Abstract
Background
Since the conception of enhanced recovery after surgery protocols, tubeless strategies have become popular. Herein, we introduce a previously unreported alternative air‐extraction strategy for patients who have undergone thoracoscopic wedge resection and explore its feasibility and safety.
Methods
Between January 2015 and June 2017, 264 consecutive patients underwent thoracoscopic wedge resection with different drainage strategies. Patients were divided according to the postoperative drainage strategies used: routine chest tube drainage (RT group), complete omission of chest tube drainage (OT group), and prophylactic air‐extraction catheter insertion procedure (PC group). Using the propensity score matching method, clinical parameters and objective operative qualities were compared among the three groups.
Results
Optimal 1:1 matching was used to form pairs of RT (n =36) and PC (n =36) groups and balance baseline characteristics among the three groups. The incidence rates of pneumothorax were 5.6% (2/36), 9.8% (5/51), and 19.4% (7/36) in the RT, OT, and PC groups, respectively (P = 0.07). Chest tube reinsertion incidence for postoperative pneumothorax was 19.4% (1/7) in the PC group and 60% (3/5) in the OT group. Other postoperative complications were comparable among these groups.
Conclusions
The prophylactic air‐extraction strategy may be an alternative procedure for selected patients. Remedial air extraction may reduce the occurrence of chest tube reinsertion for pneumothorax patients, but further investigation is required.
Keywords: Air‐extraction strategy, pneumothorax, thoracoscopic wedge resection, tubeless
Introduction
The enhanced recovery after surgery (ERAS) protocol was first introduced in 2001, and focuses on patient recovery rather than fast discharge.1 ERAS has increased in popularity in recent years and has become the central theme of perioperative protocol in multidisciplinary works. In the future, ERAS in combination with population features and healthcare policies will result in positive changes in clinical practice.2
With the extensive implementation of low‐dose computed tomography (CT) screening, an increasing number of patients are being detected with small pulmonary nodules.3, 4 Thoracoscopic wedge resection is a minimally invasive operation, especially for patients with ground glass or suspected benign nodules.5, 6, 7, 8, 9, 10 Because of the limited resection range and ease of use, the incidence of postoperative complications after thoracoscopic wedge resection is low.6, 8, 9, 11, 12, 13 The tubeless strategy, which directly omits chest tube drainage, was first used in a thoracoscopic wedge resected population.13, 14, 15, 16, 17 However, previous studies have reported that this procedure has an increased rate of complications, especially pneumothorax (5.9–40%).15, 18, 19, 20 Thus, some investigators have suggested the use of a preset air‐extraction catheter for prophylactic or remedial air‐extraction, which we call a prophylactic air‐extraction strategy.
In the present study, we introduced this innovative strategy for patients who had undergone thoracoscopic wedge resection and retrospectively analyzed clinical data to evaluate the feasibility and safety of the prophylactic air‐extraction and other drainage strategies.
Methods
This study retrospectively analyzed the clinical data of 264 patients who underwent thoracoscopic wedge resection for pulmonary nodules at Guangdong General Hospital. The ethics committee of Guangdong General Hospital approved the study.
Study design and patients
Recruited patients met the following criteria: (i) underwent lung wedge resection between January 2015 and June 2017 at Guangdong General Hospital; (ii) with sufficient information from clinical records; and (iii) with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of ≤ 2.
After surgery, patients were observed in a monitored setting for one to five days. A chest roentgenogram was repeated before and after chest tube removal, and blood tests were taken as required. The following independent variables were ascertained by reviewing the medical records: age, gender, smoking history, ECOG PS score, nodule size, nodule location, and adhesion in surgery. The surgeon evaluated pleural adhesion, which refers to fibrous bands in the thoracic cavity, during surgery.
Postoperative factors included pneumothorax, pleural effusion, lung infection, chest tube reinsertion, postoperative Numerical Rating Scale (NRS) score, and perioperative death. Pneumothorax was defined as the apex (top) of the lung (a line) surpassing 3 cm and was diagnosed by chest roentgenography.21 Pleural effusion was defined as blunting of the costophrenic angle on the chest roentgenogram. Postoperative pain on day 1 after surgery was evaluated using the NRS.
Preset air‐extraction catheter procedure
After thoracoscopic wedge resection, patients underwent an underwater air‐tightness test, which was checked by the insertion of a tube immersed in water and the gradual inflation of the remaining lung to ensure no obvious air leaks. The patients were categorized into the following three groups based on the postoperative drainage strategies used: routine chest tube drainage (RT), omission of chest tube drainage (OT), and prophylactic air‐extraction catheter insertion procedure (PC) (Fig 1). In the RT group, a 20 Fr or 24 Fr chest tube for drainage was inserted. In the OT group, the incision was directly closed after two‐lung ventilation. In the PC group, a two‐lumen central venous catheter (20 cm 7 Fr) was inserted into the second intercostal space before directly closing the incision (Video S1, Fig 2).
Figure 1.
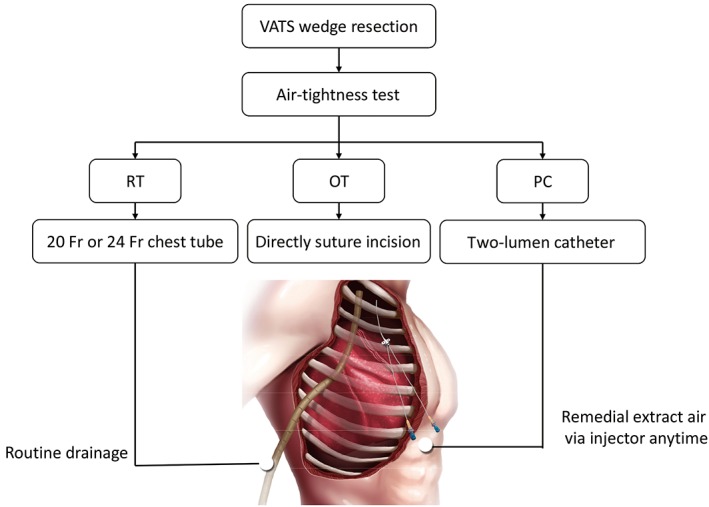
The core procedure of different drainage strategies. OT, complete omission of chest tube drainage; PC, prophylactic air‐extraction catheter insertion procedure; RT, routine chest tube drainage VATS, video‐assisted thoracoscopic surgery.
Figure 2.
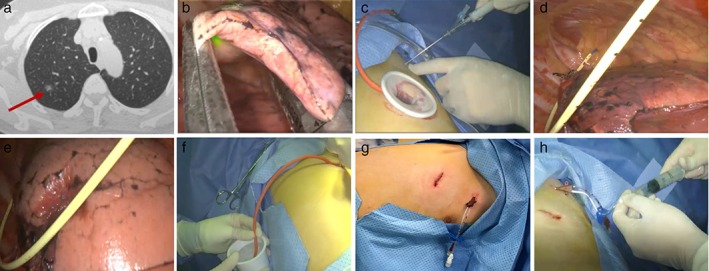
The prophylactic air‐extraction strategy procedure. (a) A 1.1 cm mixed ground glass nodule in the right upper lobe; (b) uniportal video‐assisted thoracoscopic surgery (VATS) wedge resection; (c) a puncture into the second intercostal space; (d) preset two‐lumen central venous catheter; (e) gradual inflation of the remaining lung; (f) air‐tightness test when suturing; (g) the incision after suturing; and (h) air‐extraction via injector.
In the PC group, if the chest roentgenogram revealed a pneumothorax on postoperative day 1, the air‐extraction catheter strategy was performed using an injector through the preset catheter.
Statistical analyses
To avoid confounding baseline differences between the RT and PC groups, we performed our analyses in all patients after propensity score matching. Patients in the RT and PC groups were matched by propensity scores using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The propensity score of an individual was calculated according to the covariates of the objective and subjective clinical parameters using a logistic regression model. We applied 1:1 nearest neighbor matching without replacement to ensure that the conditional bias was minimized. Categorical variables were compared using the χ2 test. Continuous variables were compared using the Kruskal–Wallis test. All statistical tests were two‐sided. A P value of < 0.05 was considered statistically significant.
Results
Between January 2015 and June 2017, 264 consecutive patients who underwent thoracoscopic wedge resection for pulmonary nodules were enrolled in the study. Patients were divided into the following three groups according to the different drainage strategies used: RT (n = 176), OT (n = 51), and PC (n = 37). To minimize the selection bias, optimal 1:1 propensity score matching was performed for gender, age, smoking history, ECOG PS score, nodule size, nodule location, and adhesion in surgery. The analysis is presented in Figure 3. As shown in Table 1, differences in age, ECOG PS score, and adhesion in surgery were balanced among the three groups after propensity score matching (age: before P = 0.031, after P = 0.338; ECOG PS score: before P = 0.007, after P = 0.360; nodule size: before P = 0.029, after P = 0.230; and adhesion in surgery: before P < 0.001, after P = 0.586).
Figure 3.
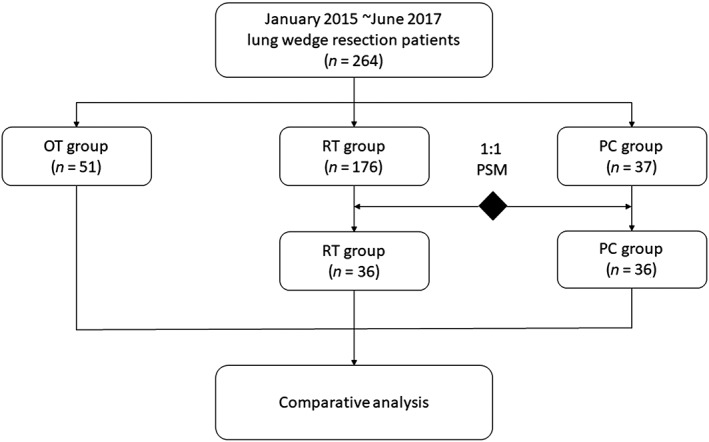
Schematic of the analysis. OT, complete omission of chest tube drainage; PC, prophylactic air‐extraction catheter insertion procedure; PSM, propensity score matching; RT, routine chest tube drainage.
Table 1.
Baseline characteristics before and after PSM in patients who underwent thoracoscopic wedge resection between 2015 and 2017
| Characteristics | Before PSM | P | After PSM | P | ||||
|---|---|---|---|---|---|---|---|---|
| RT group | OT group | PC group | RT group | OT group | PC group | |||
| Gender | 0.189 | 0.128 | ||||||
| Male | 89 (50.6%) | 27 (52.9%) | 13 (35.1%) | 12 (33.3%) | 27 (52.9%) | 13 (36.1%) | ||
| Female | 87 (49.4%) | 24 (47.1%) | 24 (64.9%) | 24 (66.7%) | 24 (47.1%) | 23 (63.9%) | ||
| Age | 57 (16~91) | 53 (24~82) | 54 (31~79) | 0.031 | 52 (16~82) | 53 (24~82) | 55.5 (31~79) | 0.338 |
| Smoking | ||||||||
| No | 149 (84.7%) | 45 (88.2%) | 36 (97.3%) | 0.110 | 35 (97.2%) | 45 (88.2%) | 35 (97.2%) | 0.137 |
| Yes | 27 (15.3%) | 6 (11.8%) | 1 (2.70%) | 1 (2.8%) | 6 (11.8%) | 1 (2.8%) | ||
| ECOG score | 0.007 | 0.360 | ||||||
| 0 | 68 (38.6%) | 32 (62.7%) | 22 (59.5%) | 17 (47.2%) | 32 (62.7%) | 21 (58.3%) | ||
| 1 | 107 (60.8%) | 18 (35.3%) | 15 (40.5%) | 19 (52.8%) | 18 (35.3%) | 15 (41.7%) | ||
| 2 | 1 (0.6%) | 1 (2.0%) | 0 (0%) | 0 (0%) | 1 (2.0%) | 0 (0%) | ||
| Nodule size (cm) | 1.5 ± 0.8 | 1.5 ± 0.8 | 1.1 ± 0.6 | 0.029 | 1.2 ± 0.7 | 1.5 ± 0.8 | 1.2 ± 0.6 | 0.230 |
| Nodule location | 0.547 | 0.278 | ||||||
| RUL | 46 (26.1%) | 16 (31.4%) | 12 (32.4%) | 8 (22.2%) | 16 (31.4%) | 11 (30.6%) | ||
| RML | 8 (4.5%) | 4 (7.8%) | 3 (8.1%) | 2 (5.6%) | 4 (7.8%) | 3 (8.3%) | ||
| RLL | 43 (24.4%) | 10 (19.6%) | 5 (13.5%) | 14 (38.9%) | 10 (19.6%) | 5 (13.9%) | ||
| LUL | 45 (25.6%) | 8 (15.7%) | 11 (29.7%) | 7 (19.4%) | 8 (15.7%) | 11 (30.6%) | ||
| LLL | 34 (19.3%) | 13 (25.5%) | 6 (16.2%) | 5 (13.9%) | 13 (25.5%) | 6 (16.7%) | ||
| Operation duration (min) | 81.8 ± 35.7 | 78.1 ± 23.6 | 69.6 ± 27.3 | 0.113 | 66.5 ± 27.5 | 78.1 ± 23.6 | 69.6 ± 23.8 | 0.034 |
| Adhesion | ||||||||
| Yes | 131 (74.4%) | 48 (94.1%) | 35 (94.6%) | < 0.001 | 35 (97.2%) | 48 (94.1%) | 34 (94.4%) | 0.586 |
| No | 45 (25.6%) | 3 (5.9%) | 2 (5.4%) | 1 (2.8%) | 3 (5.9%) | 2 (5.6%) | ||
| Pathology | 0.018 | 0.053 | ||||||
| Primary tumor | 114 (64.8%) | 22 (43.1%) | 27 (73.0%) | 23 (63.8%) | 22 (43.1%) | 26 (72.2%) | ||
| Metastatic tumor | 13 (7.4%) | 3 (5.9%) | 2 (5.4%) | 2 (5.6%) | 3 (5.9%) | 2 (5.6%) | ||
| Benign lesion | 49 (27.8%) | 26 (51.0%) | 8 (21.6%) | 11 (30.6%) | 26 (51.0%) | 8 (22.2%) | ||
Significant P values are given in bold.
ECOG, Eastern Cooperative Oncology Group; LLL, left lower lobe; LUL, left upper lobe; OT, complete omission of chest tube drainage; PC, prophylactic air‐extraction catheter insertion procedure; PSM, propensity score matching; RLL, right lower lobe; RML, right middle lobe; RT, routine chest tube drainage; RUL, right upper lobe.
Table 2 compares the summarized postoperative statistics for these groups. No significant differences were identified in all three groups in terms of pneumothorax, pleural effusion, lung infection, chest tube reinsertion, postoperative NRS score, hospitalization days, and perioperative death. The incidence of pneumothorax was the most common postoperative complication (14/123, 11.4%). A significant correlation was found between the RT, OT, and PC groups (5.6%, 9.8%, and 19.4%, respectively; P = 0.07); however, only one patient (1/7, 14.3%) among the seven patients with pneumothorax in the PC group underwent chest tube reinsertion compared to three patients (3/5, 60%) in the RT group, which suggests that the air extraction catheter strategy in the PC group was efficient in reducing the incidence of chest tube reinsertion.
Table 2.
Perioperative characteristics of each group
| Characteristics | RT group (n = 36) | OT group (n = 51) | PC group (n = 36) | P |
|---|---|---|---|---|
| Pneumothorax (a line ≥ 3 cm) | 2 (5.6%) | 5 (9.8%) | 7 (19.4%) | 0.07 |
| Pleural effusion | 0 (0%) | 1 (2.0%) | 0 (0%) | 0.41 |
| Lung infection | 1 (2.8%) | 0 (0%) | 0 (0%) | 0.19 |
| Perioperative death | 1 (2.8%) | 0 (0%) | 0 (0%) | 0.19 |
| NRS score | 3.4 ± 1.1 | 2.3 ± 0.9 | 2.3 ± 0.8 | < 0.001 |
| Chest tube reinsertion | 1 (2.8%) | 3 (5.9%) | 1 (2.8%) | 0.696 |
| Postoperative hospitalization | 4.8 ± 2.9 | 3.3 ± 1.5 | 2.9 ± 1.4 | < 0.001 |
Significant P values are given in bold.
LRT, routine chest tube drainage; NRS, Numerical Rating Scale; OT, complete omission of chest tube drainage; PC, prophylactic air‐extraction catheter insertion procedure; RT, routine chest tube drainage.
Figure 4 shows the indication for prophylactic air‐extraction catheter strategies for thoracoscopic wedge resected patients. One patient underwent thoracoscopic wedge resection of a 0.9 cm pure ground‐glass nodule in the right upper lobe using a two‐lumen catheter. The chest roentgenogram on postoperative day 1 showed massive pneumothorax (a line = 4.6 cm) (Fig 4a). After 500 ml of air was extracted using an injector, reexamination via roentgenography showed that the pneumothorax had improved (a line = 1.8 cm) (Fig 4b). The patient was discharged on postoperative day 3.
Figure 4.
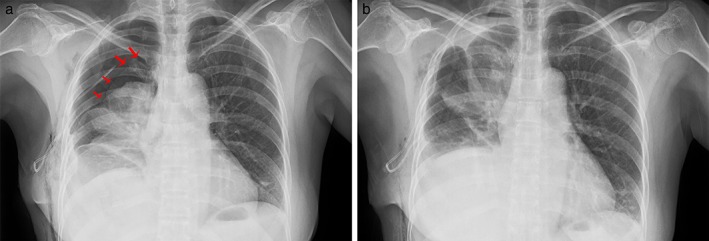
Chest roentgenogram of a patient who underwent air extraction using the prophylactic air‐extraction catheter insertion procedure. Chest roentgenogram on (a) postoperative day 1 shows massive pneumothorax (the red arrow shows the pneumothorax line) and (b) after the remedial air‐extraction strategy.
Discussion
Enhanced recovery after surgery describes a multimodal perioperative care program.1, 22 Tubeless strategies are a major area of interest within the field of ERAS for thoracic surgery.23 Recently, there has been considerable interest in the literature on the omission of chest tube drainage for certain patients, suggesting that the omission of chest tube drainage for thoracoscopic wedge resected patients could be an alternative, less invasive option.15 However, the complete removal of drainage might increase the rate of complications, especially pneumothorax, and some patients may need chest tube reinsertion.14, 15, 19 In our study we used a prophylactic air‐extraction strategy for thoracoscopic wedge resected patients. Our results showed that this strategy could be an alternative procedure and may reduce the incidence of chest tube reinsertion in patients with pneumothorax.
With the improvement of surgical techniques and thoracoscopic instruments, omission of chest tube drainage has gradually been adapted for thoracoscopic wedge resection. Nevertheless, some complications (mainly pneumothorax) still occur. According to previous studies on tubeless strategies, the incidence rate of chest tube reinsertion is 2.9–4.8%, and it is approximately 50% in pneumothorax patients.15, 20 The main goal of this prophylactic air‐extraction strategy is to avoid chest tube reinsertion. Moreover, the preset catheter can remove remnant air, thereby facilitating patient recovery. The most prominent problem of the two‐lumen catheter is plugging, as demonstrated by one patient in the PC group who required chest tube reinsertion. This could be avoided by following a few important rules: (i) the puncture needle should incline to the head during the puncture process; (ii) the distal portion of the catheter should be kept close to the top of the chest; (iii) the patient should be in a sitting position and should be breathing deeply when pumping; and (iv) once the catheter is blocked, the catheter should be pulled out a little or some air should be pumped into the thorax.
Our findings showed comparable results of the prophylactic air‐extraction strategy and routine chest tube drainage. In our study, the incidence rate of postoperative pneumothorax was 9.8% in the OT group and 19.4% in the PC group, which are consistent with previous reports.18, 19, 20 However, on the other hand, patients in the tubeless strategy groups (OT + PC groups) had reduced postoperative pain based on NRS scores compared to the RT group (RT vs. OT vs. PC: 3.4 ± 1.1 vs. 2.3 ± 0.9 vs. 2.3 ± 0.8, respectively; P < 0.001). In addition, the tubeless strategy groups also had fewer postoperative hospitalization days (RT vs. OT vs. PC: 4.8 ± 2.9 vs. 3.3 ± 1.5 vs. 2.9 ± 1.4, respectively; P < 0.001). These results corroborate the findings of the previous studies. A list of potential advantages and disadvantages of the different drainage strategies is summarized in Table 3.
Table 3.
Comparison of the different drainage strategies
| Characteristics | Routine chest tube | Omission of chest tube | Prophylactic air‐extraction strategy |
|---|---|---|---|
| Pneumothorax or pleural effusion | Low risk, real‐time observation | High risk, patients with resistant pneumothorax may need chest tube reinsertion | High risk, remedial air extraction may decrease the occurrence of chest tube reinsertion |
| Wound satisfaction | Acceptable | Satisfied | Satisfied |
| Pain assessment | Moderate or severe pain | Pain free or without discomfort | Pain free or without discomfort |
| Subcutaneous emphysema | High risk | Low risk | Low risk |
| Postoperative hospitalization | Long | Short | Short |
| Lung infection | Low risk | Low risk | Low risk |
As this is a preliminary feasibility study, we acknowledge several limitations, including the retrospective design and subjective bias. To resolve this, we have since designed a randomized comparative study (TBL‐1, NCT03230019) to clarify the safety and benefits of this prophylactic air‐extraction strategy.
In conclusion, the prophylactic air‐extraction strategy may be an alternative air‐extraction procedure for selected thoracoscopic wedge resected patients. This remedial air extraction strategy may decrease the incidence of chest tube reinsertion in patients with pneumothorax; however, further investigation of this strategy is warranted.
Disclosure
No authors report any conflict of interest.
Supporting information
Video S1. Prophylactic air‐extraction strategy for thoracoscopic wedge resection.
Acknowledgments
We express our deepest gratitude to the anonymous reviewers for their careful work and thoughtful suggestions that have substantially improved this paper. This work was supported by grants from the National Natural Science Foundation of China (Grant NO.81673031), the Research Fund from Guangzhou Science and Technology Bureau (Grant No. 201704020161), the Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (Grant No. 2012A061400006), and the Special Fund for Research in the Public Interest from National Health and Family Planning Commission of PRC (Grant No.201402031).
References
- 1. Ljungqvist O. ERAS–enhanced recovery after surgery: Moving evidence‐based perioperative care to practice. JPEN J Parenter Enteral Nutr 2014; 38: 559–66. [DOI] [PubMed] [Google Scholar]
- 2. Wang JY, Hong X, Chen GH, Li QC, Liu ZM. Clinical application of the fast track surgery model based on preoperative nutritional risk screening in patients with esophageal cancer. Asia Pac J Clin Nutr 2015; 24: 206–11. [DOI] [PubMed] [Google Scholar]
- 3. Saghir Z, Dirksen A, Ashraf H et al CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: Status after five annual screening rounds with low‐dose CT. Thorax 2012; 67: 296–301. [DOI] [PubMed] [Google Scholar]
- 4. National Lung Screening Trial Research Team , Aberle DR, Adams AM et al Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altorki NK, Kamel MK, Narula N et al Anatomical segmentectomy and wedge resections are associated with comparable outcomes for patients with small cT1N0 non‐small cell lung cancer. J Thorac Oncol 2016; 11: 1984–92. [DOI] [PubMed] [Google Scholar]
- 6. Nakamura H, Taniguchi Y, Miwa K et al Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non‐small cell lung cancer. J Thorac Cardiovasc Surg 2011; 59: 137–41. [DOI] [PubMed] [Google Scholar]
- 7. Yoshida J, Nagai K, Yokose T et al Limited resection trial for pulmonary ground‐glass opacity nodules: fifty‐case experience. J Thorac Cardiovasc Surg 2005; 129: 991–6. [DOI] [PubMed] [Google Scholar]
- 8. Grills IS, Mangona VS, Welsh R et al Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non‐small‐cell lung cancer. J Clin Oncol 2010; 28: 928–35. [DOI] [PubMed] [Google Scholar]
- 9. Shennib H, Bogart J, Herndon JE et al Video‐assisted wedge resection and local radiotherapy for peripheral lung cancer in high‐risk patients: The Cancer and Leukemia Group B (CALGB) 9335, a phase II, multi‐institutional cooperative group study. J Thorac Cardiovasc Surg 2005; 129: 813–8. [DOI] [PubMed] [Google Scholar]
- 10. Detterbeck FC, Homer RJ. Approach to the ground‐glass nodule. Clin Chest Med 2011; 32: 799–810. [DOI] [PubMed] [Google Scholar]
- 11. Endo C, Sagawa M, Sakurada A, Sato M, Kondo T, Fujimura S. Surgical treatment of stage I non‐small cell lung carcinoma. Ann Thorac Cardiovasc Surg 2003; 9: 283–9. [PubMed] [Google Scholar]
- 12. Rotman JA, Plodkowski AJ, Hayes SA et al Postoperative complications after thoracic surgery for lung cancer. Clin Imaging 2015; 39: 735–49. [DOI] [PubMed] [Google Scholar]
- 13. Li S, Jiang L, Ang KL et al New tubeless video‐assisted thoracoscopic surgery for small pulmonary nodules. Eur J Cardiothorac Surg 2017; 51: 689–93. [DOI] [PubMed] [Google Scholar]
- 14. Ueda K, Hayashi M, Tanaka T, Hamano K. Omitting chest tube drainage after thoracoscopic major lung resection. Eur J Cardiothorac Surg 2013; 44: 225–9. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe A, Watanabe T, Ohsawa H et al Avoiding chest tube placement after video‐assisted thoracoscopic wedge resection of the lung. Eur J Cardiothorac Surg 2004; 25: 872–6. [DOI] [PubMed] [Google Scholar]
- 16. Refai M, Brunelli A, Salati M, Xiumè F, Pompili C, Sabbatini A. The impact of chest tube removal on pain and pulmonary function after pulmonary resection. Eur J Cardiothorac Surg 2012; 41: 820–2. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez‐Rivas D, Aymerich H, Bonome C, Fieira E. From open operations to nonintubated uniportal video‐assisted thoracoscopic lobectomy: Minimizing the trauma to the patient. Ann Thorac Surg 2015; 100: 2003–5. [DOI] [PubMed] [Google Scholar]
- 18. Yang SM, Wang ML, Hung MH, Hsu HH, Cheng YJ, Chen JS. Tubeless uniportal thoracoscopic wedge resection for peripheral lung nodules. Ann Thorac Surg 2017; 103: 462–8. [DOI] [PubMed] [Google Scholar]
- 19. Luckraz H, Rammohan KS, Phillips M et al Is an intercostal chest drain necessary after video‐assisted thoracoscopic (VATS) lung biopsy? Ann Thorac Surg 2007; 84: 237–9. [DOI] [PubMed] [Google Scholar]
- 20. Hung MH, Cheng YJ, Chan KC et al Nonintubated uniportal thoracoscopic surgery for peripheral lung nodules. Ann Thorac Surg 2014; 98: 1998–2003. [DOI] [PubMed] [Google Scholar]
- 21. Baumann MH, Strange C, Heffner JE et al Management of spontaneous pneumothorax: An American College of Chest Physicians Delphi consensus statement. Chest 2001; 119: 590–602. [DOI] [PubMed] [Google Scholar]
- 22. Fearon KC, Ljungqvist O, Von Meyenfeldt M et al Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005; 24: 466–77. [DOI] [PubMed] [Google Scholar]
- 23. Mueller XM, Tinguely F, Tevaearai HT, Ravussin P, Stumpe F, von Segesser LK. Impact of duration of chest tube drainage on pain after cardiac surgery. Eur J Cardiothorac Surg 2000; 18: 570–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Prophylactic air‐extraction strategy for thoracoscopic wedge resection.


