Abstract
Nivolumab, an anti‐PD‐1 antibody, has been shown to be effective in many cancers, such as malignant melanoma and lung cancer; however, nivolumab therapy can result in pseudoprogression. Diffuse alveolar hemorrhage (DAH) is persistent or recurrent pulmonary hemorrhage as a result of drugs, autoimmune diseases, or infections. DAH with pseudoprogression during nivolumab administration has rarely been reported. Herein, we describe our experience with one such case. A 41‐year‐old woman exhibited bloody sputum and ground glass opacities in the lungs along with tumor growth during nivolumab therapy for multiple lung metastases of malignant melanoma. We diagnosed DAH with pseudoprogression as a result of nivolumab and administered steroid therapy. The DAH subsequently improved and the tumor shrank. This case illustrates that nivolumab can cause DAH with pseudoprogression, which can be controlled by steroid therapy. Thus, if bloody sputum and ground glass opacities in the lungs are observed with tumor growth during nivolumab administration, steroid therapy should be considered to control DAH with pseudoprogression.
Keywords: Diffuse alveolar hemorrhage, immuno‐checkpoint inhibitor, lung metastasis, nivolumab, pseudoprogression
Introduction
Immune‐checkpoint inhibitors, such as anti‐PD‐1 antibodies, have changed treatment for patients with various cancers. Nivolumab, an anti‐PD‐1 antibody, has been shown to be effective in many cancers, such as malignant melanoma and lung cancer.1, 2, 3 However, its use can result in pseudoprogression, and in some cases, the tumor temporarily increases and then shrinks; therefore, it is difficult to judge whether treatment should be continued.4 In melanoma, pseudoprogression has been observed in 4–8.9% of patients treated with immune‐checkpoint inhibitors.5, 6, 7
Diffuse alveolar hemorrhage (DAH) is persistent or recurrent pulmonary hemorrhage as a result of drugs, autoimmune diseases, or infections.8 Bloody sputum, cough, and respiratory distress are observed in DAH. In chest computed tomography (CT), ground glass opacities (GGO) and consolidations are shown in the lungs.8 Bronchoalveolar lavage (BAL) is useful for diagnosis, and steroid therapy is often performed; however, this may lead to severe respiratory failure and death.9 DAH with pseudoprogression during nivolumab administration has rarely been reported in the literature. Herein, we describe our experience with a 41‐year‐old female patient who developed DAH with pseudoprogression, and provide a literature review.
Case report
A 41‐year‐old woman underwent surgery to treat left femoral malignant melanoma. Two years later, lung metastasis of malignant melanoma was observed. She began treatment with nivolumab (2 mg/kg, every 3 weeks). After one and two months of treatment, the size of the metastatic lung lesions increased slightly and GGOs were faintly observed around the tumor. Notably, although the possibility of pseudoprogression was considered, treatment was continued (Fig 1a–c). Three months after the initiation of treatment, bloody sputum and respiratory distress occurred. On examination, the patient's body temperature was 37.3 °C and oxygen saturation on room air was 93%. Laboratory tests showed a white blood cell count of 11 600/μL with 89% neutrophils and 6% lymphocytes, a lactate dehydrogenase (LDH) level of 818 IU/L (normal < 222 IU/L), a C‐reactive protein level of 11.85 mg/dL, and a KL‐6 level of 106 U/mL (normal < 500 U/mL). On chest CT, an increased number of lung metastatic lesions and GGOs were observed in both lungs. GGOs were found around the lung metastatic lesions, as well as at sites devoid of lesions (Fig 1d). BAL fluid revealed a progressively bloody return from the right upper lobe; analysis of the fluid revealed a cell count of 25.8 × 105 cells/ml (50.6% neutrophils, 32.2% lymphocytes, 15.3% macrophages, and 1.0% eosinophils) (Fig 2). No pulmonary pathogens or serum autoantibodies were identified; moreover, no melanoma cells were detected in the BAL fluid. We diagnosed nivolumab‐induced DAH. Nivolumab was discontinued and methylprednisolone pulse therapy (1 g/day) was administered for three days, followed by prednisolone therapy (40 mg/body).
Figure 1.
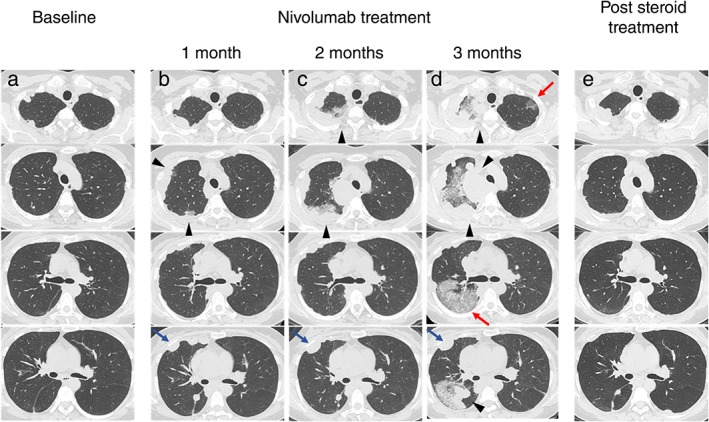
(a) Chest computed tomography showing multiple lung metastases before nivolumab therapy. (b,c) A slight increase in the size of the lung metastatic lesions and the appearance of nearby ground glass opacities (GGOs) (triangle) are observed after one and two months of therapy. A slight increase in the size of lung metastatic lesions without GGOs is also observed (blue arrows) (d) There are multiple lung metastases and increased GGOs (triangles), as well as the emergence of new GGOs in areas without lung metastases (red arrows). (e) Disappearance of GGOs and reduction of multiple lung metastases after steroid therapy.
Figure 2.

Bronchoalveolar lavage fluid showed a progressively bloody return from the right upper lobe.
The GGOs in both lungs disappeared one month after commencing steroids, and prednisolone was gradually reduced over two months. Many of the lung metastases shrank. Five months after commencing nivolumab, the lung metastases worsened and the patient died.
Discussion
This case illustrates that: (i) nivolumab can cause DAH with pseudoprogression, and (ii) DAH with pseudoprogression can be effectively treated with steroid therapy.
DAH occurs as a result of drugs, autoimmune diseases, or infections; bloody sputum is observed in many cases.8 Typical imaging findings include focal or diffuse GGOs and/or consolidations and BAL is performed for definitive diagnosis.8 In this case, bloody sputum and GGOs in both lungs were observed with pseudoprogression during nivolumab administration, and alveolar bleeding was observed in BAL fluid. The test results showed no sign of autoimmune diseases or infections and there was no direct bleeding from the tumor, indicated by shadows in areas without tumors.
In cases of DAH caused by drugs, the causative drug should be discontinued. In addition, if alveolar hemorrhage is associated with immunity, higher doses of methylprednisolone are effective.9 In this case, nivolumab was discontinued, high‐dose methylprednisolone was administered, and the patient improved.
Pseudoprogression is suspected to be a result of the infiltration of immune cells, such as cytotoxic T lymphocytes, around tumors, as well as edema and necrosis.10 It is possible that such an immune reaction causes damage to the small blood vessels of the lung, which leads to the occurrence of DAH. In addition, because GGOs appear at sites where no tumor exists, the immune reaction may also occur at these same sites. This report offers evidence that steroid therapy is effective. If tumor growth and DAH are observed, this may indicate pseudoprogression. During immunotherapy, pseudoprogression is a frustrating clinical problem, and our report may help to solve it.
In conclusion, nivolumab can cause DAH with pseudoprogression, and this condition can be effectively treated with steroid therapy. If bloody sputum and GGOs in the lungs are observed with tumor growth during nivolumab administration, steroid therapy should be considered to control DAH with pseudoprogression.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We would like to thank Editage (http://www.editage.jp) for English language editing.
References
- 1. Robert C, Long GV, Brady B et al Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–30. [DOI] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozaki T, Shindoh J, Miura Y et al Serial pseudoprogression of metastatic malignant melanoma in a patient treated with nivolumab: a case report. BMC Cancer 2017; 17: 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Topalian SL, Sznol M, McDermott DF et al Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32: 1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodi FS, Hwu WJ, Kefford R et al Evaluation of immune‐related response criteria and RECIST v1.1 in patients with advanced melanoma treated with Pembrolizumab. J Clin Oncol 2016; 34: 1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolchok JD, Hoos A, O'Day S et al Guidelines for the evaluation of immune therapy activity in solid tumors: Immune‐related response criteria. Clin Cancer Res 2009; 15: 7412–20. [DOI] [PubMed] [Google Scholar]
- 8. Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest 2010; 2013: 1164–71. [DOI] [PubMed] [Google Scholar]
- 9. Krause ML, Cartin‐Ceba R, Specks U, Peikert T. Update on diffuse alveolar hemorrhage and pulmonary vasculitis. Immunol Allergy Clin North Am 2012; 32: 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiou VL, Burotto M. Pseudoprogression and immune‐related response in solid tumors. J Clin Oncol 2015; 33: 3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


