Abstract
Background
In esophageal cancer, nutritional challenges are extremely common. Malignant obstruction resulting from esophageal cancer (EC) is often treated by the insertion of expandable stents, but little is known as to the role and evolution of sarcopenia in this patient population. The aim of this article was to determine the effects of body mass parameters on survival of advanced EC patients who received a stent for palliation of malignant obstruction.
Methods
This was a retrospective observational study of 238 EC patients who had a stent inserted for palliation of malignant obstruction between 2005 and 2013. Skeletal muscle mass was calculated from abdominal computed tomography scans, and the patients were divided into sarcopenic and non‐sarcopenic groups. A follow‐up computed tomography scan was available in 118 patients. The primary outcome was survival, and complication rates and the need for an alternative enteral feeding route were secondary outcomes.
Results
Sarcopenia occurred in 199 (85%) patients. Median survival was 146 (range: 76–226) days in the sarcopenia group and 152 (range: 71–249) days in the non‐sarcopenic group (P = 0.61). Complication rates between the groups were not significantly different (P = 0.85). In Cox regression analysis, the skeletal muscle index was inversely correlated with overall survival (hazard ratio 0.98, 95% confidence interval 0.97–0.99; P = 0.033).
Conclusions
Sarcopenia, defined by consensus thresholds, at the time of stent insertion cannot effectively predict poor survival in this patient cohort, but a lower skeletal muscle index correlates with poor prognosis as a continuous variable.
Keywords: Esophageal neoplasm, esophageal stent, sarcopenia, swallowing disorder
Introduction
Esophageal cancer (EC) is a major cause of cancer mortality and morbidity worldwide, with approximately 455 800 new cases diagnosed and 400 200 deaths annually.1
EC patients commonly present with symptoms of dysphagia and weight loss.2 These symptoms are associated with a higher mortality from EC.3 Sarcopenia represents a new means to measure the degree of body composition changes and cancer cachexia.4, 5 The definition of sarcopenia is a skeletal muscle index (SMI) of ≤ 39 cm2/m2 for women and ≤ 55 cm2/m2 for men by international validated consensus.5, 6 Measurement of SMI can involve axial computed tomography (CT) imaging studies. In EC patients who undergo esophagectomy, sarcopenia has been linked to poor long‐term outcome and postoperative respiratory complications,7, 8 and is also associated with poor long‐term outcomes in liver, pancreatic, breast, urothelial, and colorectal cancers.9, 10, 11, 12, 13 Sarcopenia is related to the dose‐limiting toxicity of chemotherapeutic agents in hepatocellular and renal cell carcinomas and breast cancer.14, 15, 16, 17, 18
Advanced EC often causes malignant obstruction, which is widely treated with palliative insertion of a self‐expandable metallic stent (SEMS) and quickly and efficiently relieves dysphagia.19, 20 Use of covered SEMS and bare metal stents has been deemed safe by prospective randomized trials.21, 22
The purpose of this study was to assess the prognostic significance of sarcopenia at the time of stent insertion in patients receiving a SEMS for palliation of malignant obstruction from advanced EC, and the effect of body mass loss on survival during follow‐up.
Methods
This was a retrospective cohort study. The Helsinki University Hospital (HUH) academic review board granted an institutional study permit for the review of relevant medical records.
Data collection and study parameters
Data was collected retrospectively from HUH medical records. Patients who received a stent for palliation of malignant obstruction caused by uncurable advanced EC between 2005 and 2013 were candidates for inclusion. The availability of electronic medical records regarding stenting data dictated the selected time period. The timeframe for an acceptable CT image was ± two weeks from stent insertion. A second CT image, if available, served to assess body composition changes over time. The acceptable time frame for this CT image was two to six months after SEMS insertion. No patients were lost to follow‐up.
We collected the following data from our center's medical records: demographic parameters, patients’ comorbidity status, details of stent insertion, details of tumor type and location, oncologic therapy administered, time of death, and details related to stent complications.
The primary end point was overall survival (OS), defined as the number of days from stent insertion to death. Secondary outcomes were the need for alternative enteral feeding route, that is, percutaneous endoscopic gastrostomy (PEG) or jejunal feeding tube, and complications resulting from the stent insertion.
Stent‐insertion protocol
Indications for stenting were dysphagia and evidence of obstructive tumor growth in gastroscopy. The stents were placed under propofol sedation, without intubation, using the standard technique over guidewires and with the help of fluoroscopy. Tumor location, as well as the length and severity of the stricture, determined the size and location of the stent. Stent position was confirmed endoscopically and radiologically.
Oncological treatments and follow‐up
Patients who received a stent before or during definitive or neoadjuvant chemotherapy or chemoradiation therapy were followed‐up in the medical oncology unit as standard during their treatments and were evaluated for the need for surgery by the esophageal surgery unit after the completion of therapy. These patients underwent follow‐up imaging to assess the tumor response to treatments. Patients that were not fit for definitive treatments were followed in a non‐standardized manner depending on the treatment regimen administered (chemotherapy, chemoradiation, radiation or surveillance). These patients underwent follow‐up imaging to either monitor the progress of the disease or to evaluate the effect of oncological treatments.
Image analysis
We selected a single image on the level of L3, with both transverse processes visible and imported it to OsirIX Version 3.3 (32‐bit Pixmeo, OsirIX, Sarl, Switzerland). Abdominal muscles were delineated by the use of a semi‐automated selection of region of interest (ROI). The Hounsfield Unit (HU) threshold range for skeletal muscle was −29 to +150. When required, images were manually corrected using the propulsion and brush tools in OsirIX. The cross‐sectional total muscle area (TMA) at the level of L3 was assessed, and the skeletal muscle index (SMI) was calculated by TMA/(height2); the resulting unit of measurement was cm2/m2. Sarcopenia was defined as an SMI of ≤ 39 cm2/m2 for women and ≤ 55 cm2/m2 for men, as recommended by international validated consensus statement.5
Statistical analysis
All statistical analysis was performed using R Project (R Core Team, 2016, R Foundation for Statistical Computing, Vienna, Austria, URL: https://www.r-project.org/). Variables are presented as mean and standard deviation for normally distributed variables and as median and interquartile range (IQR) for non‐normally distributed variables. Normality of variables was calculated using the Shapiro–Wilks test. Variables were compared using the Mann–Whitney U test for non‐normal continuous variables and the Student's t‐test for normally distributed continuous variables. Correlations were measured by Pearson product moment correlation. The Kaplan–Meier method was used for survival analysis. Cox multivariate regression analysis was performed using the backwards elimination method with a P value limit of 0.2. The limit for statistical significance of P values was set at 0.05.
Results
A total of 279 patients were identified and their records were analyzed for inclusion; 41 patients had missing data and/or inadequate CT scans and were thus excluded, resulting in a study population of 238 patients.
Table 1 shows the characteristics and muscle parameters of the study population (n = 238), and the subgroups. A total of 199 (83.6%) patients had sarcopenia at the time of stenting. There were no statistically significant differences in age, gender, weight loss within three months of stenting, Charlson Comorbidity Index (CCI), tumor histology, location, tumor node metastasis (TNM) stage, or amount of previous or post‐stent oncological treatment between the groups. Statistically significant differences were observed in weight and height, body mass index (BMI), TMA, and SMI; 36 (15.1%) patients had missing data in the weight loss variable. Follow‐up lasted until death for all 234 patients. The median duration between the diagnosis of cancer and stenting was 19 days (range: 7–39). In 30 (12.6%) patients, the delay between diagnosis and stent insertion was 180 days. Most patients (n = 196, 82.4%) were not candidates for definitive treatment (surgery or chemoradiation), the disease was thought to be incurable in 114 patients (47.9%), 72 patients (30.3%) were evaluated as unable to tolerate curative intent treatment because of poor performance status, and 10 patients (4.2%) refused curative treatment. The mean SMI for men was 39.4 cm2/m2 and for women was 35.3 cm2/m2.
Table 1.
Characteristics of the patient population
| Characteristic | All | Sarcopenia | No sarcopenia | P | |||
|---|---|---|---|---|---|---|---|
| (n = 238) | (n = 199) | (n = 39) | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age, years | 67.52 | ±10.895 | 68.12 | ±10.481 | 64.31 | ±12.561 | 0.35 |
| Gender | N | % | N | % | N | % | 0.408 |
| Male | 159 | 66.8% | 142 | 71.4% | 17 | 43.6% | |
| Female | 79 | 33.2% | 57 | 28.6% | 22 | 56.4% | |
| Esophageal cancer histology | 0.102 | ||||||
| SCC | 110 | 46.2% | 94 | 47.2% | 14 | 35.9% | |
| AC | 108 | 44.5% | 88 | 44.2% | 22 | 56.4% | |
| Other | 20 | 9.3% | 18 | 8.6% | 3 | 7.7% | |
| Primary tumor location | 0.315 | ||||||
| Proximal third | 22 | 9.2% | 20 | 10.1% | 2 | 5.1% | |
| Middle third | 114 | 47.9% | 96 | 48.2% | 18 | 46.2% | |
| Distal third | 102 | 42.9% | 83 | 41.7% | 19 | 48.7% | |
| TNM stage | |||||||
| T2 | 2 | 0.8% | 2 | 1.0% | 0 | 0% | 0.807 |
| T3 | 130 | 54.6% | 108 | 54.3% | 22 | 56.4% | |
| T4 | 106 | 44.5% | 89 | 44.7% | 17 | 43.6% | |
| N0 | 25 | 10.5% | 20 | 10.1% | 5 | 12.8% | 0.770 |
| N1 | 139 | 58.4% | 115 | 57.8% | 24 | 12.1% | |
| N2 | 51 | 21.4% | 45 | 22.6% | 6 | 15.4% | |
| N3 | 23 | 9.7% | 19 | 9.5% | 4 | 10.3% | |
| M1 | 94 | 39.5% | 76 | 38.2% | 18 | 46.2% | 0.453 |
| Recurrent tumor† | 6 | 2.5% | 6 | 3.0% | 0 | 0% | 0.589 |
| Previous oncological treatment | 0.205 | ||||||
| Chemotherapy | 7 | 2.9% | 5 | 2.5% | 2 | 5.1% | |
| Radiation | 8 | 3.4% | 7 | 3.5% | 1 | 2.6% | |
| Chemoradiation | 18 | 7.6% | 18 | 9.0% | 0 | 0% | |
| Oncological treatments after stent | 0.054 | ||||||
| Chemotherapy | 53 | 22.3% | 42 | 21.1% | 11 | 28.2% | |
| Radiation | 61 | 25.6% | 48 | 24.1% | 13 | 33.3% | |
| Chemoradiation | 41 | 17.2% | 37 | 18.6% | 4 | 10.3% | |
| Muscle parameters | median | IQR | median | IQR | median | IQR | |
| TMA | 104.5 | 81.9–132.4 | 101.7 | 80.1–128.7 | 134.8 | 115.1–173.8 | < 0.001 |
| SMI | 36.3 | 29.0–45.3 | 35.2 | 27.8–40.7 | 52.8 | 44.3–55.9 | < 0.001 |
| Weight, kg | 63 | 55–74 | 62 | 54–72 | 75 | 61.6–88 | < 0.001 |
| Height, cm | 173 | 164–179 | 173 | 165–179 | 168.5 | 158–175.8 | 0.003 |
| BMI kg/m2 | 21.7 | 19.2–24.5 | 21 | 18.5–23.7 | 26.4 | 23.5–30.7 | < 0.001 |
| Weight loss, kg | 10 | 7,5–17 | 10 | 7,5–17 | 10 | 7.3–17.3 | 0.806 |
| CCI | 7 | 5–9 | 7 | 5–9 | 7 | 6–11.0 | 0.384 |
All six were recurrences after definitive chemotherapy or chemoradiation treatment.
AC, adenocarcinoma; BMI, body mass index; CCI, Charlson Comorbidity Index; IQR, interquartile range; SCC, squamous cell carcinoma; SD, standard deviation; SMI, skeletal muscle index; TMA, total muscle area; TNM, tumor node metastasis.
No significant differences between the groups as to stent type, location, size, or complications were observed (Table 2). The overall frequency of complications was 31.1% (n = 74). The most common complication was migration of the stent, which occurred in 29 patients (12.2%). No fatal complications were observed in this cohort.
Table 2.
Stent characteristics
| Characteristics | All (n = 238) | Sarcopenia (n = 199) | No sarcopenia (n = 39) | P | |||
|---|---|---|---|---|---|---|---|
| Stent type | 0.425 | ||||||
| Ultraflex | 218 | 91.6 | 181 | 91 | 37 | 94.9 | |
| Polyflex | 7 | 2.9 | 6 | 3 | 1 | 2.6 | |
| Microtec | 3 | 1.3 | 4 | 2 | 0 | 0 | |
| Antireflux | 4 | 1.7 | 3 | 1.5 | 0 | 0 | |
| Other | 6 | 2.5 | 5 | 2.5 | 1 | 2.6 | |
| Beginning stent location | 134 | 56.3 | 114 | 57.3 | 20 | 51.3 | 0.547 |
| Below 30 cm | |||||||
| Above 30 cm | 104 | 43.7 | 85 | 42.7 | 19 | 48.7 | |
| Stent Size | 0.435 | ||||||
| 15 cm | 37 | 15.5 | 33 | 16.6 | 4 | 10.3 | |
| 12 cm | 95 | 39.9 | 80 | 40.2 | 15 | 38.5 | |
| 10 cm | 84 | 35.3 | 68 | 34.2 | 16 | 41 | |
| Other | 10 | 4.2 | 9 | 4.5 | 1 | 2.6 | |
| N/A† | 12 | 5 | 9 | 4.5 | 3 | 7.7 | |
| Complications | 0.85 | ||||||
| Any | 74 | 31.1 | 61 | 30.7 | 13 | 33.3 | |
| Migration | 29 | 12.2 | 24 | 12.1 | 5 | 12.8 | |
| Bleeding | 6 | 2.5 | 23 | 11.6 | 2 | 5.1 | |
| Fistula | 25 | 10.5 | 7 | 3.5 | 1 | 2.6 | |
| Perforation | 8 | 3.4 | 6 | 3 | 2 | 5.1 | |
| Infection | 10 | 4.2 | 8 | 4 | 2 | 5.1 | |
Not specified in operative report.
A total of 119 (49.6%) patients had an adequate second CT scan. The indications for CT scanning were: monitoring of oncological treatment response in 68 (57.6%), other oncological follow‐up in 32 (27.1%), and acute disease in 18 (25.3%) patients. The median interval to the second scan was 91 days (range: 61–113); 108 patients (91.5%) were sarcopenic, and 10 (8.5%) were non‐sarcopenic in this group. Table 3 shows the characteristics of these groups. Age, TMA, and SMI significantly differed between the groups. Of the 119 patients, 90 (75.6%) had sarcopenia in both CT imaging studies, 19 (8%) had no sarcopenia in the initial imaging but later developed sarcopenia, 6 (2.5%) initially had sarcopenia but had no sarcopenia in the second CT scan, and 4 (1.7%) had no sarcopenia in either of the imaging studies. The mean SMI for this population was 36.5 cm2/m2 for men and 32.1 cm2/m2 for women; the differences between mean pre‐stent and post‐stent insertion were statistically significant for men (P = 0.016), but not for women (P = 0.050).
Table 3.
Characteristics of follow‐up patients
| Characteristics | All (n = 118) | Sarcopenia (n = 108) | No sarcopenia (n = 10) | P | |||
|---|---|---|---|---|---|---|---|
| Age, years | 65.8 | ±9.9 | 66.4 | ±9.7 | 60.1 | ±10.4 | 0.043 |
| Gender | 0.371 | ||||||
| Male | 80 | 67% | 72 | 66% | 8 | 80% | |
| Female | 39 | 33% | 37 | 34% | 2 | 20% | |
| Weight, kg | 65 | 55–77 | 64 | 55–77.5 | 69 | 60–74.8 | 0.612 |
| Height, cm | 173 | 165–179 | 173 | 164.5–179 | 173 | 164.5–178.3 | 0.799 |
| BMI, kg/m2 | 22.3 | 20.0–24.9 | 22 | 19.6–24.7 | 23.5 | 21.7–25.6 | 0.427 |
| Weight loss, kg | 10 | 7–17 | 10 | 7.5–17 | 10 | 5–15 | 0.58 |
| CCI | 6.8 | ±2.1 | 6.8 | ±1.6 | 7.1 | ±1.6 | 0.725 |
| Muscle parameters | |||||||
| TMA†, m2 | 101.6 | 85.1–119.1 | 97.1 | 83.2–116.4 | 131.9 | 115.9–161.7 | 0.001 |
| SMI‡, m2/cm2 | 35.4 | 29.6–40.0 | 33.5 | 28.8–38.3 | 45.3 | 40.0–53.4 | 0.000 |
| SMI Change | −4.1 | −8.9‐(−0.3) | −4.3 | −9.1‐(−0.8) | −1.3 | −5.4‐1.3 | 0.073 |
Cross‐sectional total muscle area (TMA) at the level of L3.
Skeletal muscle index (SMI) = TMA/(height2).
BMI, body mass index; CCI, Charlson Comorbidity Index.
The median OS was 146 days (range: 73–226) from stent insertion. The median OS of the sarcopenic patients was 146 days (range: 76–226) and 152 (range: 71–249) in patients without sarcopenia. Figure 1a shows Kaplan–Meier survival analysis between the groups with no significant difference (logrank P = 0.61). The presence of sarcopenia was associated with a later need for PEG or a jejunal feeding tube (P = 0.012).
Figure 1.
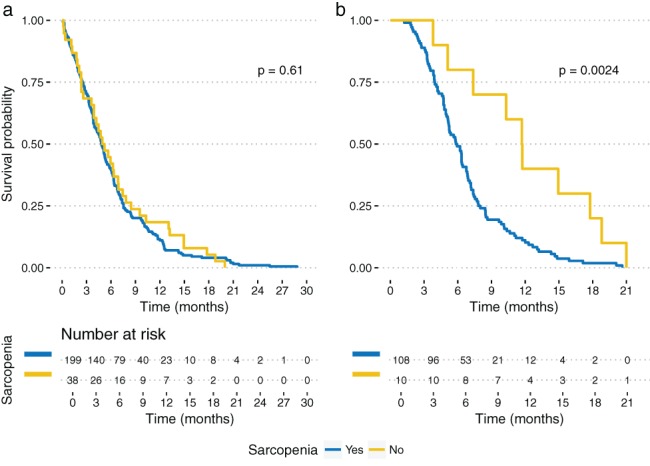
Kaplan–Meier survival curve for overall survival between patients with and without sarcopenia at (a) initial and (b) follow‐up imaging.
The median OS in the group with sarcopenia in the second imaging was 177 days (range: 119–236) and 352 (range: 243–512) in those without sarcopenia. Figure 1b shows Kaplan–Meier analysis between these groups (logrank P = 0.0024).
The median OS of patients who received a follow‐up CT scan was 189 days (range: 125–257), whereas the median OS in patients who did not was 97 days (range: 38–189) (P = 0.001).
Two groups were formed based on the percentage SMI change (ΔSMI) in the follow‐up period. The median ΔSMI (−11.6%) served as a cutoff value. There were 59 patients in each group. The median OS in group 1 (ΔSMI over median) was 190 days (range: 140–325) and the median OS in group 2 (ΔSMI under median) was 178 (range: 117–235). Figure 2 shows the Kaplan–Meier analysis of OS between these groups (P = logrank P = 0.018). ΔSMI was correlated with OS as a continuous variable (P = 0.013, r = 0.21).
Figure 2.
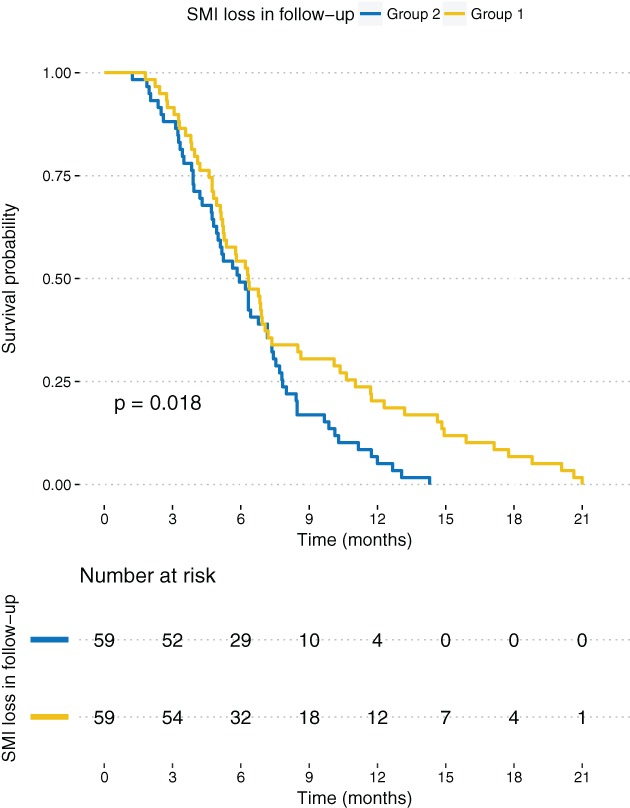
Kaplan–Meier survival curve of overall survival between patients with greater than median skeletal muscle index (ΔSMI) loss (−11.6%, group 2) versus patients with less than median ΔSMI loss (group 1).
Backwards‐eliminating Cox proportional hazards analysis was performed to elucidate the relationship between SMI and OS. Predictors included in the initial model were age, gender, BMI, weight loss, CCI, EC histology, clinical TNM status, previous treatments, treatments after stenting, non‐curative intent of treatments, location of EC, and SMI. There were 238 events with 6 covariates in the final model, resulting in an event per predictor variable ratio of 39, well over the suggested limit of 10.23 Table 4 shows the final Cox model. SMI, age, treatment after stent, and CCI were significantly associated with OS in the final model, whereas clinical N‐stage and non‐curative intent of treatment did not reach significance but could not be excluded from the model because P < 0.2. A higher CCI score produced a higher risk of death (hazard ratio [HR] 1.13, 95% confidence interval [CI] 1.06–1.20; P < 0.001) whereas both patient age (HR 0.98, 95% CI 0.96–0.99; P < 0.001) and SMI (HR 0.98, 95% CI 0.97–0.99; P = 0.033) were inversely correlated with OS. Treatment received after stenting lowered the risk of death (HR 0.56, 955 CI 0.41–0.76; P < 0.001).
Table 4.
Multivariate Cox regression analysis of overall survival
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age | 0.98 | 0.96–0.99 | < 0.001 |
| CCI† | 1.13 | 1.06‐1.20 | < 0.001 |
| Skeletal muscle index | 0.98 | 0.97–0.99 | 0.033 |
| cN | 0.87 | 0.74–1.04 | 0.122 |
| Non‐curative intent | 0.75 | 0.51–1.08 | 0.123 |
| Treatment after stent† | 0.56 | 0.41–0.76 | < 0.001 |
Chemotherapy, radiation, or chemoradiation.
CCI, Charlson Comorbidity Index; CI, confidence interval; HR, hazard ratio.
Discussion
Sarcopenia, measured by muscle area from an abdominal CT scan at the level of the L3 lumbar vertebra, was not significantly associated with poorer survival (P = 0.61). An association existed between sarcopenia in a later imaging study, taken on average 91 days after the initial CT, and poorer survival (P = 0.0024). Multivariate analysis showed an inverse correlation between SMI and OS (HR 0.98, 95% CI 0.97–0.99; P = 0.033), meaning that a more pronounced loss of muscle mass had a negative effect on survival. These results indicate that although the traditionally defined sarcopenia cutoffs do not produce a statistically significant correlation with OS, SMI has predictive value as a variable and a lower SMI is correlated with poor survival.
There were no statistical differences in the clinical T, N, or M‐stages between patients with and without sarcopenia in our study, indicating that other factors contributed to the amount of sarcopenia aside from the tumor stage. Sarcopenic patients received fewer oncological treatments after stent insertion (P = 0.054), but this result was not statistically significant; this may be explained by the fact that physicians were more conservative when offering treatment options to sarcopenic patients because of their overall poor performance status.
There was a statistically significantly correlation between sarcopenia and a later need for an alternative feeding route (P = 0.012). This implies that sarcopenia might impact clinical decision‐making when the success of stenting and the need for further interventions are considered. Recurrent tumor growth through the stent may also lead the clinician to seek an alternative permanent solution to the enteral feeding route. Some patients may also prefer the PEG insertion to stenting. In cases of more aggressive tumor metabolism and inflammation, interventions to maintain an enteral feeding route may become futile, and these patients may be unable to overcome their catabolic state by enteral feeding alone.
Researchers are increasingly interested in sarcopenia in cancer. The incidence of malnutrition, dysphagia, and weight loss among EC patients is among the highest of all cancers.2, 3 These symptoms are correlated with sarcopenia incidence.24 Sarcopenia can also cause dysphagia in the elderly, with OS in EC patients who underwent esophageal resection negatively affected by sarcopenia.7 To our knowledge, no data exists on the effect of sarcopenia or SMI in EC patients who receive a stent for palliation of dysphagia. However, poor nutrition evaluated by weight, BMI, and albumin concentration is linked to poorer survival in advanced or recurrent EC patients who receive stents.19 Sarcopenia may reflect more aggressive tumor biology, systemic inflammation, and metabolic activity.25 It cannot be assessed by clinical measurements, because many sarcopenic patients have a normal BMI.25, 26, 27 Sarcopenia can even coexist with obesity,10, 12, 24 making abdominal CT scans necessary and a widely used method to precisely measure the degree of sarcopenia.6, 7, 8, 9, 11, 12, 13, 14, 15, 17, 28, 29 The magnitude of the effect of SMI on OS is small per measurement unit (HR 0.98), similar to patient age, but as this is a cumulative per unit of muscle mass, in our opinion it is clinically significant. Only 6 (5,1%) patients in the follow‐up population were cured of their sarcopenia post‐stenting, and the mean SMI dropped both in men and women during follow‐up. The percentage of muscle mass lost (ΔSMI) during follow‐up produced clearly stratified survival curves when the median ΔSMI (−11.6%) was used as a threshold. In our study, the treatment received after stenting seemed to correlate with better OS, but this should be interpreted carefully, as patients offered treatments after stenting tend to have better performance status than those who are not offered any additional treatment.
Our study has a number of limitations, including its retrospective design. Groups were neither matched nor randomized. There is a risk of a type II error because of the low expected OS in this patient population and our relatively small control population. Furthermore, our institute has no specific follow‐up program after stent insertion for patients at this stage of the disease; the frequency of follow‐up CT imaging was low and its timing varied, in part to the lack of systemic follow‐up and in part to the overall poor survival of this patient group.
Our results may indicate that in the setting of end‐stage EC, lower muscle mass predicts poor prognosis. Sarcopenia defined by traditional skeletal muscle mass thresholds does not correlate with prognosis in this patient group.
Disclosure
No authors report any conflict of interest.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Daly JM, Fry WA, Little AG et al Esophageal cancer: Results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000; 190: 562–72. [DOI] [PubMed] [Google Scholar]
- 3. Wikman A, Johar A, Lagergren P. Presence of symptom clusters in surgically treated patients with esophageal cancer: Implications for survival. Cancer 2014; 120: 286–93. [DOI] [PubMed] [Google Scholar]
- 4. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013; 10: 90–9. [DOI] [PubMed] [Google Scholar]
- 5. Fearon K, Strasser F, Anker SD et al Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011; 12: 489–95. [DOI] [PubMed] [Google Scholar]
- 6. Blum D, Stene GB, Solheim TS et al Validation of the consensus‐definition for cancer cachexia and evaluation of a classification model‐‐a study based on data from an international multicentre project (EPCRC‐CSA). Ann Oncol 2014; 25: 1635–42. [DOI] [PubMed] [Google Scholar]
- 7. Tamandl D, Paireder M, Asari R, Baltzer PA, Schoppmann SF, Ba‐Ssalamah A. Markers of sarcopenia quantified by computed tomography predict adverse long‐term outcome in patients with resected oesophageal or gastro‐oesophageal junction cancer. Eur Radiol 2016; 26: 1359–67. [DOI] [PubMed] [Google Scholar]
- 8. Ida S, Watanabe M, Yoshida N et al Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol 2015; 22: 4432–7. [DOI] [PubMed] [Google Scholar]
- 9. Balentine CJ, Enriquez J, Fisher W et al Intra‐abdominal fat predicts survival in pancreatic cancer. J Gastrointest Surg 2010; 14: 1832–7. [DOI] [PubMed] [Google Scholar]
- 10. Prado CM, Lieffers JR, McCargar LJ et al Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population‐based study. Lancet Oncol 2008; 9: 629–35. [DOI] [PubMed] [Google Scholar]
- 11. Voron T, Tselikas L, Pietrasz D et al Sarcopenia impacts on short‐ and long‐term results of hepatectomy for hepatocellular carcinoma. Ann Surg 2015; 261: 1173–83. [DOI] [PubMed] [Google Scholar]
- 12. Fukushima H, Yokoyama M, Nakanishi Y, Tobisu K, Koga F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One 2015; 10: e0115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Psutka SP, Carrasco A, Schmit GD et al Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer‐specific and all‐cause mortality. Cancer 2014; 120: 2910–8. [DOI] [PubMed] [Google Scholar]
- 14. Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose‐limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 2010; 21: 1594–8. [DOI] [PubMed] [Google Scholar]
- 15. Mir O, Coriat R, Blanchet B et al Sarcopenia predicts early dose‐limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One 2012; 7: e37563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huillard O, Mir O, Peyromaure M et al Sarcopenia and body mass index predict sunitinib‐induced early dose‐limiting toxicities in renal cancer patients. Br J Cancer 2013; 108: 1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prado CM, Baracos VE, McCargar LJ et al Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009; 15: 2920–6. [DOI] [PubMed] [Google Scholar]
- 18. Prado CM, Lima IS, Baracos VE et al An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemoth Pharm 2011; 67: 93–101. [DOI] [PubMed] [Google Scholar]
- 19. Lecleire S, Di Fiore F, Antonietti M et al Undernutrition is predictive of early mortality after palliative self‐expanding metal stent insertion in patients with inoperable or recurrent esophageal cancer. Gastrointest Endosc 2006; 64: 479–84. [DOI] [PubMed] [Google Scholar]
- 20. Burstow M, Kelly T, Panchani S et al Outcome of palliative esophageal stenting for malignant dysphagia: A retrospective analysis. Dis Esophagus 2009; 22: 519–25. [DOI] [PubMed] [Google Scholar]
- 21. Vakil N, Morris AI, Marcon N et al A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. Am J Gastroenterol 2001; 96: 1791–6. [DOI] [PubMed] [Google Scholar]
- 22. Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med 1993; 329: 1302–7. [DOI] [PubMed] [Google Scholar]
- 23. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007; 165: 710–8. [DOI] [PubMed] [Google Scholar]
- 24. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM et al Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dodson S, Baracos VE, Jatoi A et al Muscle wasting in cancer cachexia: Clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 2011; 62: 265–79. [DOI] [PubMed] [Google Scholar]
- 26. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002; 50: 889–96. [DOI] [PubMed] [Google Scholar]
- 27. Fearon KC, Voss AC, Hustead DS, Cancer Cachexia Study Group . Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 2006; 83: 1345–50. [DOI] [PubMed] [Google Scholar]
- 28. Sheetz KH, Zhao L, Holcombe SA et al Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus 2013; 26: 716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan BH, Brammer K, Randhawa N et al Sarcopenia is associated with toxicity in patients undergoing neo‐adjuvant chemotherapy for oesophago‐gastric cancer. Eur J Surg Oncol 2015; 41: 333–8. [DOI] [PubMed] [Google Scholar]


