Abstract
Biopsy has been used to diagnose thoracic diseases for more than a century. Percutaneous needle biopsy plays a crucial role in the diagnosis, staging, and treatment planning for tumors in the lungs, thoracic wall, hilum, and mediastinum. With the continuous improvement in imaging techniques, the range of clinical applications for percutaneous needle biopsy is also expanding. It has become important to improve Chinese professionals’ and technicians’ understanding of percutaneous transthoracic needle biopsy (PTNB) in order to standardize operating procedures and to strengthen perioperative management. However, there is currently no Chinese expert consensus that provides systematic standardization and guidance for PTNB in clinical practice. The Committee of Chinese Society of Interventional Oncology (CSIO) of the Chinese Anti‐Cancer Association (CACA) initiated a Chinese multidisciplinary expert consensus on PTNB. The consensus includes image‐guided methods, indications, contraindications, multidisciplinary team recommendations, biopsy procedures, daytime/outpatient biopsy, complications, pathological examination, and management of negative results.
Keywords: Consensus, guideline, percutaneous biopsy, transthoracic cancer
Introduction
Biopsy has been used to diagnose thoracic diseases for more than a century. Depending on how specimens are collected, thoracic tumor biopsy can be divided into bronchoscopic transbronchial biopsy, percutaneous needle biopsy, thoracoscopic biopsy, and open chest biopsy. Percutaneous transthoracic needle biopsy (PTNB) is a type of biopsy performed under the guidance of imaging equipment. With the continuous improvement in imaging techniques, the range of clinical applications for percutaneous needle biopsy is also expanding. From its earliest use in pathological diagnosis to the classification of tissue subtypes and genetic diagnosis, the clinical need for percutaneous needle biopsy is increasing. It has become especially important to improve Chinese professionals’ and technicians’ understanding of PTNB in order to standardize operating procedures and strengthen perioperative management. As early as 2003, the British Thoracic Society (BTS) published guidelines on percutaneous lung needle biopsy.1 In 2016, the Chinese Thoracic Society and Chinese Alliance Against Lung Cancer published the “Chinese expert consensus statement on issues related to small specimen sampling of lung cancer,"2 which categorized the key issues in common clinical practice of percutaneous needle biopsy of the lungs and discussed the corresponding solutions. However, there is currently no Chinese expert consensus that provides systematic standardization and guidance for PTNB in clinical practice. Therefore, the Committee of Chinese Society of Interventional Oncology (CSIO) of the Chinese Anti‐Cancer Association (CACA) initiated the development of a Chinese expert consensus on PTNB. Built upon updated evidence from the BTS Guidelines, the “Chinese expert consensus statement on issues related to small specimen sampling of lung cancer,” and opinions from a panel of multidisciplinary experts, a Chinese expert consensus on PTNB relevant to China's national conditions has been developed. This consensus does not include bronchoscopic transbronchial biopsy, thoracoscopic biopsy, or open chest biopsy. The Committee of CSIO of CACA will update the content of this consensus based on technological advancements, such as changes in diagnostic and tissue sampling methods, as well as the emergence of new evidence.
Literature search
Literature search strategy
English‐language electronic databases, including PubMed, Embase, and the Cochrane Library were searched using the following terms: needle, needles, fine‐needle, fine needle, biopsy, rebiopsy, lung, chest, pleura, pleural, thoracic, pulmonary, thorax, mediastinum, and mediastinal. Similarly, Chinese‐language databases, China National Knowledge Infrastructure (CNKI), VIP (Weipu), and Wanfang, were searched using the terms: lung cancer, thoracic wall tumor, pleura tumor, mediastinum tumor, biopsy, and needle biopsy. References cited in the retrieved articles were included in the supplementary search. The literature search was conducted for articles published from the date when the databases were first established to June 2017 (Fig 1).
Figure 1.
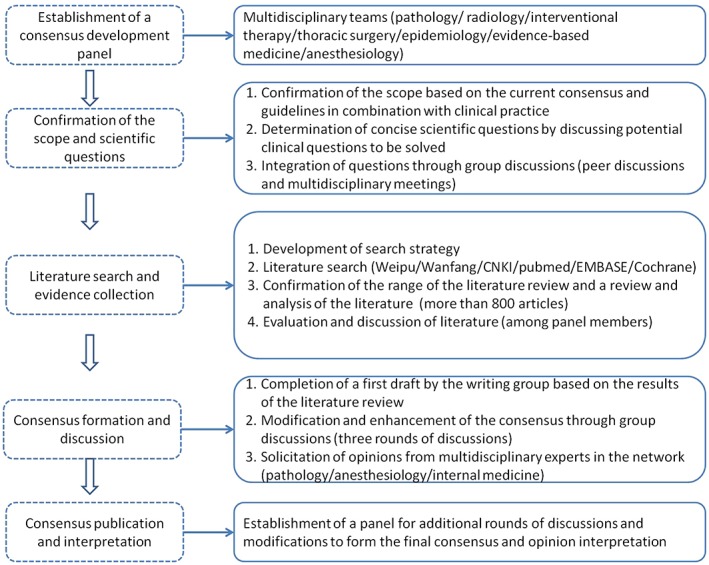
Consensus development methods and processes.
Inclusion criteria
The following publication types were included: (i) original articles; (ii) systematic reviews; (iii) meta‐analyses; and (iv) consensus or guidelines related to percutaneous needle biopsy of pulmonary nodules or masses, or a comparison of percutaneous needle biopsy and other specimen collection methods, such as bronchoscopy.
Exclusion criteria
The following publication types were excluded: (i) studies in which PTNB was not performed; (ii) articles that were not published in English or Chinese; (iii) studies published in national journals from non‐English‐speaking countries in which the full text was not available; and (iv) conference posters, conference abstracts, and seminars.
Overview
Percutaneous needle biopsy plays a crucial role in the diagnosis, staging, and treatment planning for tumors in the lungs, thoracic wall, hilum, and mediastinum. Needles used for percutaneous biopsy can be categorized as aspiration or cutting needles according to their sample‐collection principles. Aspiration needles allow the collection of high‐quality cytological specimens for disease diagnosis, whereas cutting needles are used to collect histological specimens because of their larger needle diameters compared to aspiration needles. Based on the type of biopsy needle, percutaneous needle biopsy can be divided into two main categories: fine needle aspiration (FNA) and core needle biopsy (CNB).
Methods for imaging guidance
Image‐guided methods for PTNB include X‐ray fluoroscopy, C‐arm cone‐beam computed tomography (CT), CT or CT fluoroscopy, ultrasound, and magnetic resonance imaging (MRI). Guidance methods should be selected based on factors such as lesion size, location, visibility, relationship to surrounding important anatomical structures, available imaging equipment, and operator preferences.3, 4, 5, 6
X‐ray fluoroscopy
X‐ray fluoroscopy is a traditional guidance method primarily used for needle biopsy of peripheral lung diseases and larger lesions.7, 8 This method cannot display clear vascular anatomy around the lesion and is gradually being replaced by CT guidance.9
C‐arm cone‐beam computed tomography (CT)
C‐arm cone‐beam CT may also be used for image‐guided PTNB.10 The advantages of this approach include low radiation dose and the capacity to simulate real‐time guidance, but the density resolutions of the images are inferior to those of conventional CT.
CT
Computed tomography is widely used because it produces images with high spatial and density resolutions. Chest CT scans can clearly show the lesion size and depth, as well as the relationships between the lesion and the ribs, mediastinum, interlobar fissures, and blood vessels. This information assists the design of safe needle tracks and also allows for the early detection of complications.6 CT has become the primary choice and most common guidance method for PTNB. Contrast‐enhanced CT can assist the identification of necrotic and solid areas in the tumor and help to determine the blood supply around the lesion. These properties may improve the positive rates of biopsy and reduce the probability of postoperative complications. CT fluoroscopy can provide near real‐time imaging, which allows physicians to manipulate the needle based on patient breathing. It also helps needle insertion into moving nodules and the avoidance of rib puncture,11 which shortens procedure time and reduces complications. This is particularly advantageous for elderly patients12 or patients with poor cooperativity. However, this method can significantly increase the radiation exposure to the patient and operating physicians.11, 13 The learning curve for CT‐guided biopsy is short, but obtaining sufficient tissues with pathologic diagnostic value, especially from small or moving lesions, requires meticulousness, the mastery of relevant techniques, and sufficient practical experience.
Ultrasound
Ultrasound can be used to monitor the insertion process, angle, and depth of the biopsy needle in real time.14, 15 It can accurately locate the needle tip and help to avoid damage to nearby structures. It is usually used for the biopsy of superficial lesions adjacent to the thoracic wall.16 Contrast‐enhanced ultrasound can clearly show tumor blood vessels and necrotic lesions,17, 18, 19, 20, 21 which can partially reduce the false‐negative rates of needle biopsy.11, 22
Magnetic resonance imaging
Compared to other imaging techniques, MRI has a superior resolution for tissues and multiplanar imaging capability. It produces near real‐time imaging without radiation. The use of respiratory gating technology to capture images can shorten the scan time. MRI has unique advantages in defining thoracic blood vessels and in guiding biopsies for tumors in the mediastinum, hilum, and thoracic wall. MRI‐guided percutaneous needle biopsy of thoracic tumors has been reported in the literature.23, 24 However, MRI‐related consumables and equipment need to be compatible with magnets. MRI is also expensive and requires a longer operation duration. MRI‐guided needle biopsy can be performed in hospital units with available resources.
Indications
Isolated nodules or masses, multiple nodules or masses, and pulmonary consolidation that require characterization;
Focal consolidation that cannot be confirmed by bronchoscopy, sputum cytology, or sputum culture;
Ground‐glass nodules suspicious for malignancy;
Malignant lesions that require classification of histology or molecular pathology (rebiopsy);
Re‐evaluation of local histology or molecular pathology after disease progression or relapse (rebiopsy);
Other conditions, such as hilar mass with failed or negative results of bronchoscopic biopsy, undiagnosed mediastinal mass, and mediastinal lymph nodes suspicious for malignancy.
Contraindications
Absolute contraindications
Severe cardiopulmonary insufficiency, such as severe pulmonary arterial hypertension;
Uncorrectable coagulopathy.
Relative contraindications
Anatomical or functional lung isolation;
Prominent infectious lesions on the needle path;
Pulmonary bullae, chronic obstructive pulmonary disease, emphysema, or pulmonary fibrosis;
Mechanical ventilation (ventilator).
Multidisciplinary team construction
Preoperative multidisciplinary panel discussion is recommended. Percutaneous needle biopsy should be performed by or under the guidance of an experienced surgeon.25, 26 Well‐trained doctors and nurses are needed to manage preoperative, intraoperative, and postoperative procedures. The presence of a cytopathologist during surgery can improve diagnostic accuracy. If necessary, anesthetic intervention should be considered to ensure successful completion of the surgery.
Biopsy procedures
Preoperative evaluation and management
Prior to surgery, patient details such as medical history, medication history, and allergy history must be obtained and a physical examination must be performed. Patient cardiopulmonary function and ability to cooperate, such as the ability to hold their breath or stay still, should be assessed. Contrast‐enhanced chest CT or contrast‐enhanced chest MRI should be conducted prior to surgery to determine the lesion location, shape, size, and relationship to surrounding organs, blood vessels, and nerves to design the needle track. Patients suspected via chest CT examination to have hydatid cysts or vascular malformations should not undergo biopsy.3 Prior to surgery, it is recommended that all patients undergo complete blood count, coagulation testing,27 infection screening (such as hepatitis B virus, hepatitis C virus, syphilis, and human immunodeficiency virus [HIV]), electrocardiogram, blood biochemistry, and blood type testing. In specific populations with a high risk of increasing capillary fragility, the construction of vascular elasticity diagrams is also recommended. For patients with underlying lung diseases, such as chronic obstructive pulmonary disease and emphysema, pulmonary function tests are recommended to assess the oxygenation capacity and functional residual capacity of the patients.
Discontinuation of anticoagulant and antiplatelet drugs and re‐examination of complete blood count and coagulation functions prior to surgery are recommended, as follows: (i) warfarin should be replaced with low molecular weight heparin 1 week prior to surgery and low molecular weight heparin should be discontinued 24 hours before surgery; (ii) aspirin and clopidogrel should be discontinued for at least 7 days before surgery28 and (iii) biopsy can be performed only if the platelet count is > 50 × 109/L and the international normalized ratio (INR) is < 1.5. For biopsies in patients receiving anti‐angiogenic drugs, discontinuing the drug according to the in vivo elimination half‐life of the drug is recommended. For example, it is recommended that bevacizumab be discontinued 6 weeks prior to surgery.
Formulating a biopsy plan
Patient imaging data must be carefully reviewed prior to surgery. A biopsy plan should be formulated based on lesion size, location, anatomic relationships, image‐guiding method, and operator experience. Multidisciplinary expert discussion is recommended for patients with relative contraindications or in special circumstances.
The needle track should be designed on the premise that vital organs and skeletal structures, such as ribs and scapula, are avoided, while concurrently avoiding bullae, great vessels, the trachea, and interlobar fissures. Additionally, the distance between the lesion and needle puncture point in the pleura and the traversal of normal lung tissues should be minimized.
Informed consent
Prior to surgery, patients and their authorized representatives should be informed of the purpose, benefits, and possible risks of the surgery, as well as any available treatment alternatives. Written informed consent must be obtained from the patient or their authorized representative (see Appendix I–III for details).
Preoperative preparations
Preoperative psychological and spiritual counseling are recommended to alleviate patient anxiety and tension. Patients should be trained to breathe calmly and to coordinate their breathing during surgery. Venous access catheters should be implanted prior to surgery and monitored by electrocardiograph.
Anesthesia and sedation
Percutaneous transthoracic needle biopsy requires patients to maintain a certain level of consciousness to cooperate during surgery and monitoring; therefore, local anesthesia is routinely used.4 Basic anesthesia and sedation may be considered for patients with anxiety or cooperative difficulties during surgery. However, patients must be conscious enough to follow and complete instructions relevant to the surgery.
Steps for biopsy
Selection of needle puncture point
Simulation by CT or other imaging techniques should be performed prior to surgery. The shortest needle track should be selected on the premise that important anatomical structures, such as bones, blood vessels, and the trachea, are avoided.
Local anesthesia
After local skin disinfection, sterile drapes are covered. Local anesthesia should then be performed using a 1–2% lidocaine solution. The dose of anesthesia may be adjusted accordingly based on the patient's response, anesthetic efficacy, and needle depth.
Needle puncture and acquisition of specimens
Using CT‐guided needle puncture as an example, multiple needle passes are recommended. Based on positions identified by CT, the biopsy needle should first be inserted into the parietal pleura for local anesthesia, followed by insertion into the lung tissues. The correct positioning should be confirmed by image scans. If the needle advances appropriately along the track, the needle can puncture the lesion directly. The biopsy site should be selected based on lesion characteristics. For larger lesions, central areas with ischemic necrosis should be avoided. For cavitary lesions, biopsy specimens should be obtained from solid tissues.
Coaxial technique
The coaxial technique allows multiple biopsy specimens to be obtained with only one puncture and causes less trauma.29 In cases of pneumothorax or hemothorax, the coaxial channels can be used to remove accumulated gas or blood, or to inject drugs, which allows the immediate treatment of complications. The protective effects of the coaxial channels may partially reduce the risk of implantation metastasis along the needle track.
Postoperative monitoring
A complete chest CT scan is recommended immediately after CT‐guided biopsy to observe the presence of complications, such as pneumothorax and hemorrhage. Treatment should be initiated if necessary. Patients who do not require treatment can be transferred to the ward or observation room to monitor vital signs, blood oxygen saturation levels, etc. Patients should be reminded to minimize activities that may increase chest pressure, such as coughing and talking. A chest X‐ray examination is recommended within 24 hours after surgery. If the patient's conditions have changed, additional chest X‐ray or chest CT scanning should be performed in a timely manner.
Daytime/outpatient biopsy
For patients who have been preoperatively evaluated as low‐risk for needle biopsy, completion of biopsy at a daytime or outpatient clinic may be considered. If no abnormalities are observed within four hours of the outpatient biopsy, patients may leave the clinic after the chest X‐ray has been reviewed. Follow‐up must be conducted for at least 24 hours after the biopsy. Patients should be reminded to visit the hospital without delay if they experience any abnormalities, discomfort, or symptoms.30
Complications and treatment
The most common complications of PTNB include pneumothorax, hemorrhage, and pleural reaction. Complications such as systemic air embolism, cardiac tamponade, and tumor implantation along the needle track are relatively rare. The mortality rate of PTNB is between 0.02% and 0.15%.31 The leading causes of death include acute or pulmonary hemorrhage, cardiac arrest, and air embolism.
Pneumothorax
Pneumothorax is a common postoperative complication of PTNB. The incidence rate of pneumothorax reported in the literature ranges from 2.4% to 60% (average 20%), and 5–18% of patients with pneumothorax require thoracic drainage catheters.25, 32, 33, 34 Factors attributable to the increased incidence of pneumothorax and/or the increased requirement of drainage catheters include: tall and thin body types, old age, smoking, underlying lung diseases (e.g. emphysema or chronic obstructive pulmonary disease),13, 35 deeply located lesions, lesions with small diameters,36, 37, 38 a biopsy needle not perpendicular to the pleural section, multiple punctures at the pleura,39, 40 the needle track traverses intrapulmonary fissures or pulmonary bullae,41 and long operative duration. Pneumothorax generally occurs within an hour after surgery,42 but some patients may develop delayed pneumothorax (24 hours or later) after surgery.43 If pneumothorax is not treated properly, subcutaneous emphysema may develop.44
Treatment principles: small pneumothorax, asymptomatic, and stable pneumothorax do not require special treatment. If the pneumothorax exceeds 30%, the pneumothorax area continues to increase, or if the patient shows severe clinical symptoms, a chest tube should be inserted for suction or fluid drainage through a closed chest drainage system.
Prevention: Patients should remain quiet and avoid talking or coughing5 an appropriate needle track should be chosen, and the number of punctures should be reduced. It is not currently known whether the use of exogenously injected biogel, injectable gelatin sponge, or sterile saline to seal the needle track can effectively reduce the development of pneumothorax.45, 46, 47, 48
Hemorrhage and hemoptysis
Hemorrhage (with or without hemoptysis) is another common complication of PTNB. The incidence rate of hemorrhage reported in the literature is between 5% and 16.9%, and that of hemoptysis is between 1.25% and 7%.6, 25, 49, 50 Hemorrhage is usually self‐limited, but deaths caused by massive intrapulmonary hemorrhage have also been reported.51 Factors attributable to the increased risk of intrapulmonary hemorrhage include: distance between the lesion and the pleura,52, 53 number of biopsies,53, 54, 55 type of biopsy needle (cutting‐needle biopsy),56 lesions located in the mediastinum or next to the heart or mediastinum,57 lesions with a rich blood supply (such as metastatic renal cell carcinoma), lesions close to branches originating from dilated bronchial arteries (chronic cavitary disease),52, 57 coagulopathy, and pulmonary hypertension.
Treatment principles
Mild hemoptysis, intraparenchymal hemorrhage, bleeding along the needle track, and small hemothorax can be resolved spontaneously without special treatment.44, 58 For massive hemoptysis, it is recommended to place the patient in a lateral decubitus position ipsilateral to the bleeding site (needle puncture point facing downward) to prevent blood from being inhaled into the contralateral bronchus. The airways should remain clear and intubation may be performed if necessary. Hemostatic drugs,58, 59 blood transfusion25 and other treatments may also be applied. For massive hemothorax, draining with a thoracic catheter is recommended.60 If there is a large amount or continuous bleeding, interventional procedures or surgery should be performed in a timely manner and relevant departments should be informed to organize appropriate treatments.25
Pleural reaction
Pleural reaction refers to a series of manifestations that occur during needle puncture at the pleura, including continuous cough, dizziness, chest tightness, paleness, sweating, and even syncope, which may be related to a vasovagal reaction. Possible factors leading to pleural reaction include a thin body type, anxiety, low baseline blood glucose, and multiple punctures at the pleura.
Most patients have mild symptoms that resolve spontaneously without treatment. In severe cases, patients may experience excessive sweating, progressive decline in blood pressure, or even shock or syncope. If these occur, all procedures must be halted immediately. The patients should immediately be administered adrenaline or glucose solution based on their conditions and concurrently treated with oxygen therapy. The patients should be kept warm and their vital signs should be monitored. Precautions should be taken to prevent shock.
Systemic air embolism
Systemic air embolism can be divided into systemic venous air embolism and systemic arterial air embolism. The incidence rate of systemic air embolism is 0.02%–1.80%.31, 49, 61, 62 While most systemic venous air embolism is asymptomatic, systemic arterial air embolism is the most serious complication of lung biopsy and can lead to serious consequences, such as shock, cardiac arrest, and hemiplegia. Although systemic air embolism is rare, surgeons should be mindful of the condition because of its fatal clinical consequences.
At present, the mechanism of systemic arterial air embolism is believed to be the direct introduction of air into the pulmonary vein through the coaxial cannula61 or iatrogenic bronchopulmonary or alveolar‐pulmonary venous fistulas as a result of injury caused by puncture.61 The gas enters the pulmonary vein and refluxes into the left side of the heart, which then enters blood vessels, such as the coronary and intracranial arteries, through systemic circulation. The causes of systemic arterial air embolism may include biopsy of cavitary or vascular inflammatory lesions (such as ground glass), coughing, and positive‐pressure ventilation. Patients may be asymptomatic if the amount of air entering the chambers on the left side of the heart is relatively small, and cause no significant effect on hemodynamics.60, 63 In coronary air embolism, patients may experience a transient loss of consciousness and their electrocardiography may reflect myocardial ischemia.60, 64, 65 In intracranial air embolism, patients may experience seizures65 or loss of consciousness.60, 66 CT scans can reveal the gas in the organs or blood vessels with embolism, which may serve as objective evidence for the diagnosis of air embolism.60, 63, 64, 65, 66
Treatment principles: It is crucial to rapidly identify air embolisms and to initiate treatment immediately, which may help to improve the prognosis of some patients. Once air embolism is suspected, the needle should be immediately withdrawn. The patient should be placed in the Trendelenburg position.64 If there is a relatively large amount of gas in the chambers on the left side of the heart, the patient should be placed in a right lateral decubitus position, which makes the left atrium higher than the left ventricle. This position can prevent the gas from entering the systemic circulation through the base of the left ventricle outflow tract, a situation that may lead to the aforementioned serious complications. Vital signs should be monitored closely at the same time. Oxygen masks and other rescue measures should be proactively administered. If intracranial arterial air embolism occurs and conditions allow, the patient can be transported to a hyperbaric oxygen chamber for treatment.65
Prevention: (i) The types of lesions, such as cavitary and vasculitic lesions, for needle biopsy should be carefully selected; (ii) needle biopsy on patients standing erect should be avoided; (iii) needle biopsy under positive‐pressure ventilation should be avoided; (iv) the needle core should be inserted in a timely manner without allowing long exposure of the coaxial cannula to the air; (v) iatrogenic injury, such as bleeding during surgery, should be reduced, for example, by avoiding repeated puncture; and (vi) behaviors such as coughing, deep breathing, and talking should be minimized during surgery.
Other uncommon and rare complications
Implantation metastasis along the needle track is extremely rare,31, 67, 68 with reported incidence in the literature between 0.012% and 0.061%.69 The coaxial technique can reduce the risk of its occurrence. Other rare complications include cardiac tamponade,70 intercostal artery pseudoaneurysm,71 atrial fibrillation,72 chest infections,49, 73, 74, 75 vasovagal reaction,44 and pleural metastasis.76, 77, 78, 79
Pathological examination
Specimen processing
Specimens for cytological examination should be smeared immediately after they are obtained. The samples should be smeared gently and evenly and should be wet‐fixed immediately to prevent cell degradation. Wet fixation should be performed with 95% alcohol for at least 15 minutes. Liquid‐based smear samples should immediately be placed in digestive or preservative solutions and sent to the laboratory for further processing according to the operating procedures. Both rapid on‐site evaluation of cytology (ROSE) and on‐site imprint cytology involving cytopathologists can improve diagnostic accuracy.80, 81, 82, 83, 84, 85, 86, 87, 88 Specimens for histopathological examination should be fixed immediately after they are obtained by placing the samples in a 10% neutral buffered formalin fixative. If the freshly obtained tissues are to be used for molecular testing, they should, in principle, only contain tumor components that meet the quality control requirements. The tissues obtained should be quickly frozen in liquid nitrogen storage or stored in an RNA preservation solution.
Before the samples are sent for examination, the pathology test request form should be accurately completed, with detailed descriptions of the patient's basic information, medical history, history of related examinations and treatment, preliminary clinical diagnosis, biopsy location, and the number of punctures.
Staining method
Smears prepared from FNA specimens may be used for Papanicolaou or hematoxylin and eosin staining after fixation. Samples suspicious for lymphoid or hematopoietic malignancies may be considered for air‐drying followed by Wright–Giemsa staining. Diff‐Quik staining is commonly used on‐site to evaluate the sample quality. After a diagnosis is made with liquid‐based cytology slides, the current practice is to centrifuge the remaining samples and embed as much sample as possible in paraffin wax to preserve the cells for immunocytochemistry or gene mutation testing. Significant data have confirmed that microsamples of cells can provide similar results in immunocytochemistry or gene mutation testing and histological examination. Histopathological specimens from CNB are routinely paraffin‐embedded, sectioned, and stained with hematoxylin and eosin. Immunohistochemical staining or related molecular pathology testing can be conducted if necessary. Specimens used for molecular pathology testing should have passed pathological quality control before commencing the molecular testing process.
Clinical diagnostic value
Accuracy rate
Percutaneous transthoracic needle biopsy shows high diagnostic accuracy for malignant thoracic diseases (peripheral pulmonary lesions, hilar lymph nodes, hilar masses, and mediastinal masses). The diagnostic accuracy of FNA for malignant diseases ranges from 64% to 97%,26, 89, 90 but its use in the diagnosis of benign diseases is limited, with accuracy rates between 10% and 50%.91, 92, 93, 94, 95 FNA also shows a limited capacity for the accurate classification of tumor types. The diagnostic accuracy of CNB for malignant diseases is similar to that of FNA (74–95%),96, 97, 98 but its accuracy for diagnosing benign diseases is higher than FNA.99 The factors that may affect diagnostic accuracy include lesion size and location, operator experience, choice of guidance, and on‐site cytology evaluation.
Follow‐up management of negative biopsy
The reasons for a negative biopsy may include poor patient cooperation,100, 101 very small lesions, special tumor types, and difficulties in pathological diagnosis. For patients with negative biopsy results who are highly suspected of malignant disease, rebiopsy is recommended. Patients who have not undergone rebiopsy are recommended to attend regular follow‐up imaging tests. If patients show disease progression during follow‐up, additional biopsy or surgery is recommended.
Rebiopsy
Rebiopsy, also called second biopsy, refers to a biopsy performed after a patient has a confirmed diagnosis and has received corresponding treatment based on the results of the first biopsy, but because of disease progression, additional tissues of the lesion or blood samples are required. The rebiopsied samples are used to monitor disease progression and interpret drug resistance mechanisms, which may guide the selection of follow‐up treatment plans for patients who show disease progression after targeted therapy or those who develop drug resistance. In patients with non‐small cell lung cancer diagnosed with EGFR mutations in the first biopsy, 33–63% have confirmed EGFR T790M mutations in the rebiopsy performed after they have shown disease progression following EGFR‐tyrosine kinase inhibitor therapy.102, 103, 104, 105 The timing of rebiopsy after disease progression does not affect the detection rate of T790M mutations.106 Patients who are temporarily unsuitable to undergo biopsy should be scheduled for a rebiopsy during subsequent treatment. Histological examination of the rebiopsy sample is recommended. The use of CT‐guided methods to obtain tissue has a high success rate and is relatively safe (incidences of pneumothorax and hemorrhage are 6% and 7%, respectively).107
Disclosure
No authors report any conflict of interest.
Acknowledgments
We thank the Youth Committee of Interventional Oncology, China Anti‐Cancer Association. We are also grateful for contributions from Yan Wang, Cao Fei, and Haonan Zhang.
Informed consent form for percutaneous transthoracic needle biopsy (reference only)
Name ________ Sex______ Age______ Department ______ Outpatient admission number __________ Inpatient admission number __________.
Clinical diagnosis: ___________________________Biopsy type: ________________________.
Guidance method: _________________________ Type of anesthesia: ____________________.
Main purpose:
1. Identify the pathological subtypes to guide treatment planning;
2. Genetic testing to guide the use of targeted therapy;
3. Others: ____________________.
If the patient refuses to undergo a percutaneous needle biopsy, the alternative courses of treatment include: ____________.
Potential risks and possible complications:
1. There are risks associated with all surgical procedures involving anesthesia.
2. All drugs have the potential to cause allergies or adverse effects. Mild symptoms may include nausea and rash. Severe symptoms may include anaphylactic shock and cardiorespiratory arrest and can be life‐threatening.
3. The risk of surgery may increase in patients with heart disease, hypertension, diabetes, renal insufficiency, venous thrombosis, and other related conditions. These conditions may be exacerbated during or after surgery. Patients may experience cardiovascular or cerebrovascular events or even death.
4. The potential risks and possible complications of this surgery may include, but are not limited to, the following:
1) Hemorrhage, hematoma, and local infection at the puncture site or surrounding organs and tissues, which may require hemostasis and anti‐infective treatment. Severe cases can be life‐threatening;
2) Puncture may lead to great vessel injury or tumor rupture, causing hemorrhage and shock, and may require open surgery to stop the bleeding. Severe cases can be life‐threatening;
3) Symptoms such as numbness, pain, and motor dysfunction may occur in the organs, tissues, or nerves near the puncture site;
4) Symptoms such as cough, hemoptysis, chest pain, chest tightness, and fever may occur during or after surgery;
5) Complications such as pleural reaction, pneumothorax, hemothorax, hemopneumothorax, and air embolism may occur during or after surgery. Severe cases can be life‐threatening;
6) Cardiovascular or cerebrovascular events, vasovagal reaction, atrial fibrillation, cardiac tamponade, intercostal artery pseudoaneurysm, subcutaneous emphysema, stress ulcer, and gastrointestinal bleeding may occur during or after surgery;
7) Damage to the tissues and organs other than the targets, such as normal lung tissue, trachea, thoracic wall, etc.;
8) Potential tumor metastasis along the puncture site, needle track, or traversed organs;
9) If pathological examination after the surgery suggests malignancy, immunohistochemistry may be needed to further confirm the diagnosis;
10) The pathological outcome of the first biopsy may be false‐negative and a rebiopsy should be performed if necessary;
11) The surgery may be abandoned if the initially planned procedures require modification or the surgery cannot be performed smoothly due to anatomical deformity of the lesion or other unforeseen circumstances;
12) Some image‐guided procedures may have potential risks of radiation exposure;
13) Specific risks (list according to the specific condition of the patient): ___________________________;
14) Other unpredictable medical risks and rare complications.
Statement of patients and their authorized relatives
1. I understand that based on my current disease condition and clinical diagnosis, the above surgery is necessary for the further diagnosis and treatment of my disease;
2. Before indicating my acceptance of the surgical plan, the doctor has explained to me the surgical method, anesthesia method, intraoperative and postoperative complications, and other risks and has answered my questions related to this surgery. I clearly understand all of the contents explained by the doctor. I __________ (agree/disagree) with the above surgical plan for needle biopsy, and I ________ (agree/disagree) to undertake the risks and consequences of needle biopsy;
3. In addition to the above situations, I understand that unpredictable situations may occur during surgery. I hereby authorize the doctor to contact my family immediately in the event of an unexpected situation. If the situation is urgent and my family cannot be contacted, I should be treated based on my best interests and in accordance with routine medical practice. If the above situation occurs, I believe that the medical staff will exert their maximal efforts to treat me. I have undergone sufficient mental preparation and will actively cooperate with the doctor during the treatment;
4. I authorize the doctor to handle and use the tissues or specimens obtained from the surgery, including for pathological and cytological examination purposes, and for medical waste disposal;
5. Others:__________________________________________________________________________________________.
Patient's signature: __________ Date:__________.
If the patient is unable to sign the informed consent form, their authorized relatives should sign here:
Authorized relative's signature: _________ Relationship with patient: __________ Date: _____________.
Physician's signature: _____________ Surgeon's signature: ________ Date: _____________
Types of biopsy needles
Needles used for FNA: The Chiba needle is one of the most commonly used aspiration biopsy needles. Other needles include the Tuner, Madayag, Greene, and Franseen needles.
Needles used for CNB: Cutting needles are usually used for CNB. The most common biopsy needles used for CNB include the Trucut, Temno, Bard, Fullcore, and Rotex needles. The most commonly used specification is 18 G, but 16 G and 20 G are also used.
Estimation of the amount of biopsy material
The lower limit of the amount of biopsy materials is estimated according to the amount of materials acquired by commonly used biopsy needles and the quality of pathological diagnosis.
An example using an 18G biopsy needle for a 1 cm specimen is described below. The amount of specimen acquired with biopsy needles of different specifications or for specimens with different lengths can be estimated using ratios.
| Type of biopsy | 18 G cutting biopsy needle for a semi‐cylindrical cutting | 18 G cutting biopsy needle for a full cylindrical cutting |
|---|---|---|
| First biopsy | 1 cm × 1–2 pieces (approximately 4–5 mm3/piece) | 1 cm × 1 piece (approximately 7–8 mm3/piece) |
| Rebiopsy | 1 cm × 4–5 pieces (approximately 4–5 mm3/piece) | 1 cm × 2–3 pieces (approximately 7–8 mm3/piece) |
Contributor Information
Zhi Guo, Email: cjr.guozhi@vip.163.com.
Hong Shi, Email: shihong@cma.org.
References
- 1. Henry M, Arnold T, Harvey J, Pleural Diseases Group, Standards of Care Committee, British Thoracic Society . BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003; 58 (Suppl. 2): ii39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chinese Thoracic Society . Chinese Allliance against Lung Cancer. [Chinese experts consensus on issues related to small sample collection for lung cancer]. J Intern Med China 2016; 55: 406–13 (In Chinese.). [Google Scholar]
- 3. Klein JS, Zarka MA. Transthoracic needle biopsy: An overview. J Thorac Imaging 1997; 12: 232–49. [DOI] [PubMed] [Google Scholar]
- 4. Manhire A, Charig M, Clelland C et al Guidelines for radiologically guided lung biopsy. Thorax 2003; 58: 920–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore EH. Technical aspects of needle aspiration lung biopsy: A personal perspective. Radiology 1998; 208: 303–18. [DOI] [PubMed] [Google Scholar]
- 6. Murphy JM, Gleeson FV, Flower CD. Percutaneous needle biopsy of the lung and its impact on patient management. World J Surg 2001; 25: 373–9. [DOI] [PubMed] [Google Scholar]
- 7. Laurent F, Montaudon M, Latrabe V, Bégueret H. Percutaneous biopsy in lung cancer. Eur J Radiol 2003; 45: 60–8. [DOI] [PubMed] [Google Scholar]
- 8. Nordenström B. Transthoracic needle biopsy. N Engl J Med 1967; 276: 1081–2. [DOI] [PubMed] [Google Scholar]
- 9. Klein JS, Zarka MA. Transthoracic needle biopsy. Radiol Clin North Am 2000; 38: 235–66. [DOI] [PubMed] [Google Scholar]
- 10. Choo JY, Park CM, Lee NK, Lee SM, Lee HJ, Goo JM. Percutaneous transthoracic needle biopsy of small (≤ 1 cm) lung nodules under C‐arm cone‐beam CT virtual navigation guidance. Eur Radiol 2013; 23: 712–9. [DOI] [PubMed] [Google Scholar]
- 11. Kim GR, Hur J, Lee SM et al CT fluoroscopy‐guided lung biopsy versus conventional CT‐guided lung biopsy: A prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol 2011; 21: 232–9. [DOI] [PubMed] [Google Scholar]
- 12. Cheng YC, Tsai SH, Cheng Y, Chen JH, Chai JW, Chen CCC. Percutaneous transthoracic lung biopsy: Comparison between C‐arm cone‐beam CT and conventional CT guidance. Transl Oncol 2015; 8: 258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heck SL, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy‐guided needle biopsies of lung masses. Eur Radiol 2006; 16: 1387–92. [DOI] [PubMed] [Google Scholar]
- 14. Sheth S, Hamper UM, Stanley DB, Wheeler JH, Smith PA. US guidance for thoracic biopsy: A valuable alternative to CT. Radiology 1999; 210: 721–6. [DOI] [PubMed] [Google Scholar]
- 15. Rubens DJ, Strang JG, Fultz PJ, Gottlieb RH. Sonographic guidance of mediastinal biopsy: An effective alternative to CT guidance. AJR Am J Roentgenol 1997; 169: 1605–10. [DOI] [PubMed] [Google Scholar]
- 16. Zhan P, Zhu QQ, Miu YY et al Comparison between endobronchial ultrasound‐guided transbronchial biopsy and CT‐guided transthoracic lung biopsy for the diagnosis of peripheral lung cancer: A systematic review and meta‐analysis. Transl Lung Cancer Res 2017; 6: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong Y, Mao F, Wang WP, Ji ZB, Fan PL. Value of contrast‐enhanced ultrasound in guidance of percutaneous biopsy in peripheral pulmonary lesions. Biomed Res Int 2015; 2015: 531507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Görg C, Bert T, Kring R. Contrast‐enhanced sonography of the lung for differential diagnosis of atelectasis. J Ultrasound Med 2006; 25: 35–9. [DOI] [PubMed] [Google Scholar]
- 19. Görg C, Bert T, Kring R, Dempfle A. Transcutaneous contrast enhanced sonography of the chest for evaluation of pleural based pulmonary lesions: Experience in 137 patients. Ultraschall Med 2006; 27: 437–44. [DOI] [PubMed] [Google Scholar]
- 20. Yi D, Feng M, Wen Ping W, Zheng Biao J, Fan PL. Contrast‐enhanced US‐guided percutaneous biopsy of anterior mediastinal lesions. Diagn Interv Radiol 2017; 23: 43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu J, Yang W, Wang S et al Clinical value of contrast‐enhanced ultrasound in improving diagnostic accuracy rate of transthoracic biopsy of anterior‐medial mediastinal lesions. Chin J Cancer Res 2016; 28: 617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao BS, Wu JH, Li XL, Deng J, Liao GQ. Sonographically guided transthoracic biopsy of peripheral lung and mediastinal lesions: Role of contrast‐enhanced sonography. J Ultrasound Med 2011; 30: 1479–90. [DOI] [PubMed] [Google Scholar]
- 23. Sakarya ME, Unal O, Ozbay B et al MR fluoroscopy‐guided transthoracic fine‐needle aspiration biopsy: Feasibility. Radiology 2003; 228: 589–92. [DOI] [PubMed] [Google Scholar]
- 24. Liu M, Huang J, Xu Y et al MR‐guided percutaneous biopsy of solitary pulmonary lesions using a 1.0‐T open high‐field MRI scanner with respiratory gating. Eur Radiol 2017; 27: 1459–66. [DOI] [PubMed] [Google Scholar]
- 25. Richardson CM, Pointon KS, Manhire AR, Macfarlane JT. Percutaneous lung biopsies: A survey of UK practice based on 5444 biopsies. Br J Radiol 2002; 75: 731–5. [DOI] [PubMed] [Google Scholar]
- 26. Li H, Boiselle PM, Shepard JO, Trotman‐Dickenson B, McLoud TC. Diagnostic accuracy and safety of CT‐guided percutaneous needle aspiration biopsy of the lung: Comparison of small and large pulmonary nodules. AJR Am J Roentgenol 1996; 167: 105–9. [DOI] [PubMed] [Google Scholar]
- 27. British Thoracic Society Bronchoscopy Guidelines Committee a Subcommittee of Standards of Care Committee of British Thoracic Society . British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001; 56 (Suppl. 1): i1–i21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel IJ, Davidson JC, Nikolic B et al Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image‐guided interventions. J Vasc Interv Radiol 2012; 23: 727–36. [DOI] [PubMed] [Google Scholar]
- 29. Nour‐Eldin NE, Alsubhi M, Emam A et al Pneumothorax complicating coaxial and non‐coaxial CT‐guided lung biopsy: Comparative analysis of determining risk factors and management of pneumothorax in a retrospective review of 650 patients. Cardiovasc Intervent Radiol 2016; 39: 261–70. [DOI] [PubMed] [Google Scholar]
- 30. Anzidei M, Sacconi B, Fraioli F et al Development of a prediction model and risk score for procedure‐related complications in patients undergoing percutaneous computed tomography‐guided lung biopsy. Eur J Cardiothorac Surg 2015; 48: e1–6. [DOI] [PubMed] [Google Scholar]
- 31. Tomiyama N, Yasuhara Y, Nakajima Y et al CT‐guided needle biopsy of lung lesions: A survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006; 59: 60–4. [DOI] [PubMed] [Google Scholar]
- 32. Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population‐based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: An analysis of discharge records. Ann Intern Med 2011; 155: 137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tuna T, Ozkaya S, Dirican A, Findik S, Atici AG, Erkan L. Diagnostic efficacy of computed tomography‐guided transthoracic needle aspiration and biopsy in patients with pulmonary disease. Onco Targets Ther 2013; 6: 1553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuban JD, Tam AL, Huang SY et al The effect of needle gauge on the risk of pneumothorax and chest tube placement after percutaneous computed tomographic (CT)‐guided lung biopsy. Cardiovasc Intervent Radiol 2015; 38: 1595–602. [DOI] [PubMed] [Google Scholar]
- 35. Collings CL, Westcott JL, Banson NL, Lange RC. Pneumothorax and dependent versus nondependent patient position after needle biopsy of the lung. Radiology 1999; 210: 59–64. [DOI] [PubMed] [Google Scholar]
- 36. Cox JE, Chiles C, McManus CM, Aquino SL, Choplin RH. Transthoracic needle aspiration biopsy: Variables that affect risk of pneumothorax. Radiology 1999; 212: 165–8. [DOI] [PubMed] [Google Scholar]
- 37. Kazerooni EA, Lim FT, Mikhail A, Martinez FJ. Risk of pneumothorax in CT‐guided transthoracic needle aspiration biopsy of the lung. Radiology 1996; 198: 371–5. [DOI] [PubMed] [Google Scholar]
- 38. Laurent F, Michel P, Latrabe V, Tunon de Lara M, Marthan R. Pneumothoraces and chest tube placement after CT‐guided transthoracic lung biopsy using a coaxial technique: Incidence and risk factors. AJR Am J Roentgenol 1999; 172: 1049–53. [DOI] [PubMed] [Google Scholar]
- 39. Ko JP, Shepard JO, Drucker EA et al Factors influencing pneumothorax rate at lung biopsy: Are dwell time and angle of pleural puncture contributing factors? (Published erratum appears in Radiology 2001; 220: 556). Radiology 2001; 218: 491–6. [DOI] [PubMed] [Google Scholar]
- 40. Haramati LB, Austin JH. Complications after CT‐guided needle biopsy through aerated versus nonaerated lung. Radiology 1991; 181: 778. [DOI] [PubMed] [Google Scholar]
- 41. Moreland A, Novogrodsky E, Brody L et al Pneumothorax with prolonged chest tube requirement after CT‐guided percutaneous lung biopsy: Incidence and risk factors. Eur Radiol 2016; 26: 3483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Charig MJ, Phillips AJ. CT‐guided cutting needle biopsy of lung lesions‐‐safety and efficacy of an out‐patient service. Clin Radiol 2000; 55: 964–9. [DOI] [PubMed] [Google Scholar]
- 43. Koh DM, Burke S, Davies N, Padley SPG. Transthoracic US of the chest: Clinical uses and applications. Radiographics 2002; 22: e1. [DOI] [PubMed] [Google Scholar]
- 44. Priola AM, Priola SM, Cataldi A et al Diagnostic accuracy and complication rate of CT‐guided fine needle aspiration biopsy of lung lesions: A study based on the experience of the cytopathologist. Acta Radiol 2010; 51: 527–33. [DOI] [PubMed] [Google Scholar]
- 45. Graffy P, Loomis SB, Pickhardt PJ et al Pulmonary intraparenchymal blood patching decreases the rate of pneumothorax‐related complications following percutaneous CT‐guided needle biopsy. J Vasc Interv Radiol 2017; 28: 608–13. [DOI] [PubMed] [Google Scholar]
- 46. Clayton JD, Elicker BM, Ordovas KG, Kohi MP, Nguyen J, Naeger DM. Nonclotted blood patch technique reduces pneumothorax and chest tube placement rates after percutaneous lung biopsies. J Thorac Imaging 2016; 31: 243–6. [DOI] [PubMed] [Google Scholar]
- 47. Tran AA, Brown SB, Rosenberg J, Hovsepian DM. Tract embolization with gelatin sponge slurry for prevention of pneumothorax after percutaneous computed tomography‐guided lung biopsy. Cardiovasc Intervent Radiol 2014; 37: 1546–53. [DOI] [PubMed] [Google Scholar]
- 48. Zaetta JM, Licht MO, Fisher JS, Avelar RL, Bio‐Seal Study Group . A lung biopsy tract plug for reduction of postbiopsy pneumothorax and other complications: Results of a prospective, multicenter, randomized, controlled clinical study. J Vasc Interv Radiol 2010; 21: 1235–43. [DOI] [PubMed] [Google Scholar]
- 49. Sinner WN. Complications of percutaneous transthoracic needle aspiration biopsy. Acta Radiol Diagn (Stockh) 1976; 17: 813–28. [DOI] [PubMed] [Google Scholar]
- 50. Berquist TH, Bailey PB, Cortese DA, Miller WE. Transthoracic needle biopsy: Accuracy and complications in relation to location and type of lesion. Mayo Clin Proc 1980; 55: 475–81. [PubMed] [Google Scholar]
- 51. Glassberg RM, Sussman SK. Life‐threatening hemorrhage due to percutaneous transthoracic intervention: Importance of the internal mammary artery. AJR Am J Roentgenol 1990; 154: 47–9. [DOI] [PubMed] [Google Scholar]
- 52. Yeow KM, See LC, Lui KW et al Risk factors for pneumothorax and bleeding after CT‐guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol 2001; 12: 1305–12. [DOI] [PubMed] [Google Scholar]
- 53. Loh SE, Wu DD, Venkatesh SK et al CT‐guided thoracic biopsy: Evaluating diagnostic yield and complications. Ann Acad Med Singapore 2013; 42: 285–90. [PubMed] [Google Scholar]
- 54. Wang Y, Li W, He X, Li G, Xu L. Computed tomography‐guided core needle biopsy of lung lesions: Diagnostic yield and correlation between factors and complications. Oncol Lett 2014; 7: 288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rizzo S, Preda L, Raimondi S et al Risk factors for complications of CT‐guided lung biopsies. Radiol Med 2011; 116: 548–63. [DOI] [PubMed] [Google Scholar]
- 56. Beslic S, Zukic F, Milisic S. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol Oncol 2012; 46: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fish GD, Stanley JH, Miller KS, Schabel SI, Sutherland SE. Postbiopsy pneumothorax: Estimating the risk by chest radiography and pulmonary function tests. AJR Am J Roentgenol 1988; 150: 71–4. [DOI] [PubMed] [Google Scholar]
- 58. Yuan DM, Lü YL, Yao YW et al Diagnostic efficiency and complication rate of CT‐guided lung biopsy: A single center experience of the procedures conducted over a 10‐year period. Chin Med J (Engl) 2011; 124: 3227–31. [PubMed] [Google Scholar]
- 59. Jae LI, June IH, Miyeon Y, Kwanseop L, Yul L, Hoon BS. Percutaneous core needle biopsy for small (≤ 10 mm) lung nodules: Accurate diagnosis and complication rates. Diagn Interv Radiol 2012; 18: 527–30. [DOI] [PubMed] [Google Scholar]
- 60. Yamaura H, Inaba Y, Arai Y, Matsueda K, Hatooka S. Massive intrathoracic haemorrhage after CT‐guided lung biopsy. Br J Radiol 2000; 73: 1105–7. [DOI] [PubMed] [Google Scholar]
- 61. Hare SS, Gupta A, Goncalves AT et al Systemic arterial air embolism after percutaneous lung biopsy. Clin Radiol 2011; 66: 589–96. [DOI] [PubMed] [Google Scholar]
- 62. Hiraki T, Fujiwara H, Sakurai J et al Nonfatal systemic air embolism complicating percutaneous CT‐guided transthoracic needle biopsy: Four cases from a single institution. Chest 2007; 132: 684–90. [DOI] [PubMed] [Google Scholar]
- 63. Wu YF, Huang TW, Kao CC, Lee SC. Air embolism complicating computed tomography‐guided core needle biopsy of the lung. Interact Cardiovasc Thorac Surg 2012; 14: 771–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cheng HM, Chiang KH, Chang PY et al Coronary artery air embolism: A potentially fatal complication of CT‐guided percutaneous lung biopsy. Br J Radiol 2010; 83: e83–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smit DR, Kleijn SA, de Voogt WG. Coronary and cerebral air embolism: A rare complication of computed tomography‐guided transthoracic lung biopsy. Neth Heart J 2013; 21: 464–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bou‐Assaly W, Pernicano P, Hoeffner E. Systemic air embolism after transthoracic lung biopsy: A case report and review of literature. World J Radiol 2010; 2: 193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim JH, Kim YT, Lim HK, Kim YH, Sung SW. Management for chest wall implantation of non‐small cell lung cancer after fine‐needle aspiration biopsy. Eur J Cardiothorac Surg 2003; 23: 828–32. [DOI] [PubMed] [Google Scholar]
- 68. Ayar D, Golla B, Lee JY, Nath H. Needle‐track metastasis after transthoracic needle biopsy. J Thorac Imaging 1998; 13: 2–6. [DOI] [PubMed] [Google Scholar]
- 69. Wu CC, Maher MM, Shepard JA. Complications of CT‐guided percutaneous needle biopsy of the chest: Prevention and management. AJR Am J Roentgenol 2011; 196: W678–82. [DOI] [PubMed] [Google Scholar]
- 70. Mitchell MJ, Montgomery M, Reiter CG, Culp WC Jr. Pericardial tamponade following CT‐guided lung biopsy. Cardiovasc Intervent Radiol 2008; 31 (Suppl. 2): S227–30. [DOI] [PubMed] [Google Scholar]
- 71. Melloni G, Bandiera A, Crespi G, Zannini P. Intercostal artery pseudoaneurysm after computed tomography‐guided percutaneous fine needle aspiration lung biopsy. J Thorac Imaging 2012; 27: W48–9. [DOI] [PubMed] [Google Scholar]
- 72. Liu A, Southern I, Nicol E. Atrial fibrillation and pneumothorax after transthoracic needle lung biopsy. BMJ Case Rep 2012. 10.1136/bcr.10.2011.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Voravud N, Shin DM, Dekmezian RH, Dimery I, Lee JS, Hong WK. Implantation metastasis of carcinoma after percutaneous fine‐needle aspiration biopsy. Chest 1992; 102: 313–5. [DOI] [PubMed] [Google Scholar]
- 74. Raftopoulos Y, Furey WW, Kacey DJ, Podbielski FJ. Tumor implantation after computed tomography‐guided biopsy of lung cancer. J Thorac Cardiovasc Surg 2000; 119: 1288–9. [DOI] [PubMed] [Google Scholar]
- 75. Kucharczyk W, Weisbrod GL, Cooper JD, Todd T. Cardiac tamponade as a complication of thin‐needle aspiration lung biopsy. Chest 1982; 82: 120–1. [DOI] [PubMed] [Google Scholar]
- 76. Sawabata N, Ohta M, Maeda H. Fine‐needle aspiration cytologic technique for lung cancer has a high potential of malignant cell spread through the tract. Chest 2000; 118: 936–9. [DOI] [PubMed] [Google Scholar]
- 77. Matsuguma H, Nakahara R, Kondo T, Kamiyama Y, Mori K, Yokoi K. Risk of pleural recurrence after needle biopsy in patients with resected early stage lung cancer. Ann Thorac Surg 2005; 80: 2026–31. [DOI] [PubMed] [Google Scholar]
- 78. Inoue M, Honda O, Tomiyama N et al Risk of pleural recurrence after computed tomographic‐guided percutaneous needle biopsy in stage I lung cancer patients. Ann Thorac Surg 2011; 91: 1066–71. [DOI] [PubMed] [Google Scholar]
- 79. Asakura K, Izumi Y, Yamauchi Y et al Incidence of pleural recurrence after computed tomography‐guided needle biopsy in stage I lung cancer. PLoS One 2012; 7: e42043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stewart CJ, Stewart IS. Immediate assessment of fine needle aspiration cytology of lung. J Clin Pathol 1996; 49: 839–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fassina A, Corradin M, Zardo D, Cappellesso R, Corbetti F, Fassan M. Role and accuracy of rapid on‐site evaluation of CT‐guided fine needle aspiration cytology of lung nodules. Cytopathology 2011; 22: 306–12. [DOI] [PubMed] [Google Scholar]
- 82. Austin JH, Cohen MB. Value of having a cytopathologist present during percutaneous fine‐needle aspiration biopsy of lung: Report of 55 cancer patients and metaanalysis of the literature. AJR Am J Roentgenol 1993; 160: 175–7. [DOI] [PubMed] [Google Scholar]
- 83. Pak HY, Yokota S, Teplitz RL, Shaw SL, Werner JL. Rapid staining techniques employed in fine needle aspirations of the lung. Acta Cytol 1981; 25: 178–84. [PubMed] [Google Scholar]
- 84. Miller DA, Carrasco CH, Katz RL, Cramer FM, Wallace S, Charnsangavej C. Fine needle aspiration biopsy: The role of immediate cytologic assessment. AJR Am J Roentgenol 1986; 147: 155–8. [DOI] [PubMed] [Google Scholar]
- 85. Hahn PF, Eisenberg PJ, Pitman MB, Gazelle GS, Mueller PR. Cytopathologic touch preparations (imprints) from core needle biopsies: Accuracy compared with that of fine‐needle aspirates. AJR Am J Roentgenol 1995; 165: 1277–9. [DOI] [PubMed] [Google Scholar]
- 86. Padhani AR, Scott WW Jr, Cheema M, Kearney D, Erozan YS. The value of immediate cytologic evaluation for needle aspiration lung biopsy. Invest Radiol 1997; 32: 453–8. [DOI] [PubMed] [Google Scholar]
- 87. Moghadamfalahi M, Podoll M, Frey AB, Alatassi H. Impact of immediate evaluation of touch imprint cytology from computed tomography guided core needle biopsies of mass lesions: Single institution experience. CytoJournal 2014; 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tachibana K, Nakazato Y, Tsuchida S et al Immediate cytology improves accuracy and decreases complication rate in real‐time computed tomography‐guided needle lung biopsy. Diagn Cytopathol 2013; 41: 1063–8. [DOI] [PubMed] [Google Scholar]
- 89. Tarver RD, Conces DJ Jr. Interventional chest radiology. Radiol Clin North Am 1994; 32: 689–709. [PubMed] [Google Scholar]
- 90. Westcott JL. Percutaneous transthoracic needle biopsy. Radiology 1988; 169: 593–601. [DOI] [PubMed] [Google Scholar]
- 91. Greif J, Marmor S, Schwarz Y, Staroselsky AN. Percutaneous core needle biopsy vs. fine needle aspiration in diagnosing benign lung lesions. Acta Cytol 1999; 43: 756–60. [DOI] [PubMed] [Google Scholar]
- 92. Stanley JH, Fish GD, Andriole JG et al Lung lesions: Cytologic diagnosis by fine‐needle biopsy. Radiology 1987; 162: 389–91. [DOI] [PubMed] [Google Scholar]
- 93. Greene R, Szyfelbein WM, Isler RJ, Stark P, Janstsch H. Supplementary tissue‐core histology from fine‐needle transthoracic aspiration biopsy. AJR Am J Roentgenol 1985; 144: 787–92. [DOI] [PubMed] [Google Scholar]
- 94. Khouri NF, Stitik FP, Erozan YS et al Transthoracic needle aspiration biopsy of benign and malignant lung lesions. AJR Am J Roentgenol 1985; 144: 281–8. [DOI] [PubMed] [Google Scholar]
- 95. Fraser RS. Transthoracic needle aspiration. The benign diagnosis. Arch Pathol Lab Med 1991; 115: 751–61. [PubMed] [Google Scholar]
- 96. Klein JS, Salomon G, Stewart EA. Transthoracic needle biopsy with a coaxially placed 20‐gauge automated cutting needle: Results in 122 patients. Radiology 1996; 198: 715–20. [DOI] [PubMed] [Google Scholar]
- 97. Boiselle PM, Shepard JA, Mark EJ et al Routine addition of an automated biopsy device to fine‐needle aspiration of the lung: A prospective assessment. (Published erratum appears in AJR Am J Roentgenol 1997; 169: 1755.). AJR Am J Roentgenol 1997; 169: 661–6. [DOI] [PubMed] [Google Scholar]
- 98. Moulton JS, Moore PT. Coaxial percutaneous biopsy technique with automated biopsy devices: Value in improving accuracy and negative predictive value. Radiology 1993; 186: 515–22. [DOI] [PubMed] [Google Scholar]
- 99. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for percutaneous transthoracic needle biopsy. Am Rev Respir Dis 1989; 140: 255–6. [DOI] [PubMed] [Google Scholar]
- 100. Poe RH, Kallay MC, Wicks CM, Odoroff CL. Predicting risk of pneumothorax in needle biopsy of the lung. Chest 1984; 85: 232–5. [DOI] [PubMed] [Google Scholar]
- 101. Miller JA, Pramanik BK, Lavenhar MA. Predicting the rates of success and complications of computed tomography‐guided percutaneous core‐needle biopsies of the thorax from the findings of the preprocedure chest computed tomography scan. J Thorac Imaging 1998; 13: 7–13. [DOI] [PubMed] [Google Scholar]
- 102. Ko R, Kenmotsu H, Serizawa M et al Frequency of EGFR T790M mutation and multimutational profiles of rebiopsy samples from non‐small cell lung cancer developing acquired resistance to EGFR tyrosine kinase inhibitors in Japanese patients. BMC Cancer 2016; 16: 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kawamura T, Kenmotsu H, Taira T et al Rebiopsy for patients with non‐small‐cell lung cancer after epidermal growth factor receptor‐tyrosine kinase inhibitor failure. Cancer Sci 2016; 107: 1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yu HA, Arcila ME, Rekhtman N et al Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res 2013; 19: 2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Oxnard GR, Arcila ME, Sima CS et al Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011; 17: 1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tseng JS, Su KY, Yang TY et al The emergence of T790M mutation in EGFR‐mutant lung adenocarcinoma patients having a history of acquired resistance to EGFR‐TKI: Focus on rebiopsy timing and long‐term existence of T790M. Oncotarget 2016; 7: 48059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yoon HJ, Lee HY, Lee KS et al Repeat biopsy for mutational analysis of non‐small cell lung cancers resistant to previous chemotherapy: Adequacy and complications. Radiology 2012; 265: 939–48. [DOI] [PubMed] [Google Scholar]


