Abstract
Background
Recombinant human endostatin (rh‐endostatin) plus standard chemotherapy in advanced non‐small cell lung cancer (NSCLC) patients has shown improved efficacy; however, it is unclear whether it is effective and safe when added to pemetrexed/cisplatin and used as maintenance therapy.
Methods
We retrospectively evaluated the data of untreated NSCLC patients administered rh‐endostatin plus pemetrexed/cisplatin or pemetrexed/cisplatin. The primary endpoint was progression‐free survival (PFS).
Results
Fifty‐six and 39 patients received rh‐endostatin plus pemetrexed/cisplatin and pemetrexed/cisplatin, and 34 and 29 underwent maintenance treatment, respectively. The median PFS was 10 months (95% confidence interval [CI] 5.85–14.15) in the rh‐endostatin and 8.2 months (4.04–12.36) in the chemotherapy group, but the difference was not statistically significant (P = 0.13). In patients administered maintenance treatment, rh‐endostatin plus pemetrexed was associated with prolonged PFS compared to single‐agent pemetrexed when PFS was calculated from first dosing (13.7 [9.41–17.99] vs. 8.2 [4.16–12.24]; P = 0.032); however, PFS did not differ between the groups (hazard ratio 0.618; 95% CI 0.368–1.038; P = 0.069) after adjusting for clinical factors. No difference was observed in the objective response rate between the groups (48.2% vs. 38.5%; P = 0.346), with the exception of men (62.1% vs. 33.3%; P = 0.032) or in the incidence of drug‐related or grade 3–4 adverse events.
Conclusion
In previously untreated, advanced‐stage NSCLC patients, first‐line treatment with pemetrexed/cisplatin plus rh‐endostatin did not prolong PFS or overall survival when compared to pemetrexed/cisplatin, but a trend of improved PFS was observed in patients administered maintenance rh‐endostatin plus pemetrexed.
Keywords: Anti‐angiogenesis, maintenance, non‐small cell lung cancer, rh‐endostatin
Introduction
Lung cancer remains a global health burden as the most common cancer and the leading cause of cancer‐related death, with an estimated 224 390 new cases diagnosed and 158 080 deaths per year in the United States, and an estimated 733 300 new cases diagnosed and 610 200 deaths per year in China.1, 2 Non‐small cell lung cancers (NSCLCs) comprise approximately 75–80% of lung cancers, which mainly consist of adenocarcinoma and squamous cell carcinoma. A platinum‐based, doublet chemotherapy regimen has been established as standard treatment. Recently, the introduction of pemetrexed has been found to be more effective than gemcitabine as a component of first‐line treatment for patients with non‐squamous carcinoma, particularly adenocarcinoma (median 12.6 vs. 10.9 months).3, 4
Angiogenesis plays a key role in the development of cancer.5, 6, 7 Several agents that target vascular endothelial growth factor receptor (VEGFR) have been approved for the treatment of NSCLC. In a first‐line setting, the addition of bevacizumab to chemotherapy significantly improved the clinical outcome with overall survival (OS) of 12.3 months in a Western population and 24.3 months in a Chinese population.8, 9 However, increased toxicity was observed during bevacizumab treatment and class‐related adverse events including hypertension, proteinuria, febrile neutropenia and life‐threatening pulmonary hemorrhage, particularly in squamous NSCLC.8, 9, 10 The approval of ramucirumab and nintedanib has provided new options for NSCLC patients who progressed on initial treatment, with improved OS and tolerable toxicity when combined with standard second‐line chemotherapy.11, 12, 13 More recently, anlotinib, a novel multitarget tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor 2 (VEGFR2), platelet‐derived growth factor receptor‐β (PDGFRβ), and fibroblast growth factor receptor‐1 (FGFR1), has provided significant progression‐free survival (PFS) and OS benefits as third‐line treatment.14
Endostatin is a C‐terminal fragment type of XVIII collagen that directly targets new capillary endothelial cells around a tumor. Using a yeast expression system, recombinant human endostatin (rh‐endostatin, Endostar) has been developed. Rh‐endostatin displays an increased tumor response when added to chemotherapy in NSCLC patients,15 with cardiac adverse events such as dose limited toxicity, and other mild drug‐related adverse events including fever, rash, dizziness, headache, diarrhea, fatigue, palpitation, and chest discomfort.16 A randomized, double‐blind, phase 3 study further confirmed these results, finding that rh‐endostatin plus vinorelbine/cisplatin was associated with significantly prolonged time to progression (6.3 vs. 3.6 months; P = 0.0000).17 Based on the results of such studies, rh‐endostatin was approved by the Chinese Food and Drug Administration in 2006 for the treatment of advanced NSCLC. However, no study has evaluated the efficacy and safety of rh‐endostatin when added to pemetrexed/cisplatin, and its role as maintenance therapy. Herein, we present a retrospective comparison of efficacy and safety between rh‐endostatin plus pemetrexed/cisplatin and pemetrexed/cisplatin in our department at a single center.
Methods
Patients
Retrospective analysis of data collected between November 2013 and January 2017 from a lung cancer database at the Cancer Hospital and Institute, Chinese Academy of Medical Sciences (CAMS, Beijing, China) was conducted. The eligibility criteria were as follows: pathologically or cytologically confirmed non‐squamous lung cancer; stage IIIB or IV disease (defined by American Joint Committee on Cancer Tumor Node Metastasis [TNM] staging system version 7.0); no previous systemic anticancer treatment; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 –1; at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1); and adequate bone marrow, hepatic, and renal functions. The primary endpoint was PFS, while secondary endpoints were objective response rate (ORR), disease control rate (DCR), OS, and safety.
This study was conducted in compliance with the Declaration of Helsinki and applicable local regulations. The hospital institutional review board approved the study protocol and written informed consent was obtained from each participant.
Treatment
Patients were administered pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 on day 1 by intravenous infusion (chemotherapy group) plus rh‐endostatin 7.5 mg/m2 on days 1–14 (rh‐endostatin group), every three weeks. Patients who experienced a tumor response or achieved stable disease (SD) after four to six cycles of treatment continued to receive maintenance rh‐endostatin plus pemetrexed or single‐agent pemetrexed until unacceptable adverse events or disease progression. Dose reduction or interruption of study drugs was allowed according to label recommendations. Clinical data was recorded at baseline, including age, gender, PS, biomarker analysis, disease stage, pathological subtype, and smoking status. Imaging evaluation by computed tomography (CT) scan was conducted every six to eight weeks according to clinical practice. Safety was monitored during the study period.
Treatment evaluation
All patients underwent a CT scan at the start of chemotherapy and then every six to eight weeks to evaluate the tumor response using RECIST v1.1. Safety assessments included physical examination, documentation of adverse events, electrocardiogram, and laboratory tests. Adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Tumor response was assessed every six to eight weeks using RECIST v1.1. The response‐evaluable population was defined as patients who received at least two cycles of treatment regimens with a measurable lesion.
Statistical analysis
The two groups were compared regarding basic clinical characteristics; efficacy outcomes in terms of PFS, ORR, DCR, and OS; and safety. PFS was measured as the interval between the date of first dosing and the date of disease progression or intolerable toxicity. ORR was defined as the percentage of patients who had a tumor response (complete response [CR] and/or partial response [PR]). DCR was defined as the percentage of patients who had CR and/or PR and SD. OS was measured as the interval between the date of first dosing and the date of death or last follow‐up. Survival analysis was conducted using the Kaplan–Meier method, and the differences according to treatment were compared using the log‐rank test. A Cox regression model was used for PFS and OS multivariate analyses to test the effect of independent variables such as gender, histology, disease stage, smoking status, and duration of treatment. Statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA). A P value < 0.05 was considered significant.
Results
Patient characteristics
From November 2013 to January 2017, 95 patients met the inclusion criteria and were included in the analysis. A total of 39 patients received pemetrexed/cisplatin and 56 received pemetrexed/cisplatin plus rh‐endostatin. No significant differences were observed in treatment cycles between the groups (Table S1).
Baseline characteristics and demographics were similar between the groups (Table 1). The mean ages were 54.07 and 57.41 years in the rh‐endostatin and chemotherapy groups, respectively. There were more male patients in the chemotherapy group than in the rh‐endostatin group (69.2% vs. 51.8%), but the difference was not statistically significant. All patients had lung adenocarcinoma, with the majority of patients in both arms with stage IV disease and good performance (PS score 0–1). A total of 51.3% patients in the chemotherapy group and 35.7% in the rh‐endostatin group were smokers. Test results of biomarkers in 56 patients showed EGFR mutations or ALK rearrangement. Biomarker status was also similar between the groups (Table 1).
Table 1.
Patient characteristics
| Characteristic | Chemotherapy + Endostar (n = 56) | Chemotherapy (n = 39) | P |
|---|---|---|---|
| Age (year, mean ± SD) | 54.07 ± 9.48 | 57.41 ± 11.09 | 0.119 |
| Gender | |||
| Female | 27 (48.2) | 12 (30.8) | 0.089 |
| Male | 29 (51.8) | 27 (69.2) | |
| Disease stage | |||
| IIIb | 7 (12.5) | 2 (5.1) | 0.395 |
| IV | 49 (87.5) | 37 (94.9) | |
| ECOG PS score | |||
| 0–1 | 56 (100.0) | 38 (97.4) | 0.411 |
| 2 | 0 (0.0) | 1 (2.6) | |
| Smoking status | |||
| Yes | 20 (35.7) | 20 (51.3) | 0.131 |
| No | 36 (64.3) | 19 (48.7) | |
| Presence of distinct metastasis | |||
| Yes | 55 (98.2) | 39 (100.0) | 1.000 |
| No | 1 (1.8) | 0 (0.0) | |
| Biomarker status | |||
| Unknown | 20 (35.7) | 19 (48.7) | 0.402 |
| Wild | 17 (30.4) | 10 (25.6) | |
| EGFR 19 Del | 7 (12.5) | 1 (2.6) | |
| EGFR 21 L858R | 8 (14.3) | 4 (10.3) | |
| Other EGFR mutation | 1 (1) | 1 (2.6) | |
| ALK rearrangement | 3 (5.4) | 4 (10.3) | |
Del, deletion; ECOG PS, Eastern Cooperative Oncology Group performance score; SD, standard deviation.
Efficacy
All patients experienced disease progression (n = 90) or died (n = 5). Figure 1 shows the Kaplan–Meier curve for overall PFS; the median PFS was 10 months (95% confidence interval [CI] 5.85–14.15) in the rh‐endostatin group and 8.2 months (4.04–12.36) in the chemotherapy group, but the difference was not statistically significant (P = 0.13) (Fig 1, Table 2). After adjusting for clinical factors (age, gender, disease stage, and smoking status), PFS did not differ in the overall study population between the rh‐endostatin and chemotherapy groups (hazard ratio [HR] 0.788, 95% CI 0.510–1.216; P = 0.282). The influence of the addition of maintenance on PFS is summarized in Table S2. No statistically significant difference in PFS was found between the groups in subgroup analysis, including gender, disease stage, or smoking status (Table S3). We also explored PFS in patients with identified tumor driven mutations, which was 7 months (95%CI 4.43–9.57), 9.5 months (4.24–14.76), and 17 months (15.72–18.28) for patients with EGFR 19 Del, EGFR 21 L858R, and ALK rearrangement, respectively.
Figure 1.
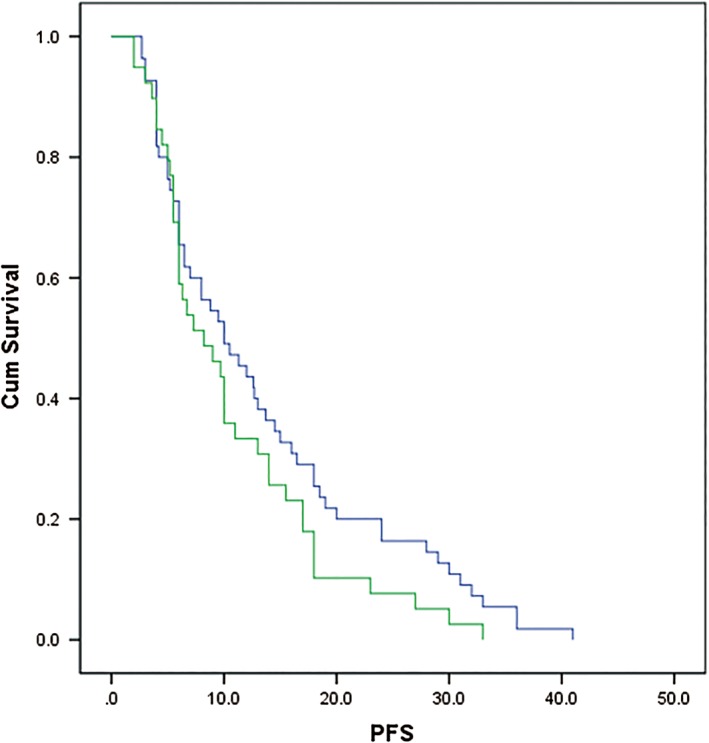
Kaplan–Meier curves for progression‐free survival (PFS). ( ) Rh‐endostatin plus chemotherapy with or without maintenance therapy and (
) Rh‐endostatin plus chemotherapy with or without maintenance therapy and ( ) chemotherapy with or without maintenance therapy.
) chemotherapy with or without maintenance therapy.
Table 2.
Efficacy of pemetrexed/cisplatin alone or with rh‐endostatin for advanced non‐small cell lung cancer
| Efficacy parameters | Pemetrexed/cisplatin (n = 39) | Rh‐endostatin + pemetrexed/cisplatin (n = 56) | P |
|---|---|---|---|
| PFS (95% CI), months | 8.2 (4.04–12.36) | 10 (5.85–14.15) | 0.13 |
| OS (95% CI), months | 36 (27.25–44.75) | 29 (25.1–32.9) | 0.775 |
| CR | 0 | 0 | — |
| PR | 27 | 15 | — |
| SD | 28 | 24 | — |
| ORR | 48.2% | 38.5% | 0.346 |
| DCR | 98.2% | 100% | 1.000 |
CI, confidence interval; CR, complete response; DCR, disease control rate; PFS, progression‐free survival; PR, partial response; ORR, overall response rate; OS, overall survival.
All patients had measurable lesions. The ORR and DCR were 48.2% and 98.2% in the rh‐endostatin group, and 38.5% and 100% in the chemotherapy group, respectively, without statistical difference (P = 0.346 and P = 1.000 for ORR and DCR, respectively) (Table 2). Similar response rates in women, non‐smokers, patients with stage IIIB or IV disease, and those with ECOG PS 0‐1 were observed between the groups (Table S2). Of note, men and smokers showed a higher response rate in the rh‐endostatin than in the chemotherapy group (Table S4).
At data cut‐off (30 September 2017), more than half (53.7%) of the patients were still alive. The median OS was 36 months (95% CI 27.25–44.75) in the rh‐endostatin group and 29 months (95% CI 25.1–32.9) in the chemotherapy group, without significant difference (P = 0.775) (Fig 2, Table 2). The influence of the addition of maintenance on OS is summarized in Table S2.
Figure 2.
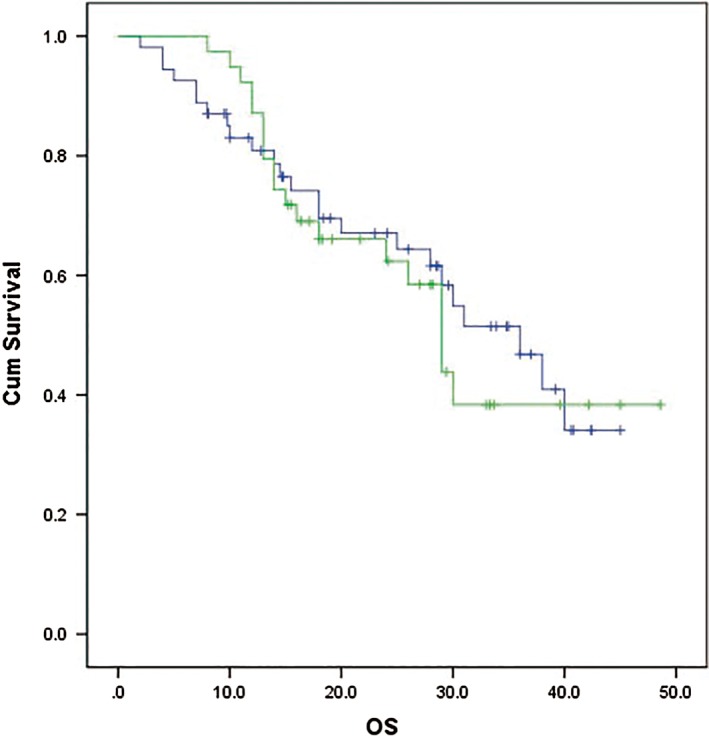
Kaplan–Meier curves for overall survival (OS). ( ) Rh‐endostatin plus chemotherapy with or without maintenance therapy, (
) Rh‐endostatin plus chemotherapy with or without maintenance therapy, ( ) chemotherapy with or without maintenance therapy, (
) chemotherapy with or without maintenance therapy, ( ) Rh‐endostatin plus chemotherapy with or without maintenance therapy‐censored, and (
) Rh‐endostatin plus chemotherapy with or without maintenance therapy‐censored, and ( ) chemotherapy with or without maintenance therapy‐censored.
) chemotherapy with or without maintenance therapy‐censored.
Safety
The overall incidence of drug‐related adverse events is listed in Table 3. There was no difference between the groups with respect to the frequency of overall drug‐related adverse events (rh‐endostatin 96.45 vs. chemotherapy 100%; P = 0.511) or grade 3 or 4 drug‐related adverse events (rh‐endostatin 19.6% vs. chemotherapy 23.1%; P = 0.686). Hematological toxicity, elevated transaminase, and gastric toxicity were the most common drug‐related adverse events, and hematological toxicity was the major grade 3–4 drug‐related adverse event in both groups.
Table 3.
Drug‐related adverse events (n/%)
| All | Grade 3–4 | |||||
|---|---|---|---|---|---|---|
| Adverse event | Endostar + pemetrexed/cisplatin (n = 56) | Pemetrexed/cisplatin (n = 39) | P | Endostar + pemetrexed/cisplatin (n = 56) | Pemetrexed/cisplatin (n = 39) | P |
| Any | 54 (96.4) | 39 (100.0) | 0.511 | 11 (19.6) | 9 (23.1) | 0.686 |
| Hematological toxicity | ||||||
| Myelosuppression | 48 (85.7) | 37 (94.9) | 0.275 | 9 (16.1) | 6 (15.4) | 1.000 |
| Thrombocytopenia | 8 (14.3) | 3 (7.7) | 0.508 | 2 (3.6) | 0 (0.0) | 0.511 |
| Hemoglobin reduction | 24 (42.9) | 20 (51.3) | 0.418 | 1 (1.8) | 2 (5.1) | 0.749 |
| Non‐hematological toxicity | ||||||
| Increased transaminase | 13 (23.2) | 9 (23.1) | 0.988 | — | — | — |
| Cardiotoxicity | 1 (1.8) | 1 (2.6) | 1.000 | 1 (1.8) | 0 (0.0) | 1.000 |
| Renal dysfunction | 2 (3.6) | 4 (10.3) | 0.374 | — | — | — |
| Pigmentation | 1 (1.8) | 0 (0.0) | 1.000 | — | — | — |
| Rash | 0 (0.0) | 1 (2.6) | 0.411 | — | — | — |
| Nausea | 50 (89.3) | 30 (76.9) | 0.104 | 2 (3.6) | 2 (5.1) | 1.000 |
| Vomiting | 48 (85.7) | 29 (74.4) | 0.165 | 2 (3.6) | 2 (5.1) | 1.000 |
| Fatigue | 3 (5.4) | 1 (2.6) | 0.883 | — | — | — |
| Neurotoxicity | 0 (0.0) | 1 (2.6) | 0.411 | — | — | — |
Discussion
Rh‐endostatin is a novel recombinant human endostatin expressed and purified in Escherichia coli. It was approved in 2006 as a component combined with vinorelbine/cisplatin for the treatment of NSCLC. Previous studies have evaluated the efficacy and safety of the combination of rh‐endostatin and platinum‐based doublet chemotherapy; however, limited data is available on the combination of rh‐endostatin and pemetrexed‐based first‐line and maintenance therapy.17, 18, 19, 20
Despite the lack of significant differences, our results showed a trend of prolonged PFS and OS in the overall population, regardless of the addition of maintenance. Rh‐endostatin plus pemetrexed/cisplatin followed by rh‐endostatin plus pemetrexed maintenance significantly improved the PFS in treatment‐naïve patients with lung adenocarcinoma; however, after adjusting for clinical factors including age, gender, disease stage, and smoking status, the difference was not statistically significant. Moreover, the addition of rh‐endostatin to pemetrexed/cisplatin was well tolerated without increased toxicity.
Several randomized studies have been performed to compare efficacy and safety between rh‐endostatin plus platinum‐based doublet chemotherapy and chemotherapy alone. The addition of rh‐endostatin was associated with prolonged time‐to‐progression when compared to chemotherapy alone (5.7–6.6 vs. 3.2–3.7 months) for the treatment of NSCLC patients, regardless of histological subtypes or the presence of previous treatment.17, 18, 19 On the contrary, no PFS benefit was shown in a multicenter phase 2 study in which 126 previously untreated advanced‐stage NSCLC patients were enrolled and randomized to receive rh‐endostatin plus paclitaxel/carboplatin or paclitaxel/carboplatin.20 Despite a numerical prolongation of survival, there was no statistically significant difference in PFS (7.1 vs. 6.3 months, respectively; P = 0.522) or OS (17.6 vs. 15.8 months, respectively; P = 0.696) between the groups. However, the study designs of the abovementioned studies did not allow us to ascertain any benefit of the combination of rh‐endostatin and pemetrexed‐based first‐line chemotherapy and the continued administration of rh‐endostatin after the end of chemotherapy. Pemetrexed‐based first‐line and maintenance chemotherapy is a relatively modern standard regimen for treatment‐naïve, advanced‐stage NSCLC patients, especially for those with the non‐squamous subtype, with a median PFS of 6.9–7.7 months.21, 22 In the current study, the median PFS for patients receiving only first‐line treatment was five months in each group, thus the addition of rh‐endostatin to pemetrexed/cisplatin did not improve PFS (P = 0.81) during the induction period. For patients receiving maintenance treatment, rh‐endostatin plus pemetrexed significantly improved PFS. Although our results were not statistically significant different because of the small patient sample, it should be noted that patients in the rh‐endostatin group tended to be younger, female, at stage IIIB disease, and non‐smokers, which could have favorably affected efficacy results. Further analysis using a Cox regression model found borderline PFS benefits in patients treated with maintenance rh‐endostatin plus pemetrexed.
Despite the lack of significant difference, the Kaplan–Meier plots show a divergent trend in OS in patients receiving maintenance therapy. It is possible that no OS differences were observed in this study because the data were premature and we had a limited sample size. In this study, the efficacy of rh‐endostatin, including survival benefit and tumor response did not differ in subgroups such as gender, disease stage, and smoking status, with the exception of tumor response in men. The lack of an association between clinical characteristics and efficacy is probably related to the limited number of patients in each subgroup.
Regarding safety, the adverse event profile of rh‐endostatin in this study was consistent with previous studies, without unexpected safety concerns.15, 16, 17, 18, 19, 20 Adding rh‐endostatin to pemetrexed/cisplatin did not increase the incidence of drug‐related or grade 3/4 adverse events, suggesting that toxicity is related to chemotherapy. No class‐related side effects of antiangiogenic therapy, such as hemorrhage, hypertension, or venous thromboembolism were observed.23 The main drug‐related adverse events of rh‐endostatin plus pemetrexed/cisplatin comprise hematological, gastrointestinal, and reversible increases in liver enzymes, which compare with the incidence of adverse events of other antiangiogenic therapies23 and are consistent with that reported for rh‐endostatin in other clinical studies15, 16, 17, 18, 19, 20
Our study has some limitations. The small number of subjects limited statistical validity. The retrospective nature of the study introduced selection bias, which resulted in an unbalanced population. Finally, the short follow‐up period meant that only half of the OS events were observed. These limitations should be considered when interpreting the results of this study.
In summary, to the best of our knowledge, this is the first study to evaluate the benefit of the continued administration of rh‐endostatin after induction chemotherapy. The combination of rh‐endostatin and pemetrexed/cisplatin was not associated with a significant improvement in PFS or OS in patients with treatment‐naïve advanced‐stage lung adenocarcinoma, regardless of the addition of maintenance. There are several possible explanations for these negative results, including the retrospective nature of the study and limited sample size. The unbalanced treatment exposure between the groups should also be noted, as fewer patients received maintenance treatment in the rh‐endostatin + pemetrexed/cisplatin group. Prospective randomized study is warranted to further investigate the clinical benefits of rh‐endostatin.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. Treatment cycles of the two groups
Table S2. Efficacy of pemetrexed/cisplatin alone or with rh‐endostatin for advanced non‐small cell lung cancer according to the presence of maintenance therapy
Table S3. Subgroup analysis of progression‐free survival by clinical characteristics
Table S4. Subgroup analysis of response rate by clinical characteristics
Acknowledgment
The authors would like to acknowledge support from the China International Medical Foundation (CIMF‐F‐H001‐283).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 3. Scagliotti GV, Parikh P, von Pawel J et al Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 3543–51. [DOI] [PubMed] [Google Scholar]
- 4. Syrigos KN, Vansteenkiste J, Parikh P et al Prognostic and predictive factors in a randomized phase III trial comparing cisplatin‐pemetrexed versus cisplatin‐gemcitabine in advanced non‐small‐cell lung cancer. Ann Oncol 2010; 21: 556–61. [DOI] [PubMed] [Google Scholar]
- 5. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29(6 Suppl 16): 15–8. [DOI] [PubMed] [Google Scholar]
- 6. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 7. Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med 1997; 3: 1222–7. [DOI] [PubMed] [Google Scholar]
- 8. Sandler A, Gray R, Perry MC et al Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. (Published erratum appears in N Engl J Med 2007; 356: 318.). N Engl J Med 2006; 355: 2542–50. [DOI] [PubMed] [Google Scholar]
- 9. Zhou C, Wu YL, Chen G et al BEYOND: A randomized, double‐blind, placebo‐controlled, multicenter, phase III study of first‐line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non‐small‐cell lung cancer. J Clin Oncol 2015; 33: 2197–204. [DOI] [PubMed] [Google Scholar]
- 10. Johnson DH, Fehrenbacher L, Novotny WF et al Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non‐small‐cell lung cancer. J Clin Oncol 2004; 22: 2184–91. [DOI] [PubMed] [Google Scholar]
- 11. Garon EB, Ciuleanu TE, Arrieta O et al Ramucirumab plus docetaxel versus placebo plus docetaxel for second‐line treatment of stage IV non‐small‐cell lung cancer after disease progression on platinum‐based therapy (REVEL): A multicentre, double‐blind, randomised phase 3 trial. Lancet 2014; 384: 665–73. [DOI] [PubMed] [Google Scholar]
- 12. Reck M, Kaiser R, Mellemgaard A et al Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non‐small‐cell lung cancer (LUME‐Lung 1): A phase 3, double‐blind, randomised controlled trial. Lancet Oncol 2014; 15: 143–55. [DOI] [PubMed] [Google Scholar]
- 13. Hanna NH, Kaiser R, Sullivan RN et al Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non‐small cell lung cancer (LUME‐Lung 2): A randomized, double‐blind, phase III trial. Lung Cancer 2016; 102: 65–73. [DOI] [PubMed] [Google Scholar]
- 14. Han B, Li K, Zhao Y et al Anlotinib as a third‐line therapy in patients with refractory advanced non‐small‐cell lung cancer: A multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018; 118: 654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang L, Wang JW, Cui CX et al [Rh‐endostatin (YH‐16) in combination with vinorelbine and cisplatin for advanced non‐small cell lung cancer: A multicenter phase II trial.]. Chin J New Drugs 2005; 14: 204–7 (In Chinese). [Google Scholar]
- 16. Yang L, Wang JW, Tang ZM. [A phase I clinical trial for recombinant human endostatin.]. Chin J New Drugs 2004; 13: 548–53 (In Chinese). [Google Scholar]
- 17. Wang J, Sun Y, Liu Y et al [Results of randomized, multicenter, double‐blind phase III trial of rh‐endostatin (YH‐16) in treatment of advanced non‐small cell lung cancer patients.]. Zhongguo Fei Ai Za Zhi 2005; 8: 283–90 (In Chinese). [DOI] [PubMed] [Google Scholar]
- 18. Sun Y, Wang JW, Liu YY et al Long‐term results of a randomized, double‐blind, and placebo‐controlled phase III trial: Endostar (rh‐endostatin) versus placebo in combination with vinorelbine and cisplatin in advanced non‐small cell lung cancer. Thorac Cancer 2013; 4: 440–8. [DOI] [PubMed] [Google Scholar]
- 19. Shi GY, Su YH. [The clinical observation of Endostar injection combined with GP regimen in advanced non‐small cell lung cancer.]. J Med Forum 2009; 30: 53–4. (In Chinese). [Google Scholar]
- 20. Han BH, Xiu QY, Wang HM et al A multicenter, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy of paclitaxel‐carboplatin alone or with endostar for advanced non‐small cell lung cancer. J Thorac Oncol 2011; 6: 1104–9. [DOI] [PubMed] [Google Scholar]
- 21. Ciuleanu T, Brodowicz T, Zielinski C et al Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non‐small‐cell lung cancer: A randomised, double‐blind, phase 3 study. Lancet 2009; 374: 1432–40. [DOI] [PubMed] [Google Scholar]
- 22. Paz‐Ares L, de Marinis F, Dediu M et al Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non‐squamous non‐small‐cell lung cancer (PARAMOUNT): A double‐blind, phase 3, randomised controlled trial. Lancet Oncol 2012; 13: 247–55. [DOI] [PubMed] [Google Scholar]
- 23. Gadgeel SM. Safety profile and tolerability of antiangiogenic agents in non‐small‐cell lung cancer. Clin Lung Cancer 2012; 13: 96–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Treatment cycles of the two groups
Table S2. Efficacy of pemetrexed/cisplatin alone or with rh‐endostatin for advanced non‐small cell lung cancer according to the presence of maintenance therapy
Table S3. Subgroup analysis of progression‐free survival by clinical characteristics
Table S4. Subgroup analysis of response rate by clinical characteristics


