Abstract
Background
The study was conducted to investigate the effectiveness and cost of computed tomography (CT)‐guided percutaneous microwave ablation (MWA) and thoracoscopic lobectomy for stage I non‐small cell lung cancer (NSCLC).
Methods
We retrospectively analyzed the data of 46 and 85 patients with stage I NSCLC treated with CT‐guided percutaneous MWA or thoracoscopic lobectomy, respectively, at our center from July 2013 to June 2015. Overall survival (OS), disease‐free survival (DFS), local control rate, hospital stay, and cost were evaluated. Survival curves were constructed using the Kaplan–Meier method and compared using the log‐rank test.
Results
The one and two‐year OS rates were 97.82% and 91.30% and 97.65% and 90.59% in the MWA and lobectomy groups, respectively. The one and two‐year DFS rates were 95.65% and 76.09% and 95.29% and 75.29%, respectively. No significant differences were observed in log‐rank analysis between the groups (P = 0.169). The hospital stays in the MWA and lobectomy groups were 6.62 ± 2.31 and 9.57 ± 3.19 days, respectively. The costs of MWA and lobectomy were US$3274.50 ± US$233.91 and US$4678.87 ± US$155.96, respectively. The differences were all significant (P = 0.003).
Conclusion
MWA and thoracoscopic lobectomy for stage I NSCLC demonstrate similar one and two‐year OS and DFS, with no significant differences between the two groups. MWA involved a shorter hospital stay and lower cost, thus should be considered a better option for patients with severe cardiopulmonary comorbidity and patients unwilling to undergo surgery.
Keywords: Disease free survival, microwave ablation, non‐small‐cell lung cancer, overall survival, surgery
Introduction
Lung cancer has the highest mortality in the world, particularly in China, and non‐small cell lung cancer (NSCLC) accounts for 80–85% of deaths.1, 2, 3 Most patients are diagnosed between the age of 35 to 75 years, with peak incidence occurring in those aged 55–65. Smoking is considered one of the most significant pathogenic factors.4 It is believed that between 70% and 87% of lung cancer cases occur in people with a smoking history. Low dose computed tomography (CT) plays an important role in the early screening and diagnosis of lung cancer.5 Other methods include sputum cytology, positron emission tomography (PET)‐CT, fiber bronchoscope, and percutaneous biopsy. At present, lobectomy is the main option to treat stage I (T1N0M0) NSCLC, with five‐year survival rates of 60–80% for stage I NSCLC patients, with or without lymph node dissection.6 Compared to open lobectomy, video‐assisted thoracoscopic surgery, also known as thoracoscopic lobectomy, is associated with longer five‐year survival, a similar distant recurrence rate, and a lower total complication rate.7 However, patients who suffer from severe cardiopulmonary comorbidity or have a poor physical condition may not tolerate surgery or general anesthesia with tracheal intubation. External beam radiation therapy delivered in standard fractionation (45–66 Gy in 1.8–2 Gy/fraction) results in local recurrence in 55–70% of patients with median survival of > 30 months and five‐year survival rates of 10–30%.8, 9, 10
Minimally invasive methods are increasingly being used to treat stage I NSCLC, such as stereotactic body radiotherapy,11, 12 brachytherapy,13 photodynamic therapy,14 and thermal ablation. As a heat‐based ablation technique, microwave ablation (MWA) can be delivered either percutaneously under CT‐guidance or via open surgical or laparoscopic approach. Because of the properties of electromagnetic waves, MWA is conducted in a shorter period of time, with an enlarged ablation zone and minimal heat sink effect to the vasculature compared to radiofrequency ablation (RFA). However, few comparative studies have compared the clinical outcomes and economical characteristics between MWA and thoracoscopic lobectomy for stage I NSCLC. The purpose of this study was to evaluate the clinical value of these two modalities, including overall survival (OS), disease‐free survival (DFS), local control, hospital stay, and cost.
Methods
From July 2013 to June 2015, 131 consecutive patients with stage I NSCLC, confirmed by enhanced CT/PET‐CT and pathology, were treated in our center. Forty‐six patients underwent CT‐guided MWA (MWA group), and thoracoscopic lobectomy was performed in 85 (lobectomy group). The hospital ethics committee approved this retrospective study, and all patients provided written informed consent.
The baseline characteristics, including gender, age, tumor size, and pathology, were comparable between the groups (Table 1). Medical history, physical examination, and recent imaging should be conducted before surgery. Multidisciplinary decisions between the Departments of Respiratory, Thoracic Surgery, Oncology, Radiation, Chemotherapy, and Interventional Radiology are necessary to select the optimal procedure. Contrast‐enhanced chest CT (within 2 weeks before MWA) is a key imaging assessment, and can reveal the tumor size, location, and relationship with neighboring vital organs, blood vessels, trachea, or bronchi (Fig 1). PET‐CT is strongly recommended to identify tumor node metastasis (TNM) stage.
Table 1.
Baseline patient characteristics
| Characteristic | MWA group (%) | Lobectomy group (%) | P * |
|---|---|---|---|
| No. of patients | 46 | 85 | — |
| Gender | |||
| Female | 24 (52.13) | 45 (52.94) | 0.061 |
| Male | 22 (47.87) | 40 (47.06) | 0.059 |
| Age (years) | |||
| < 50 | 8 (17.39) | 16 (18.82) | 0.072 |
| 50–70 | 25 (54.35) | 45 (52.94) | 0.069 |
| > 70 | 13 (28.26) | 24 (28.24) | 0.062 |
| T stage | |||
| T1a | 4 (8.70) | 8 (9.41) | 0.072 |
| T1b | 11 (23.91) | 21 (24.71) | 0.058 |
| T1c | 31 (67.39) | 56 (65.88) | 0.087 |
| Symptoms | |||
| Cough | 10 (21.74) | 19 (22.35) | 0.076 |
| Bloody sputum | 7 (15.22) | 13 (15.29) | 0.081 |
| Asymptomatic | 29 (63.04) | 53 (62.35) | 0.068 |
| Pathology | |||
| SCC | 21 (45.65) | 40 (47.06) | 0.077 |
| Adenocarcinoma | 18 (39.13) | 35 (41.18) | 0.082 |
| Large cell lung cancer | 7 (15.22) | 10 (11.76) | 0.057 |
| Pathology method | |||
| Percutaneous biology | 29 (63.04) | 55 (64.71) | 0.062 |
| Bronchoscopy | 2 (4.25) | 4 (4.71) | 0.076 |
| Sputum cytology | 15 (32.61) | 26 (30.59) | 0.071 |
Paired t‐test.
MWA, microwave ablation; SCC, squamous cell carcinoma.
Figure 1.
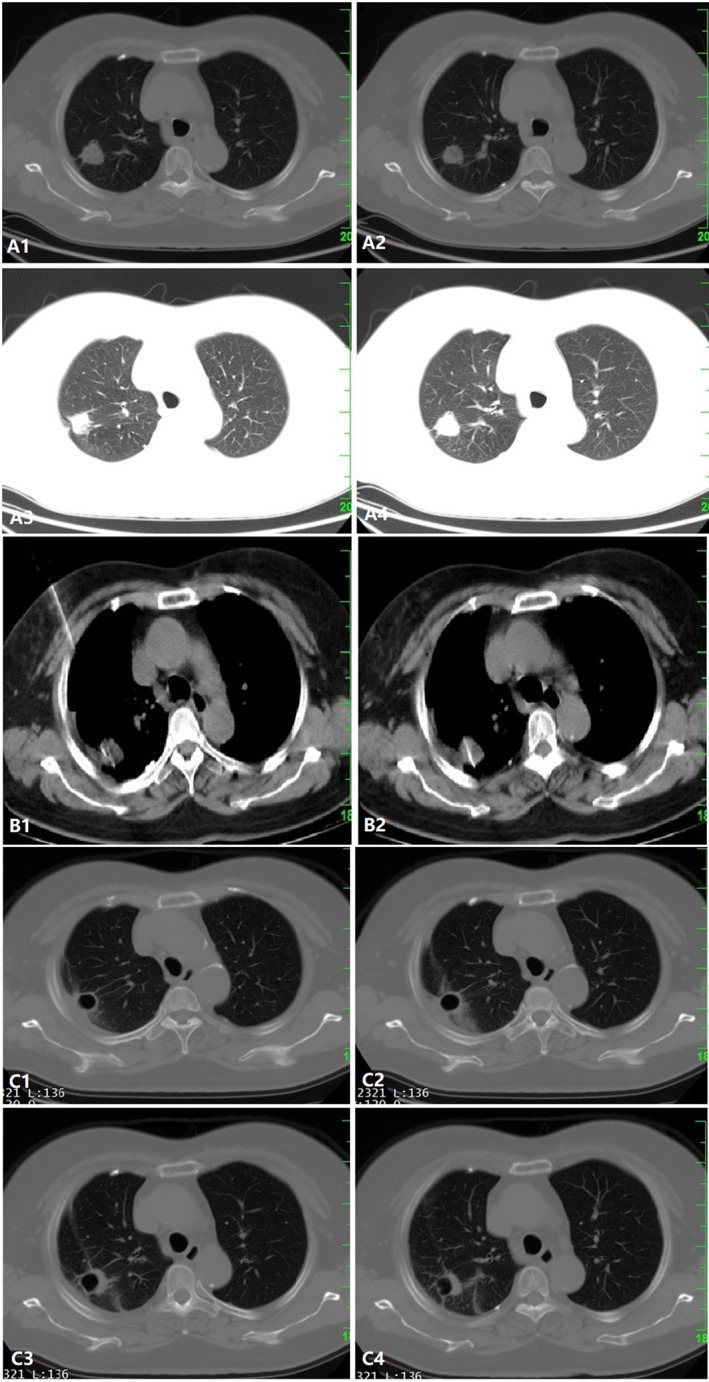
(a) A 2 cm solitary lesion is observed in the right lung (T1c). (b) The microwave ablation (MWA) probe was inserted into the lesion under computed tomography (CT)‐guidance. (c) Contrast‐enhanced CT one month after MWA demonstrated cavernous formation with fibrotic scar, indicating complete ablation.
The study inclusion criteria were: (i) a solitary solid mass demonstrated by enhanced CT or PET‐CT, with a diameter < 4 cm and without lymph node involvement or distant metastasis; (ii) NSCLC pathology was indicated by percutaneous biology, bronchoscopy biology, or sputum cytology; (iii) patients could not be a candidate for or refuse open surgery, stereotactic body radiotherapy, brachytherapy, or photodynamic therapy; (iv) an expected life span of ≥ 6 months; and (v) Karnofsky score > 70.
Exclusion criteria were: (i) massive hemoptysis; (ii) poorly controlled infection or inflammation around the lesions; (iii) puncture site infection or ulceration; (iv) pleural malignant effusions that were not well controlled; (v) an expected life span of < 6 months; (vi) severe cardiac insufficiency (New York Heart Association class III–IV) or advanced lung disease (determined by consultation with respiratory disease specialists), liver disease (Child‐Pugh class C), or kidney disease (grade 3 chronic kidney disease); (vii) severe coagulopathy (prothrombin time > 17 seconds or platelet count ≤ 60 × 109/L); and (viii) patients with implantable cardiac devices or the presence of surgical clips.15
Microwave ablation (MWA) procedure
After the patient had fasted for six hours, the procedure was performed under intravenous anesthesia with dexmedetomidine. The patient was placed in a supine or prone position, depending on the location of the tumor, as indicated by CT scan.
A contrast‐enhanced or plain CT scan was taken to confirm the number, size, and location of tumors. The puncture site, depth, and perspective were determined with caution to avoid ribs, large vessels, pulmonary fissures, and pulmonary bulla. An MWA applicator (ECO, Microwave Electronic Institute, Nanjing, China) of an appropriate length (10, 15, 18 or 20 cm) was then inserted into the lesion under CT‐guidance. The shortest puncture pathway is strongly recommended to reduce complications. After confirming via multi‐planar CT imaging that the ablation applicator was correctly positioned, ablation was performed (Fig 2). Ablation power and time were determined according to the size, geometry, and location of the lesion. A single session at one single point was usually adequate to achieve complete ablation of stage I NSCLC,16 which was demonstrated by ground‐glass opacity appearing in the CT image with a peripheral margin expanding 5 mm or more beyond the pre‐procedure tumor borders. Ablation of the withdrawal path of the applicator was necessary to reduce the possibility of tumor seeding. A whole‐lung CT scan was taken at the end of the procedure to identify any complications and assess technical success (Fig 3).
Figure 2.
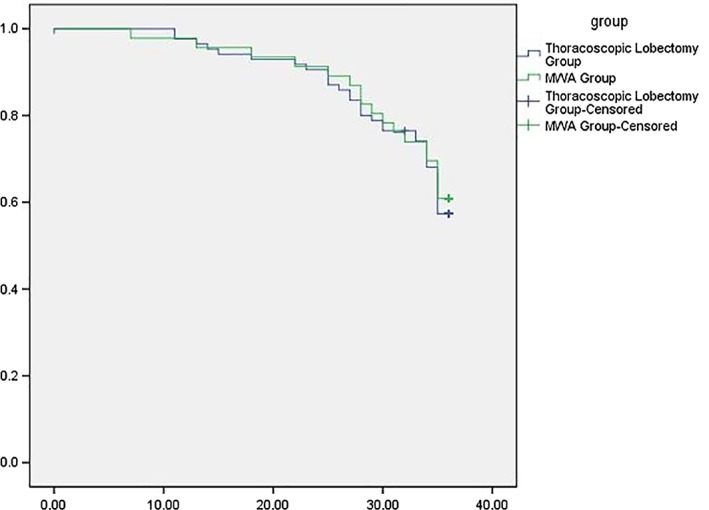
Overall survival in the microwave ablation (MWA) and thoracoscopic lobectomy groups.
Figure 3.
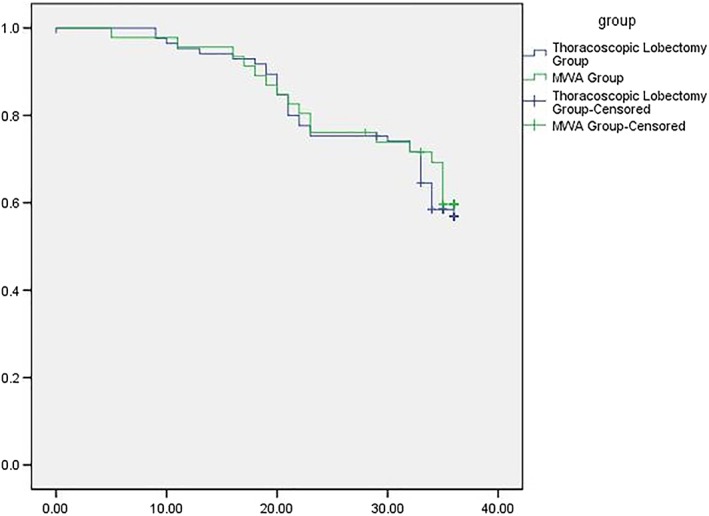
Disease‐free survival in the microwave ablation (MWA) and thoracoscopic lobectomy groups.
Patients’ vital signs, including blood pressure, heart rate, oxygen saturation, and temperature were carefully monitored during the procedure. A chest X‐ray or CT scan should be performed after 24 to 48 hours to check for complications (e.g. asymptomatic pneumothorax or pleural effusion). No chemotherapy or radiotherapy was performed after the procedure.
Thoracoscopic lobectomy procedure
The procedure was performed under general anesthesia with single‐lung ventilation, which may be accomplished with either dual‐lumen endotracheal tubes or with single‐lumen tubes and bronchial blockers. The patient was positioned in the full lateral decubitus position with slight flexion of the table at the mid‐chest level, which allows slight splaying of the ribs to improve exposure in the absence of rib spreading.
The thoracoscope was placed in the seventh or eighth intercostal space in the midaxillary line, and an anterior utility incision was placed in the fifth intercostal space (4–5 cm). Hilar dissection was performed through the anterior incision. Dissection of the pulmonary vessels and bronchi was performed in the same manner as in open surgery. Endoscopic linear staplers were used for individual vessel and bronchial ligation. After complete resection, the lobe was placed in a specimen bag for retrieval, avoiding the implantation of tumor cells into the incision. Mediastinal lymph node dissection was also performed, similar to conventional techniques.17
The patient's vital signs, including blood pressure, heart rate, oxygen saturation, and temperature were carefully monitored during the procedure. A chest X‐ray or CT scan should be performed after 24 to 48 hours to check for complications (e.g. pneumonia or pleural effusion). No chemotherapy or radiotherapy was performed after the procedure.
Follow‐up
Contrast‐enhanced chest CT was performed monthly for the first three months after the procedure. Complications, including perioperative mortality, pneumothorax, pleural effusion, infection, respiratory failure, coronary/cerebral vascular events, or postoperative bleeding requiring reoperation, were calculated.
Contrast‐enhanced chest CT scans and tumor markers were then analyzed every three months to detect any local recurrence or new pulmonary lesions. To define the distant metastasis, cerebral CT, emission CT, and abdominal ultrasound were examined 6, 12, 18, and 24 months after the procedure in all patients. A PET‐CT scan should be taken if the equipment is available.18
Efficacy assessment after MWA was carried out according to the Chinese Expert Consensus Workshop Report: Guidelines for Thermal Ablation of Primary and Metastatic Lung Tumors.16 Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was applied to assess responses after thoracoscopic lobectomy.19 OS, DFS, local control rate, hospital stay, and cost were recorded.
Statistical analysis
All statistical analyses were performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). Categorical variables are presented as numbers and percentages. Continuous data are presented as means ± standard deviations. A chi‐square test was applied to compare local control rates, and an independent t‐test to compare hospital stay and cost. Survival curves were constructed using the Kaplan–Meier method and compared by the log‐rank test. A P value of < 0.05 was considered statistically significant.
Results
Assessment of local efficacy of MWA
Contrast‐enhanced CT scans taken one month after ablation demonstrated lesion disappearance in 25 cases, complete cavernous formation in 15, and fibrotic scar in 6 cases without contrast enhancement, indicating complete ablation in the whole group.
Complications
Microwave ablation and thoracoscopic lobectomy procedures were successfully performed in all patients. Perioperative complications are listed in Table 2. In the lobectomy group, infection occurred in four cases and respiratory failure in two, with P < 0.05 compared to the MWA group. One patient died of respiratory failure and infection in the lobectomy group (1.18%). Seven cases in the MWA (15.22%) and two in the lobectomy group (2.35%) suffered from slight pneumothorax after the procedure (P < 0.05), without significant decreases in SO2. The differences in incidences of mortality, pleural effusion, coronary/cerebral vascular events, and bleeding requiring reoperation were not significant between the groups.
Table 2.
Perioperative complications
| Characteristic | MWA group (%) | Lobectomy group (%) | P * |
|---|---|---|---|
| No. of patients | 46 | 85 | — |
| Mortality | 0 (0) | 1 (1.18) | 0.062 |
| Pneumothorax | 7 (15.22) | 2 (2.35) | 0.009 |
| Pleural effusion | 4 (8.70) | 8 (9.41) | 0.074 |
| Infection | 0 (1.18) | 4 (4.71) | 0.026 |
| Respiratory failure | 0 (0) | 2 (2.35) | 0.031 |
| Coronary vascular events | 0 (0) | 0 (0) | — |
| Cerebral vascular events | 0 (0) | 1 (1.18) | 0.062 |
| Bleeding requiring reoperation | 0 (0) | 1 (1.18) | 0.062 |
Paired t‐test. MWA, microwave ablation.
Survival
Median survival in the MWA and lobectomy groups was 32.74 ± 0.94 (7–36) and 32.45 ± 0.74 (0–36) months, respectively. The one and two‐year OS rates were 97.82% and 91.30% versus 97.65% and 90.59% in the MWA and lobectomy groups, respectively (Fig 2). The one and two‐year DFS were 95.65% and 76.09% versus 95.29% and 75.29%, respectively (Fig 3). The results of log‐rank analysis of the two sets of data did not reveal any significant differences (P = 0.169).
Hospital stay and cost
The hospital stays in the MWA and lobectomy groups were 6.62 ± 2.31 and 9.57 ± 3.19 days, respectively (P = 0.016). The cost of MWA compared to lobectomy was US$3274.50 ± US$233.91 and US$4678.87 ± US$155.96, respectively (P = 0.023). The differences were all significant.
Discussion
Many studies have indicated that MWA is an effective, feasible, and minimally invasive option for the treatment of early stage NSCLC.20, 21, 22 However, little research has compared the clinical outcomes, complications, and cost between MWA and thoracoscopic lobectomy for stage I NSCLC.
The advantages of MWA over RFA include larger volumes of necrosis in a shorter procedural time, less “heat sink” effect for the better treatment of perivascular tissue, and maximization of the ablation zone size by simultaneously positioning multiple MWA antennas into larger lesions. Theoretically, MWA can achieve higher rates of local control, OS, and DFS. Similar to RFA, MWA is associated with risks of pneumothorax, pleural effusion, infection, respiratory failure, and bleeding.
Wolf et al. analyzed MWA of 82 lesions in 50 patients with lung malignancies, and reported a one‐year local control rate of 67%.23 Residual disease was observed in 26% cases. In our study, assessment of local efficacy one month after MWA indicated complete ablation in all 46 patients and one‐year DFS of 95.65%. The differences between these results are partly attributed to the larger size (mean diameter 3.5 ± 1.6 cm) of the tumors in our study.
Thoracoscopic lobectomy shows variable advantages over open surgery, including decreased blood loss, less pain, shorter hospital stay, more rapid recovery, preserved postoperative pulmonary function, and decreased inflammatory response.24, 25, 26, 27 However, this procedure should be performed under general anesthesia with tracheal intubation, takes more time, and consumes more specific instruments (such as a stapler, clips) than MWA. This may explain why the hospital stay was longer and the cost higher in the lobectomy group. Furthermore, a longer surgical duration increases the risk of complication. In the lobectomy group, infection occurred in four cases and respiratory failure in two (P < 0.05) and one patient (1.18%) died of respiratory failure and infection.
Because of the percutaneous approach, the incidence of pneumothorax in the MWA group was significantly higher than in the lobectomy group. However, no symptoms of dyspnea were exhibited, and the air was automatically absorbed after approximately a week in all cases. The one and two‐year‐OS and DFS were comparable between the groups, which indicates that MWA has a similar value to thoracoscopic lobectomy and is a curative option for stage I NSCLC.7
In conclusion, we confirm that MWA is an effective and safe option for stage I NSCLC, demonstrating similar clinical value to thoracoscopic lobectomy, with a shorter hospital stay and lower cost. MWA should be considered a better option for patients with severe cardiopulmonary comorbidity, which causes intolerance of surgery or general anesthesia with tracheal intubation, and in patients unwilling to undergo surgery.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This research was supported by National Natural Science Foundation of China (61671276), and Natural Science Foundation of Shandong Province (2014ZRE27479, ZR2018PH032, ZR2018PH033).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Zhang S et al Annual report on status of cancer in China, 2010. Chin J Cancer Res 2014; 26: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang F, Sui X, Chen X et al Sublobar resection versus lobectomy in surgical treatment of elderly patients with early‐stage non‐small cell lung cancer (STEPS): Study protocol for a randomized controlled trial. Trials 2016; 17: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Office of the Surgeon General (US), Office on Smoking and Health (US). The Health Consequences of Active Smoking: A Report of the Surgeon General. Centers for Disease Control and Prevention, Atlanta, GA: 2004. [PubMed] [Google Scholar]
- 5. Richards TB, White MC, Caraballo RS. Lung cancer screening with low‐dose computed tomography for primary care providers. Prim Care 2014; 41: 307–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Little AG, Gay EG, Gaspar LE, Stewart AK. National survey of non‐small cell lung cancer in the United States: Epidemiology, pathology and patterns of care. Lung Cancer 2007; 57: 253–60. [DOI] [PubMed] [Google Scholar]
- 7. Cai YX, Fu XN, Xu QZ, Sun W, Zhang N. Thoracoscopic lobectomy versus open lobectomy in stage I non‐small cell lung cancer: A meta‐analysis. PLoS One 2013; 8: e82366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeremic B, Classen J, Bamberg M. Radiotherapy alone in technically operable, medically inoperable, early‐stage (I/II) non‐small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2002; 54: 119–30. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong JG, Minsky BD. Radiation therapy for medically inoperable stage I and II non‐small cell lung cancer. Cancer Treat Rev 1989; 16: 247–55. [DOI] [PubMed] [Google Scholar]
- 10. Kaskowitz L, Graham MV, Emami B, Halverson KJ, Rush C. Radiation therapy alone for stage I non‐small cell lung cancer. Int J Radiat Oncol Biol Phys 1993; 27: 517–23. [DOI] [PubMed] [Google Scholar]
- 11. Timmerman R, Papiez L, McGarry R et al Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non‐small cell lung cancer. Chest 2003; 124: 1946–55. [DOI] [PubMed] [Google Scholar]
- 12. McGarry RC, Papiez L, Williams M, Whitford T, Timmerman RD. Stereotactic body radiation therapy of early‐stage non‐small‐cell lung carcinoma: Phase I study. Int J Radiat Oncol Biol Phys 2005; 63: 1010–5. [DOI] [PubMed] [Google Scholar]
- 13. Santos R, Colonias A, Parda D et al Comparison between sublobar resection and 125Iodine brachytherapy after sublobar resection in high‐risk patients with stage I non‐small‐cell lung cancer. Surgery 2003; 134: 691–7. [DOI] [PubMed] [Google Scholar]
- 14. Simone CB II, Friedberg JS, Glatstein E et al Photodynamic therapy for the treatment of non‐small cell lung cancer. J Thorac Dis 2012; 4: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simon CJ, Dupuy DE. Current role of image‐guided ablative therapies in lung cancer. Expert Rev Anticancer Ther 2005; 5: 657–66. [DOI] [PubMed] [Google Scholar]
- 16. Ye X, Fan W, Chen JH et al Chinese expert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors. Thorac Cancer 2015; 6: 112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yim AP, Izzat MB, Liu HP, Ma CC. Thoracoscopic major lung resections: An Asian perspective. Semin Thorac Cardiovasc Surg 1998; 10: 326–31. [DOI] [PubMed] [Google Scholar]
- 18. Pereira PL, Salvatore M, Cardiovascular and Interventional Radiological Society of Europe (CIRSE) . Standards of practice: Guidelines for thermal ablation of primary and secondary lung tumors. (Published erratum appears in Cardiovasc Intervent Radiol 2012; 35: 444). Cardiovasc Intervent Radiol 2012; 35: 247–54. [DOI] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J et al New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 20. Yang X, Ye X, Zheng A et al Percutaneous microwave ablation of stage I medically inoperable non‐small cell lung cancer: Clinical evaluation of 47 cases. J Surg Oncol 2014; 110: 758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ridge CA, Solomon SB, Thornton RH. Thermal ablation of stage I non‐small cell lung carcinoma. Semin Intervent Radiol 2014; 31: 118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones GC, Kehrer JD, Kahn J et al Primary treatment options for high risk/medically inoperable early stage NSCLC patients. Clin Lung Cancer 2015; 16: 413–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolf FJ, Grand DJ, Machan JT, DiPetrillo TA, Mayo‐Smith WW, Dupuy DE. Microwave ablation of lung malignancies: Effectiveness, CT findings, and safety in 50 patients. Radiology 2008; 247: 871–9. [DOI] [PubMed] [Google Scholar]
- 24. Burfeind WR, D'Amico TA. Thoracoscopic lobectomy. Oper Tech Thorac Cardiovasc Surg 2004; 9: 98–114. [Google Scholar]
- 25. Li WWL, Lee RLM, Lee TW et al The impact of thoracic surgical access on early shoulder function: Video‐assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg 2003; 23: 390–6. [DOI] [PubMed] [Google Scholar]
- 26. Nomori H, Ohtsuka T, Horio H, Naruke T, Suemasu K. Difference in the impairment of vital capacity and 6‐minute walking after a lobectomy after a lobectomy performed by thoracoscopic surgery, an anterior limited thoracotomy, an anteroaxillary thoracotomy, and a posterolateral thoracotomy. Surg Today 2003; 33: 7–12. [DOI] [PubMed] [Google Scholar]
- 27. Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: A comparison of VATS and conventional procedure. Ann Thorac Surg 2001; 72: 362–5. [DOI] [PubMed] [Google Scholar]


