PD‐L1 and TGF‐β expression and the prevalence of CD8+ TILs and evaluated their progonostic roles in pretreated patients with advanced TETs.
Methods
Patients and samples
A total of 33 patients diagnosed with advanced TETs between 2006 and 2014 at the Thoracic Medical Oncology Department of Peking University Cancer Hospital were included in this study. Thirteen patients had stage III/IV invasive thymoma and 20 patients had stage IV thymic carcinoma. The inclusion criteria were: (i) histopathological confirmation of advanced TETs; (ii) adequate pre‐treated samples from original tumor sites or from remote metastatic sites for immunohistochemistry (IHC) staining; (iii) complete follow‐up information for overall survival (OS) analysis; and (iv) in 17 patients with advanced thymic carcinoma receiving first‐line chemotherapy with (or without) radiotherapy, complete treatment history and evaluation information for progression‐free survival (PFS), objective response rate (ORR), and disease control rate (DCR) analysis. The relevant clinical data were retrospectively reviewed from the patients’ charts. This study was performed according to the principles of the Declaration of Helsinki and was approved by the independent ethics committees of Peking University Cancer Hospital.
Immunohistochemistry
Formalin‐fixed, paraffin‐embedded blocks of all patients included in the study were obtained from the Department of Pathology, Peking University Cancer Hospital. The blocks were sectioned at a thickness of 4 μm and deparaffinized. Firstly, the sections were incubated in xylene for 20 minutes, followed by 100%, 95%, and 75% ethanol, and then washed with double‐distilled H2O. Secondly, the sections were boiled in 10 mM sodium citrate buffer for 15 minutes at 121°C to retrieve the antigen. The sections were incubated in 3% peroxidase for 10 minutes and then washed with phosphate buffered saline followed by double‐distilled H2O. Afterwards, the sections were blocked by phosphate buffered saline containing 5% bovine serum albumin at room temperature for 20 minutes, followed by exposure to the primary antibodies for 18 hours at 4°C. The primary antibodes used in this study were CD8 (C8/144B, 1:100), PD‐L1 (ab58810, 1:200), and TGF‐β (ab66043, 1:100; Abcam, Cambridge, MA, USA), which were diluted in antibody diluent (SignalStain Antibody Diluent #8112, Cell Signaling Technology, Danvers, MA, USA) for use. The sections were then incubated with a general secondary antibody (GT Vision TM III, GK500705, Gene Tech Company Limited, Shanghai, China) at room temperature for 30 minutes, and visualized by dextran polymer‐conjugated horseradish‐peroxidase and 3,3′‐diaminobenzidine chromogen, followed by counterstaining with hematoxylin solution.
Expression score evaluation
The IHC scoring of CD8+ TILs was determined by the density of cells with positive CD8 staining, as previously described (graded on a scale of 0–4: 0 = no or sporadic cells; 1 = moderate numbers of cells; 2 = abundant occurrence of cells; and 3 = highly abundant occurrence of cells).18 The median value was used as a cutoff point to separate the patients into two groups with either high or low levels of CD8+ T cell infiltration.
The IHC scoring of PD‐L1 and TGF‐β expression was assessed according to previous standards using a visual grading system based on the extent (the percentage of positive tumor cells graded on a scale of 0–4: 0 < 5%, 1 = 5–25%, 2 = 26–50%, 3 = 51–75%, 4 > 75%) and the intensity of staining (graded on a scale of 1–3: 1 = weak staining, 2 = moderate staining, 3 = strong staining).19 The percentage of positive tumor cells and the staining intensity were then multiplied to produce an individual staining score for each sample, ranging from 0 (no positive tumor cells) to 12 (> 75% of tumor cells with intense staining). The median value of all scores was chosen as the cutoff value for dividing protein expression into high and low.
Images were acquired at magnifications of 10× and 40×. All IHC results were examined by two independent pathologists blinded to the patients’ clinical and pathological data to minimize inter‐observer variability. Any discrepancies in the results were examined by a third blinded pathologist who made a final diagnosis.
Evaluation of efficacy and patient status
The efficacy of treatments and patient status were evaluated based on three parameters: (i) OS, (ii) PFS, and (iii) ORR and DCR. Tumor response was assessed according to Response Evaluation Criteria in Solid Tumors version 1.1 at week 9 and every six weeks thereafter until disease progression. During the follow‐up period, patients were contacted every three months to assess survival. OS was calculated from the date of the beginning of first‐line treatment to the date of death. Data were updated as of 1 March 2015. PFS was defined as the duration between the start of therapy and tumor progression (locoregional recurrence and/or distant metastasis) or death from any cause. Patients were assessed radiographically after they had received at least two cycles of chemotherapy, and the duration of response was analyzed from the date of the first cycle to the confirmation of disease progression. ORR was defined as complete response plus partial response. DCR was defined as complete response plus partial response plus stable disease.
Statistical methods
SPSS version 22 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The Fisher's exact test was applied to assess the differences between categorical variables. The Kaplan–Meier method was used to plot the survival curves for OS and PFS. Univariate analysis was performed using the log‐rank test to test the difference between groups. A larger multivariate model was not used because of limitations in the sample size. A two‐sided P value < 0.05 was considered statistically significant.
Results
Expression of PD‐L1, transforming growth factor‐β (TGF‐β), and CD8 in advanced thymic epithelial tumors (TETs)
The characteristics of the patient sample are summarized in Table 1. We did not perform CD8 staining in patients with advanced invasive thymoma because it is relatively difficult to distinguish dysregulated T cells from auto‐reactive T cells.
Table 1.
Characteristics of patients with advanced thymic carcinoma and advanced invasive thymoma
| Patient characteristics | Advanced thymic carcinoma | Advanced invasive thymoma | |||
|---|---|---|---|---|---|
| No. | No. | ||||
| Median age (range), years | 50 (28–67) | % | 49 (35–66) | % | Fisher's exact test |
| Age (years) | |||||
| < 60 | 16 | 80.0 | 11 | 84.6 | P = 1 |
| ≥ 60 | 4 | 20.0 | 2 | 15.4 | |
| Gender | |||||
| Male | 13 | 65.0 | 6 | 46.2 | P = 0.47 |
| Female | 7 | 35.0 | 7 | 53.8 | |
| ECOG PS | |||||
| 0 | 11 | 55.0 | 5 | 38.5 | P = 0.5684 |
| 1 | 8 | 45.0 | 8 | 61.5 | |
| 2 | 1 | 5.0 | 0 | 0 | |
| Smoking status | |||||
| Yes | 7 | 35.0 | 9 | 69.2 | P = 0.08 |
| No | 13 | 65.0 | 4 | 30.8 | |
| Masaoka stage | |||||
| IIIa | 0 | 0 | 1 | 7.7 | P = 0.2658 |
| IIIb | 0 | 0 | 1 | 7.7 | |
| IVa | 2 | 10.0 | 2 | 15.4 | |
| IVb | 18 | 90.0 | 9 | 69.2 | |
| Histology/WHO classification | |||||
| Squamous cell carcinoma | 20 | 100.0 | NA | NA | |
| Low grade | 20 | 100.0 | NA | NA | |
| Type A | NA | NA | 2 | 6.9 | |
| Type AB | NA | NA | 3 | 10.3 | |
| Type B | NA | NA | 4 | 13.8 | |
| Unknown | NA | NA | 4 | 13.8 | |
ECOG PS, Eastern Cooperative Oncology Group performance status; NA, not available; WHO, World Health Organization.
PD‐L1 staining appeared both on the membrane and in the cytoplasm of the TET cells; in addition, there were four cases with nuclear staining of PD‐L1. PD‐L1 levels were relatively higher in patients with advanced thymic carcinoma compared to patients with advanced invasive thymoma (65.0% [13/20] vs. 46.2% [6/13]) (Table 2, Fig 1). TGF‐β staining was primarily observed inside the TET cells within tumor nests. Patients with thymic carcinoma exhibited significantly higher TGF‐β expression than patients with invasive thymoma (65.0% [13/20] vs. 15.4% [2/13]; P = 0.011) (Table 2, Fig 2). In advanced thymic carcinoma biopsies, 11 out of 20 cases (55.0%) presented high CD8 expression, while 9 out of 20 cases (45.0%) exhibited a relatively low level (Table 2, Fig 3).
Table 2.
PD‐L1, TGF‐β, and CD8 immunohistochemistry results
| Advanced thymic carcinoma n(%) | Advanced invasive thymoma n(%) | Fisher's exact test | |
|---|---|---|---|
| PD‐L1 | |||
| High score | 13 (65.0%) | 6 (46.2%) | P = 0.472 |
| Low score | 7 (35.0%) | 7 (53.8%) | |
| TGF‐β | |||
| High score | 13 (65.0%) | 2 (15.4%) | P = 0.011 |
| Low score | 7 (35.0%) | 11 (84.6%) | |
| CD8 | |||
| Low score | 9 (45.0%) | — | |
| High score | 11(55.0%) | — |
TGF‐β, transforming growth factor‐β.
Figure 1.
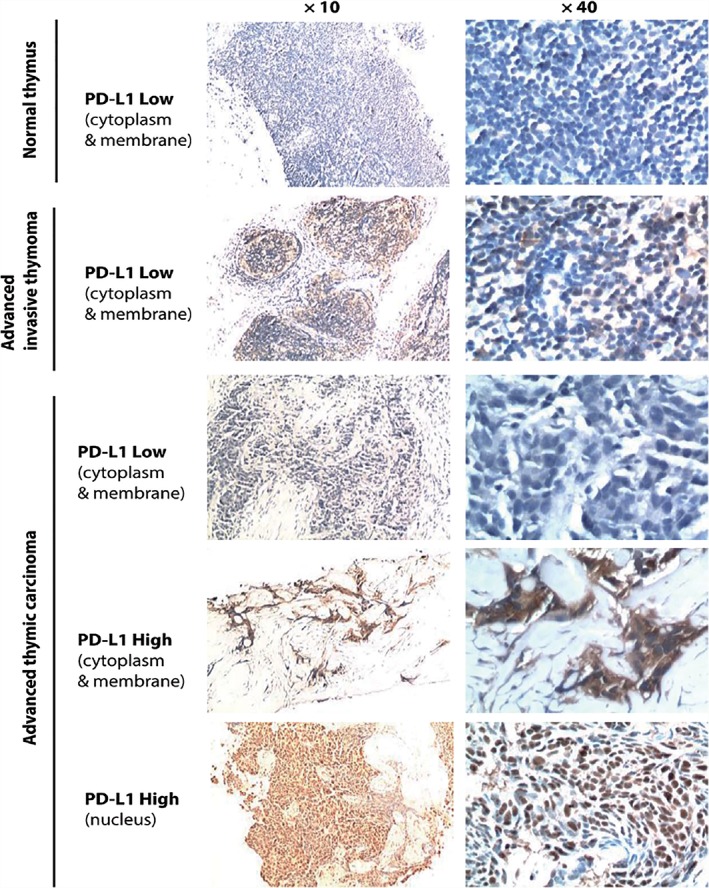
Representative images of PD‐L1 staining in normal thymus tissue and advanced thymic epithelial tumor (TET) pre‐treated specimens. The median PD‐L1 immunohistochemistry expression score was 2. High PD‐L1 expression was identified with a score ≥ 2, while low expression was < 2. In TET specimens, PD‐L1 staining was mainly observed on the membrane and cytoplasm of the tumor cells, with some samples also showing positive nucleus staining. A weak basal level of PD‐L1 expression could be observed on the surface and in the cytoplasm of non‐thymocytes in the normal thymus tissue.
Figure 2.
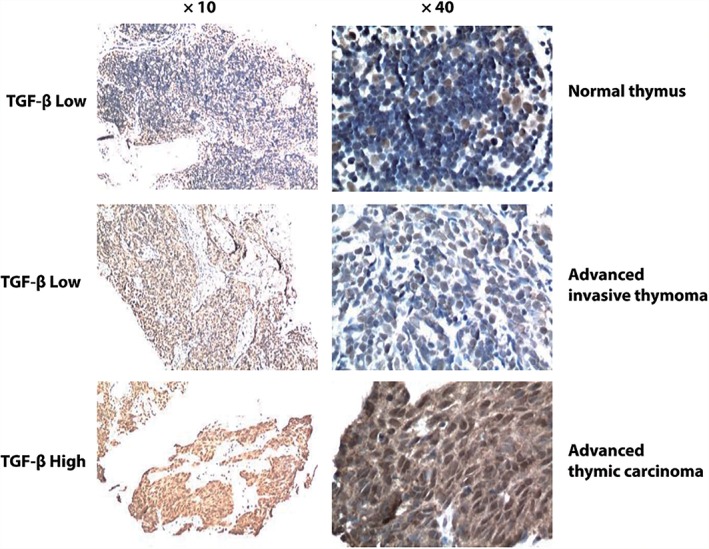
Representative images of transforming growth factor‐β (TGF‐β) staining in normal thymus tissue and advanced thymic epithelial tumor (TET) pre‐treatment specimens. The median TGF‐β immunohistochemistry expression score is 4. High TGF‐β expression was identified with a score > 4, while low expression was ≤ 4. Basal level TGF‐β staining could be observed in the non‐thymocytes located in the medulla of normal thymus tissue.
Figure 3.
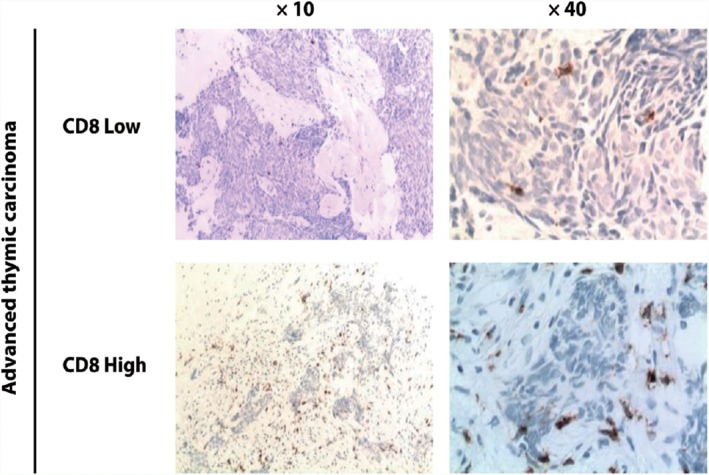
Representative images of CD8 staining in pre‐treatment advanced thymic carcinoma specimens. CD8 expression assayed by immunohistochemistry was used to evaluate the prevalence of CD8+ tumor‐infiltrating lymphocytes in advanced thymic carcinoma specimens. The median CD8 immunohistochemistry expression score was 1. A high level of CD8+ T cell infiltration was identified with a score ≥ 1 (moderate or abundant numbers of cells), while a low level of infiltration was < 1 (no or sporadic cells).
Effect of PD‐L1 and TGF‐β expression on the number of tumor‐infiltrating CD8+ cytotoxic lymphocytes
We investigated the relationship between PD‐L1/TGF‐β expression and the number of infiltrated CD8+ T cells in advanced thymic carcinoma patients. Based on IHC results, 5 of 7 (71.4%) patients with low PD‐L1/TGF‐β expression exhibited a high level of CD8 staining (Fig 4) and 7 of 13 (53.8%) patients with high PD‐L1/TGF‐β expression showed a low level of CD8 staining, although the difference was not statistically significant between the groups (P = 0.374) (Table 3). These data suggest that high PD‐L1/TGF‐β expression in the tumor may lead to a reduction of CD8+ T cell infiltration.
Figure 4.
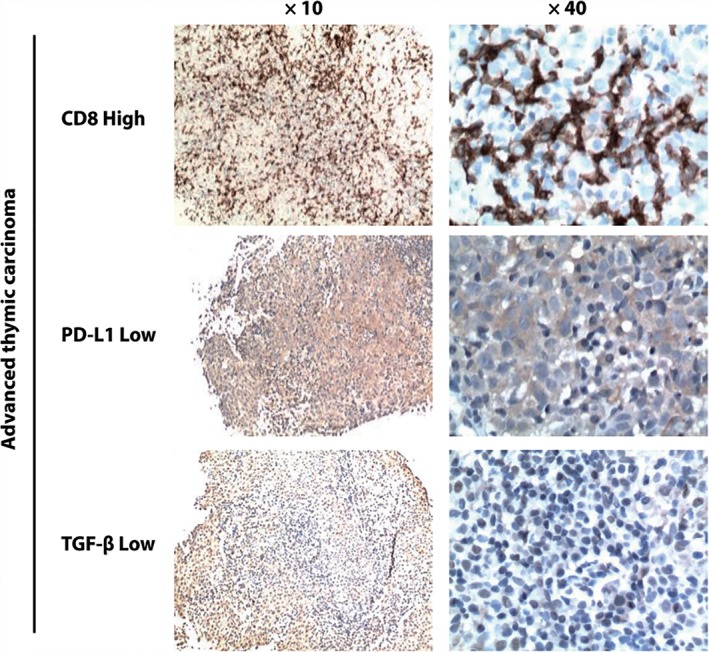
PD‐L1, transforming growth factor‐β (TGF‐β), and CD8 protein expression patterns in pre‐treated advanced thymic carcinoma specimens. A potential inverse relationship is observed between PD‐L1/TGF‐β and tumor‐infiltrating CD8+ T cells. A high CD8+ T cell tumor‐infiltrating pattern is observed in samples with a low level of PD‐L1/TGF‐β expression.
Table 3.
PD‐L1, TGF‐β, and CD8+ expression in advanced thymic carcinoma patients
| CD8+ | ||||
|---|---|---|---|---|
| Low | High | Total | Fisher's exact test | |
| PD‐L1 | ||||
| Low | 2 (28.6%) | 5 (71.4%) | 7 (100%) | P = 0.374 |
| High | 7 (53.8%) | 6 (46.2%) | 13 (100%) | |
| TGF‐β | ||||
| Low | 2 (28.6%) | 5 (71.4%) | 7 (100%) | P = 0.374 |
| High | 7 (53.8%) | 6 (46.2%) | 13 (100%) | |
| Total | 9 (45.0%) | 11 (55.0%) | 20 (100%) |
TGF‐β, transforming growth factor‐β.
Prognostic value of PD‐L1, TGF‐β, and CD8+ T cells for predicting overall survival in patients with advanced TETs
Kaplan–Meier survival analysis was performed on all patient samples (Table 4, Fig 5) and separately in advanced thymic carcinoma and advanced invasive thymoma patient samples (Table 5, Fig 6).
Table 4.
Univariate analysis of PD‐L1 and TGF‐β for predicting OS in patients with advanced TETs
| Advanced TETs | ||||
|---|---|---|---|---|
| Expression | N (n = 33) | Median OS (months) | 95% CI | P |
| PD‐L1 | ||||
| Low | 14 | 42.6 | 0.0–98.3 | 0.186 |
| High | 19 | 29.5 | 20.0–39.0 | |
| TGF‐β | ||||
| Low | 18 | 62.9 | 15.6–110.1 | 0.052 |
| High | 15 | 29.5 | 18.6–40.4 | |
CI, confidence interval; OS, overall survival; TETs, thymic epithelial tumors; TGF‐β, transforming growth factor‐β.
Figure 5.
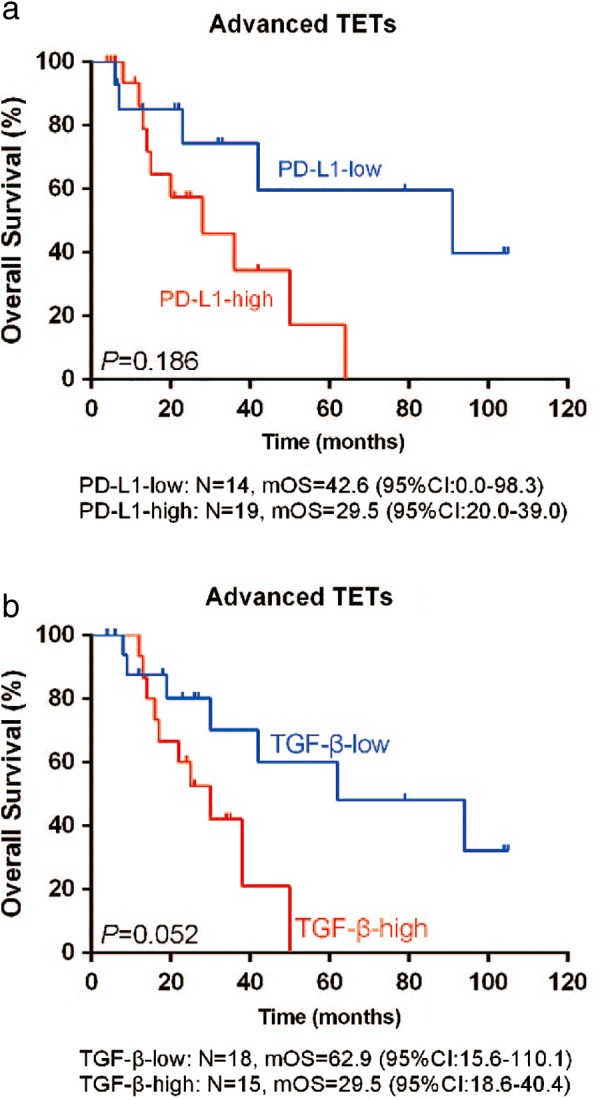
Kaplan–Meier plots with the log‐rank test for overall survival (OS) of patients with advanced thymic epithelial tumors (TETs) according to (a) PD‐L1 and (b) transforming growth factor‐β (TGF‐β) expression. There was a trend of favorable OS in patients with low PD‐L1 or TGF‐β expression, although the differences were not significant (P = 0.186 and P = 0.052, respectively). CI, confidence interval; mOS, median OS.
Table 5.
Univariate analysis of PD‐L1, TGF‐β, and CD8+ for predicting OS in patients with advanced thymic carcinoma or advanced invasive thymoma
| Advanced thymic carcinoma | Advanced invasive thymoma | |||||||
|---|---|---|---|---|---|---|---|---|
| Expresssion | N | Median OS (months) | 95% CI | P | N | Median OS (months) | 95% CI | P |
| PD‐L1 | ||||||||
| Low | 7 | NE | NE | 0.262 | 7 | 42.6 | 7.3–77.9 | 0.711 |
| High | 13 | 29.5 | 20.8–38.2 | 6 | NE | NE | ||
| TGF‐β | ||||||||
| Low | 7 | 62.9 | 9.3–116.5 | 0.054 | 11 | 91.3 | 21.9–160.7 | 0.734 |
| High | 13 | 29.5 | 7.1–51.9 | 2 | 25.4 | NE | ||
| CD8+ | ||||||||
| Low | 9 | 15.1 | 0.0–36.4 | 0.154 | ||||
| High | 11 | 50.7 | 18.2–83.2 | |||||
CI, confidence interval; OS, overall survival; NE, not evaluable; TGF‐β, transforming growth factor‐β.
Figure 6.
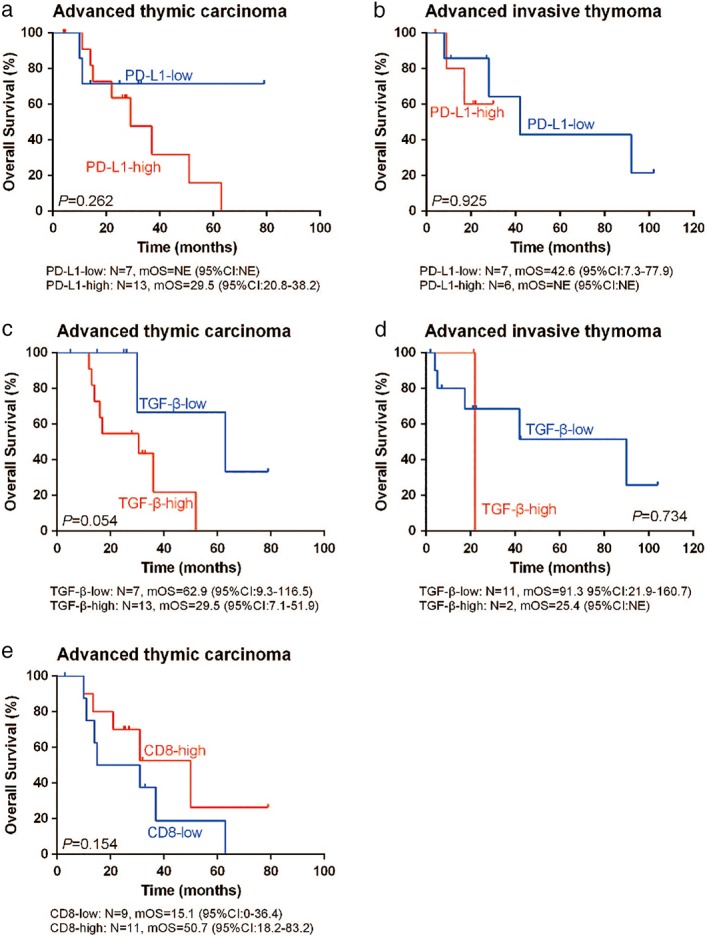
Kaplan–Meier plots with the log‐rank test for overall survival (OS) of patients with (a,c,e) advanced thymic carcinoma and (b,d) advanced invasive thymoma according to (a,b) PD‐L1, (c,d) transforming growth factor‐β (TGF‐β), and (e) CD8 expression. There was a trend of better OS in advanced thymic carcinoma patients with low TGF‐β or high CD8 expression (P = 0.054 and P = 0.154, respectively). Evaluation of the prognostic function of PD‐L1 for OS in advanced thymic carcinoma and invasive thymoma was limited by the relatively small sample size in this study, thus that the median OS (mOS) could not be estimated in subgroups, as shown in (a) and (b). CI, confidence interval.
Among all patients, the median OS (mOS) in patients with high PD‐L1 expression was shorter than in patients with low PD‐L1 expression (mOS: 29.5 months, [95% confidence interval, CI 20.0–39.0] vs. 42.6 months [95% CI 0–98.3]; P = 0.186) (Fig 5a). The mOS in patients with high TGF‐β expression was also shorter than in patients with low TGF‐β expression (29.5 months [95% CI 18.6–40.4] vs. 62.9 months [95% CI 15.6–110.1]; P = 0.052) (Fig 5b). Although there were no significant differences in these results, patients with low PD‐L1 or TGF‐β expression may have better OS than the whole TET population (P = 0.186 and P = 0.052, respectively). Further analysis of advanced thymic carcinoma samples demonstrated a correlation between TGF‐β and OS, but this result was not statistically significant. The mOS in patients with high or low TGF‐β expression was 29.5 months (95% CI 7.1–51.9) and 62.9 months (95% CI 9.3–116.5), respectively (P = 0.054) (Fig 6). In contrast, high CD8 expression correlated with better OS in advanced thymic carcinoma compared to low CD8 expression (50.7 months [95% CI 18.2–83.2] vs. 15.1 months [95% CI 0.0–36.4], respectively; P = 0.154) (Fig 6). These data suggest that PD‐L1, TGF‐β, and CD8 protein expression may be used as prognostic factors for OS in patients with advanced thymic carcinoma.
Prognostic value of CD8+, PD‐L1, and TGF‐β for predicting progression‐free survival in advanced thymic carcinoma for first‐line therapy
Most thymic carcinoma patients (17/20) received first‐line chemotherapy, and 9 of them also subsequently received radiotherapy. Thymic carcinoma patient charateristics are summarized in Table 6. Kaplan–Meier survival analysis was performed (Fig 7).
Table 6.
Characteristics of advanced thymic carcinoma patients administered first‐line treatment
| Variable | N (n = 17) | % |
|---|---|---|
| Gender | ||
| Male | 12 | 70.6 |
| Female | 5 | 29.4 |
| Age, years | ||
| < 60 | 13 | 76.5 |
| ≥ 60 | 4 | 23.5 |
| ECOG PS | ||
| 0 | 9 | 52.9 |
| 1 | 8 | 47.1 |
| CD8+ | ||
| Low | 7 | 41.2 |
| High | 10 | 58.8 |
| PD‐L1 | ||
| Low | 5 | 29.4 |
| High | 12 | 70.6 |
| TGF‐β | ||
| Low | 5 | 29.4 |
| High | 12 | 70.6 |
| First‐line chemo best response | ||
| PR | 4 | 23.5 |
| SD | 13 | 76.5 |
| RT after first‐line chemo | ||
| − | 8 | 47.1 |
| + | 9 | 52.9 |
Chemo, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; PR, partial response; RT, radiotherapy; SD, stable disease; TGF‐β, transforming growth factor‐β.
Figure 7.
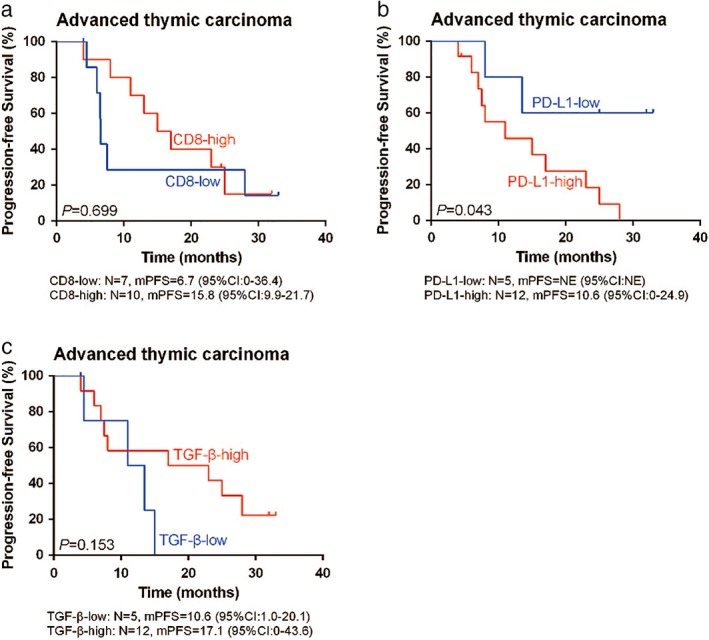
Kaplan–Meier curves with log‐rank test for progression‐free survival (PFS) after first‐line treatment of patients with advanced thymic carcinoma according to (a) CD8, (b) PD‐L1, and (c) transforming growth factor‐β (TGF‐β) expression. Significantly superior PFS after first‐line treatment was achieved in patients with low PD‐L1 expression (P = 0.043). No significance of TGF‐β (P = 0.153) or CD8 (P = 0.699) expression with regard to PFS was observed.
The median PFS (mPFS) was significantly shorter in thymic carcinoma patients with high PD‐L1 expression compared to patients with low PD‐L1 expression (mPFS: 10.6 months [95% CI 0‐24.9] vs. NE [95% CI 0‐NE]; P = 0.043) (Fig 7b). However, we did not find a significant correlation between TGF‐β or CD8+ and PFS in advanced thymic carcinoma patients (Fig 7a,c).
Correlation of CD8+, PD‐L1, and TGF‐β with response to first‐line therapy in advanced thymic carcinoma
Advanced thymic carcinoma patient characteristics are shown in Table 7. Objective reponses were observed in 3/10 (30.0%) patients with high CD8 expression. By contrast, objective reponses were only observed in 1/7 (14.3%) patients with low CD8 expression. Furthermore, the ORR was 40.0% (2/5) with low TGF‐β expression versus 16.7% (2/12) with high TGF‐β expression. These data showed that CD8 or TGF‐β expression may be correlated with reponse to first‐line therapy in patients with advanced thymic carcinoma, although there were no statistically significant differences (P = 0.603 and P = 0.538).
Table 7.
Biomarkers and best response of first‐line chemotherapy in patients with advanced thymic carcinoma (n = 17)
| First‐line chemo response | Fisher's exact test | |||
|---|---|---|---|---|
| Expression | PR | SD | Total | |
| CD8+ | ||||
| Low | 1 (14.3%) | 6 (85.7%) | 7 (100%) | P = 0.603 |
| High | 3 (30.0%) | 7 (70.0%) | 10 (100%) | |
| Total | 4 (23.5%) | 13 (76.5%) | 17 (100%) | |
| PD‐L1 | ||||
| Low | 1 (20.0%) | 4 (80.0%) | 5 (100%) | P = 1.000 |
| High | 3 (25.0%) | 9 (75.0%) | 12 (100%) | |
| Total | 4 (23.5%) | 13 (76.5%) | 17 (100%) | |
| TGF‐β | ||||
| Low | 2 (40.0%) | 3 (60.0%) | 5 (100%) | P = 0.538 |
| High | 2 (16.7%) | 10 (83.3%) | 12 (100%) | |
| Total | 4 (23.5%) | 13 (76.5%) | 17 (100%) | |
Chemo, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; PR, partial response; RT, radiotherapy; SD, stable disease; TGF‐β, transforming growth factor‐β.
Discussion
Immune checkpoint blockades (ICBs), including antibodies to PD‐1 or PD‐L1 and CTLA‐4 have shown clinical efficacy in most malignances.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 However, some cancer patients still cannot not benefit from current ICB therapy.2, 3 Therefore, determining the predictive biomarkers to better identify patients more likely to respond to ICBs is urgent for clinical practice.
Our results show that patients with advanced thymic carcinoma exhibited relatively higher PD‐L1 or TGF‐β expression than patients with advanced invasive thymoma, consistent with the results of previous studies.31, 32, 33 However, other studies have reported that PD‐L1 expression is relatively higher in thymoma than in thymic carcinoma patients.34, 35 The challenges in defining cutoff values of PD‐L1, distribution of intratumoral heterogeneity, platform uniformity testing, and dynamic changes may explain the differences between studies.36 Although PD‐L1 was more frequently detected in advanced thymic carcinoma compared to advanced invasive thymoma patients in our study, the difference was not statistically significant, suggesting that PD‐L1 might play a role in both malignancies.
Transforming growth factor‐β is an important cytokine for promoting cancer progression and suppressing the host immune response against cancer.37, 38 It regulates various types of cells in the tumor microenvironment to provide favorable conditions for tumor growth. TGF‐β negatively regulates cytotoxic CD8+ cells through two mechanisms: (i) inhibiting CTL clonal expansion and proliferation in vivo; and (ii) suppressing the expression of cytotoxic proteins in T cells through transcriptional repression.17 TGF‐β also activates CD4+ regulatory T cells (Treg) to promote self‐tolerance and promote cancer.39, 40 Treg cells can also produce TGF‐β to suppress the expression of stimulating receptors on natural killer cells.40, 41 TGF‐β can negatively regulate B cell proliferation and antibody production.42, 43
The higher expression of TGF‐β in patients with advanced thymic carcinoma suggests that TGF‐β might play an important role in the pathogenesis of thymic cancer. Similar to our findings, a very different expression pattern of c‐kit and IGF‐1R overexpression between thymoma and thymic carcinoma (2% c‐kit overexpression in thymoma vs. 79% in thymic carcinoma; and 4% IGF‐1R expression in thymoma vs. 37% in thymic carcinoma) has been reported.2 In fact, the identification of c‐kit overexpression has led to the use of c‐kit inhibitors (sunitinib) in clinical trials for the treatment of chemotherapy‐refractory thymic malignancies.44 This suggests that TGF‐β expression could be exploited as a therapeutic target for thymic carcinoma.
We further found that low PD‐L1 and TGF‐β expression was numerically associated with a high level of CD8 staining. This is consistent with the counteracting effects between the function of PD‐L1/ TGF‐β expressed by tumor cells and the number of infiltrated CTLs in the tumor microenvironment. Tumor infiltrated CTLs are known to secrete cytolytic proteins and cytokines to kill cancer cells. To inhibit the function of infiltrated CTLs, PD‐L1, and TGF‐β are highly expressed by cancer cells to induce T cell exhaustion and “shut‐off” the cytotoxicity program of CTLs.16, 17 Furthermore, PD‐L1 and TGF‐β have been shown to inhibit T cell clonal expansion and proliferation.16, 17 The defined immunosuppressive functions of PD‐L1 and TGF‐β could explain their inverse relationship of expression with the number of CD8+ TILs, as indicated by our results.
The prognostic roles of PD‐L1, TGF‐β, and CD8 have been studied in various cancer types.37, 38, 45, 46 However, their prognostic value in thymoma and thymic carcinoma remains unclear. Our results demonstrate that high TGF‐β expression is numerically associated with poor OS in thymic carcinoma. This observation is consistent with previous reports of the immunosuppressive role of TGF‐β in the tumor microenviroment.37, 38 We further found that high PD‐L1 expression may be associated with poor PFS, which is consistent with the results of several previously published papers. PD‐L1 overexpression is reported to be associated with poor clinical outcomes in non‐small cell lung cancer (NSCLC),19 gastric,47, 48 colorectal,49 and esophageal cancers.50 However, there are significant inconsistencies regarding the prognostic impact of PD‐L1 on survival in patients with TETs.31, 32, 35, 51, 52 Padda et al. concluded that high PD‐L1 expression was associated with poorer OS in patients with advanced TETs,31 consistent with our findings. In contrast, another two papers reported improved OS in TET patients with high PD‐L1 expression.33, 35 The challenges in defining a cutoff value of PD‐L1, distribution of intratumoral heterogeneity, platform uniformity testing, and dynamic changes may explain these differences. Furthermore, a larger sample size is warranted for further investigation.
Our results indicate that higher CD8+ TILs may be associated with better OS in patients with advanced thymic carcinoma, consistent with the results of previous reports, which showed that T cell infiltration was likely a prognostic indicator for better survival in melanomas, NSCLC, and colorectal, breast, and ovarian cancers.5, 6, 11 In melanomas, patients with a higher amount of T cells exhibited better survival.5 In NSCLC, CD8+ T cell infiltration in both early and late‐stage patients was associated with better prognosis.8, 9 In colorectal cancer, active CD8+ T cells in the tumor mass and stromal region provided significant prognostic value of a better clinical outcome.53, 54
In this study, we investigated the association between PD‐L1, TGF‐β, and CD8 expression with the response to first‐line treatment of chemotherapy in patients with advanced thymic carcinoma. Our results show that patients with higher CD8+ TILs or lower TGF‐β expression were likely to acquire higher ORRs. This trend was consistent with accumulating evidence that the therapeutic effect of chemotherapy is partially dependent on the immune system of patients.55 Chemotherapy‐induced “immunogenic” cancer cell death leads to an increase of “eat me” signals, cytokines, and danger signals in the microenvironment, which triggers the activities of antigen‐presenting cells for engulfing cancer cells. The phagocytosis of target cancer cells by dendritic cells is followed by presentation of the cancer‐associated antigen to T cells to activate adaptive immunity. Activated TILs recognize cancer cells by specific antigens and release cytokines and cytolytic proteins to kill cancer cells. As a result, an active immune response in patients can largely support the therapeutic effect of chemotherapy. The relationship between chemotherapy and immunity may explain the observed trend in our study that higher CD8+ TILs or lower TGF‐β expression appeared to correlate with reponse to first‐line chemotherapy.
Anti‐PD‐1/PD‐L1 antibodies, such as nivolumab and atezolizumab approved by the United States Food and Drug Administration (FDA) are now standard therapies for a range of solid tumours.28, 56, 57, 58, 59 More patients achieve a durable response to immunotherapy for a year or more.60, 61, 62, 63 Because the immune response plays an important synergistic role in chemotherapy, the restoration of active immunity during chemotherapy may be beneficial to cancer patients. Pre‐clinical mouse models have illustrated significantly higher therapeutic effects of chemotherapy in immunocompetent animals compared to immunodeficient mice.64 Clinical trials, including FDA‐approved nivolumab and other PD‐1/PD‐L1 inhibitors combined with chemotherapy, are underway to investigate the therapeutic efficacy and clinical benefits. Recently, 22.5% of patients with recurrent thymic carcinoma who had progressed after at least one line of chemotherapy with pembrolizumab achieved an objective response, and the proportion of PD‐L1 positive patients was significantly higher in the responding group than in the non‐responding group.65 This suggests that PD‐L1 may be a useful biomarker to predict the efficacy of immunotherapy in patients with thymic carcinoma.
Several limitations of our study need to be addressed. First, clinical validation was based on a retrospective setting and the limited sample size might cause statistical bias. Second, insufficient information on treatment outcomes may have influenced the ultimate clinical outcomes to some degree. The findings of this study warrant further investigation.
In conclusion, PD‐L1, TGF‐β, and CD8+ CTLs may be used as potential prognostic factors for clinical outcome in advanced TETs, especially in advanced thymic carcinoma. The results of this study support a clinical trial of immunotherapy in this rare tumor type and warrant further evaluation.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We wish to thank Zhongwu Li for examination of the IHC results. We also thank Tongfu Xu (an employee of 3D Medicines Inc.) for revising our manuscript.
References
- 1. Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010; 5 (10 Suppl 4): S260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lamarca A, Moreno V, Feliu J. Thymoma and thymic carcinoma in the target therapies era. Cancer Treat Rev 2013; 39: 413–20. [DOI] [PubMed] [Google Scholar]
- 3. Marx A, Rieker R, Toker A, Länger F, Ströbel P. Thymic carcinoma: Is it a separate entity? From molecular to clinical evidence. Thorac Surg Clin 2011; 21: 25–31. [DOI] [PubMed] [Google Scholar]
- 4. Kelly RJ. Thymoma versus thymic carcinoma: Differences in biology impacting treatment. J Natl Compr Canc Netw 2013; 11: 577–83. [DOI] [PubMed] [Google Scholar]
- 5. Hillen F, Baeten CI, van de Winkel A et al Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother 2008; 57: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexe G, Dalgin GS, Scanfeld D et al High expression of lymphocyte‐associated genes in node‐negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res 2007; 67: 10669–76. [DOI] [PubMed] [Google Scholar]
- 7. Mahmoud SM, Paish EC, Powe DG et al Tumor‐infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29: 1949–55. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Shibli KI, Donnem T, Al‐Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non‐small cell lung cancer. Clin Cancer Res 2008; 14: 5220–7. [DOI] [PubMed] [Google Scholar]
- 9. Kawai O, Ishii G, Kubota K et al Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008; 113: 1387–95. [DOI] [PubMed] [Google Scholar]
- 10. Mlecnik B, Tosolini M, Kirilovsky A et al Histopathologic‐based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29: 610–8. [DOI] [PubMed] [Google Scholar]
- 11. Kusuda T, Shigemasa K, Arihiro K, Fujii T, Nagai N, Ohama K. Relative expression levels of Th1 and Th2 cytokine mRNA are independent prognostic factors in patients with ovarian cancer. Oncol Rep 2005; 13: 1153–8. [PubMed] [Google Scholar]
- 12. Sato E, Olson SH, Ahn J et al Intraepithelial CD8+ tumor‐infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102: 18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang L, Conejo‐Garcia JR, Katsaros D et al Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348: 203–13. [DOI] [PubMed] [Google Scholar]
- 14. Momtaz P, Postow MA. Immunologic checkpoints in cancer therapy: Focus on the programmed death‐1 (PD‐1) receptor pathway. Pharmgenomics Pers Med 2014; 7: 357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD‐L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD‐L1 blockade. Proc Natl Acad Sci USA 2002; 99: 12293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death‐1 ligand 1 interacts specifically with the B7‐1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27: 111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang L, Pang Y, Moses HL. TGF‐beta and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31: 220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dahlin AM, Henriksson ML, Van Guelpen B et al Colorectal cancer prognosis depends on T‐cell infiltration and molecular characteristics of the tumor. Mod Pathol 2011; 24: 671–82. [DOI] [PubMed] [Google Scholar]
- 19. Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD‐L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011; 28: 682–8. [DOI] [PubMed] [Google Scholar]
- 20. Hodi FS, O'Day SJ, McDermott DF et al Improved survival with ipilimumab in patients with metastatic melanoma. (Published erratum appears in N Engl J Med 2010; 363: 1290.). N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. [DOI] [PubMed] [Google Scholar]
- 22. Motzer RJ, Escudier B, McDermott DF et al Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015; 373: 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bauml J, Seiwert TY, Pfister DG et al Pembrolizumab for platinum‐ and cetuximab‐refractory head and neck cancer: Results from a single‐arm, phase II study. J Clin Oncol 2017; 35: 1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ansell SM, Lesokhin AM, Borrello I et al PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenberg JE, Hoffman‐Censits J, Powles T et al Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaufman HL, Russell J, Hamid O et al Avelumab in patients with chemotherapy‐refractory metastatic Merkel cell carcinoma: A multicentre, single‐group, open‐label, phase 2 trial. Lancet Oncol 2016; 17: 1374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le DT, Durham JN, Smith KN et al Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science 2017; 357: 409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El‐Khoueiry AB, Sangro B, Yau T et al Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open‐label, non‐comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muro K, Chung HC, Shankaran V et al Pembrolizumab for patients with PD‐L1‐positive advanced gastric cancer (KEYNOTE‐012): A multicentre, open‐label, phase 1b trial. Lancet Oncol 2016; 17: 717–26. [DOI] [PubMed] [Google Scholar]
- 30. Robert C, Schachter J, Long GV et al Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–32. [DOI] [PubMed] [Google Scholar]
- 31. Padda SK, Riess JW, Schwartz EJ et al Diffuse high intensity PD‐L1 staining in thymic epithelial tumors. J Thorac Oncol 2015; 10: 500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katsuya Y, Fujita Y, Horinouchi H, Ohe Y, Watanabe SI, Tsuta K. Immunohistochemical status of PD‐L1 in thymoma and thymic carcinoma. Lung Cancer 2015; 88: 154–9. [DOI] [PubMed] [Google Scholar]
- 33. Yokoyama S, Miyoshi H, Nakashima K et al Prognostic value of programmed death ligand 1 and programmed death 1 expression in thymic carcinoma. Clin Cancer Res 2016; 22: 4727–34. [DOI] [PubMed] [Google Scholar]
- 34. Katsuya Y, Horinouchi H, Asao T et al Expression of programmed death 1 (PD‐1) and its ligand (PD‐L1) in thymic epithelial tumors: Impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer 2016; 99: 4–10. [DOI] [PubMed] [Google Scholar]
- 35. Arbour KC, Naidoo J, Steele KE et al Expression of PD‐L1 and other immunotherapeutic targets in thymic epithelial tumors. PLoS One 2017; 12: e0182665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor‐based immunotherapy. Lancet Oncol 2016; 17: e542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li MO, Wan YY, Sanjabi S, Robertson AKL, Flavell RA. Transforming growth factor‐beta regulation of immune responses. Annu Rev Immunol 2006; 24: 99–146. [DOI] [PubMed] [Google Scholar]
- 38. Wahl SM, Wen J, Moutsopoulos N. TGF‐beta: A mobile purveyor of immune privilege. Immunol Rev 2006; 213: 213–27. [DOI] [PubMed] [Google Scholar]
- 39. Nakamura K, Kitani A, Strober W. Cell contact‐dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface‐bound transforming growth factor beta. J Exp Med 2001; 194: 629–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghiringhelli F, Ménard C, Terme M et al CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor‐beta‐dependent manner. J Exp Med 2005; 202: 1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF‐beta1 secretion and down‐modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 2004; 172: 7335–40. [DOI] [PubMed] [Google Scholar]
- 42. Kehrl JH, Thevenin C, Rieckmann P, Fauci AS. Transforming growth factor‐beta suppresses human B lymphocyte Ig production by inhibiting synthesis and the switch from the membrane form to the secreted form of Ig mRNA. J Immunol 1991; 146: 4016–23. [PubMed] [Google Scholar]
- 43. Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res 2006; 66: 7741–7. [DOI] [PubMed] [Google Scholar]
- 44. Thomas A, Rajan A, Berman A et al Sunitinib in patients with chemotherapy‐refractory thymoma and thymic carcinoma: An open‐label phase 2 trial. Lancet Oncol 2015; 16: 177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu P, Wu D, Li L, Chai Y, Huang J. PD‐L1 and survival in solid tumors: A meta‐analysis. PLoS One 2015; 10: e0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour‐infiltrating lymphocytes in cancer: A systematic review with meta‐analysis. Br J Cancer 2011; 105: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death‐1 ligand‐1 (PD‐L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006; 108: 19–24. [DOI] [PubMed] [Google Scholar]
- 48. Hou J, Yu Z, Xiang R et al Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7‐H1 in the tumor tissues of gastric cancer. Exp Mol Pathol 2014; 96: 284–91. [DOI] [PubMed] [Google Scholar]
- 49. Shi SJ, Wang LJ, Wang GD et al B7‐H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One 2013; 8: e76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen L, Deng H, Lu M et al B7‐H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol 2014; 7: 6015–23. [PMC free article] [PubMed] [Google Scholar]
- 51. Tiseo M, Damato A, Longo L et al Analysis of a panel of druggable gene mutations and of ALK and PD‐L1 expression in a series of thymic epithelial tumors (TETs). Lung Cancer 2017; 104: 24–30. [DOI] [PubMed] [Google Scholar]
- 52. Weissferdt A, Fujimoto J, Kalhor N et al Expression of PD‐1 and PD‐L1 in thymic epithelial neoplasms. Mod Pathol 2017; 30: 826–33. [DOI] [PubMed] [Google Scholar]
- 53. Pagès F, Berger A, Camus M et al Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353: 2654–66. [DOI] [PubMed] [Google Scholar]
- 54. Galon J, Costes A, Sanchez‐Cabo F et al Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313: 1960–4. [DOI] [PubMed] [Google Scholar]
- 55. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune‐based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale‐based combined treatments against cancer. Cell Death Differ 2014; 21: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Muro K, Chung HC, Shankaran V et al Pembrolizumab for patients with PD‐L1‐positive advanced gastric cancer (KEYNOTE‐012): a multicentre, open‐label, phase 1b trial. The Lancet Oncology 2016; 17 (6): 717–26. [DOI] [PubMed] [Google Scholar]
- 57. Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site: When a biomarker defines the indication. N Engl J Med 2017; 377: 1409–12. [DOI] [PubMed] [Google Scholar]
- 58. Larkin J, Chiarion‐Sileni V, Gonzalez R, B et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Balar AV, Weber JS. PD‐1 and PD‐L1 antibodies in cancer: Current status and future directions. Cancer Immunol Immunother 2017; 66: 551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weber JS, Kudchadkar RR, Yu B et al Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab‐refractory or ‐naive melanoma. J Clin Oncol 2013; 31: 4311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Topalian SL, Sznol M, McDermott DF et al Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32: 1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brahmer JR, Drake CG, Wollner I et al Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee Y, Auh SL, Wang Y et al Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009; 114: 589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Giaccone G, Kim C, Thompson J et al Pembrolizumab in patients with thymic carcinoma: A single‐arm, single‐centre, phase 2 study. Lancet Oncol 2018; 19: 347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


